Abstract
Gastric cancer is a common type of cancer worldwide, and has a poor prognosis, in part due to the low rates of early diagnosis and the limited treatment methods available. Apolipoprotein E (ApoE) is involved in exogenous cholesterol transport and may be important in enabling tumor cells to fulfill their high cholesterol requirements. A number of reports have indicated that ApoE affects the development and prognosis of gastric cancer. Therefore, the aim of the present study was to investigate the genes and transcription factors that interact with ApoE during the development of gastric cancer. Using gene expression profiling, the BioGRID database and the transcriptional regulatory element database, gene expression and regulatory networks in gastric cancer tissues and adjacent normal tissues were analyzed. The data demonstrated that eight genes associated with ApoE were differentially expressed, with six of these upregulated and two downregulated. Functionally, these genes were involved in the JAK-STAT cascade, acute-phase response, acute inflammatory response, and the steroid hormone response. Among these ApoE-associated genes, expression of the signal transducer and activator of transcription 2 (STAT2) and STAT3 transcription factors was upregulated. To the best of our knowledge, this is the first study to demonstrate the network of ApoE-related genes and transcription factors in gastric cancer. Additional studies are required in order to confirm these data and to translate the results into the identification of clinical biomarkers and novel treatment strategies for gastric cancer.
Keywords: gastric cancer, apolipoprotein E, gene expression profile, BioGRID database, transcriptional regulatory element database
Introduction
Gastric cancer is the fourth most common type of cancer and the second-highest cause of cancer-related mortality, worldwide (1). Approximately 8% of newly diagnosed malignant tumors originate in the stomach, and >700,000 people succumb to gastric cancer annually (2). Epidemiological studies have indicated that the risk factors for gastric cancer include family history, age, gender, consumption of salt-preserved foods and dietary nitrites, gastrectomy, Helicobacter pylori (H. pylori) infection, smoking and alcohol. Several treatment modalities for gastric cancer are available, including surgery, chemotherapy, radiotherapy and immunotherapy. However, the prognosis for patients with gastric cancer remains relatively poor. The global five-year survival rate is ~20%, which is likely to be due to diagnosis at an advanced stage of disease and the limited treatment options that are available. Therefore, there is a requirement to identify novel biomarkers to aid with the early diagnosis and treatment of gastric cancer.
Previous studies have demonstrated that aberrant cellular metabolism is a hallmark of tumorigenesis and cancer progression (3–5). Accumulating evidence indicates that in vivo tumors and tumor cell lines undergo abnormal changes in cholesterol metabolism (6,7). In theory, rapidly dividing cancer cells utilize two major mechanisms, in order to fulfill their cholesterol requirements (8). Cellular requirements may be met by either de novo cholesterol biosynthesis, or by uptake of exogenous lipoprotein-associated cholesterol and cholesteryl esters. De novo cholesterol biosynthesis is under tight feedback regulation in normal cells and tissues (9). However, during tumorigenesis, this mechanism is altered, which is likely to be a reflection of the increased cholesterol requirements of actively dividing tumor cells. Cholesterol metabolism shifts during neoplasia, and absorption of exogenous cholesterol increases because it is important for neoplasia due to the increased demand of the tumor cells (10).
Apolipoprotein E (ApoE), secreted by hepatic and extrahepatic cells, affects cholesterol transport, lipid metabolism and protein synthesis, by binding to the low-density lipoprotein receptor and the ApoE receptor on lipid particles. ApoE participates in other cellular functions, including tissue repair, immune response and regulation, and cell growth and differentiation (11,12). Numerous studies have shown that ApoE expression is associated with a number of types of tumors and tumor cell lines (13). Recently, ApoE has been identified as a potential tumor-associated marker for gastric cancer, due to its elevated protein expression relative to that of normal controls (14).
Although ApoE is known to be overexpressed in gastric cancer, its associated genes and transcription factors remain to be identified. The present study used Affymetrix Exon Arrays to identify differential gene expression profiles in gastric cancer tissues and adjacent normal tissues. Transcription factor-gene regulatory networks were constructed through integration of the transcriptional regulatory element database (TRED) (15), the BioGRID database and gene expression profiling, using Cytoscape software version 3.0.1 (Ontario Genomics Institute, Toronto, Canada). ApoE associated genes and its transcription factor-gene regulatory network were systematically identified. The aim of the study was to provide insight into the pathogenesis of gastric cancer, and to identify biomarkers in order to improve the diagnosis and treatment of this disease.
Materials and methods
Sample collection
Five pairs of gastric cancer tissues and adjacent noncancerous tissues were obtained at the First Hospital of Jilin University (Changchun, China). This study was approved by the First Hospital of Jilin University review board and each patient provided written informed consent. All tissues were snap-frozen and stored in liquid nitrogen within 20 min of resection. TNM cancer staging and histological classification were performed by a pathologist, according to the World Health Organization (WHO) criteria (16).
RNA isolation and microarray hybridization and scanning
Briefly, a total of 15 mg RNA was extracted from each tissue sample, using TRIzol™ (Invitrogen Life Technologies, Carlsbad, CA, USA), followed by purification using the RNeasy Mini kit (Qiagen, Düsseldorf, Germany), according to the manufacturer's instructions. The A260/A280 ratio was determined by a UV2800 ultraviolet spectrophotometer (Unico, Dayton, NY, USA). RNA samples with ratios of 1.8–2.0 were considered highly purified.
RNA samples were analyzed using the GeneChip Human Exon 1.0 ST Array (Affymetrix, Santa Clara, CA, USA), according to the protocol detailed in the GeneChip Expression Analysis Technical Manual (P/N 900223). Briefly, 1 mg of RNA template was reverse transcribed into cDNA, followed by endonuclease digestion and labeling with the DNA labeling reagent provided by Affymetrix. The labeled samples were mixed with hybridization cocktail and hybridized to the microarray at 45°C with centrifugation at 1 × g for 17 h. The array was washed and stained on the GeneChip Fluidics Station 450, using the appropriate fluidics solutions, prior to insertion into the Affymetrix autoloader carousel. Arrays were scanned using the GeneChip Scanner 3000 with GeneChip Operating Software. All instruments, chips and reagents were obtained from Affymetrix.
Analysis of differentially expressed genes
In order to analyze the arrays and extract raw signal data, GeneChip Operating Software was used. After importing raw signal data, the Limma algorithm, linear models and empirical Bayes methods were utilized to analyze the data and identify differentially expressed genes. Stringent criteria were used in order to prevent very small fold changes from being judged as differentially expressed as a result of small residual standard deviations. The resulting P-values were adjusted using the Benjamini and Hochberg false discovery rate (BH-FDR) algorithm (17). Gene expression was considered to be significantly different if both FDR values were <0.05, limiting the FDR to ≤5%, and gene expression exhibited a ≥2-fold change between cancer and the corresponding normal tissues; that is, log2FC >1 or log2FC <-1, and P-value <0.05.
Construction of a transcription factor gene network using gene expression profiling, TRED and the BioGRID database
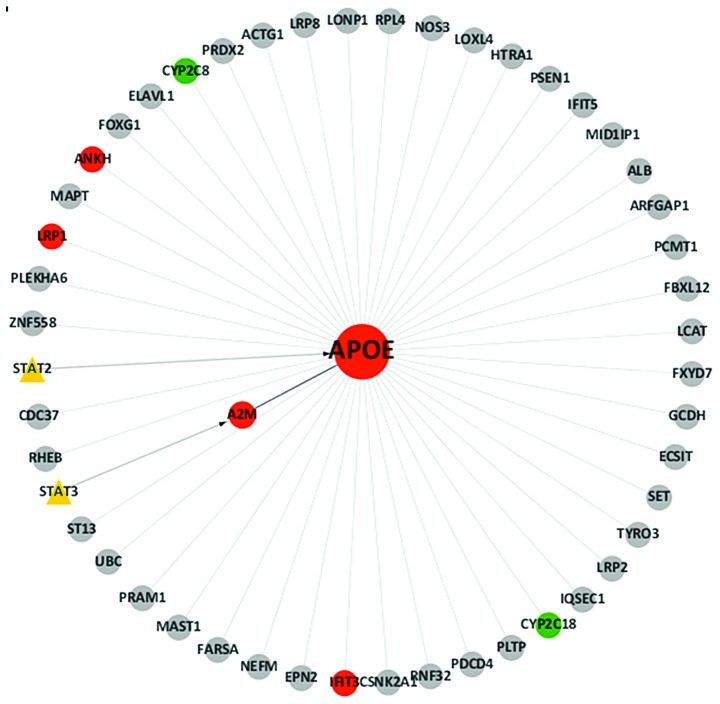
A transcription factor (TF)-gene network was constructed, based on gene expression profiling, TRED and the BioGRID database, using cytoscape software, according to the regulatory interactions and the differential expression values of each TF and gene. Attributing associations among all genes and TFs created the adjacent matrix. The resulting analysis is presented in Fig. 1.
Figure 1.
TF gene network of eight differentially expressed genes related to apolipoprotein E in gastric cancer tissues. Red circles indicate upregulated genes, green circles indicate downregulated genes and grey circles indicate genes with unchanged expression. Yellow triangles indicate TFs. The direction of the arrow is from the regulatory source to the target. TF, transcription factor.
Functional enrichment analysis of genes
The database for annotation, visualization and integrated discovery (DAVID) functional annotation software was applied to analyze the functional enrichment of aberrant genes. The ‘GENETIC_ASSOCIATION_DB_DISEASE_CLASS’ and ‘GENE ONTOLOGY’ options provided information about disease association enrichment and functional enrichment of gene clusters. The ‘GENETIC_ASSOCIATION_DB_DISEASE_CLASS’ was selected in order to identify disease class enrichment, and ‘GOTERM_MF_FAT’ was used to identify functional enrichment, with the Benjamini method for determining a significant enrichment score ≥1.3.
Results and Discussion
In order to identify differentially expressed genes in gastric cancer, Affymatrix Exon Arrays containing 17,800 human genes were utilized, and five pairs of gastric tumor tissues and adjacent normal tissues were analyzed. A total of 1,224 genes demonstrated a ≥2-fold change in expression in tumor tissues relative to that of adjacent normal tissues (data not shown). Among these differentially expressed genes, 730 were upregulated while 495 were downregulated. Specifically, the expression of ApoE was greater (log2FC=1.345) in gastric cancer tissues compared with that adjacent normal tissues (P<0.01). A previous study demonstrated that ApoE was upregulated in gastric cancer tissues, and that its overexpression was associated with a poor prognosis in patients with gastric cancer (18). Therefore, ApoE-associated genes and its regulatory network in gastric cancer tissues, were further investigated using in silico analyses.
In order to identify genes and TFs associated with ApoE-overexpression, the BioGRID database was utilized. Additionally, TRED was used to analyze cis- and trans-regulatory elements that have been identified in mammals. Thus, integration of the analyses from the gene expression profiling, and the BioGRID and TRED databases was used to analyze ApoE-associated genes. The results demonstrated that eight genes, namely signal transducer and activator of transcription 2 (STAT2), STAT3, low-density lipoprotein receptor-related protein 1 (LRP1), α2-macroglobulin (A2M), interferon-induced protein with tetratricopeptide repeats 3 (IFIT3), ankylosis progressive homolog (ANKH), and the cytochrome P450 genes, CYP2C8 and CYP2C18, were associated with ApoE and were differentially expressed, with six of these found to be upregulated and two downregulated (Table I). Ultimately, TF-gene regulatory networks centered on ApoE, were established for the gastric cancer tissues examined in the present study (Fig. 1).
Table I.
Summary of eight differentially expressed genes related to apolipoprotein E in gastric cancer tissues.
| Gene symbol | log2FC | Diseases | Entrez gene name | Location | Family |
|---|---|---|---|---|---|
| STAT2 | 1.205043 | Antigen Presentation, Cancer, Cardiovascular Disease, Connective Tissue Disorders, Dermatological Diseases and Conditions, Endocrine System Disorders, Gastrointestinal Disease, Genetic Disorder, Hematological Disease, Hepatic System Disease, Immunological Disease, Infection Mechanism, Infectious Disease, Inflammatory Disease, Inflammatory Response, Neurological Disease, Ophthalmic Disease, Organismal Injury and Abnormalities, Reproductive System Disease, Respiratory Disease, Skeletal and Muscular Disorders | Signal transducer and activator of transcription 2 1,91 kDa | Nucleus | Transcription regulator |
| STAT3 | 1.179164 | Antigen Presentation, Cancer, Cardiovascular Disease, Connective Tissue Disorders, Dermatological Diseases and Conditions, Endocrine System Disorders, Gastrointestinal Disease, Genetic Disorder, Hematological Disease, Hepatic System Disease, Immunological Disease, Infection Mechanism, Infectious Disease, Inflammatory Disease, Inflammatory Response, Neurological Disease, Ophthalmic Disease, Organismal Injury and Abnormalities, Reproductive System Disease, Respiratory Disease, Skeletal and Muscular Disorders | Signal transducer and activator of transcription 3 1,91 kDa | Nucleus | Transcription regulator |
| A2M | 1.450473 | Antigen Presentation, Endocrine System Disorders, Genetic Disorder, Infectious Disease, Inflammatory Disease, Inflammatory Response, Metabolic Disease, Neurological Disease | α2-macroglobulin | Extracellular space | Transporter |
| IFIT3 | 1.575557 | Cancer, Organismal Injury and Abnormalities, Reproductive System Disease | Interferon-induced protein with tetratricopeptide repeats 3 | Cytoplasm | Other |
| ANKH | 1.040222 | Connective Tissue Disorders, Developmental Disorder, Genetic Disorder, Inflammatory Disease, Organismal Injury and Abnormalities, Skeletal and Muscular Disorders | Ankylosis, progressive homolog (mouse) | Plasma membrane | Transporter |
| LRP1 | 1.320753 | Antigen Presentation, Cancer, Cardiovascular Disease, Endocrine System Disorders, Genetic Disorder, Hematological Disease, Inflammatory Response, Metabolic Disease, Neurological Disease, Nutritional Disease, Organismal Injury and Abnormalities, Psychological Disorders, Skeletal and Muscular Disorders | Low density lipoprotein-related protein 1 (α2-macroglobulin receptor) | Plasma membrane | Transmembrane receptor |
| CYP2C8 | −1.774129 | (Unknown or not applicable) | Cytochrome P450, family 2, subfamily C, polypeptide 8 | Cytoplasm | Enzyme |
| CYP2C18 | −2.802344 | (Unknown or not applicable) | Cytochrome P450, family 2, subfamily C, polypeptide 18 | Cytoplasm | Enzyme |
In addition, DAVID was utilized to provide a functional enrichment analysis of these eight differentially expressed genes. Using DAVID, the JAK-STAT cascade, acute-phase response, acute inflammatory response and steroid hormone response genes were identified as significantly enriched (Table II). These eight aberrantly expressed genes were categorized into immune, pharmacogenomic, metabolic and cardiovascular disease classes (Table III).
Table II.
Functional enrichment analysis of genes in the regulatory network.
| Category | Term | P-value | Genes | Fold enrichment | Benjamini |
|---|---|---|---|---|---|
| GO:0007259 | JAK-STAT cascade | 1.4×10−2 | STAT3, STAT2 | 115.62 | 8.8×10−1 |
| GO:0006953 | Acute-phase response | 1.5×10−2 | A2M, STAT3 | 112.73 | 6.6×10−1 |
| GO:0002526 | Acute inflammatory response | 3.6×10−2 | A2M, STAT3 | 46.014 | 8.3×10−1 |
| GO:0048545 | Steroid hormone response | 6.9×10−2 | A2M, STAT3 | 23.4 | 9.3×10−1 |
STAT, signal transducer and activation of transcription; A2M, α2-macroglobulin.
Table III.
Disease class enrichment analysis of genes in the TF-gene regulatory network.
| Term | P-value | Genes | Fold enrichment | Benjamini |
|---|---|---|---|---|
| Immune | 5.8×10−2 | A2M, LRP1, ANKH, STAT3 | 3.053 | 3.0×10−1 |
| Pharmacogenomic | 6.0×10−2 | LRP1, CYP2C8, STAT3 | 5.5436 | 2.2×10−1 |
| Metabolic | 6.6×10−2 | A2M, LRP1, CYP2C8, ANKH | 2.9038 | 1.8×10−1 |
| Cardiovascular | 2.5×10−3 | A2M, LRP1, CYP2C8, ANKH, STAT3 | 0.02926 | 2.9×10−2 |
TF, transcription factor; A2M, α2-macroglobulin; LRP1, low-density lipoprotein receptor-related protein 1; ANKH, ankylosis progressive homolog; STAT, signal transducer and activator of transcription; CYP, cytochrome P450 enzyme.
LRP1, also termed ApoE-specific lipoprotein receptor, or ApoE receptor, is a cell-surface protein that is involved in the metabolism of cholesterol, by mediating the endocytosis of ApoE-containing lipoproteins from plasma into cells. ApoE secreted from cancer cells, suppresses invasion and metastatic endothelial recruitment by engaging LRP1 and LRP8 receptors, respectively. The function of LRP1 in the regulation of tumor growth has been well documented. Studies have shown that increased LRP1 expression correlates with high levels of invasion (19) and, conversely, silencing of LRP1 prevents the spread of malignant cells (20). In gliomas, LRP1 expression in tumors greatly exceeded that in normal brain tissues (21) and its expression was found to be correlated with tumor aggressiveness (22). The abundant expression of LRP1 mRNA suggests that it may be involved in the uptake of ApoE phospholipid discoidal particles or ApoE-enriched high-density lipoprotein in gastric cancer.
LRP1 is an A2M receptor. Recent studies have demonstrated that A2M may also regulate cell signal transduction via LRP1 (23–25). A2M is responsible for the binding and inactivation of plasma proteases, as well as the transport of various cytokines, growth factors and hormones (26). ApoE is non-covalently bound to A2M in human plasma, and therefore, the present in silico analysis of ApoE binding may provide insights into the pathogenic and intracellular role of ApoE in cancer cells. In addition, A2M has previously been reported as a candidate biomarker for the early diagnosis in numerous types of cancers, including gastric cancer (27–29).
Additional genes, including IFIT3, ANKH, CYP2C18 and CYP2C8, were demonstrated to be correlated with ApoE expression in the present study, and have also previously been linked to ApoE expression in Alzheimer's disease (30). IFIT3 inhibits cell migration and shows marked antiproliferative effects (31). Overexpression of IFIT3 has been shown to induce tumor proliferation, angiogenesis and chemoresistance in pancreatic carcinoma cells (32). ANKH is a transmembrane protein that transports intracellular pyrophosphate to the extracellular milieu (33) and has been demonstrated to be overexpressed in bladder cancer (34) and small cell lung cancer cell lines (35). CYP450 has been shown to be downregulated in hepatocytes in response to inflammation and infection (36). Local chronic inflammation is hypothesized to contribute to tumorigenesis, particularly in gastric cancer that is associated with H. pylori infection (37). The present study also indicated that CYP2C18 and CYP2C8 were downregulated in gastric cancer tissues. A separate study demonstrated that CYP2C18 was associated with the development of gastric cancer (38).
In order to identify ApoE regulatory TFs, TRED was used. The results demonstrated that ApoE may be regulated directly by STAT2, or indirectly by STAT3. In order to gain an improved understanding of the regulatory network, a brief framework of the network was configured (Fig. 1). STAT2 and STAT3 are members of the signal transducer and activator of transcription family. STAT3 is involved in selectively inducing and maintaining a procarcinogenic inflammatory microenvironment that promotes tumor cell transformation (39). The JAK-STAT signaling pathway is known to regulate genes that are involved in the regulation of cell proliferation, differentiation and apoptosis, by transducing signals from the cell membrane to the nucleus (40). Targeting the JAK-STAT3 signaling pathway, and specifically STAT3, has been hypothesized to be a potential therapeutic strategy for cancer (41). STAT2 has been identified as a novel contributor to carcinogensis, and may increase the gene expression and secretion of proinflammatory mediators, thereby activating the oncogenic STAT3 signaling pathway (42). The current data demonstrated significantly increased levels of STAT3 and STAT2 in gastric cancer tissues. However, further investigation of the importance of JAK-STAT activation in this disease is required.
The present study demonstrated that a combination of interaction discovery experiments and computational analyses from diverse biological data, may help to identify causative genes in gastric cancer. In particular, ApoE as was identified as a potential biomarker of this disease. Although further studies are required, these findings indicate a role for ApoE in the development of gastric cancer.
Acknowledgements
The authors would like to thank all those who participated in this study. Particular thanks is extended to Dr Quan Lin (The First Hospital of Jilin University), for assistance with sample collection and to the five anonymous patients involved in this study. The authors would also like to thank Medjaden Bioscience ltd. for editing and proofreading this manuscript.
References
- 1.Cui J, Chen Y, Chou WC, Sun L, Chen L, Suo J, Ni Z, Zhang M, Kong X, Hoffman LL, et al. An integrated transcriptomic and computational analysis for biomarker identification in gastric cancer. Nucleic Acids Res. 2011;39:1197–1207. doi: 10.1093/nar/gkq960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee HJ, Song IC, Yun HJ, Jo DY, Kim S. CXC chemokines and chemokine receptors in gastric cancer: From basic findings towards therapeutic targeting. World J Gastroentero. 2014;20:1681–1693. doi: 10.3748/wjg.v20.i7.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Hsu PP, Sabatini DM. Cancer Cell Metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Ríos-Marco P, Martín-Fernández M, Soria-Bretones I, Ríos A, Carrasco MP, Marco C. Alkylphospholipids deregulate cholesterol metabolism and induce cell-cycle arrest and autophagy in U-87 MG glioblastoma cells. Biochim Biophys Acta. 2013;1831:1322–1334. doi: 10.1016/j.bbalip.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Pussinen PJ, Karten B, Wintersperger A, Reicher H, McLean M, Malle E, Sattler W. The human breast carcinoma cell line HBL-100 acquires exogenous cholesterol from high-density lipoprotein via CLA-1 (CD-36 and LIMPII analogous 1)-mediated selective cholesteryl ester uptake. Biochem J. 2000;349:559–566. doi: 10.1042/0264-6021:3490559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Weille J, Fabre C, Bakalara N. Oxysterols in cancer cell proliferation and death. Biochem Pharmacol. 2013;86:154–160. doi: 10.1016/j.bcp.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Tosi MR, Tugnoli V. Cholesteryl esters in malignancy. Clin Chim Acta. 2005;359:27–45. doi: 10.1016/j.cccn.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein JL, Brown MS. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- 10.Coleman PS, Chen LC, Sepp-Lorenzino L. Cholesterol Metabolism and Tumor Cell Proliferation. In: Bittman R, editor. Subcellular Biochemistry. Vol. 28. Plenum Press; New York: 1997. pp. 363–435. (Cholesterol: Its Functions and Metabolism in Biology and Medicine). [DOI] [PubMed] [Google Scholar]
- 11.Weisgraber KH, Rall SC, Jr, Mahley RW. Human E apoprotein heterogeneity. Cysteine-arginine interchanges in the amino acid sequence of the apo-E isoforms. J Biol Chem. 1981;256:9077–9083. [PubMed] [Google Scholar]
- 12.Rall SC, Jr, Weisgraber KH, Innerarity TL, Mahley RW. Structural basis for receptor binding heterogeneity of apolipoprotein E from type III hyperlipoproteinemic subjects. Proc Natl Acad Sci USA. 1982;79:4696–4700. doi: 10.1073/pnas.79.15.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Roz A, Bard JM, Valin S, Huvelin JM, Nazih H. Macrophage apolipoprotein E and proliferation of MCF-7 breast cancer cells: Role of LXR. Anticancer Res. 2013;33:3783–3789. [PubMed] [Google Scholar]
- 14.Sakashita K, Tanaka F, Zhang X, Mimori K, Kamohara Y, Inoue H, Sawada T, Hirakawa K, Mori M. Clinical significance of ApoE expression in human gastric cancer. Oncol Rep. 2008;20:1313–1319. [PubMed] [Google Scholar]
- 15.Jiang C, Xuan Z, Zhao F, Zhang MQ. TRED: A transcriptional regulatory element database, new entries and other development. Nucleic Acids Res. 2007;35(Database Issue):D137–D140. doi: 10.1093/nar/gkl1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zurleni T, Gjoni E, Ballabio A, Casieri R, Ceriani P, Marzoli L, Zurleni F. Sixth and seventh tumor-node-metastasis staging system compared in gastric cancer patients. World J Gastrointest Surg. 2013;5:287–293. doi: 10.4240/wjgs.v5.i11.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan YD, Xu H. A general method for accurate estimation of false discovery rates in identification of differentially expressed genes. Bioinformatics. 2014;30:2018–2025. doi: 10.1093/bioinformatics/btu124. [DOI] [PubMed] [Google Scholar]
- 18.De Feo E, Simone B, Persiani R, Cananzi F, Biondi A, Arzani D, Amore R, D'Ugo D, Ricciardi G, Boccia S. A case-control study on the effect of Apolipoprotein E genotypes on gastric cancer risk and progression. BMC Cancer. 2012;12:494. doi: 10.1186/1471-2407-12-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song H, Li Y, Lee J, Schwartz AL, Bu G. Low-density lipoprotein receptor-related protein 1 promotes cancer cell migration and invasion by inducing the expression of matrix metalloproteinases 2 and 9. Cancer Res. 2009;69:879–886. doi: 10.1158/0008-5472.CAN-08-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dedieu S, Langlois B, Devy J, Sid B, Henriet P, Sartelet H, Bellon G, Emonard H, Martiny L. LRP-1 silencing prevents malignant cell invasion despite increased pericellular proteolytic activities. Mol Cell Biol. 2008;28:2980–2995. doi: 10.1128/MCB.02238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopes MB, Bogaev CA, Gonias SL, VandenBerg SR. Expression of alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein is increased in reactive and neoplastic glial cells. FEBS Lett. 1994;338:301–305. doi: 10.1016/0014-5793(94)80288-2. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M, Ikeda K, Ohshima K, Tsugu H, Kimura H, Tomonaga M. Increased expression of low density lipoprotein receptor-related protein/alpha2-macroglobulin receptor in human malignant astrocytomas. Cancer Res. 1997;57:2799–2805. [PubMed] [Google Scholar]
- 23.Barcelona PF, Luna JD, Chiabrando GA, Juarez CP, Bhutto IA, Baba T, McLeod DS, Sánchez MC, Lutty GA. Immunohistochemical localization of low density lipoprotein receptor-related protein 1 and alpha (2)-Macroglobulin in retinal and choroidal tissue of proliferative retinopathies. Exp Eye Res. 2010;91:264–272. doi: 10.1016/j.exer.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonacci GR, Cáceres LC, Sánchez MC, Chiabrando GA. Activated alpha(2)-macroglobulin induces cell proliferation and mitogen-activated protein kinase activation by LRP-1 in the J774 macrophage-derived cell line. Arch Biochem Biophys. 2007;460:100–106. doi: 10.1016/j.abb.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Qiu Z, Strickland DK, Hyman BT, Rebeck GW. alpha 2-Macroglobulin exposure reduces calcium responses to N-methyl-D-aspartate via low density lipoprotein receptor-related protein in cultured hippocampal neurons. J Biol Chem. 2002;277:14458–14466. doi: 10.1074/jbc.M112066200. [DOI] [PubMed] [Google Scholar]
- 26.Andersson M, Jönsson U, Olsson A. A slow form of alpha-2-macroglobulin in diseased and healthy dogs. J Comp Pathol. 2002;127:37–44. doi: 10.1053/jcpa.2002.0564. [DOI] [PubMed] [Google Scholar]
- 27.Hudler P, Kocevar N, Komel R. Proteomic approaches in biomarker discovery: New perspectives in cancer diagnostics. ScientificWorldJournal. 2014;2014:260348. doi: 10.1155/2014/260348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahn HS, Shin YS, Park PJ, Kang KN, Kim Y, Lee HJ, Yang HK, Kim CW. Serum biomarker panels for the diagnosis of gastric adenocarcinoma. Br J Cancer. 2012;106:733–739. doi: 10.1038/bjc.2011.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanas JS, Hocker JR, Cheung JY, Larabee JL, Lerner MR, Lightfoot SA, Morgan DL, Denson KD, Prejeant KC, Gusev Y, et al. Biomarker identification in human pancreatic cancer sera. Pancreas. 2008;36:61–69. doi: 10.1097/mpa.0b013e3180d0a738. [DOI] [PubMed] [Google Scholar]
- 30.Soler-López M, Zanzoni A, Lluís R, Stelzl U, Aloy P. Interactome mapping suggests new mechanistic details underlying Alzheimer's disease. Genome Res. 2011;21:364–376. doi: 10.1101/gr.114280.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fensterl V, Sen GC. The ISG56/IFIT1 gene family. J Interf Cytok Res. 2011;31:71–78. doi: 10.1089/jir.2010.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camaj P, Ischenko I, Seeliger H, Arnold G, Jauch KW, Bruns CJ. Overexpression of the gene IFIT3 enhances tumor growth, angiogenesis, metastasing and chemoresistance of the pancreas carcinoma cells. In: Schumpelick V, Bruch HP, Schackert HK, editors. Deutsche Gesellschaft für Chirurgie. Vol. 38. Springer; Berlin Heidelberg: 2009. pp. 17–18. (Chirurgisches Forum und DGAV Forum 2009). [Google Scholar]
- 33.Kirsch T, Kim HJ, Winkles JA. Progressive ankylosis gene (ank) regulates osteoblast differentiation. Cells Tissues Organs. 2009;189:158–162. doi: 10.1159/000151725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng M, Simon R, Mirlacher M, Maurer R, Gasser T, Forster T, Diener PA, Mihatsch MJ, Sauter G, Schraml P. TRIO amplification and abundant mRNA expression is associated with invasive tumor growth and rapid tumor cell proliferation in urinary bladder cancer. Am J Pathol. 2004;165:63–69. doi: 10.1016/S0002-9440(10)63275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang A, Zheng C, Hou M, Lindvall C, Wallin KL, Angström T, Yang X, Hellström AC, Blennow E, Björkholm M, et al. Amplification of the telomerase reverse transcriptase (hTERT) gene in cervical carcinomas. Genes Chromosomes Cancer. 2002;34:269–275. doi: 10.1002/gcc.10071. [DOI] [PubMed] [Google Scholar]
- 36.Aitken AE, Morgan ET. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos. 2007;35:1687–1693. doi: 10.1124/dmd.107.015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaoka Y, Graham DY. Helicobacter pylori virulence and cancer pathogenesis. Future Oncol. 2014;10:1487–1500. doi: 10.2217/fon.14.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu K, Chen F. Identification of significant pathways in gastric cancer based on protein-protein interaction networks and cluster analysis. Genet Mol Biol. 2012;35:701–708. doi: 10.1590/S1415-47572012005000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saturnino C, Palladino C, Napoli M, Sinicropi MS, Botta A, Sala M, Carcereri de Prati A, Novellino E, Suzuki H. Synthesis and biological evaluation of new N-alkylcarbazole derivatives as STAT3 inhibitors: Preliminary study. Eur J Med Chem. 2013;60:112–119. doi: 10.1016/j.ejmech.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Silver-Morse L, Li WX. JAK-STAT in heterochromatin and genome stability. JAKSTAT. 2013;2:e26090. doi: 10.4161/jkst.26090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bournazou E, Bromberg J. Targeting the tumor microenvironment: JAK-STAT3 signaling. JAKSTAT. 2013;2:e23828. doi: 10.4161/jkst.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gamero AM, Young MR, Mentor-Marcel R, Bobe G, Scarzello AJ, Wise J, Colburn NH. STAT2 contributes to promotion of colorectal and skin carcinogenesis. Cancer Prev Res (Phila) 2010;3:495–504. doi: 10.1158/1940-6207.CAPR-09-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]