Abstract
The involvement of the central nervous system (CNS) is rare in acute promyelocytic leukemia (APL). The present study reported the case of a 34-year-old male patient with APL that possessed a rare point mutation (p.Asn841Gly, c.2523C>A) in the tyrosine kinase domain of the FMS-like tyrosine kinase 3 (FLT3) gene and a novel Wilm tumor gene mutation (c.1209_1210insT/p.K404X). The patient suffered central nervous system and systemic relapses twice during systemic and intrathecal chemotherapy. At present, the patient is undergoing alternative induction and consolidation therapies, including the administration of FLT3 inhibitor, tetraarsenic tetrasulfide and novel cytotherapy, and is prepared for salvage allogeneic hematopoietic stem cell transplantion (allo-HSCT). The present study indicated that patients with APL that are at a high risk of relapse and unfavorable gene mutations should receive immediate allo-HSCT, whenever possible.
Keywords: acute promyelocytic leukemia, central nervous system relapse, multiple relapses, FLT3-TKD mutation, WT1 mutation, prognosis
Introduction
Acute promyelocytic leukemia (APL) is a distinctive subtype of acute myeloid leukemia that exhibits specific cytogenetic features characterized by the reciprocal translocation between chromosomes 15 and 17, which leads to the formation of the PML-RARα fusion gene. With the encoded gene product, this fusion gene interferes with the maturation process of myeloid cells and finally results in phenotypic alterations in the target cells. Prior to receiving all-trans retinoic acid (ATRA)-based regimens, the complete remission rate for APL is 75–80% and the five year disease-free survival rate is 35–45% (1). Despite a high complete remission rate of 95–95% and significant improvement in the five-year disease free survival rate (to 74%) of patients with APL following treatment with ATRA and arsenic trioxide (ATO) (1), certain patients inevitably relapse, even subsequent to consolidation or maintenance therapies. Similar to other variants of myeloid leukemia, the most frequent location of relapse for APL is the bone marrow (BM); however, an increasing frequency of extramedullary (EM) recurrence, mainly in the CNS and skin, has been reported (2). Factors associated with CNS relapse include advanced patient age, B-cell antigen receptor isoform (3), development of differentiation syndrome, presence of leukocytosis at presentation and occurrence of hemorrhage in the CNS during induction therapy (3). The present study reports the case of a patient with APL.
The patient exhibited a rare point mutation (p.Asn841Gly, c.2523C>A) in the tyrosine kinase domain (TKD) of the FMS-like tyrosine kinase 3 (FLT3-TKD) gene and a novel Wilm tumor (WT1) gene mutation (c.1209_1210insT/p.K404X). The patient suffered two CNS and systemic relapses during systemic and intrathecal chemotherapy.
Case report
A 34-year-old Chinese man presented to the Department of Hematology of West China Hospital, Sichuan University (Chengdu, China) with a fever and chills in July 2011. The initial laboratory evaluations revealed a white blood cell (WBC) count of 55.61×109 cells/l (normal range, 4–10×109 cells/l), with 85% abnormal promyelocytes (normal proportion, 0%), a hemoglobin (HB) concentration of 111 g/l (normal range, 120–160 g/l) and a platelet (PLT) count of 62×109 cells/l (normal range, 100–300×109 cells/l). The prothrombin time (PT; normal range, 9.6–12.8 sec) and activated partial thromboplastin time (APTT; normal range, 20–40 sec) at the time of admission were 17.2 sec and 23.6 sec, respectively. The PT international normalized ratio (PT INR) was 1.61 (normal range, 0.86–1.14) and the thrombin time (TT) was 24.7 sec (normal range, 14–22 sec). The fibrinogen level (FIB) was 0.65 g/l (normal range, 2–4g/l) and the D-dimer value was 35.56 mg/l fibrinogen equivalent units (FEU; normal range, <0.55mg/l FEU), which indicated the presence of disseminated intravascular coagulation (DIC). The BM aspirate containing hypergranular promyelocytes was consistent with the diagnosis of APL. Immunophenotyping of the BM sample revealed that leukemic cells were positive for the expression of cluster of differentiation (CD)13, CD33 and CD117, but did not express CD34, CD14, CD36, CD5, CD7, CD56 or CD19. Human leukocyte antigen-DR and CD64 were expressed at a low level. The gene mutational analysis revealed the presence of the PML-RARα fusion gene, which was the result of a t (15;17) translocation.
The (ATRA; 20 mg twice a day) therapy was initiated immediately subsequent to the suspected diagnosis of APL. The presence of the PML-RARα fusion gene was then rapidly confirmed in the BM sample. Daunorubicin (DNR; 60 mg/d for 3 consecutive days) and ATO (10 mg/d for 22 days) were added to the induction therapy regimen. With nearly 40 days of induction therapy, the BM aspirate indicated morphological complete remission, but the PML-RARα fusion gene remained detectable. Following an additional two cycles of the standard darubicin and Ara-C (IDA) regimen (20 mg idarubicin on day 1 and10 mg idarubicin on days 2 and 3; 150 mg Ara-C on days 1–7) and one cycle of the intermediate-dose Ara-C regimen (40 mg DNR on days 1 and 2; 1,800 mg Ara-C on days 1–4) as consolidation therapy, the patient achieved complete molecular remission. Subsequently, regimens containing ATO (10 mg/day ATO for 14 consecutive days), ATRA (10 mg ATRA three times a day for 28 days), 6-mercaptopurine (6-MP; 50 mg 6-MP three times a day, for 28 days) and methotrexate (MTX; 15 mg MTX weekly for 4 weeks) were administered in succession as maintenance therapy every three months. The cerebrospinal fluid (CSF) was also monitored and intrathecal prophylaxis of dexamethasone, MTX or Ara-C was performed regularly. Although allogenic hematopoietic stem cell transplantation (allo-HSCT) was then considered as an appropriate approach to improve the outcome of the patient, a fully matched unrelated donor was not available and the patient was unwilling to undergo haplo-identical transplantation.
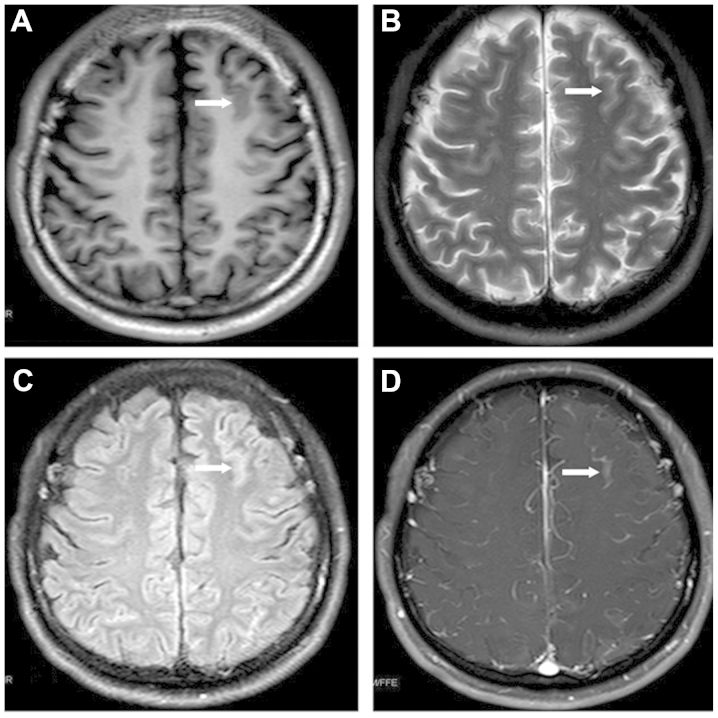
Routine CSF monitoring 20 months subsequent to the diagnosis of APL revealed an elevated nucleated cell count (526×106 cells/l) and an increased CSF total protein level (0.52 g/l), with an elevated CSF pressure of 240 mm H2O. The atypical promyelocytes that expressed CD117 and CD33, but did not express CD5, were detected by flow cytometric analysis. The patient exhibited no symptoms of CNS involvement at that time. Magnetic resonance imaging (MRI) revealed leukemic linear infiltration at the frontal lobe (Fig. 1) and cerebellar tonsillar hernia. The patient was therefore treated with 4 cycles of intrathecal chemotherapy composed of 5 mg dexamethasone, 15 mg MTX and 50 mg Ara-C, and 14 cycles of whole-brain and spinal cord radiotherapy, followed by one cycle of the intermediate-dose Ara-C regimen (10 mg idarubicin on days 1 and 2; 1,500 mg Ara-C on days 1–4). CSF analysis eventually revealed a normal result, and the blood and BM examinations also indicated complete hematological remission at that time. Within three months of the CNS relapse and the administration of the corresponding therapies, the bone marrow aspirate indicated complete molecular remission, with the PML-RARα fusion gene being undetectable. MRI revealed that the lesions within the frontal lobe had diminished.
Figure 1.
Magnetic resonance imaging revealing the leukemic linear infiltration in the frontal lobe that was detected in the present patient with acute promyelocytic leukemia (arrows). (A) T1WI; (B) T2WI; (C) fluid attenuation inversion recovery; (D) gadolinium-enhanced. WI, weighted image.
However, the patient developed systemic relapse with persistent hyperleukocytosis and DIC only five months subsequent to the first CNS relapse. Laboratory tests revealed a WBC count of 1.59×109 cells/l with 75% abnormal promyelocytes, APTT of 68.7 sec, TT of 27.6 sec, FIB level of 0.73 g/l, and D-dimer value of 29.73 mg/l FEU. The administration of 20 mg DNR daily for 6 consecutive days, 20 mg ATRA twice a day, and 10 mg ATO daily was used as combined re-induction therapy, and the patient achieved complete hematological remission again in almost one month, demonstrating normal blood and BM tests and the PML-RARα fusion gene was undetectable again. The patient subsequently received four cycles of consolidation therapy, including the standard IDA regimen and routine intrathecal chemotherapy.
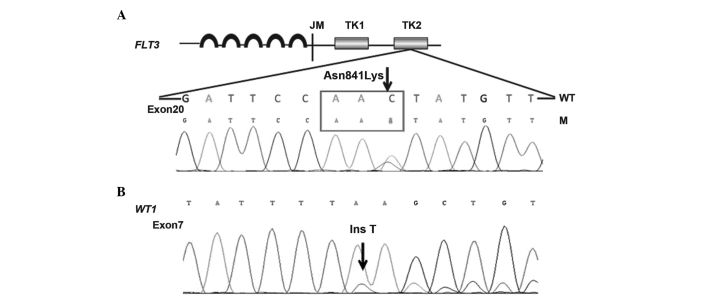
Possible genetic changes were suspected due to the CNS and systemic relapses observed in the present patient. Therefore, 11 prognosis-associated genes, consisting of CEBPA, DNAT3A, FLT3-ITD/TKD, IDH1, IDH2, KIT, KRAS, NPM1, NRAS, TET2 and WT1, were retrospectively analyzed. A rare point mutation in the FLT3-TKD gene (p.Asn841Gly, c.2523C>A) and a novel mutation of the WT1 gene (c.1209_1210insT/p.K404X) were detected in the preserved bone marrow and blood samples of the patient throughout the course of disease (Fig. 2).
Figure 2.
(A) FLT3-TK domain mutation (p.Asn841Gly, c.2523C>A) and (B) WT1 mutation (c.1209_1210insT/p.K404X) were detected in the peripheral blood and bone marrow samples. The double peaks (arrows) demonstrated the point and insertion mutations. FLT3, FMS-like tyrosine kinase 3; TK, tyrosine kinase; WT1, Wilm tumor; WT, wild type; M, mutation; JM, juxtamembrane; Ins T, insertion T basic group.
The patient suffered systemic relapse and DIC again 16 months after the intial CNS relapse. Laboratory tests revealed a HB level of 126 g/l, PLT count of 36×109 PLT/l, WBC count of 2.43×109 cells/l, PT of 18.5 sec, PT INR of 1.61, APTT of 41.9 sec, TT of 36.7 sec, FIB level of 0.50 g/l and D-dimer value of 38 mg/l FEU. The BM smear revealed that 17.5% of promyelocytic cells were abnormal, with a PML-RARα gene expression rate of 16.39%. The patient received combined re-induction therapy regimens consisting of 10 mg IDA on days 1–4, 20 mg ATRA twice a day, and 10 mg ATO once a day for nearly 40 days. The patient achieved hematological remission after this time period, with the PML-RARα gene being expressed in 12.81% of BM cells and no symptoms of CNS involvement being exhibited. However, routine CSF examination revealed an elevated nucleated cell count of 185×106 cells/l, and 37.15% of cells were revealed to possess the immunophenotype of abnormal promyelocytes, as determined by flow cytometry. Considering the relapse and refractory character of the present patient with APL and the possible resistance of the APL to conventional therapy, alternative induction and consolidation treatments composed of targeted FLT3 inhibitor, tetraarsenic tetrasulfide, intrathecal chemotherapy and novel cytotherapy were administered in attempt to achieve an improved therapeutic response. At present, the patient is being prepared for ensuing salvage allo-HSCT.
Discussion
The long-term survival of the majority of patients with APL improves subsequent to ATRA and ATO mediated treatment, but certain patients inevitably relapse during the follow-up period. The majority of post-remission relapses occur in the bone marrow; however, ~3% present at EM sites, mainly the CNS and skin (2). CNS relapse associated factors include advanced age, specific BCR isoform (3), development of differentiation syndrome, leukocytosis at presentation and hemorrhage in the CNS during induction therapy (3). In previous studies, treatment of APL with ATRA (4) and the occurrence of retinoic acid syndrome (5) were considered to predispose patients to EM relapse, but this was not reported by other studies (6). Additionally, an insufficient ATRA dose may fail to yield an effective concentration in the EM sites, which is also a possible contributor to subsequent relapse (7). With respect to potential risk factors, certain studies have indicated that FLT3-ITD mutations and an increased expression of adhesion molecules, including CD56 (3), may promote leukemic infiltration in the CNS. In the present study, although the notable leukocytosis at presentation may be responsible for the EM recurrence in the current patient, the potential impact on the clinicobiological characteristics of APL from two identified genetic mutations also requires consideration.
FLT3, also termed CD135, is a member of the class III tyrosine kinase receptor family, which also includes c-FMS, c-KIT and PDGFR. FLT3 plays an important role in hematopoietic malignancies and the FLT3 gene encodes a 993-amino acid protein in humans, which comprises an immunoglobulin-like extracellular ligand-binding domain, a transmembrane domain, a juxtamembrane dimerization domain and a cytoplasmic domain with a split tyrosine kinase motif. Two major forms of gain-of-function mutations of FLT3, internal tandem duplication (ITD) and point mutations within the activation loop of the tyrosine kinase domain (TKD), were found in 21–38% and 9–20% of APL cases, respectively (8–13). Among these mutations, Asp835Tyr (D835Y) is the most frequent FLT3-TKD mutation [13.2% in monocytic variant (M5); 11.8% in micro granular variant (M3v)] and results in constitutive tyrosine phosphorylation, which boosts neoplastic proliferation (14). In contrast to common mutations, the rare mutation Asn841Gly (A841G), which is located in the activation loop of FLT3-TKD2, was detected in the present study. The activation loop usually forms a hydrogen bond network together with adjacent amino acid residues; however, the impact of Asn841Gly on the conformational change of the activation loop, which leads to phosphatase activity variation in FLT3-TKD has not been elucidated. In addition, a novel WT1 mutation (c.1209_1210insT/p.K404X), was also detected in the present patient, but little is known about the prevalence of WT1 mutations and their significance in APL; limited information was found in a previous study, in which 4 out of 103 APL patients carrying WT1 mutations were predisposed to leukemia relapse (15). Although WT1 mutations act as well-recognized indicators of poor prognosis, independent of chromosomal aberrations in acute myeloid leukemia, this is not the same in FLT3-TKD, and their prognostic significance in APL remains controversial (10,13,16–18). The majority of the studies revealed that certain mutations indicated inferior traits of APL. Since there is little information regarding the association between genetic mutations and extramedullary disease (EMD) in patients with APL (19), the possibility that the rare genetic mutations observed in the present patient may be responsible for the multiple CNS and systemic relapses could not be excluded.
At present, there has been no concensus on prophylaxis for CNS relapse in APL (3,20). It is recommended that intrathecal chemotherapy should be performed subsequent to the first complete remission in patients at high CNS relapse risk, including those with an elevated WBC count, low platelet count, promyelocytes expressing CD56, differentiation syndrome and high serum lactate dehydrogenase (3,21). The polymerase chain reaction and fluorescence in situ hybridization analyses of the CSF should also be performed to detect any subclinical CNS involvement, which may indicate a requirement for intrathecal chemotherapy (3). The present patient was administered with regular intrathecal prophylaxis of dexamethasone, MTX and Ara-C and routine monitoring of CSF examination. CNS relapse was confirmed by flow cytometry of the CSF without presenting any clinical manifestations each time.
Despite the impressive complete remission and survival results of APL that have been achieved, the prognosis for CNS relapse of APL patients is generally poor (1). In a previous study, 20/31 patients relapsed. The duration of remission ranged between 1 and 144 months, with a median of 5 months (22). The present study was reported 20 months subsequent to the initial diagnosis. As it is challenging to avoid CNS relapse and subsequent systemic relapse in patients with APL that possess molecular abnormalities and leucocytosis, regardless of routine prophylaxis for CNS involvement, the remaining approach for APL patients with high CNS relapse risks may be allo-HSCT immediately subsequent to achieving the first hematological remission.
In summary, the present study reported the case of a patient with APL that experienced multiple CNS relapses and systemic relapses, and harbored unfavorable gene mutations. The present study highlights the importance of carefully monitoring EMD during follow-up, which may be a clinical biomarker of impending systemic relapses, and the importance of risk stratification with mutational analysis for APL. However, additional preclinical studies investigating these novel mutations and the interpretation of the mutation status of the genes during APL progression continue to be required. In addition, it appears inevitable that patients with APL that possess leucocytosis and unfavorable gene mutations relapse even following treatment with ARTA, ATO and anthracycline-containing systemic therapy in combination with intrathecal prophylaxis, which indicates that the patients with APL that possess a high relapse risk should receive immediate allo-HSCT whenever possible.
Acknowledgements
The authors thank Dr Chun-rong Tong from the Department of Immunotherapy and Wen Teng from the Department of Laboratory Medicine (Lu Daopei Hematology and Oncology Center, Beijing, China) for their helpful discussions and technical assistance.
References
- 1.Wang ZY, Chen Z. Acute promyelocytic leukemia: From highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 2.de Botton S, Sanz MA, Chevret S, Dombret H, Martin G, Thomas X, Mediavilla JD, Recher C, et al. (European APL Group; PETHEMA Group). Extramedullary relapse in acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Leukemia. 2006;20:35–41. doi: 10.1038/sj.leu.2404006. [DOI] [PubMed] [Google Scholar]
- 3.Colovic N, Tomin D, Vidovic A, et al. Central nervous system relapse in CD56+, FLT3/ITD+ promyelocytic leukemia. Med Oncol. 2012;29:260–262. doi: 10.1007/s12032-011-9834-y. [DOI] [PubMed] [Google Scholar]
- 4.Wiernik PH, De Bellis R, Muxi P, Dutcher JP. Extramedullary acute promyelocytic leukemia. Cancer. 1996;78:2510–2514. doi: 10.1002/(SICI)1097-0142(19961215)78:12<2510::AID-CNCR10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 5.Ko BS, Tang JL, Chen YC, et al. Extramedullary relapse after all-trans retinoic acid treatment in acute promyelocytic leukemia - the occurrence of retinoic acid syndrome is a risk factor. Leukemia. 1999;13:1406–1408. doi: 10.1038/sj.leu.2401495. [DOI] [PubMed] [Google Scholar]
- 6.Specchia G, Lo Coco F, Vignetti M, et al. Extramedullary involvement at relapse in acute promyelocytic leukemia patients treated or not with all-trans retinoic acid: A report by the Gruppo Italiano Malattie Ematologiche dell'Adulto. J Clin Oncol. 2001;19:4023–4028. doi: 10.1200/JCO.2001.19.20.4023. [DOI] [PubMed] [Google Scholar]
- 7.Ohno R, Asou N, Ohnishi K. Treatment of acute promyelocytic leukemia: Strategy toward further increase of cure rate. Leukemia. 2003;17:1454–1463. doi: 10.1038/sj.leu.2403031. [DOI] [PubMed] [Google Scholar]
- 8.Callens C, Chevret S, Cayuela JM, et al. (European APL Group). Prognostic implication of FLT3 and Ras gene mutations in patients with acute promyelocytic leukemia (APL): A retrospective study from the European APL Group. Leukemia. 2005;19:1153–1160. doi: 10.1038/sj.leu.2403790. [DOI] [PubMed] [Google Scholar]
- 9.Kuchenbauer F, Schoch C, Kern W, et al. Impact of FLT3 mutations and promyelocytic leukemia-breakpoint on clinical characteristics and prognosis in acute promyelocytic leukemia. Br J Haematol. 2005;130:196–202. doi: 10.1111/j.1365-2141.2005.05595.x. [DOI] [PubMed] [Google Scholar]
- 10.Barragán E, Montesinos P, Camos M, et al. (PETHEMA Group; HOVON Group). Prognostic value of FLT3-mutations in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline monochemotherapy. Haematologica. 2011;96:1470–1477. doi: 10.3324/haematol.2011.044933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chillón MC, Santamaría C, García-Sanz R, Balanzategui A, Sarasquete ME, Alcoceba M, Marín L, Caballero MD, Vidriales MB, Ramos F, Bernal T, et al. Long FLT3 internal tandem duplications and reduced PML-RARα expression at diagnosis characterize a high-risk subgroup of acute promyelocytic leukemia patients. Haematolgica. 2010;95:745–751. doi: 10.3324/haematol.2009.015073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gale RE, Hills R, Pizzey AR, Kottaridis PD, Swirsky D, Gilkes AF, Nugent E, Mills KI, Wheatley K, Solomon E, et al. (NCRI Adult Leukaemia Working Party). Relationship between FLT3 mutation status, biologic characteristics and response to targeted therapy in acute promyelocytic leukemia. Blood. 2005;106:3768–3776. doi: 10.1182/blood-2005-04-1746. [DOI] [PubMed] [Google Scholar]
- 13.Schnittger S, Bacher U, Haferlach C, Kern W, Alpermann T, Haferlach T. Clinical impact of FTL3 mutation load in acute promyelocytic leukemia with t(15;17)/PML-RARA. Haematologica. 2011;96:1799–1807. doi: 10.3324/haematol.2011.049007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacher U, Haferlach C, Kern W, Haferlach T, Schnittger S. Prognostic relevance of FTL3-TKD mutations in AML: The combination matters - an analysis of 3082 patients. Blood. 2008;111:2527–2737. doi: 10.1182/blood-2007-05-091215. [DOI] [PubMed] [Google Scholar]
- 15.Gaur GC, Ramadan SM, Cicconi L, Noguera NI, Luna I, Such E, et al. Analysis of mutational status, SNP rs16754, and expression levels of Wilms tumor 1 (WT1) gene in acute promyelocytic leukemia. Ann Hematol. 2012;91:1855–1860. doi: 10.1007/s00277-012-1546-7. [DOI] [PubMed] [Google Scholar]
- 16.Hou HA, Huang TC, Lin LI, Liu CY, Chen CY, Chou WC, Tang JL, Tseng MH, Huang CF, Chiang YC, et al. WT1 mutation in 470 adult patients with acute myeloid leukemia: Stability during disease evolution and implication of its incorporation into a survival scoring system. Blood. 2010;115:5222–5231. doi: 10.1182/blood-2009-12-259390. [DOI] [PubMed] [Google Scholar]
- 17.Shih LY, Kuo MC, Liang DC, Huang CF, Lin TL, Wu JH, Wang PN, Dunn P, Lai CL. Internal tandem duplication and Asp835 mutations of the FMS-like tyrosine kinase 3 (FLT3) gene in acute promyelocytic leukemia. Cancer. 2003;98:1206–1216. doi: 10.1002/cncr.11636. [DOI] [PubMed] [Google Scholar]
- 18.Kutny MA, Moser BK, Laumann K, Feusner JH, Gamis A, Gregory J, Larson RA, Powell BL, Stock W, Willman CL, et al. FLT3 mutation status is a predictor of early death in pediatric acute promyelocytic leukemia: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2012;59:662–667. doi: 10.1002/pbc.24122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tashiro H, Shirasaki R, Oka Y, Sugao T, Mizutani-Noguchi M, Yamamoto T, Akiyama N, Kawasugi K, Shirafuji N. FLT3 internal tandem duplication is associated with a high relapse rate and central nervous system involvement in acute promyelocytic leukemia cases: Single institutional analysis. Eur J Haematol. 2011;86:272–273. doi: 10.1111/j.1600-0609.2010.01559.x. [DOI] [PubMed] [Google Scholar]
- 20.Montesinos P, Díaz-Mediavilla J, Debén G, Prates V, Tormo M, Rubio V, Pérez I, Fernández I, Viguria M, Rayón C, et al. Central nervous system involvement at first relapse in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline monochemotherapy without intrathecal prophylaxis. Haematologica. 2009;94:1242–1249. doi: 10.3324/haematol.2009.007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai S, Nannya Y, Arai S, Yoshiki Y, Takahashi T, Kurokawa M. Molecular or cytogenetic monitoring and preemptive therapy for central nervous system relapse of acute promyelocytic leukemia. Haematologica. 2010;95:169–171. doi: 10.3324/haematol.2009.015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bae SH, Ryoo HM, Cho HS, Lee JL, Lee KH, Hyun MS. Meningeal relapse in a patient with acute promyelocytic leukemia: A case report and review of the literature. J Korean Med Sci. 2004;19:311–314. doi: 10.3346/jkms.2004.19.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]