Abstract
Background
Emergence from anaesthesia is often accompanied by signs of delirium, including fluctuating mental status and inattention. The evolution of these signs of delirium requires investigation since delirium in the post-anaesthesia care unit (PACU) may be associated with worse outcomes.
Methods
Adult patients emerging from anaesthesia were assessed for agitated emergence in the operating room using the Richmond Agitation-Sedation Scale (RASS). The Confusion Assessment Method for the Intensive Care Unit was then used to evaluate delirium signs at PACU admission and during PACU stay at 30 min, 1 h, and discharge. Signs consistent with delirium were classified as hyperactive vs hypoactive based upon a positive CAM-ICU assessment and the concomitant RASS score. Multivariable logistic regression was utilized to assess potential risk factors for delirium during PACU stay including age, American Society of Anesthesiologists classification, and opioid and benzodiazepine exposure.
Results
Among 400 patients enrolled, 19% had agitated emergence. Delirium signs were present at PACU admission, 30 min, 1 h, and PACU discharge in 124 (31%), 59 (15%), 32 (8%), and 15 (4%) patients, respectively. In patients with delirium signs, hypoactive signs were present in 56% at PACU admission and in 92% during PACU stay. Perioperative opioids were associated with delirium signs during PACU stay (P=0.02).
Conclusions
A significant proportion of patients develop delirium signs in the immediate postoperative period, primarily manifesting with a hypoactive subtype. These signs often persist to PACU discharge, suggesting the need for structured delirium monitoring in the PACU to identify patients potentially at risk for worse outcomes in the postoperative period.
Keywords: anaesthesia, complications, delirium
Delirium is an acute brain organ dysfunction characterized by changes in level of consciousness, inattention, and disorganized thinking. Delirium can manifest with hyperactive signs (i.e. hyperactive subtype with agitation and restlessness) or with hypoactive signs (i.e. hypoactive subtype with lethargy and inattentiveness). It is extremely common throughout the hospital, with 60–80% of mechanically ventilated patients and 20–50% of patients with a lower severity of illness developing delirium at some point during their hospital course.1–3 Studies in surgical patients focusing on delirium in the first few postoperative
Editor's key points.
Limited evidence suggests that early postoperative delirium is associated with worse outcome.
The authors determined the incidence of emergence agitation and delirium among 400 adult patients.
On post-anaesthesia care unit admission, 31% of patient had signs of delirium.
Further studies are needed to confirm an association with adverse outcomes.
days4–14 have found that this brain organ dysfunction is independently associated with increased length of stay, higher cost of care, prolonged cognitive impairment, and increased mortality, similar to that reported in general hospital patients.4,15–18 The course of this brain dysfunction in the immediate postoperative period, however, is not well characterized.
Recent data suggest that even early postoperative delirium – at post-anaesthesia care unit (PACU) discharge – may be associated with worse outcomes14 and that delirium in the PACU is likely predictive of further delirium in the postoperative course.13 Unfortunately, delirium diagnosis in this period is confounded by the fact that emergence from general anaesthesia often presents with signs similar to delirium with alterations in mental status, inattentiveness, and disorganized thinking. We hypothesized that delirium signs would be most common immediately after general anaesthesia and decrease over time, that signs would persist in a significant number of patients at PACU discharge, that hypoactive features (often underdiagnosed in this setting) would be frequent, and that certain patient and anaesthetic characteristics (e.g. age, drug exposure) would predict increased probability of delirium signs. We, therefore, performed a prospective cohort study of patients undergoing general anaesthesia and assessed them for level of arousal using the Richmond Agitation-Sedation Scale (RASS)19 and for delirium signs using the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU)1 at multiple time points immediately after general anaesthesia.
Methods
Study design and patient population
This prospective observational study was approved by the Vanderbilt University Institutional Review Board with waiver of consent due to the non-interventional nature of the study. The principle investigator (EC), experienced in CAM-ICU1 delirium assessments, first trained five PACU nurse investigators in delirium assessment. We included non-cardiac surgery patients admitted to the PACU at Vanderbilt University Medical Center after general anaesthesia with volatile anaesthetics. These patients were assigned by the charge nurse (in the usual round-robin, rotation fashion) to the PACU nurse investigators, as is standard PACU practice of assigning patients to PACU nurses/beds. Thus, this was a prospective convenience sample study of patients assigned randomly to our five nurse investigators. Exclusion criteria included non-English speaking or deaf patients and those with a history of severe dementia, anoxic brain injury, or neuromuscular disorders as documented in the patient's medical record by his/her treating physicians.
Data collection
Data collected included de-identified demographics (age, gender, race, American Society of Anesthesiologists [ASA] classification), health history (comorbid diseases, chronic alcohol or illicit drug use, and smoking status), details of anaesthetic course (length of anaesthetic exposure, inhaled agent used, induction agent and dose, and lowest intraoperative vital signs [temperature, oxygen saturation, systolic and diastolic blood pressure]), perioperative medications (benzodiazepines, opioids, ketamine), PACU vital signs (lowest temperature, oxygen saturation, systolic and diastolic blood pressure), blood product administration, Aldrete scores,20 and verbal pain scores.
Assessments and definitions
A multidisciplinary focus group of anaesthesiologists, intensivists, and nurses (including but not restricted to the authors) determined face valid definitions for agitated emergence and PACU delirium signs based on review of literature and expert opinion. Agitated emergence was defined as agitation after discontinuation of the inhaled anesthetic based on RASS score of +1 to +4 as reported by in-room anaesthesia providers. Patients were assessed for delirium signs using the CAM-ICU performed by the trained nurse investigators at PACU admission, at 30 min, at 1 h, and at discharge from the PACU. The CAM-ICU has been validated against the Diagnostic and Statistical Manual of Mental Disorders-IV21 in both ventilated and non-ventilated verbal patients1,22–24 and can be rapidly and accurately performed by bedside nurses. The CAM-ICU has a higher specificity than sensitivity for delirium when used in the PACU25; thus, we expected fewer false positives than false negatives, thereby taking a conservative approach to the determination of the incidence of delirium signs in our cohort. Delirium signs were defined as being present if patients were CAM-ICU positive at any time point. Hyperactive delirium signs were defined as a RASS score of +1 to +4 (i.e. agitated patient) accompanying a positive CAM-ICU. Hypoactive delirium signs were defined as a RASS score of −3 to 0 (i.e. somnolent or calm patient) accompanying a positive CAM-ICU. Postoperative delirium (not assessed in this study) was defined as delirium that continued beyond the PACU or occurred in the hospital ward or the ICU.
Statistical analysis
Descriptive data are presented as medians (with interquartile ranges [IQR]) and percentages where applicable. We used a multivariable logistic regression model to study the associations of perioperative risk factors with the occurrence of delirium signs during PACU stay (dependent variable). For the regression model alone, having PACU delirium signs was classified as a positive CAM-ICU at 30 min, at 1 h, or at PACU discharge (thus excluding the assessment upon arrival to the PACU for this was considered continuation of emergence from general anaesthesia). The following a priori defined risk factors were assessed: age, ASA classification, opioid exposure (fentanyl equivalents), and benzodiazepine exposure (midazolam equivalents). A sensitivity analysis was performed adjusting for anaesthetic duration in addition to these above mentioned risk factors, as a recent study demonstrated surgical duration to be associated with postoperative delirium.14 In the statistical model, opioid doses were transformed using their cube root to reduce the influence of extreme outliers, and continuous variables were modelled using restricted cubic splines to allow for nonlinear associations. Benzodiazepine exposure was categorized into three categories (0, 0.5–2, and >2 mg) owing to sparseness of data.
Per standard recommendations, each degree of freedom for our multivariable model required 10 cases of delirium in order to reliably fit the model. Thus, a multivariable model with a complexity of 7 degrees of freedom would require at least 70 patients with delirium.26 We anticipated our PACU delirium signs rate to be approximately 20% in this cohort of general surgical patients, thus our study had a planned enrollment of 400 subjects into the cohort to be able to evaluate the risk factors of interest. Because missing data rarely occur entirely randomly, excluding such patients may have biased our results.27 Thus, we used multiple imputation to account for missing variables at time of modelling, which occurred in 9 patients (2.3%). We calculated optimization to assess potential overfitting. Smaller optimization is indicative of less overfitting, and optimization <0.20 indicates a model with good predictive ability (lack of overfitting). Optimization in our PACU delirium model was 0.14, confirming that the model was not overfit. We used R software version 3.0.1 (www.r-project.org) for all statistical analyses. We used REDCap, a secure online database, supported in part by a National Institutes of Health grant (UL1 TR000445 from NCATS/NIH).
Results
Between December 2010 and February 2012, 400 patients were enrolled in this convenience sample prospective cohort. Patient, anaesthesia, and surgery characteristics are presented in Table 1. Median (interquartile [IQR]) age was 57 (44, 67) years, ASA classification was 3 (2, 3), anaesthetic duration was 140 (87, 207) min, and 55% of the cohort was male.
Table 1.
Description of Baseline Characteristics. *Median (interquartile range) unless specified. †Total preoperative, intraoperative, and post-anaesthesia care unit administration. Abbreviations: ASA, American Society of Anesthesiologists; PACU, post-anaesthesia care unit
| Variable* | n=400 |
|---|---|
| Age (years) | 57 (44, 67) |
| Sex ratio (M/F) | 220/180 |
| ASA Classification | 3 (2, 3) |
| 1 | 4% |
| 2 | 44% |
| 3 | 49% |
| 4 | 3% |
| Comorbid Disease (% of patients) | |
| Malignancy | 33% |
| Diabetes | 17% |
| Cardiovascular Disease | 13% |
| Congestive Heart Failure | 4% |
| Liver Disease | 3% |
| Mild Dementia | 1% |
| Surgical Operation (% of patients) | |
| Urology | 24% |
| General Surgery | 22% |
| Orthopedic | 19% |
| Neurosurgery | 7% |
| Vascular | 5% |
| Other | 23% |
| Anaesthetic Duration (minutes) | 140 (87, 207) |
| Inhalation Agent (% of patients) | |
| Sevoflurane | 53% |
| Desflurane | 35% |
| Isoflurane | 12% |
| Benzodiazepines (midazolam equivalents, mg)† | 2 (0, 2) |
| Opioids (fentanyl equivalents, μg)† | 383 (200, 554) |
| Aldrete Score | |
| PACU admission | 8 (8, 8) |
| PACU discharge | 10 (9, 10) |
| Verbal Pain Score | |
| PACU admission | 0 (0, 3) |
| PACU discharge | 0 (0, 2) |
Agitated emergence was present in 75 (19%) patients (Table 2). Of these 75 patients, 45 (60%) were also CAM-ICU positive at PACU admission, with 25 (33%), 12 (16%), and six (8%) continuing to be CAM-ICU positive at 30 min, at 1 h, and at PACU discharge, respectively. Thus, 30 patients (8%) had agitated emergence only without subsequent delirium signs.
Table 2.
Emergence and PACU Delirium Signs. Abbreviations: CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; PACU, post-anaesthesia care unit. Delirium assessments were performed at PACU admission, at 30 minutes, at 1 hour, and at PACU discharge using CAM-ICU.1 Hypoactive delirium was defined as a Richmond Agitation-Sedation Scale19 score of −3 to 0 accompanying a positive CAM-ICU. Hyperactive delirium was defined as a Richmond Agitation-Sedation Scale score of +1 to +4 accompanying a positive CAM-ICU
| Outcome | n=400 |
|---|---|
| Agitated Emergence | 75 (19%) |
| Delirium signs at PACU admission (N) | 124 (31%) |
| Hypoactive Subtype | 56% |
| Hyperactive Subtype | 44% |
| Delirium signs during PACU stay (N) | 65 (16%) |
| Hypoactive Subtype | 92% |
| Hyperactive Subtype | 8% |
| CAM-ICU positive at 30 min (N) | 59 (15%) |
| CAM-ICU positive at 1 h (N) | 32 (8%) |
| CAM-ICU positive at PACU discharge (N) | 15 (4%) |
Overall, 124 (31%) patients had delirium signs (positive CAM-ICU) at PACU admission, 56% of whom had hypoactive features based upon concomitant RASS score (Table 2). At PACU admission, median Aldrete scores were 8 (7, 8) in patients with concurrent delirium signs vs 8 (8, 9) in patients without signs. Median verbal pain scores at PACU admission in patients with vs without concurrent delirium signs were 0 (0, 2) vs 0 (0, 3).
Excluding the CAM-ICU evaluation at PACU admission, delirium signs were present during PACU stay in 65 (16%) patients overall and in 59 (15%), 32 (8%), and 15 (4%) patients at 30 min, at 1 h, and at PACU discharge, respectively (Table 2). Of these 65 patients with delirium signs during PACU stay, 92% had hypoactive features. At PACU discharge, median Aldrete scores were 9 (8, 9.8) in patients with concurrent delirium signs vs 10 (9, 10) in patients without signs. Median verbal pain scores at PACU discharge in patients with vs without concurrent delirium signs were 1 (0, 3.5) vs 0 (0, 2).
In our multivariable regression model, we found total perioperative opioid administration (fentanyl equivalents) to be independently associated with delirium signs during PACU stay (P=0.02, Table 3) after adjusting for relevant covariates. This association was nonlinear and is demonstrated in Fig. 1. The additional risk factors studied, including age, ASA classification, and benzodiazepine exposure, did not have significant associations with delirium signs during PACU stay in this cohort. Sensitivity analysis demonstrated that anaesthetic duration was also independently associated with delirium signs during PACU stay (P<0.001).
Table 3.
Risk factors for delirium signs during PACU Stay. *Total preoperative, intraoperative, and post-anaesthesia care unit administration. †Nonlinear association. Abbreviations: ASA, American Society of Anesthesiologists; PACU, post-anaesthesia care unit. Multivariable logistic regression was used to study the association of potential risk factors with signs of delirium during PACU stay, defined as a positive Confusion Assessment Method for the Intensive Care Unit1 at 30 min, at 1 h, or at discharge from the PACU. Perioperative opioid administration was significantly associated with delirium signs during PACU stay. The additional risk factors studied did not have significant associations with delirium signs during PACU stay. Given the nonlinear associations between opioid administration and delirium signs, a single odds ratio cannot represent the relationship and hence is not presented (see Fig. 1)
| Variable, n=65 | P-value |
|---|---|
| Age (yrs) | 0.12 |
| ASA Classification | 0.20 |
| Benzodiazepines (midazolam equivalents, mg)* | 0.24 |
| Opioids (fentanyl equivalents, μg)* | 0.02† |
Fig 1.
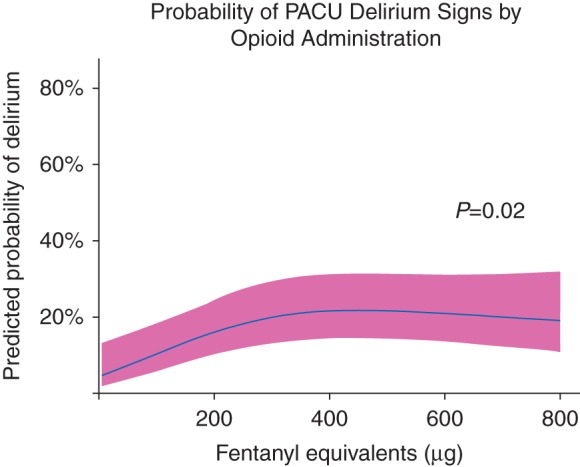
Probability of PACU Delirium Signs by Opioid Administration. Total preoperative, intraoperative, and post-anaesthesia care unit (PACU) opioid administration was independently associated with delirium signs during PACU stay, defined as a positive Confusion Assessment Method for the ICU1 at 30 min, at 1 h, or at discharge from the PACU. The solid line demonstrates the predicted probability of developing PACU delirium signs according to fentanyl equivalents received, with the pink ribbon indicating the 95% confidence interval. This association was most meaningful when examined between the 5th (50 μg fentanyl) and 50th (383 μg fentanyl) percentiles of opioid administration (common dose ranges for opioids in patients undergoing general anaesthesia) in our cohort, such that a patient receiving opioids equivalent to 383 μg fentanyl would have over 6 times the adjusted odds of developing delirium signs during PACU stay than a patient receiving 50 μg fentanyl (odds ratio [OR] 6.2, 95% CI 1.7, 22.1). Alternatively, when comparing the 50th to the 95th percentiles of opioid administration within our cohort, a patient receiving 383 μg fentanyl would have similar adjusted odds of developing delirium signs during PACU stay than a patient receiving 850 μg fentanyl (OR 0.88, 95% CI 0.45, 1.71). The lack of association at the higher doses could reflect a ceiling effect of the role of opioids in contributing to delirium signs or be a manifestation of fewer patients receiving such large doses.
Discussion
In this study, 19% of patients had agitated emergence from general anaesthesia. Furthermore using a structured delirium assessment tool at multiple time points immediately following general anaesthesia, we found a high incidence of delirium signs at arrival to the PACU and then continuing during the PACU stay, with a significant proportion of these patients manifesting signs consistent with hypoactive delirium. While the percentage of patients with delirium signs decreased over the PACU stay, 4% of patients still had persistent signs of delirium at PACU discharge despite meeting discharge criteria, indicating that these patients would not have been detected without delirium screening. Given that the volume of major surgery is greater than 230 million cases per year worldwide28 and that PACU delirium is likely predictive of postoperative delirium,13 this represents a substantial number of patients who leave the PACU with altered brain function and at risk for persistent delirium and its associated complications in the postoperative period. Of the risk factors studied, perioperative opioid administration was independently associated with delirium signs during PACU stay.
Studies evaluating patterns of abnormal emergence from general anaesthesia have focused on emergence delirium with reported prevalence rates of approximately 5%.29,30 In our study, 19% of our patients emerged from general anaesthesia with agitation (as measured by a sedation-agitation scale), while 31% of our patients had signs consistent with delirium (measured with a highly specific delirium monitoring instrument) at admission to the PACU. Whether these delirium-like signs on PACU arrival are manifestations of delirium or are just “normal” emergence from general anaesthesia can be debated. Conversely, while there are no standard definitions of when emergence from an anaesthetic is considered complete, it is important to note that delirium signs persisted in about one-tenth of our patients 1 h after arriving in the PACU and in 4% of patients at PACU discharge, when they had met their Aldrete score20 for readiness for discharge. Attention needs to be focused on early diagnosis and management of such patients, as recent data have shown that delirium at PACU discharge predicts postoperative delirium and potentially a decline in cognitive function and increased institutionalization after hospital discharge.13,14 Furthermore, these patients with delirium signs at PACU discharge may have a form of persistent delirium, as opposed to rapidly reversible sedative-related delirium, portending worse outcomes.31
Our incidence of delirium signs was lower than the 45% reported in a recent cohort by Neufeld and colleagues14,25 which examined postoperative delirium in 91 patients >70 yr old by performing neuropsychiatric evaluations after patients had completed recovery from anaesthesia (Aldrete score20 ≥9 and at least 45 min of elapsed PACU time). The elderly patient population studied was likely at higher risk for developing delirium signs than our population, whose median age was 57 yr old. In addition, the CAM-ICU has a higher specificity than sensitivity for delirium in low severity of illness patients, especially in the PACU25,32; thus, we likely underdiagnosed delirium signs in our cohort. While neuropsychiatric evaluations by specifically trained personnel using the Diagnostic and Statistical Manual of Mental Disorders21 are generally held as the reference standard, they are time- and resource-intensive. We chose the CAM-ICU over the CAM33 secondary to its ease of use and practicality for assessment in the PACU setting (e.g. rapid patient turnover, frequent physiologic changes, occasional nonverbal patient), given the CAM-ICU can be performed by trained nursing staff at the bedside in less than 2 min. Furthermore, in promoting delirium screening in the PACU, a concise tool such as the CAM-ICU or brief CAM (bCAM)34 is more likely to be adopted and utilized on a daily basis.
We found opioid administration to be associated with delirium signs during PACU stay after adjusting for covariates such as age, ASA classification, and duration of anaesthesia. In our cohort, pain scores at both PACU admission and PACU discharge were overall low and clinically comparable in patients with vs without concurrent delirium, suggesting pain was an unlikely cause of delirium in our cohort. One may assume that higher opioid doses contributed to additional sedation, thus potentially contributing to hypoactive delirium features, but the association between opioid administration and delirium signs was most meaningful at lower opioid doses (Fig. 1). The lack of association at higher doses could reflect a ceiling effect of the role of opioids in contributing to delirium signs. Prior studies have not shown a consistent association of opioid analgesia with perioperative delirium, with some suggesting increased risk2,35–37 and others suggesting either no association38 or a reduction in delirium rates.7 Thus while it is important to achieve adequate pain control in the perioperative setting, it is also imperative to recognize that overzealous administration of these medications may contribute to delirium signs.
Previously reported risk factors of postoperative delirium (in hospital wards or ICUs) seem to be predominantly related to the preoperative medical conditions, severity of surgical insult, and sedative and analgesic drug exposure.35,36,39–45 While both young and old age have been associated with emergence delirium,30 our cohort had a median age of 57 yr, with only a few patients <40 or >65 yr old, potentially negating the impact of age on delirium signs in our study. Additionally, 93% of our cohort had an ASA classification of 2 or 3, making it difficult to study any significant differences based on ASA classification. We did not assess the relationship of surgical insult with delirium signs, and we did not find any relationship between benzodiazepine use and delirium signs as previously reported,29,30,36,46 possibly reflecting the low dose and usage of benzodiazepines currently in our practice (96% of patients received 0–2 mg of midazolam).
This study has both strengths and limitations. We were able to detect signs of delirium in patients immediately after general anaesthesia, including both hyperactive and hypoactive features, with a structured delirium assessment tool. Though this was a single academic center study, our large cohort size with a wide range of non-cardiac surgical operations should be applicable to a broader patient population. Our incidence of PACU delirium signs was lower than predicted, limiting our regression model and increasing the potential for hidden confounders. A larger cohort would have enabled us to study additional factors (e.g. postoperative nausea and vomiting, use of regional nerve blockade) while also increasing our power to detect associations that might be significant. We did conduct a sensitivity analysis that demonstrated a significant association between increased anaesthetic duration and increased risk of delirium in the PACU; however, this result needs to be interpreted with caution as this model was potentially overfit. Although neither delirium assessments nor cognitive status evaluations were performed preoperatively, all patients met the criteria for consenting adults preoperatively. Without specific testing, however, we do not know if any of the patients with delirium signs postoperatively were delirious prior to general anesthetic exposure or had evidence of mild cognitive impairment, both of which could have increased their risk for postoperative delirium signs. We did, however, exclude patients with known severe dementia or other neurocognitive disease or who were unable to sign their informed consent for surgery.
We used the RASS to assess for agitated emergence since performance of CAM-ICU assessments at emergence from general anaesthesia is not clinically feasible, and we did not perform pain score evaluations on patients at the time of emergence. While agitated emergence may be secondary to pain, the majority of patients with agitated emergence based upon RASS in our study were also CAM-ICU positive at admission to the PACU. Furthermore, there were no differences in pain scores in patients with vs without delirium signs at PACU admission. Thus, they possessed signs of brain dysfunction such as inattention or disorganized thinking, suggesting the hyperactive features upon emergence were not due to pain in the majority of these patients. This is in agreement with recent data that demonstrate moderate sensitivity and high specificity of abnormal RASS measurements for delirium.47,48 Delirium assessments were completed by the same bedside nurse investigators who were also providing bedside care, a common and practical approach used in other hospital settings, but also a potential source of bias since the delirium assessments were not blinded. Finally, we did not evaluate the patients for delirium after they were discharged from the PACU or assess hospital or long-term outcomes. Therefore, we cannot comment on the continued trajectory of delirium signs further in the postoperative period and the effects they may have on patient outcomes.
Conclusions
In conclusion, in this prospective observational study of patients undergoing general anaesthesia, we found delirium signs in the immediate postoperative period to be common, with the incidence highest upon arrival to the PACU and decreasing during the PACU stay, though some patients had persistent delirium signs at discharge from the PACU. Hypoactive features were common and far more prevalent during PACU stay compared to hyperactive features. Routine delirium monitoring in the PACU may therefore be important, since delirium at discharge from the PACU is likely associated with worse outcomes. Additional research is needed to assess if the mortality or morbidity associated with delirium in hospitalized and critical care patients is applicable to patients with delirium signs in the immediate postoperative period.
Authors' contributions
E.B.C.: study design, data collection, data analysis, manuscript preparation. P.P.P, A.J.G, A.K.S, E.W.E, C.G.H.: study design, data analysis, manuscript preparation. C.M.T, C.A.L, J.M.W, D.M.N.: data collection, manuscript preparation.
Declaration of interest
No conflicts of interest.
Funding
Dr. Pandharipande is supported by the National Institutes of Health HL111111 (Bethesda, Maryland, USA). Dr. Ely is supported by the VA Clinical Science Research and Development Service and the National Institutes of Health AG035117, AG027472, and HL111111 (Bethesda, Maryland, USA). Dr. Hughes is supported by a Foundation for Anesthesia Education and Research (Rochester, Minnesota, USA) Mentored Research Training Grant and National Institutes of Health HL111111 (Bethesda, Maryland, USA).
References
- 1.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001; 286: 2703–10 [DOI] [PubMed] [Google Scholar]
- 2.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma 2008; 65: 34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med 2007; 33: 66–73 [DOI] [PubMed] [Google Scholar]
- 4.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med 2012; 367: 30–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franco K, Litaker D, Locala J, Bronson D. The cost of delirium in the surgical patient. Psychosomatics 2001; 42: 68–73 [DOI] [PubMed] [Google Scholar]
- 6.Litaker D, Locala J, Franco K, Bronson DL, Tannous Z. Preoperative risk factors for postoperative delirium. Gen Hosp Psychiatry 2001; 23: 84–9 [DOI] [PubMed] [Google Scholar]
- 7.Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci 2003; 58: 76–81 [DOI] [PubMed] [Google Scholar]
- 8.Marcantonio ER, Goldman L, Orav EJ, Cook EF, Lee TH. The association of intraoperative factors with the development of postoperative delirium. Am J Med 1998; 105: 380–4 [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen LS, Steentoft A, Rasmussen H, et al. Benzodiazepines and postoperative cognitive dysfunction in the elderly. Br J Anaesth 1999; 83: 585. [DOI] [PubMed] [Google Scholar]
- 10.Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth 2009; 103 (Suppl. 1): i41–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flink BJ, Rivelli SK, Cox EA, et al. Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology 2012; 116: 788–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radtke FM, Franck M, Lendner J, Kruger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth 2013; 110: i98–105 [DOI] [PubMed] [Google Scholar]
- 13.Sharma PT, Sieber FE, Zakriya KJ, et al. Recovery room delirium predicts postoperative delirium after hip-fracture repair. Anesth Analg 2005; 101: 1215–20 [DOI] [PubMed] [Google Scholar]
- 14.Neufeld KJ, Leoutsakos JM, Sieber FE, et al. Outcomes of early delirium diagnosis after general anesthesia in the elderly. Anesth Analg 2013; 117: 471–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med 2001; 27: 1892–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004; 291: 1753–62 [DOI] [PubMed] [Google Scholar]
- 17.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med 2010; 38: 1513–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998; 13: 234–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002; 166: 1338–44 [DOI] [PubMed] [Google Scholar]
- 20.Aldrete JA. Modifications to the postanesthesia score for use in ambulatory surgery. J Perianesth Nurs 1998; 13: 148–55 [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edn, text revision. Washington, DC: American Psychiatric Association, 2000 [Google Scholar]
- 22.Gusmao-Flores D, Figueira Salluh JI, Chalhub RA, Quarantini LC. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care 2012; 16: R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin SM, Liu CY, Wang CH, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med 2004; 32: 2254–9 [DOI] [PubMed] [Google Scholar]
- 24.van Eijk MM, van Marum RJ, Klijn IA, de WN, Kesecioglu J, Slooter AJ. Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med 2009; 37: 1881–5 [DOI] [PubMed] [Google Scholar]
- 25.Neufeld KJ, Leoutsakos JS, Sieber FE, et al. Evaluation of two delirium screening tools for detecting post-operative delirium in the elderly. Br J Anaesth 2013; 111: 612–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrell F. Regression Modeling Strategies: with Applications to Linear Modes, Logistic Regression, and Survival Analysis. New York: Springer, 2001 [Google Scholar]
- 27.Little RJ, D'Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med 2012; 367: 1355–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008; 372: 139–44 [DOI] [PubMed] [Google Scholar]
- 29.Lepouse C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth 2006; 96: 747–53 [DOI] [PubMed] [Google Scholar]
- 30.Radtke FM, Franck M, Hagemann L, Seeling M, Wernecke KD, Spies CD. Risk factors for inadequate emergence after anesthesia: emergence delirium and hypoactive emergence. Minerva Anestesiol 2010; 76: 394–403 [PubMed] [Google Scholar]
- 31.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med 2014; 189: 658–65 [DOI] [PubMed] [Google Scholar]
- 32.van Eijk MM, van den Boogaard M, van Marum RJ, et al. Routine use of the confusion assessment method for the intensive care unit: a multicenter study. Am J Respir Crit Care Med 2011; 184: 340–4 [DOI] [PubMed] [Google Scholar]
- 33.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990; 113: 941–8 [DOI] [PubMed] [Google Scholar]
- 34.Han JH, Wilson A, Vasilevskis EE, et al. Diagnosing delirium in older emergency department patients: validity and reliability of the delirium triage screen and the brief confusion assessment method. Ann Emerg Med 2013; 62: 457–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brouquet A, Cudennec T, Benoist S, et al. Impaired mobility, ASA status and administration of tramadol are risk factors for postoperative delirium in patients aged 75 years or more after major abdominal surgery. Ann Surg 2010; 251: 759–65 [DOI] [PubMed] [Google Scholar]
- 36.Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA 1994; 272: 1518–22 [PubMed] [Google Scholar]
- 37.Krenk L, Rasmussen LS, Hansen TB, Bogo S, Soballe K, Kehlet H. Delirium after fast-track hip and knee arthroplasty. Br J Anaesth 2012; 108: 607–11 [DOI] [PubMed] [Google Scholar]
- 38.Sieber FE, Mears S, Lee H, Gottschalk A. Postoperative opioid consumption and its relationship to cognitive function in older adults with hip fracture. J Am Geriatr Soc 2011; 59: 2256–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ansaloni L, Catena F, Chattat R, et al. Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br J Surg 2010; 97: 273–80 [DOI] [PubMed] [Google Scholar]
- 40.Koebrugge B, van Wensen RJ, Bosscha K, Dautzenberg PL, Koning OH. Delirium after emergency/elective open and endovascular aortoiliac surgery at a surgical ward with a high-standard delirium care protocol. Vascular 2010; 18: 279–87 [DOI] [PubMed] [Google Scholar]
- 41.Mu DL, Wang DX, Li LH, et al. High serum cortisol level is associated with increased risk of delirium after coronary artery bypass graft surgery: a prospective cohort study. Crit Care 2010; 14: R238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noimark D. Predicting the onset of delirium in the post-operative patient. Age Ageing 2009; 38: 368–73 [DOI] [PubMed] [Google Scholar]
- 43.Olin K, Eriksdotter-Jonhagen M, Jansson A, Herrington MK, Kristiansson M, Permert J. Postoperative delirium in elderly patients after major abdominal surgery. Br J Surg 2005; 92: 1559–64 [DOI] [PubMed] [Google Scholar]
- 44.Salata K, Katznelson R, Scott BW, Carroll J, Lindsay TF, Djaiani G. Endovascular versus open approach to aortic aneurysm repair surgery: rates of postoperative delirium. Can J Anaesth 2012; 59: 556–61 [DOI] [PubMed] [Google Scholar]
- 45.Vasilevskis EE, Han JH, Hughes CG, Ely EW. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol 2012; 26: 277–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taipale PG, Ratner PA, Galdas PM, et al. The association between nurse-administered midazolam following cardiac surgery and incident delirium: An observational study. Int J Nurs Stud 2012; 49: 1064–73 [DOI] [PubMed] [Google Scholar]
- 47.Chester JG, Beth HM, Rudolph JL. Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med 2012; 7: 450–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tieges Z, McGrath A, Hall RJ, MacLullich AM. Abnormal level of arousal as a predictor of delirium and inattention: an exploratory study. Am J Geriatr Psychiatry 2013; 21: 1244–53 [DOI] [PubMed] [Google Scholar]


