Abstract
Introduction
Postoperative delirium is common in older patients. Despite its prognostic significance, the pathophysiology is incompletely understood. Although many risk factors have been identified, no reversible factors, particularly ones potentially modifiable by anaesthetic management, have been identified. The goal of this prospective cohort study was to investigate whether intraoperative hypotension was associated with postoperative delirium in older patients undergoing major non-cardiac surgery.
Methods
Study subjects were patients >65 years of age, undergoing major non-cardiac surgery, who were enrolled in an ongoing prospective observational study of the pathophysiology of postoperative delirium. Intraoperative blood pressure was measured and predefined criteria were used to define hypotension. Delirium was measured by the Confusion Assessment Method on the first two postoperative days. Data were analysed using t-tests, two-sample proportion tests and ordered logistic regression multivariable models, including correction for multiple comparisons.
Results
Data from 594 patients with a mean age of 73.6 years (sd 6.2) were studied. Of these 178 (30%) developed delirium on day 1 and 176 (30%) on day 2. Patients developing delirium were older, more often female, had lower preoperative cognitive scores, and underwent longer operations. Relative hypotension (decreases by 20, 30, or 40%) or absolute hypotension [mean arterial pressure (MAP)<50 mm Hg] were not significantly associated with postoperative delirium, nor was the duration of hypotension (MAP<50 mm Hg). Conversely, intraoperative blood pressure variance was significantly associated with postoperative delirium.
Discussion
These results showed that increased blood pressure fluctuation, not absolute or relative hypotension, was predictive of postoperative delirium.
Keywords: arterial blood pressure, delirium, hypotension
Aging of the general population results in a greater number of older patients undergoing major surgery. Older patients tend to have more co-morbidities, more severe diseases, and poorer clinical outcomes after surgery. In particular, postoperative delirium is more common in older patients.1–6 This is significant because delirium is associated with longer hospital stays, poor functional outcomes, higher health care costs, and increased long-term mortality.6–14
The pathophysiology of postoperative delirium is unclear and, at present, no definitive preventive or therapeutic measure
Editor's key points.
Although postoperative delirium is associated with adverse outcomes, its pathophysiology remains poorly understood.
In particular, the role of intraoperative hypotension is controversial.
The authors analysed blood pressure records from patients enrolled in a large prospective observational trial.
Blood pressure variability, rather than the severity and duration of hypotension, was predictive of delirium.
is known.6,10,15–17 The cause of delirium is thought to be multifactorial, with an interrelationship between predisposing and precipitating factors.10,18,19
One of the factors that remains controversial is the possible role of intraoperative control of blood pressure in the production of postoperative delirium. In particular, it has been debated6,20 whether intraoperative hypotension is a major predictor of postoperative delirium. A recent clinical trial recommended the avoidance of intraoperative hypotension as part of a multimodal intervention strategy to decrease postoperative delirium.21 However, the literature on this topic has produced conflicting results: some studies have suggested a role for intraoperative hypotension in the development of postoperative delirium,3,20,22,23 whereas others have not.24,25
Cerebral autoregulation plays an important role in maintaining appropriate blood flow to the brain.26 According to the model described by Lassen,25 in the presence of a constant concentration of carbon dioxide, cerebral perfusion is kept relatively constant (within a cerebral perfusion pressure of 50–150 mm Hg), despite changes in mean arterial pressure (MAP).26–30 Using this model and assuming a normal intracranial pressure (ICP) of 0–15 mm Hg in the supine position,26–28,31 a reduction in cerebral perfusion below 50 mm Hg may lead to cerebral hypoperfusion and affect brain function. Both cerebral perfusion pressure and cerebral autoregulation are known to be compromised in patients with decreased elasticity of the arterial wall, such as those with essential hypertension.32–34 Although age alone has not been shown to influence cerebral autoregulation,35 older patients may be more likely to have impaired autoregulation because of coexisting conditions such as hypertension,32–34 diabetes,36 a history of smoking,37 hypercapnia,30,38,39 or obstructive sleep apnoea.40 Other variables encountered during anaesthesia, such as patient position,41,42 changes in the autonomic nervous system,43 or vasodilatation by medication,44,45 may also affect cerebral autoregulation. While there is an ongoing scientific debate regarding the mechanism of cerebral autoregulation,29,44 the thresholds based on this model are generally used in clinical practice.28
Accordingly, the goal of this study was to test the hypothesis that intraoperative hypotension and/or fluctuations in blood pressure increased the occurrence of postoperative delirium in older patients undergoing major non-cardiac surgery.
Methods
Patient recruitment
The study was a prospective observational cohort study that was approved by the University of California, San Francisco Committee on Human Research. Written informed consent was obtained preoperatively from each study patient. This study was part of a larger ongoing study to evaluate the pathophysiology of postoperative delirium. Inclusion criteria included English-speaking patients ≥65 years of age undergoing elective, non-cardiac surgery requiring anaesthesia, who were anticipated to stay in the hospital for longer than 48 h. Excluded were patients who were unable to provide informed consent (such as those who were incapable of understanding and providing informed consent to the procedure and the research study) or those who were expected to remain intubated postoperatively, precluding cognitive assessments. A subset of 215 patients in this study was included in a previously published study that did not consider intraoperative blood pressure measurements.46
Preoperative assessment
Trained research assistants conducted the preoperative interviews <48 h before surgery. Depressive symptoms, pain, and functional status were evaluated and the Telephone Interview of Cognitive Status (TICS), modified from the Mini Mental State Examination,47,48 was administered to measure baseline cognitive status.
Clinical management
Intraoperative anaesthetic and blood pressure management were recorded, but not standardised. Vital signs, the type of anaesthesia, and the types and dosages of anaesthetic agents were measured. The data were extracted from either a paper record (N=489, 91%) or an automated anaesthesia record (N=51, 9%) that was introduced during the study period. The treating anaesthesiologists were blinded to the study hypothesis to minimise potential bias in management. Postoperatively, all patients were monitored with pulse oximetry, and supplemental oxygen was administered to keep O2 saturation >95%.
Measurement of intraoperative blood pressure
Each patient received standard monitoring as per guidelines from the American Society of Anaesthesiologists. Baseline values for heart rate and blood pressure were determined immediately prior to surgery by averaging two preoperative values that were obtained while the patient was awake and had not received premedication. Intraoperatively, blood pressure was measured continuously in patients who had invasive arterial monitoring. For those monitored non-invasively, blood pressure was measured with a minimal frequency of every 5 min. Anaesthesia records between June 2001 and April 2006 were recorded on paper. These records contained a graphical representation of the patient's systolic and diastolic blood pressures (SBP and DBP, respectively), heart rate, and oxygen saturation levels recorded every 5 min. Graphical data were electronically converted to digital records (DigitizeIt, version 1.5.7, Cologne, Germany; http://www.digitizeit.de). According to a personal communication from the manufacturer and programmer (Ingo Bormann), the error rate of these data is mainly limited by the resolution of the scans. Since we used 200 dpi TIFF images with a resolution of 398 pixels for the blood pressure graph range from 30 to 210 mm Hg, the error of this methodology was calculated as 180 mm Hg/398 pixels=0.45 mm Hg. Data after April 2006 were obtained from an automated anaesthesia recordkeeping system that recorded vital signs every 60 s (PICIS Anaesthesia Manager, versions 7.2 and 8.1, Wakefield, MA, USA). The raw data values were manually filtered based on (1) blood pressure values beyond <40 or >300 mm Hg for SBP and <30 or >150 mm Hg for DBP and (2) the presence of a pulse pressure <10 mm Hg. The goal of manual data filtering was to purge artefacts due to disconnections during blood samples, dampening or flushing of the arterial line from the automatic recorded blood pressure data, etc.
Definition of hypotension
Several definitions of intraoperative blood pressure changes were evaluated: (1) relative intraoperative hypotension, defined as a 20, 30, or 40% decrease below the patient's preoperative baseline for either SBP or MAP,49 and (2) an absolute blood pressure decrease below a MAP of 50 mm Hg.26–28 This threshold was chosen to be slightly below the traditional lower limit of cerebral autoregulation (assuming a normal ICP of 0–15 in the supine position),26–28,31 under the assumption that less stringent hypotensive thresholds would have less impact on postoperative delirium.
Subgroup analysis
The above analytic procedures were repeated in a subgroup of the patient population defined as high risk for postoperative delirium based on predisposing or precipitating factors established in previous studies.14,50–56 We defined high-risk patients as any of the following: >80 years of age, ASA class 3 or 4, surgical risk of 357 (see below), intraoperative oxygen desaturation <95% for >10 min, preoperative diagnosis of hypertension, and a history of stroke, transient ischaemic attack, or vascular disease.
Definition of blood pressure fluctuation
Fluctuation in a patient's blood pressure during surgery was quantified by calculating the variance of the patient's blood pressure record during surgery. Variance is a measure of the data spread. Consequently, a patient whose blood pressure fluctuates more during surgery, commonly known as blood pressure lability, will have a larger variance compared with a patient whose blood pressure remains relatively constant. Blood pressure fluctuation was calculated according to the formula: variance =, where xi is a patient's blood pressure at a particular time point, is the mean of the patient's blood pressure, and n is the number of blood pressure measurements. The mean variance was calculated by averaging the variance for patients in the delirious vs the non-delirious groups.
Delirium assessment
To determine the presence of delirium, research assistants conducted structured interviews preoperatively and on the first two postoperative days between the hours of 9 am and 12 pm, using the Confusion Assessment Method (CAM).58 (The CAM was developed as a screening instrument based on operationalization of the Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised (DSM-III-R) criteria for use by non-psychiatry clinicians in high-risk settings. Based on a structured interview, the CAM algorithm consists of four clinical criteria: acute onset and fluctuating course, inattention, disorganised thinking, and altered level of consciousness. To define delirium, both the first and second criteria have to be present, plus either criterion three or four. The CAM has a sensitivity of 94–100%, a specificity of 90–95%, a high interobserver reliability, and convergent agreement with four other mental status tests.6,41) The staff was trained in CAM use based on a detailed manual.58 Preoperative cognitive status was measured by TICS.
To ensure consistency in the evaluation, each patient was evaluated by the same research assistant for all three interviews. All cases of incident delirium were validated by a second investigator. Because the goal of our study was to investigate the effects of intraoperative blood pressure changes on postoperative delirium, we focused on delirium measurement on the first 2 days only. If hypotension were deleterious to the brain, the effects would likely occur early, hence our focus on the early postoperative period. Additionally, this would minimize missing data since many patients were discharged home after the second postoperative day.
Measurement of other covariates
Associations of several potential covariates with postoperative delirium had been determined in prior research.46,59,60 Covariates obtained during the preoperative interviews included age, history of central nervous system disorders, including stroke and transient ischaemic attack, and the Charlson co-morbidity index.61 Functional status was measured using the Activities of Daily Living (ADL)62 and the Instrumental Activities of Daily Living (IADL).63 Other factors, including type of surgery, ASA classification, duration of anaesthesia, and intraoperative blood loss were obtained from chart review. Surgical risk was determined according to the guidelines from the American College of Cardiology and American Heart Association update for perioperative cardiovascular evaluation for non-cardiac surgery.57 For general anaesthesia, a balanced anaesthesia technique was typically used.
Statistical analysis
We tested whether episodes of relative or absolute intraoperative hypotension or fluctuations in blood pressure were associated with cases of delirium. Bivariate analyses included two-sample t-tests for continuous variables and two-sample proportion tests for dichotomous comparisons. The False Discovery Rate (FDR)64 was used to control the type I error rate for bivariate comparisons relating to our hypothesis (Tables 2–4).
Table 2.
Comparison of blood pressure between patients with and without delirium on postoperative day 1. Data are given as mean (sd). P-values calculated by two-sample t-tests. P adj. refers to the P-value after adjustment for multiple comparisons according to the False Discovery Rate48 (FDR). MAP, mean arterial pressure; SBP, systolic blood pressure
| Total (N=540) | No delirium day 1 (N=362) | Delirium day 1 (N=178) | P-value | P adj. | |
|---|---|---|---|---|---|
| Baseline MAP, mm Hg | 86.05 (16.49) | 86.00 (16.51) | 86.51 (16.49) | 0.919 | 0.919 |
| Baseline SBP, mm Hg | 125.50 (24.70) | 127.20 (24.24) | 124.65 (25.57) | 0.273 | 0.410 |
| Mean MAP, mm Hg | 83.16 (9.07) | 82.65 (8.85) | 84.19 (9.45) | 0.071 | 0.213 |
| Lowest MAP, mm Hg | 62.67 (8.64) | 62.39 (8.84) | 63.26 (8.19) | 0.256 | 0.410 |
| Mean SBP, mm Hg | 121.76 (13.05) | 120.50 (12.70) | 124.32 (13.39) | 0.002 | 0.0154 |
| Lowest SBP, mm Hg | 92.48 (12.83) | 91.91 (12.87) | 93.64 (12.68) | 0.140 | 0.299 |
Table 4.
Mean intraoperative blood pressure fluctuation in patients with and without postoperative delirium. Data are given as mean (sd). P-values calculated by two-sample t-tests. P adj. refers to the P-value after adjustment for multiple comparisons according to the False Discovery Rate48 (FDR). MAP, mean arterial pressure; SBP, systolic blood pressure
| Delirium | No delirium | P-value | P adj. | |
|---|---|---|---|---|
| MAP day 1 | 123.33 (73.31) | 112.35 (66.95) | 0.129 | 0.299 |
| MAP day 2 | 127.83 (71.57) | 111.01 (68.02) | 0.010 | 0.049 |
| SBP day 1 | 274.85 (166.94) | 241.51 (150.12) | 0.031 | 0.114 |
| SBP day 2 | 283.60 (162.76) | 237.43 (152.27) | 0.002 | 0.015 |
Multivariable ordered logistic regression models were used to examine the effects of patient's SBP and MAP variance on the number of days of postoperative delirium while controlling for other risk factors. All variables in Table 1 were considered for the multivariable models; backwards variable selection was used to select the final model.
Table 1.
Descriptive statistics for patients with and without delirium on postoperative day 1. Data are given as n (%) unless stated otherwise. ASA, American Society of Anesthesiologists; IADL, Instrumental Activities of Daily Living; SBP, systolic blood pressure; TIA, transient ischaemic attack; TICS, Telephone Interview of Cognitive Status
| Total (N=540) | No delirium day 1 (N=362) | Delirium day 1 (N=178) | P-value | |
|---|---|---|---|---|
| Age, mean (sd) (range), years | 73.61 (6.18) (64–96) | 73.05 (6.02) (64–96) | 74.71 (6.37) (65–91) | <0.001 |
| Gender, female | 269 (50.6) | 191 (53.9) | 106 (60.0) | <0.001 |
| Race, white | 463 (87.0) | 315 (88.9) | 148 (83.1) | 0.289 |
| TICS | 32.17 (4.07) | 32.89 (3.52) | 30.68 (4.78) | <0.001 |
| TICS difference after 30 days | 0.443 (2.80) | 0.343 (2.74) | 0.755 (3.08) | 0.384 |
| Midazolam premedication | 338 (58.4) | 228 (59.2) | 100 (55.9) | 0.509 |
| General anaesthesia | 411 (70.6) | 272 (70.4) | 126 (70.9) | 0.914 |
| Length of surgery, h | 4.98 (2.35) | 4.73 (2.27) | 5.39 (2.70) | <0.01 |
| Surgery type (see Supplementary S2) | 0.026 | |||
| Orthopaedic (14.7%) | 302 (53.4%) | 188 (50.3%) | 109 (61.6%) | |
| Abdominal/Thoracic (32.5%) | 184 (32.5%) | 126 (33.4%) | 51 (28.8%) | |
| Other surgery (14.1%) | 80 (14.1%) | 60 (16.0%) | 17 (9.6%) | |
| Preoperative haemoglobin, g dl−1 | 13.02 (1.61) | 15.81 (27.79) | 12.80 (1.67) | 0.034 |
| Estimated blood loss | 713.12 (1254.92) | 651.03 (1294.24) | 833.38 (1163.63) | <0.001 |
| Resting SBP, mm Hg | 146.47 (47.37) | 146.94 (56.1) | 146.25 (21.7) | 0.834 |
| Resting MAP, mm Hg | 97.61 (17.5) | 98.10 (19.5) | 96.90 (13.1) | 0.390 |
| ASA class 1 or 2 | 254 (47.7%) | 171 (48.3%) | 75 (42.1%) | 0.135 |
| History of stroke | 21 (4.0) | 16 (4.5%) | 5 (2.8%) | 0.517 |
| History of TIA | 25 (4.7) | 18 (5.1%) | 7 (4.0%) | 0.753 |
| Charlson score | 1.54 (1.72) | 1.53 (1.68) | 1.54 (1.78) | 0.972 |
| IADL dependency >0 | 212 (39.5) | 148 (30.4%) | 65 (36.4%) | 0.391 |
Sample size justification
The sample size calculation was based on two-sample t-tests for detecting differences in the proportions of postoperative delirium from 15% to 40% between the groups with hemodynamic changes vs those without (power=0.8, α=0.05). The sample size was estimated to be a minimum of 182 patients for the entire group. Post hoc analysis demonstrated that a sample size of >60 000 patients would be necessary to show a difference between the groups (postoperative delirium rates of 30% vs 31%, respectively).
Results
A total of 594 patients were included in the study. Sixteen subjects did not have delirium evaluations on day 1 and were excluded from all further analyses. An additional 38 subjects had missing delirium assessments on day 2. To determine whether these study subjects with incomplete data were systematically different from the patients included in this study, we performed additional analyses of the demographics (Supplementary S1). No significant differences were found. All subsequent analyses were performed on the remaining 540 subjects.
Table 1 provides an overview of patient characteristics for the 540 patients stratified by delirium on postoperative day 1. Patients who developed delirium postoperatively were significantly older and more likely to be female. These patients had lower cognitive status as measured by preoperative TICS. However, there was no significant difference in TICS measured at 30 days after surgery. The use of midazolam premedication and general anaesthesia (vs regional anaesthesia) did not affect the rate of postoperative delirium. Patients with delirium had significantly longer operative durations and underwent procedures with significantly higher surgical risk. There was a significant difference between different types of surgery, with a highest percentage of patients with delirium in the orthopaedic surgery group. Significantly lower preoperative haemoglobin values and higher estimated blood loss were noted in the delirium group (Table 1). Of note, resting blood pressure was not significantly different between groups, nor were ASA status, history of stroke or transient ischaemic attack, Charlson co-morbidity index, or IADL scores. Delirium was measured on postoperative day 2 as well; however, results were very similar to day 1 and therefore are not shown.
Incidence of delirium
On postoperative day 1, delirium was present in 178/540 patients (33%); 167/540 (31%) patients developed delirium on day 2. Among the patients with delirium on day 1, 114/178 (64%) continued to meet criteria for delirium on day 2, while 53167 (32%) patients had delirium on the second day only.
Haemodynamic changes
After FDR adjustment, the mean baseline and mean lowest preoperative blood pressure values (both MAP and SBP) between patients with and without postoperative delirium were not significantly different (Fig. 1; Table 2). For intraoperative values, only overall mean SBP between patients with and without delirium was significantly different, with a higher value in patients in the delirium group.
Fig 1.
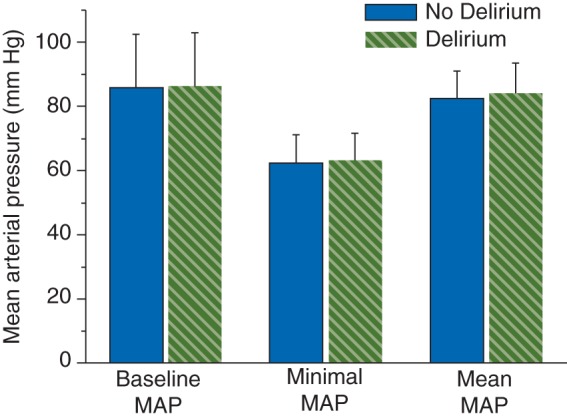
Delirium on day 1 and mean arterial blood pressure (MAP). There was no significant difference in baseline MAP, minimal MAP and mean MAP between patients with and without delirium.
During surgery we observed an intraoperative MAP decrease of >20% below the preoperative baseline for >5 min in 50% of patients, a 30% decrease in 30% of patients, and a 40% decrease in 12% of patients (Table 3). These results were similar for patients regardless of whether they developed delirium, as we found no significant differences after FDR adjustment. Decreases in blood pressure to a MAP of <50 mm Hg occurred in 6% of patients. There were no significant differences in the rates of postoperative delirium between patients with and without a MAP decrease below 50 mm Hg and for the time spent at a MAP of <50 mm Hg. Calculations were also done for a MAP decrease below 55 mm Hg,65 with unchanged results (data not shown).
Table 3.
Relative and absolute hypotension in patients with and without delirium on postoperative day 1. The upper row provides the number of patients in each category with the corresponding percentage underneath. P-values calculated by two-sample proportion tests. P adj. refers to the P-value after adjustment for multiple comparisons according to the False Discovery Rate48 (FDR). MAP, mean arterial blood pressure; decrease, decrease below baseline values
| Total (N=540) | No delirium day 1 (N=362) | Delirium day 1 (N=178) | P-value | P adj. | |
|---|---|---|---|---|---|
| MAP decrease 20% | 269 | 178 (49.2%) | 91 (51.1%) | 0.671 | 0.774 |
| MAP decrease 30% | 164 | 109 (30.1%) | 55 (30.9%) | 0.852 | 0.913 |
| MAP decrease 40% | 61 | 43 (11.9%) | 18 (10.1%) | 0.534 | 0.667 |
| MAP <50 mm Hg | 32 | 24 (6.6%) | 8 (4.5%) | 0.302 | 0.412 |
| Time MAP <50 mm Hg (average per minute) | 0.00061 (0.0048) | 0.00036 (0.0018) | 0.00074 (0.0057) | 0.252 | 0.409 |
The impact of a blood pressure decrease of 20, 30, or 40% and an absolute MAP of <50 mm Hg on postoperative delirium was compared in subjects classified as high risk (see Methods section). There was no association between blood pressure decrease and the incidence of delirium for any of the aforementioned high-risk subgroups (Supplementary S3). We further investigated a potential influence of vasopressor administration and the presence of an arterial line, the latter being a sign of a higher risk assigned by the treating anaesthesiologist. There were no significant differences in the rates of postoperative delirium in patients with vs without vasopressor administration or with vs without the use of an arterial line (Supplementary S4).
When comparing intraoperative blood pressure fluctuations (Table 4), we found a larger variance in those with delirium after surgery. This trend was present for MAP and SBP and delirium on day 1 and 2. After FDR adjustment, the difference in the MAP and SBP variances in patients that developed delirium on day 2 maintained statistical significance. The mean SBP variance for 1 day was 248.0 (sd 162.8) mm Hg2 and the mean variance for 2 days was 294.7 (165.8) mm Hg2 (P=0.031); the mean variance for MAP was 114.2 (69.3) mm Hg2 for 1 day of delirium and 131.1 (73.8) mm Hg2 (P=0.073) for 2 days of delirium. This trend was similarly observed when using delirium data from the chart review rather than CAM testing (data not shown).
To determine if increased blood pressure variance was associated with the covariates of postoperative delirium, ordered logistic regression analysis was performed (Supplementary S5, Table S1). Increased SBP and MAP variances were significantly associated with increased age (SBP and MAP variance), female gender (SBP, MAP), and lower TICS score (SBP) and significantly decreased with surgery duration (SBP, MAP). The analysis for the aforementioned high-risk groups (Supplementary S5, Table S2) showed significantly higher blood pressure variance in patients with ASA class 3 or 4 (SBP, MAP), preoperative hypertension (SBP) and intraoperative desaturation. There was no correlation between TICS and blood pressure variance (r=−0.11 for SBP and −0.08 for MAP).
The effect of blood pressure variance on postoperative delirium was additionally investigated using multivariable ordered logistic regression models (Table 5). The variances of SBP or MAP effects were statistically significant even after controlling for other risk factors. The other significant variables were TICS, length of surgery, gender, and type of surgery. Increases in SBP or MAP variances were associated with an increased risk of delirium even while controlling for other potential factors. Decreases in TICS, longer surgery duration, females, and orthopaedic surgeries were also associated with an increased risk of delirium.
Table 5.
Multivariable ordered logistic regression models predicting the number of days of delirium (0, 1, or 2). The table reports the results for the model using the variance of MAP as a predictor and the results for the model using the variance of SBP as a predictor. MAP, mean arterial pressure; SBP, systolic blood pressure
| Odds ratio | 95% CI | P-value | |
|---|---|---|---|
| MAP variance, mm Hg2 (per 10 units) | 1.038 | (1.010–1.067) | 0.008 |
| Age | 1.040 | (1.007–1.074) | 0.018 |
| Gender | 1.709 | (1.170–2.506) | 0.006 |
| TICS | 0.585 | (0.848–0.937) | 0.000 |
| Surgery duration | 1.236 | (1.134–1.135) | 0.000 |
| Surgery type | |||
| Orthopaedic (reference) | — | — | — |
| Abdominal/thoracic | 0.691 | (0.454–1.044) | 0.081 |
| Other | 0.333 | (0.168–0.630) | 0.001 |
| SBP variance, mm Hg2 (per 10 units) | 1.018 | (1.005–1.030) | 0.004 |
| Age | 1.038 | (1.005–1.072) | 0.024 |
| Gender | 1.686 | (1.151–2.475) | 0.007 |
| TICS | 0.895 | (0.851–0.940) | 0.000 |
| Surgery duration | 1.234 | (1.132–1.347) | 0.000 |
| Surgery type | |||
| Orthopaedic (reference) | — | — | — |
| Abdominal/thoracic | 0.696 | (0.457–1.052) | 0.087 |
| Other | 0.328 | (0.165–0.618) | 0.001 |
Data documentation
A sensitivity analysis was performed to determine if the type of data documentation and reading (manual vs automatic) impacted the results. A separate analysis was run on each data subset. The subset results were very similar to the full data results in terms of the direction of the effect and effect size, suggesting that the methods of blood pressure measurements did not have a significant influence on the results. These results are presented in Supplementary S6.
Discussion
Our investigation of a large cohort of older patients did not demonstrate a significant association between intraoperative hypotension and postoperative delirium. This result was true for both relative and absolute changes in blood pressure. However, we did find a relationship between an increase in intraoperative fluctuations in MAP and SBP in patients who subsequently developed postoperative delirium. This variance was one of the independent predictors of postoperative delirium, even after controlling for other covariates of delirium. The significance of our investigation is two-fold. First, our results add to the body of data on the possible role of intraoperative hypotension in the development of postoperative delirium. Three smaller studies3,20,22 and a prospective study in 818 consecutive critically ill patients66 concluded that, among other factors, hypotension, anaemia, and respiratory diseases are predictors of delirium. Hypotension in the larger study was defined as symptomatic, or SBP <80 mm Hg.66 Another study in 100 consecutive patients >65 years of age undergoing colorectal surgery determined that the risk of postoperative delirium was associated with intraoperative hypotension, higher infusion volume, and more blood loss.3 Intraoperative hypotension was defined as any documented MAP ≤60 mm Hg or the prolonged use of a vasoactive substance. Other investigators studied 90 elderly patients undergoing urological surgery and reported hypotensive episodes being one of the independent predictors of postoperative delirium.20 Hypotension was defined as an SBP decline to <90 mm Hg requiring pressors or fluids resuscitation.
However, the results of other studies did not support these conclusions. In 1341 patients >50 years of age who were undergoing major elective non-cardiac surgery, Marcantonio and collegues24 found postoperative delirium in 117 patients (9%). The route of anaesthesia and intraoperative hemodynamic complications were not associated with delirium. Hypotension in this study was defined as an SBP <66% of preoperative baseline or <90 mm Hg requiring pressors or fluid resuscitation. Delirium did correlate with greater intraoperative blood loss, more postoperative blood transfusions, and postoperative haematocrit <30%. Supporting these data, a larger multicentre study found no association between intraoperative hypotension and either short- or long-term postoperative cognitive dysfunction.25 Hypotension was defined as a MAP of ≤60% and ≤50% of the reference value before surgery for ≥30 min during or in the first 24 h after surgery.
Our results are novel, as they may explain the apparent discrepancies in these prior studies in which heterogeneous definitions of hypotension were used in different patient populations and settings. We investigated absolute and relative hypotension and blood pressure variability in one older population undergoing relatively common non-cardiac surgical procedures. While absolute and relative hypotension was not associated with postoperative delirium, our data show a significantly larger fluctuation in blood pressure, especially in SBP, in patients with postoperative delirium. It is important to note that our calculation of variance in blood pressure included both increases and decreases in blood pressure, which were not considered in any of the previous studies. After controlling for covariates, variance in blood pressure remained a significant factor for the development of postoperative delirium. Multivariate analysis confirmed that preoperative cognitive status and length of surgery, in combination with hemodynamic variance, contributed to postoperative delirium.
Variance or lability in blood pressure can be affected by a multitude of factors, including intraoperative blood loss, intravascular volume shifts, surgical stimulation, or the administration of anaesthetic agents and vasoactive drugs. Other factors, such as age, may contribute indirectly to larger intraoperative fluctuations in intravascular volume and blood rheology in some patients. Furthermore, patient compliance in taking antihypertensive medications, for example, may affect variability in blood pressure from visit to visit, the latter demonstrated in an outpatient setting as being associated with cardiovascular and stroke risk.67,68 Therefore, using blood pressure variance rather than blood pressure decrease alone as a risk predictor allows the inclusion of additional patient-related and intraoperative factors that contribute to blood pressure values during surgery.
In our cohort, women had a higher incidence of postoperative delirium, probably because more women in our study underwent orthopaedic surgery, which has previously been shown to be associated with a higher risk of postoperative delirium.69 The additional demographic variables that were associated with postoperative delirium, such as age, length of surgery, and estimated blood loss (Table 1), were also reported in previous studies.3,23,70 We further investigated whether patients with greater intraoperative variance in blood pressure and postoperative delirium were more likely to present with vascular disease or pre-existing central nervous system disorder, but this association was not supported by our data (Supplementary S5, Table S2).
One of the main results in our study is the lack of association between intraoperative hypotension and postoperative delirium. In our study, few patients had decreases in blood pressure of >40% or MAP values of <50 mm Hg. With this low incidence of marked hypotension, studies that target very long or very profound episodes of hypotension would need to be very large, as the use of small sample sizes may result in sampling errors. Conversely, repeated episodes of hypotension of smaller amplitude were not considered in previous studies but would be reflected in our analysis of blood pressure variance.
In addition, good practice in anaesthesia includes tight control of blood pressure, especially in patients with multiple co-morbidities. Therefore intraoperative blood pressure is not an independent variable or a mere consequence of co-morbidities, but depends as well on the type of surgery and the practices of the anaesthesiologist. The influence of the latter may be enhanced (or obscured) by reporting bias.71 However, our additional analysis did not reveal an influence due to the method of documentation. Although we did not find significant differences between automatically recorded and digitized records, the digitization of paper records itself may have introduced small errors in our transcribed blood pressure data. However, these small errors would likely not affect the reported results.
Another potential cause of discrepancies in study results could be differences in patient populations between studies. Even though our subgroup analysis (Supplementary S3) did not reveal significant differences between groups, some patient populations may be more sensitive to intraoperative hypotension and /or more likely to experience delirium. The negative result of our subgroup analysis indicates, however, that large studies with detailed physiologic data, such as more sensitive measurements of pre-existing cerebral vascular disease, may be necessary to elucidate mechanisms underlying such interrelationships.
Our study has several possible limitations. Because we only measured delirium once daily on the first two postoperative days, we might have missed episodes that occurred after our interviews or late in the postoperative period. However, any effect of significant variability in intraoperative blood pressure is likely to be more immediate than delayed, and the aetiology of delirium occurring in the late postoperative period probably differs from that occurring in the early postoperative period. For these reasons, we believe that measurement beyond the second postoperative day would probably not affect the present results. An additional limitation of our work, as in other studies, was that we selected the clinical marker blood pressure because of its ease of measurement, direct clinical applicability, and interpretability as a de facto surrogate marker of cerebral perfusion. Although direct measurements of cerebral blood flow, such as Doppler exams, are available, these measurements are discontinuous and are not commonly used in clinical practice.
In summary, we conclude that in the setting of active control of blood pressure by the anaesthesiologists, decreases in blood pressure during surgery were not associated with a significant increase in the risk of postoperative delirium. Rather, fluctuations in blood pressure during surgery, represented by changes in blood pressure variance, were associated with early postoperative delirium.
Supplementary material
Supplementary Material is available at British Journal of Anaesthesia online.
Authors' contributions
J.H.: manuscript preparation, data analysis. G.D.P.: data analysis, manuscript preparation. T.T.: patient recruitment, data collection. L.S.: study design, data analysis, manuscript preparation. J.L.: study design, patient recruitment, manuscript preparation.
Funding
This work was supported by funding from the Anesthesia Patient Safety Foundation (Indianapolis, IN, USA), and the National Institutes of Health of the United States (NIH 1RO1AG031795-05) (to J.M.L.).
Declaration of interest
None declared.
Supplementary Material
Acknowledgements
The authors want to thank the research assistants of the Leung lab for their help in patient recruitment and data handling.
References
- 1.Cole MG. Delirium in elderly patients. Am J Geriatr Psychiatry 2004; 12: 7–21 [PubMed] [Google Scholar]
- 2.Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg 2009; 249: 173–8 [DOI] [PubMed] [Google Scholar]
- 3.Patti R, Saitta M, Cusumano G, Termine G, Di Vita G. Risk factors for postoperative delirium after colorectal surgery for carcinoma. Eur J Oncol Nurs 2011; 15: 519–23 [DOI] [PubMed] [Google Scholar]
- 4.Jankowski CJ, Trenerry MR, Cook DJ, et al. Cognitive and functional predictors and sequelae of postoperative delirium in elderly patients undergoing elective joint arthroplasty. Anesth Analg 2011; 112: 1186–93 [DOI] [PubMed] [Google Scholar]
- 5.Kalisvaart KJ, Vreeswijk R, de Jonghe JF, van der Ploeg T, van Gool WA, Eikelenboom P. Risk factors and prediction of postoperative delirium in elderly hip-surgery patients: implementation and validation of a medical risk factor model. J Am Geriatr Soc 2006; 54: 817–22 [DOI] [PubMed] [Google Scholar]
- 6.Monk TG, Price CC. Postoperative cognitive disorders. Curr Opin Crit Care 2011; 17: 376–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCusker J, Cole MG, Dendukuri N, Belzile E. Does delirium increase hospital stay? J Am Geriatr Soc 2003; 51: 1539–46 [DOI] [PubMed] [Google Scholar]
- 8.Rockwood K, Cosway S, Carver D, Jarrett P, Stadnyk K, Fisk J. The risk of dementia and death after delirium. Age Ageing 1999; 28: 551–6 [DOI] [PubMed] [Google Scholar]
- 9.Quinlan N, Rudolph JL. Postoperative delirium and functional decline after noncardiac surgery. J Am Geriatr Soc 2011; 59(Suppl 2): S301–4 [DOI] [PubMed] [Google Scholar]
- 10.Guenther U, Radtke FM. Delirium in the postanaesthesia period. Curr Opin Anaesthesiol 2011; 24: 670–5 [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998; 13: 234–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Keeffe S, Lavan J. The prognostic significance of delirium in older hospital patients. J Am Geriatr Soc 1997; 45: 174–8 [DOI] [PubMed] [Google Scholar]
- 13.Curyto KJ, Johnson J, TenHave T, Mossey J, Knott K, Katz IR. Survival of hospitalized elderly patients with delirium: a prospective study. Am J Geriatr Psychiatry 2001; 9: 141–7 [PubMed] [Google Scholar]
- 14.Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA 1990; 263: 1097–101 [PubMed] [Google Scholar]
- 15.Steiner LA. Postoperative delirium. Part 2: detection, prevention and treatment. Eur J Anaesthesiol 2011; 28: 723–32 [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Li HL, Wang DX, et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial. Crit Care Med 2012; 40: 731–9 [DOI] [PubMed] [Google Scholar]
- 17.Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc 2005; 53: 1658–66 [DOI] [PubMed] [Google Scholar]
- 18.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 1996; 275: 852–7 [PubMed] [Google Scholar]
- 19.Brauer C, Morrison RS, Silberzweig SB, Siu AL. The cause of delirium in patients with hip fracture. Arch Intern Med 2000; 160: 1856–60 [DOI] [PubMed] [Google Scholar]
- 20.Tognoni P, Simonato A, Robutti N, et al. Preoperative risk factors for postoperative delirium (POD) after urological surgery in the elderly. Arch Gerontol Geriatr 2011; 52: e166–9 [DOI] [PubMed] [Google Scholar]
- 21.Bjorkelund KB, Hommel A, Thorngren KG, Gustafson L, Larsson S, Lundberg D. Reducing delirium in elderly patients with hip fracture: a multi-factorial intervention study. Acta Anaesthesiol Scand 2010; 54: 678–88 [DOI] [PubMed] [Google Scholar]
- 22.Echigoya Y, Kato H. Causes of postoperative delirium after abdominal surgery in elderly patients. Masui 2007; 56: 932–6 [PubMed] [Google Scholar]
- 23.Gottesman RF, Hillis AE, Grega MA, et al. Early postoperative cognitive dysfunction and blood pressure during coronary artery bypass graft operation. Arch Neurol 2007; 64: 1111–4 [DOI] [PubMed] [Google Scholar]
- 24.Marcantonio ER, Goldman L, Orav EJ, Cook EF, Lee TH. The association of intraoperative factors with the development of postoperative delirium. Am J Med 1998; 105: 380–4 [DOI] [PubMed] [Google Scholar]
- 25.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 1998; 351: 857–61 [DOI] [PubMed] [Google Scholar]
- 26.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev 1959; 39: 183–238 [DOI] [PubMed] [Google Scholar]
- 27.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 1990; 2: 161–92 [PubMed] [Google Scholar]
- 28.Dagal A, Lam AM. Cerebral autoregulation and anesthesia. Curr Opin Anaesthesiol 2009; 22: 547–52 [DOI] [PubMed] [Google Scholar]
- 29.Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol 2014; 592: 841–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Przybylowski T, Bangash MF, Reichmuth K, Morgan BJ, Skatrud JB, Dempsey JA. Mechanisms of the cerebrovascular response to apnoea in humans. J Physiol 2003; 548: 323–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andresen M, Juhler M. Intracranial pressure following complete removal of a small demarcated brain tumor: a model for normal intracranial pressure in humans. J Neurosurg 2014; 121: 797–801 [DOI] [PubMed] [Google Scholar]
- 32.Immink RV, van den Born BJ, van Montfrans GA, Koopmans RP, Karemaker JM, van Lieshout JJ. Impaired cerebral autoregulation in patients with malignant hypertension. Circulation 2004; 110: 2241–5 [DOI] [PubMed] [Google Scholar]
- 33.Strandgaard S. Autoregulation of cerebral blood flow in hypertensive patients. The modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug-induced hypotension. Circulation 1976; 53: 720–7 [DOI] [PubMed] [Google Scholar]
- 34.Eames PJ, Blake MJ, Panerai RB, Potter JF. Cerebral autoregulation indices are unimpaired by hypertension in middle aged and older people. Am J Hypertens 2003; 16: 746–53 [DOI] [PubMed] [Google Scholar]
- 35.Carey BJ, Eames PJ, Blake MJ, Panerai RB, Potter JF. Dynamic cerebral autoregulation is unaffected by aging. Stroke 2000; 31: 2895–900 [DOI] [PubMed] [Google Scholar]
- 36.Kim YS, Davis SC, Truijen J, Stok WJ, Secher NH, van Lieshout JJ. Intensive blood pressure control affects cerebral blood flow in type 2 diabetes mellitus patients. Hypertension 2011; 57: 738–45 [DOI] [PubMed] [Google Scholar]
- 37.Boyajian RA, Otis SM. Acute effects of smoking on human cerebral blood flow: a transcranial Doppler ultrasonography study. J Neuroimaging 2000; 10: 204–8 [DOI] [PubMed] [Google Scholar]
- 38.Mandell DM, Han JS, Poublanc J, et al. Selective reduction of blood flow to white matter during hypercapnia corresponds with leukoaraiosis. Stroke 2008; 39: 1993–8 [DOI] [PubMed] [Google Scholar]
- 39.Iwabuchi T, Kutsuzawa T, Ikeda K, Nakamura T. Effects of blood gases on the pressure-flow relationships in canine cerebral circulation. Stroke 1973; 4: 65–72 [DOI] [PubMed] [Google Scholar]
- 40.Urbano F, Roux F, Schindler J, Mohsenin V. Impaired cerebral autoregulation in obstructive sleep apnea. J Appl Physiol (1985) 2008; 105: 1852–7 [DOI] [PubMed] [Google Scholar]
- 41.Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth 2005; 17: 463–9 [DOI] [PubMed] [Google Scholar]
- 42.Yadeau JT, Casciano M, Liu SS, et al. Stroke, regional anesthesia in the sitting position, and hypotension: a review of 4169 ambulatory surgery patients. Reg Anesth Pain Med 2011; 36: 430–5 [DOI] [PubMed] [Google Scholar]
- 43.Hamner JW, Tan CO, Tzeng YC, Taylor JA. Cholinergic control of the cerebral vasculature in humans. J Physiol 2012; 590: 6343–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drummond JC. The lower limit of autoregulation: time to revise our thinking? Anesthesiology 1997; 86: 1431–3 [DOI] [PubMed] [Google Scholar]
- 45.Tzeng YC, Chan GS, Willie CK, Ainslie PN. Determinants of human cerebral pressure-flow velocity relationships: new insights from vascular modelling and Ca2+ channel blockade. J Physiol 2011; 589: 3263–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM. Postoperative delirium: the importance of pain and pain management. Anesth Analg 2006; 102: 1267–73 [DOI] [PubMed] [Google Scholar]
- 47.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol 1988; 1: 111–7 [Google Scholar]
- 48.Brandt J, Folstein SE, Folstein MF. Differential cognitive impairment in Alzheimer’s disease and Huntington’s disease. Ann Neurol 1988; 23: 555–61 [DOI] [PubMed] [Google Scholar]
- 49.Leung JM, O'Kelly BF, Mangano DT. Relationship of regional wall motion abnormalities to hemodynamic indices of myocardial oxygen supply and demand in patients undergoing CABG surgery. Anesthesiology 1990; 73: 802–14 [DOI] [PubMed] [Google Scholar]
- 50.Lee HB, Mears SC, Rosenberg PB, Leoutsakos JM, Gottschalk A, Sieber FE. Predisposing factors for postoperative delirium after hip fracture repair in individuals with and without dementia. J Am Geriatr Soc 2011; 59: 2306–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rockwood K. Acute confusion in elderly medical patients. J Am Geriatr Soc 1989; 37: 150–4 [DOI] [PubMed] [Google Scholar]
- 52.Elie M, Cole MG, Primeau FJ, Bellavance F. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med 1998; 13: 204–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogers MP, Liang MH, Daltroy LH, et al. Delirium after elective orthopedic surgery: risk factors and natural history. Int J Psychiatry Med 1989; 19: 109–21 [DOI] [PubMed] [Google Scholar]
- 54.Schor JD, Levkoff SE, Lipsitz LA, et al. Risk factors for delirium in hospitalized elderly. JAMA 1992; 267: 827–31 [PubMed] [Google Scholar]
- 55.Ansaloni L, Catena F, Chattat R, et al. Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br J Surg 2010; 97: 273–80 [DOI] [PubMed] [Google Scholar]
- 56.Flink BJ, Rivelli SK, Cox EA, et al. Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology 2012; 116: 788–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Anesth Analg 2002; 94: 1052–64 [DOI] [PubMed] [Google Scholar]
- 58.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990; 113: 941–8 [DOI] [PubMed] [Google Scholar]
- 59.Leung JM, Sands LP, Mullen EA, Wang Y, Vaurio L. Are preoperative depressive symptoms associated with postoperative delirium in geriatric surgical patients? J Gerontol A Biol Sci Med Sci 2005; 60: 1563–8 [DOI] [PubMed] [Google Scholar]
- 60.Leung JM, Sands LP, Vaurio LE, Wang Y. Nitrous oxide does not change the incidence of postoperative delirium or cognitive decline in elderly surgical patients. Br J Anaesth 2006; 96: 754–60 [DOI] [PubMed] [Google Scholar]
- 61.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83 [DOI] [PubMed] [Google Scholar]
- 62.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963; 185: 914–9 [DOI] [PubMed] [Google Scholar]
- 63.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9: 179–86 [PubMed] [Google Scholar]
- 64.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995; 57: 289–300 [Google Scholar]
- 65.Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119: 507–15 [DOI] [PubMed] [Google Scholar]
- 66.Aldemir M, Ozen S, Kara IH, Sir A, Bac B. Predisposing factors for delirium in the surgical intensive care unit. Crit Care 2001; 5: 265–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010; 375: 895–905 [DOI] [PubMed] [Google Scholar]
- 68.Rothwell PM. Does blood pressure variability modulate cardiovascular risk? Curr Hypertens Rep 2011; 13: 177–86 [DOI] [PubMed] [Google Scholar]
- 69.Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc 2006; 54: 1578–89 [DOI] [PubMed] [Google Scholar]
- 70.Parikh SS, Chung F. Postoperative delirium in the elderly. Anesth Analg 1995; 80: 1223–32 [DOI] [PubMed] [Google Scholar]
- 71.Benson M, Junger A, Fuchs C, et al. Using an anesthesia information management system to prove a deficit in voluntary reporting of adverse events in a quality assurance program. J Clin Monit Comput 2000; 16: 211–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


