Abstract
Atypical teratoid/rhabdoid tumor (AT/RT) is an aggressive embryonic brain tumor that is predominantly found in young children. The present study reports the unusual case of an adult male AT/RT patient with a history of another type of tumor, hereditary multiple exostoses (EXT or HME), who survived for 18 years. The patient's first and chief complaint was a 1-month history of progressive projectile vomiting. The patient was admitted twice for treatment, and on the second admission, a craniotomy was performed to remove a intracranial mass. However, no radiotherapy or chemotherapy treatment was administered. Pathology revealed monotonous medium- to large-sized neoplastic cells. The patient succumbed to a pulmonary infection and respiratory failure 14 days after the initial treatment. The prognosis for AT/RT is extremely poor; furthermore, the patient presented with another tumor. There may have been an association between the two tumors that worsened the clinical phenotype and prognosis of this patient. Additionally, symptomatic treatment for this condition is insufficient; early surgery and radiotherapy may be more useful for preventing the aggressive progression of these tumors.
Keywords: atypical teratoid/rhabdoid tumor, hereditary multiple exostoses
Introduction
Intracranial atypical teratoid/rhabdoid tumor (AT/RT) is a rare malignant embryonic neoplasm that usually occurs in children aged <3 years and accounts for a total incidence of 1–2% of all brain tumors in children and >10% of central nervous system tumors in infants (1). The clinical course is dismal, with a median survival time from diagnosis to mortality that spans only a few months (2). The tumor is typically associated with a chromosome 22q11.2 mutation or deletion, leading to the loss of nuclear expression of integrase interactor 1; in adults, the clinical presentation varies with tumor location (1). Hereditary multiple exostoses (EXT) is the most common benign bone tumor and is an autosomal dominant disorder, which is characterized by the formation of cartilage-capped bone projections (exostoses) localized mainly in the juxta-epiphyseal region of the long bones. EXT is associated with two loci, 8q24.1 (EXT1) and 11p11-p13 (EXT2) (3,4). The condition is characterized by multiple osteochondromas and is usually painless. The present study reports an adult case of intracranial AT/RT with a history of EXT and investigates the possible association between the two tumors. Written informed consent was obtained from the patient in accordance with the Declaration of Helsinki. The Ethics Committee of the Xiangya Hospital of Central South University (Changsha, Hunan, China) approved all experiments described in the study.
Case report
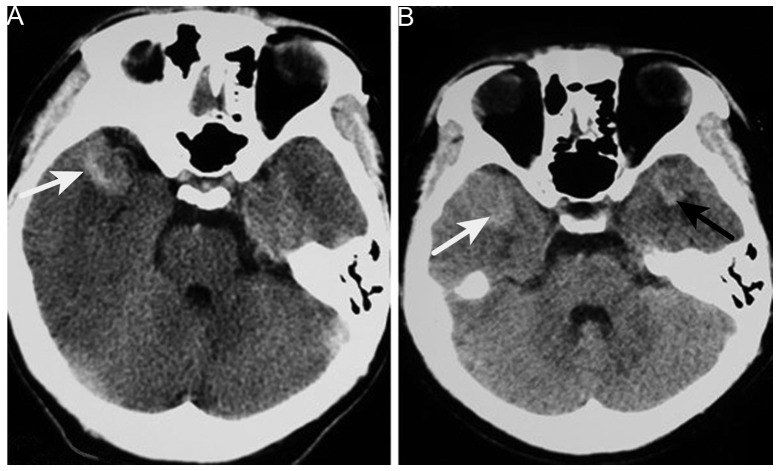
On October 11 2012, an 18-year-old male was first admitted to the Xiangya Hospital of Central South University following one month of progressive projectile vomiting. At this time, the patient did not complain of a headache or stomach discomfort. Brain computed tomography (CT) scans that had been obtained a few days after the symptoms arose revealed a hyperintense lesion on the right side of the temporal lobe; which was suspected to be an intracranial hemorrhage at the time. However, a month of corresponding therapy did not relieve the vomiting. The patient's past medical history included EXT detected at 10 years old, and the current examination noted two protuberant bony deformities over the right scapula and distal humerus. Upon admission, the neurological examination results were normal. Hematological and biochemical examination, and cerebrospinal fluid routine testing were normal; the opening pressure was 100 mm H2O (normal range, 80–180 mm H2O). Repeat CT of the brain revealed no changes in the CT value of the lesion (Fig. 1). Brain magnetic resonance imaging (MRI) revealed an abnormally low signal intensity on T1- and T2-weighted images and a high signal intensity on gadolinium-enhancing T1 in the right lateral fissure and adjacent temporal region (Fig. 2). Based on these results, the patient was diagnosed with an intracranial tumor. Surgery was suggested for removing the mass, which the patient refused, only accepting carbamazepine (0.1 g, 3 times/day, October 17–23, 2012) and ondansetron (8 mg, 2 times/day, October 12–16, 2012; 8 mg, 4 times/day, October 17–20, 2012) therapy. Upon leaving the hospital, the vomiting had been alleviated.
Figure 1.
Brain computed tomography. (A) High-density tumor in the right anterior temporal region (white arrow). (B) Image obtained 6 months later showing another tumor in the opposite temporal region (black arrow).
Figure 2.
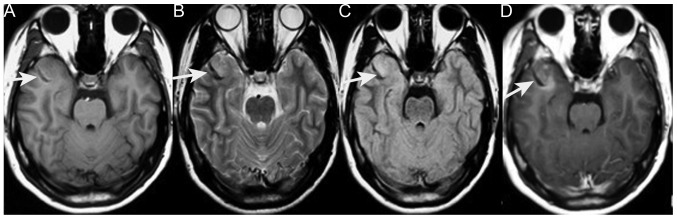
Images obtained during the first admission. (A) Axial T1-weighted MRI of a low-signal mass in the right temporal region (white arrow). (B) T2-weighted MRI of a low-intensity signal mass (white arrow). (C) Fluid-attenuated inversion recovery MRI of a low-intensity signal mass (white arrow). (D) Axial T1-weighted contrast-enhanced MRI of the mass with mild enhancement (white arrow). MRI, magnetic resonance imaging.
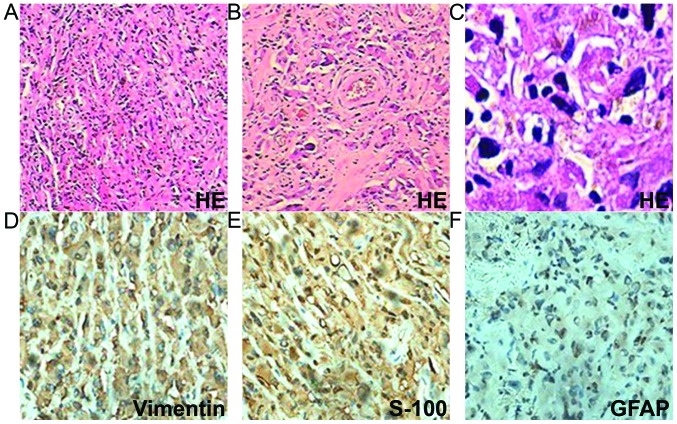
However, 6 months later, the patient was admitted again due to worsening vomiting and new symptoms of bilateral blurred vision, and severe neck and waist pain. A neurological examination revealed sixth cranial nerve palsy and bilateral papilledema. The lumbar puncture had an opening pressure of >400 mm H2O. Cerebrospinal fluid testing revealed nothing abnormal and no cancer cells. Brain CT revealed a new hyperintense lesion in the left temporal region (Fig. 1). Repeat MRI revealed two enhancing masses, with a low intensity signal on T1-weighted imaging and a heterogeneous signal on T2-weighted imaging, which had progressed more quickly compared with the lesion detected 6 months earlier. Moderate peripheral edema was also observed (Fig. 3). Digital subtraction angiography disclosed diffuse poor filling of all venous sinuses during the venous phase. Surgery was performed, but the patient's condition precluded the use of radiotherapy or chemotherapy. CT angiography revealed that the tumors were 2.9×1.9 cm (left temporal region) and 2.2×1.9 cm (right temporal region); surgical microscopy revealed surrounding strong angiogenic adhesion. The tumor pathology revealed sheets of monotonous medium- to large-sized neoplastic cells, with abundant eosinophilic cytoplasm and clear eccentric nucleus with prominent nucleoli. The tumors were positive for vimentin, glial fibrillary acidic protein and S-100 (Fig. 4). The Ki-67 labeling index was >2%, and the tumors were negative for cluster of differentiation (CD)138, CD38, pan-cytokeratin, desmin, muscle cell actin (HHF35), melanoma marker (HMB45), leukocyte common antigen, melan-A and myogenin. The diagnosis of intracranial AT/RT was verified, however, the patient succumbed to pulmonary infection at 14 days post-surgery.
Figure 3.
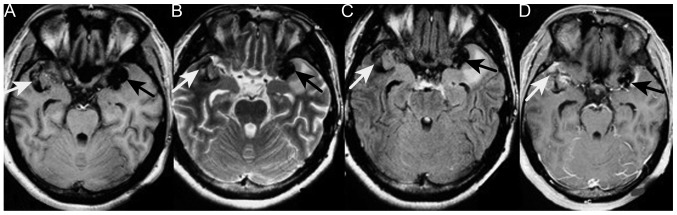
Images obtained during the second admission. A new tumor was present in the left temporal region (black arrow); the tumor on the right was larger than observed 6 months earlier (white arrow). The two tumors were symmetrical around the lateral fissure. (A) T1-weighted MRI of the low-intenisty signal tumors. (B) T2-weighted MRI of the heterogeneous tumors. (C) Fluid-attenuated inversion recovery MRI of the tumor. (D) T1-weighted contrast MRI of moderate heterogeneous enhancement. The tumors, the one on the left in particular, show moderate edema and marked calcification. MRI, magnetic resonance imaging.
Figure 4.
Tumor pathology. (A) Sheets of tumor cells (H&E staining; magnification, x40). (B and C) Rhabdoid cells with eccentric prominent nuclei and abundant eosinophilic cytoplasm (H&E staining; magnification, x200 and x400, respectively). (D) Tumor cells demonstrating diffuse cytoplasmic immunoreactivity for vimentin (magnification, x400). (E) Tumor cells demonstrating cytoplasmic immunoreactivity for S-100 (magnification, x400). (F) Tumor cells demonstrating cytoplasmic immunoreactivity for GFAP (magnification, x400). H&E, hematoxylin and eosin; GFAP, glial fibrillary acidic protein.
Discussion
The case reported in the present study is of a rare adult supratentorial AT/RT, associated with the chief complaint of vomiting and nausea. The patient's symptoms and physical examination results cannot be fully attributed to the mass itself and are more associated with cranial hypertension. To the best of our knowledge, this is the first description of an AT/RT tumor of the brain and comorbidity with EXT. Upon first admission, our primary consideration was intracranial chondrosarcoma. CT and MRI for intracranial chondrosarcoma also often disclose a mass lesion that includes areas of calcification and heterogeneous contrast enhancement. Malignant transformation of exostoses into chondrosarcoma occurs in 5% of cases (5). Brain metastasis is exceptionally rare, with few documented cases; lung and breast cancer are the primary sources of brain metastasis, together accounting for nearly two-thirds of total cases (6). Suspicion of malignant transformation is indicated by growth of the tumor following puberty, the presence of pain, a >1-cm thick cartilaginous cap in adults, or hints from radiography, bone scintigraphy (7), and enhanced CT or MRI. In the present case, the lesion did not increase in size and bone scintigraphy did not reveal focally increased radiotracer accumulation. There was no enhancement of the scapula tumor and no cardiopulmonary neoplasm on enhanced chest CT. With the exception of metastatic chondrosarcoma, intracranial primary chondrosarcoma mostly originates from skull base synchondroses and tends to grow extradurally. These neoplasms include classic, myxoid and mesenchymal chondrosarcoma (8). The peak incidence of classic chondrosarcoma is in patients in their sixth decade, where the tumor typically arises in the skull base. Mesenchymal chondrosarcoma tends to occur in the second and third decades of life, and is typically supratentorial, but most often located in the frontoparietal region and attached to the meninges, particularly the falx cerebri. The tumor has a biphasic pattern of undifferentiated mesenchymal cells and well-differentiated cartilage, and the typical transition between the two states is clear. Myxoid chondrosarcoma is the rarest form, and reported locations include the petrous bone, posterior fossa and falx cerebri. Histologically, the tumor is characterized by prominent mucinous supporting stroma. Intracranial primary chondrosarcoma has a tendency to locate in the skull base or the falx cerebri. However, the tumor in the present case was near neither the skull nor the falx cerebri. The differential diagnosis included oligodendroglioma, germinoma and other tumors containing calcification.
The present case was an uncommon example of an AT/RT tumor with typical histological features, but presenting in the supratentorial compartment of an adult. The most notable aspect was that the patient had a history of EXT. From the literature, we infer that the two tumors may be genetically correlated. Several studies reported that AT/RT was associated with insulin-like growth factor II (IGF2) and IGF receptor type 1, and that autocrine/paracrine stimulation of cell growth by IGF2, which is on chromosome 11p15 and near EXT2, may be a mechanism involved in AT/RT tumorigenesis (9–12). Therefore, AT/RT can theoretically occur together with skeletal dysplasia. However, given the scarcity of cases and limitations of the experimental infrastructure, conclusions cannot be derived at present. Instead, the current case report is presented to improve the approach to diagnosing AT/RT and aid more patients.
References
- 1.Shonka NA, Armstrong TS, Prabhu SS, Childress A, Choi S, Langford LA, Gilbert MR. Atypical teratoid/rhabdoid tumors in adults: a case report and treatment-focused review. J Clin Med Res. 2011;3:85–92. doi: 10.4021/jocmr535w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lafay-Cousin L, Hawkins C, Carret AS, Johnston D, Zelcer S, Wilson B, Jabado N, Scheinemann K, Eisenstat D, Fryer C, et al. Central nervous system atypical teratoid rhabdoid tumours: The Canadian Paediatric Brain Tumour Consortium experience. Eur J Cancer. 2012;48:353–359. doi: 10.1016/j.ejca.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Solomon L. Hereditary multiple exostosis. J Bone Joint Surg. 1963;45:292–304. [Google Scholar]
- 4.Li Y1, Wang D, Wang W, Wang J, Li H, Wang J, Wang X, Fu Q. Identification of four novel EXT1 and EXT2 mutations in five Chinese pedigrees with hereditary multiple exostoses. Genet Test Mol Biomarkers. 2009;13:825–830. doi: 10.1089/gtmb.2009.0083. [DOI] [PubMed] [Google Scholar]
- 5.Altay M, Bayrakci K, Yildiz Y, Erekul S, Saglik Y. Secondary chondrosarcoma in cartilage bone tumors: Report of 32 patients. J Orthop Sci. 2007;12:415–423. doi: 10.1007/s00776-007-1152-z. [DOI] [PubMed] [Google Scholar]
- 6.Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, Massagué J. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156:1002–1016. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sánchez-Rodríguez V, Medina-Romero F, Gómez Rodríguez-Bethencourt MÁ, González Díaz MA, González Soto MJ, Alarcó Hernández R. Value of the bone scintigraphy in multiple osteochrondromatosis with sarcomatous degeneration. Rev Esp Med Nucl Imagen Mol. 2012;31:270–274. doi: 10.1016/j.remn.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Im SH, Kim DG, Park IA, Chi JG. Primary intracranial myxoid chondrosarcoma: Report of a case and review of the literature. J Korean Med Sci. 2003;18:301–307. doi: 10.3346/jkms.2003.18.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogino S, Cohen ML, Abdul-Karim FW. Atypical teratoid/rhabdoid tumor of the CNS: Cytopathology and immunohistochemistry of insulin-like growth factor-II, insulin-like growth factor receptor type 1, cathepsin D, and Ki-67. Mod Pathol. 1999;12:379–385. [PubMed] [Google Scholar]
- 10.Tessema M, Länger F, Bock O, Seltsam A, Metzig K, Hasemeier B, Kreipe H, Lehmann U. Down-regulation of the IGF-2/H19 locus during normal and malignant hematopoiesis is independent of the imprinting pattern. Int J Oncol. 2005;26:499–507. [PubMed] [Google Scholar]
- 11.Lopez-Gines C, Cerda-Nicolas M, Kepes J, Donat J, Gil-Benso R, Llombart-Bosch A. Complex rearrangement of chromosomes 6 and 11 as the sole anomaly in atypical teratoid/rhabdoid tumors of the central nervous system. Cancer Genet Cytogenet. 2000;122:149–152. doi: 10.1016/S0165-4608(00)00289-2. [DOI] [PubMed] [Google Scholar]
- 12.Narendran A, Coppes L, Jayanthan A, Coppes M, Teja B, Bernoux D, George D, Strother D. Establishment of atypical-teratoid/rhabdoid tumor (AT/RT) cell cultures from disseminated CSF cells: A model to elucidate biology and potential targeted therapeutics. J Neurooncol. 2008;90:171–180. doi: 10.1007/s11060-008-9653-y. [DOI] [PubMed] [Google Scholar]