Abstract
Primary pancreatic lymphoma (PPL) is a rare entity, most likely to be clinically misdiagnosed as pancreatic cancer. The cure rate of PPL is higher compared with that of pancreatic adenocarcinoma. This is the case report of a 57-year-old male patient who was hospitalized with complaints of abdominal pain, weight loss and jaundice. The radiological evaluation revealed a pancreatic head mass and, following endoscopic ultrasound-guided fine-needle aspiration biopsy, the tumor was diagnosed as diffuse large B-cell lymphoma. The final diagnosis was PPL, and the patient went into remission after receiving three cycles of treatment with rituximab, doxorubicin, cyclophosphamide, vincristine and prednisolone (R-CHOP regimen). Therefore, PPL should be considered in the differential diagnosis of pancreatic masses and its management differs from that of other types of pancreatic tumor.
Keywords: primary pancreatic lymphoma, diffuse large B-cell lymphoma, pancreas, endoscopic ultrasound
Introduction
Two main types of lymphoma have been described to date, namely Hodgkin's and non-Hodgkin's lymphomas. Hodgkin's lymphomas rarely disseminate to the extralymphatic organs; by contrast, non-Hodgkin's lymphomas (NHLs) often invade extralymphatic organs (1). The most common histological type of primary pancreatic lymphoma (PPL) is NHL. The gastrointestinal (GI) tract is the most common site of extranodal NHL, accounting for 15–20% of all NHL cases (2). Although secondary involvement of the pancreas is often observed in cases of GI lymphoma, PPL is an extremely rare disease that may mimic pancreatic carcinoma. Under 2% of all extranodal malignant lymphomas and 0.5% of all pancreatic masses are PPLs (2). Behrns et al (1) defined the diagnostic criteria of PPL as follows: Mass predominantly located within the pancreas, with grossly involved lymph nodes confined to the peripancreatic region, no palpable superficial lymphadenopathy, no hepatic or splenic involvement, no mediastinal nodal enlargement on chest radiography and normal white blood cell count (1). Clinically, PPL is most likely to be misdiagnosed as pancreatic cancer (3). The cure rate of PPL is higher compared with that of pancreatic adenocarcinoma (2). This is the case report of a patient with a pancreatic head mass, diagnosed as diffuse large B-cell lymphoma following endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA) biopsy. Written informed consent was obtained from the patient.
Case report
A 57-year-old male patient was admitted with complaints of abdominal pain and 15% weight loss over the last 3 months. Jaundice, nausea and vomiting were added to the complaints over the last 2 weeks. The physical examination revealed jaundice, cachexia and abdominal tenderness. Organomegaly or lymphadenopathy were not detected. The patient had no family history of cancer, was not a smoker and had no history of alcohol abuse.
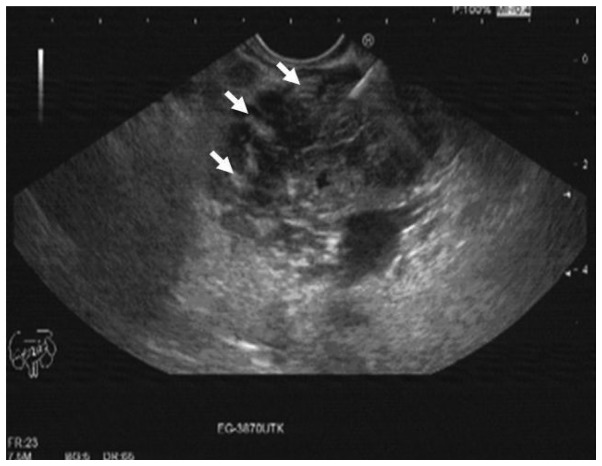
The laboratory test results revealed indirect hyperbilirubinemia (total bilirubin, 16.4 mg/dl; conjugated bilirubin, 14.3 mg/dl), hyperamylasemia (817 U/l) and hyperlipasemia (2,249 U/l). The liver function enzymes were normal; however, the γ-glutamyl transferase (GGT), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) levels were increased to 333, 585 and 3,325 U/l, respectively. The erythrocyte sedimentation rate was increased (94 mm/h) and the tumor markers were normal. The β2-microglobulin level of the patient was 9.42 mg/l (normal range, 1.09–2.53 mg/l). The abdominal computed tomography (CT) revealed a 4×3-cm hypodense lesion in the head of the pancreas. Compression of the duodenum and common bile duct by the mass and multiple pathological lymphadenopathies were also observed (Fig. 1A and B). The EUS revealed increased lobularity of the pancreas with hyperechoic bands, irregularity of the main pancreatic duct and dilation of the common bile duct and biliary tree (Fig. 2). The pathological evaluation of EUS-guided FNA biopsy (with a 22 G needle) revealed that the tumor was CD20-positive and CD3- and pancytokeratin-negative, with a high Ki-67 proliferation index (70%), findings consistent with diffuse large B-cell lymphoma (Fig. 3A and B). The final diagnosis was PPL and the patient went into remission after receiving three cycles of treatment with rituximab, doxorubicin, cyclophosphamide, vincristine and prednisolone (R-CHOP regimen).
Figure 1.
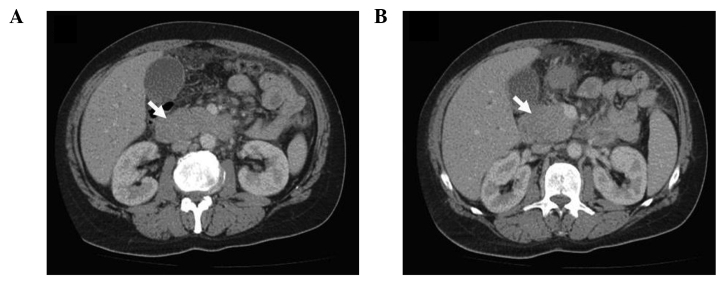
Abdominal computed tomography images. (A) Diffuse enlargement of the pancreas. (B) Tumor formation at the head of the pancreas.
Figure 2.
Endoscopic ultrasonography images (transduodenal view). Increased lobularity with hyperechoic bands.
Figure 3.
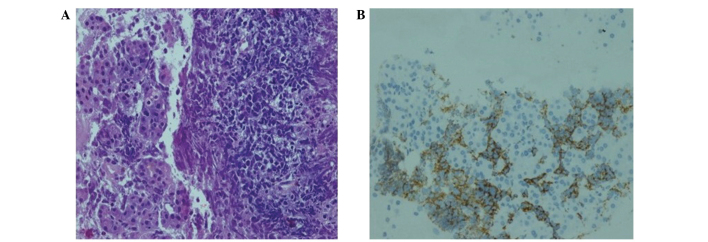
Histopathological examination. (A) Irregularly-shaped, atypical lymphocyte infiltration (hematoxylin and eosin staining; magnification, x50). (B) CD20-positive atypical lymphocytic cells (immunohistochemistry; magnification, x25).
Discussion
PPL is a rare entity. The clinical manifestations and radiological findings of PPL should be differentiated from those of chronic pancreatitis, including autoimmune cases, and occupying lesions, such as pancreatic carcinoma. PPL accounts for <1% of all pancreatic lesions (3). In a previous review, among 207 cases of malignant pancreatic tumors, only 3 cases (1.5%) were pancreatic lymphomas (4). Volmar et al (5) evaluated the pathological results of 1,050 FNA biopsies of pancreatic lesions and reported that only 14 cases (1.3%) were pancreatic lymphomas. Pancreatic lymphoma is a disease exhibiting a male predominance (male:female ratio, 7:1) (2). The age of the patients and duration of the symptoms are usually similar to those of pancreatic adenocarcinoma (2). The most common presenting symptom reported is abdominal pain (83%), followed by abdominal mass (58%), weight loss (50%), jaundice (37%), acute pancreatitis (12%), small-bowel obstruction (12%) and diarrhea (12%) (3). Frequent symptoms of NHL, such as fever, chills and night sweats, are rare in PPL (6). The patient in this study exhibited abdominal pain, weight loss, jaundice, nausea, vomiting and acute pancreatitis on admission. The majority of PPLs occur in the head of the pancreas, although this tumor may also be found in the body and tail (3). In a previousstudy, >50% of the patients presented with an epigastric mass, the diameter of which was >6 cm in 70% of the cases (7).
The laboratory tests for PPL are non-specific. Tumor burden, β2-microglobulin levels >2 mg/l and high LDH levels are poor prognostic markers (8). In the present case, the levels of total and conjugated bilirubin, amylase, lipase, GGT, ALP, LDH and β2-microglobulin were increased. Although anectodal cases with increased carbohydrate antigen 19-9 (CA19-9) levels have been reported, the CA19-9 level was normal in the present case. Imaging is crucial for the diagnosis of PPL. Transabdominal ultrasonography, EUS, CT and magnetic resonance imaging are well-established modalities for evaluating pancreatic lesions (8). Two different morphological patterns of pancreatic involvement are observed, namely a localized, well-circumscribed tumor pattern and a diffuse enlargement pattern, infiltrating or replacing the majority of the pancreatic gland (9), as in the present case. Certain radiological findings may help differentiate PPL from the more common pancreatic adenocarcinoma: Bulky, localized pancreatic head tumor without significant Wirsung duct dilatation, enlarged lymph nodes below the level of the renal veins, and invasive and infiltrating growth through to the retroperitoneal or upper abdominal organs and the GI tract (9).
The presence of calcification or necrosis rules out NHL. Peripancreatic adenopathy, diffusely increased lobularity, hyperechoic bands and enlargement of the pancreas with minimally dilated pancreatic and intrahepatic bile ducts were observed. Imaging techniques may suggest PPL, but are unable to distinguish between PPL and pancreatic adenocarcinoma (6,10). Therefore, the definitive diagnosis of PPL is based on the cyto/histological examination (6). Alternatively, a laparoscopy or laparotomy may be performed to conduct a biopsy of the pancreatic mass or lymph nodes (10). EUS-guided tissue sampling of the pancreatic mass is the optimal approach, as it is highly accurate. Sampling with a core needle is also possible using EUS. In the present case, diagnosis was based on EUS-guided FNA biopsy.
The treatment of PPL consists of chemotherapy or radiotherapy (3). Behrns et al (1) reported that the median survival of PPL patients treated by chemotherapy or radiotherapy alone was 13 and 22 months, respectively, whereas it was ≤26 months with combined chemoradiotherapy. Therefore, the first treatment choice for PPL should be a combination of chemotherapy and radiotherapy, rather than surgery. Due to the advances in EUS-guided biopsy techniques, surgery is preferred only when EUS-guided FNA is not available, or when the diagnosis is not possible by FNA biopsy. It has already been proven that pancreatic resection alone does not improve the survival rate of PPL (3). Three cycles of R-CHOP were administered to the patient in this case, who responded well, achieving remission.
In conclusion, we presented a rare case of PPL presenting with abdominal pain, weight loss and obstructive jaundice, diagnosed by EUS-guided FNA. PPL should be considered in the differential diagnosis of pancreatic masses and its management differs from that of other types of pancreatic tumors.
Glossary
Abbreviations
- PPL
primary pancreatic lymphoma
- GI
gastrointestinal
- NHL
non-Hodgkin's lymphoma
- CT
computed tomography
- EUS
endoscopic ultrasound
- FNA
fine-needle aspiration
References
- 1.Behrns KE, Sarr MG, Strickler JG. Pancreatic lymphoma: Is it a surgical disease? Pancreas. 1994;9:662–667. doi: 10.1097/00006676-199409000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Haji AG, Sharma S, Majeed KA, Vijaykumar DK, Pavithran K, Dinesh M. Primary pancreatic lymphoma: Report of three cases with review of literature. Indian J Med Paediatr Oncol. 2009;30:20–23. doi: 10.4103/0971-5851.56331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hai Lin, Li SD, Hu XG, Li ZS. Primary pancreatic lymphoma: Report of six cases. World J Gastroenterol. 2006;12:5064–5067. doi: 10.3748/wjg.v12.i31.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reed K, Vose PC, Jarstfer BS. Pancreatic cancer: 30 year review (1947 to 1977) Am J Surg. 1979;138:929–933. doi: 10.1016/0002-9610(79)90324-6. [DOI] [PubMed] [Google Scholar]
- 5.Volmar KE, Routbort MJ, Jones CK, Xie HB. Primary pancreatic lymphoma evaluated by fine-needle aspiration: Findings in 14 cases. Am J Clin Pathol. 2004;121:898–903. doi: 10.1309/UAD9PYFUA82X9R9U. [DOI] [PubMed] [Google Scholar]
- 6.Arcari A, Anselmi E, Bernuzzi P, Bertè R, Lazzaro A, Moroni CF, Trabacchi E, Vallisa D, Vercelli A, Cavanna L. Primary pancreatic lymphoma. Report of five cases. Haematologica. 2005;90:ECR09. [PubMed] [Google Scholar]
- 7.Tuchek JM, De Jong SA, Pickleman J. Diagnosis, surgical intervention and prognosis of primary pancreatic lymphoma. Am Surg. 1993;59:513–518. [PubMed] [Google Scholar]
- 8.Tondini C, Giardini R, Bozzetti F, Valagussa P, Santoro A, Bertulli R, Balzarotti M, Rocca A, Lombardi F, Ferreri AJ. Combined modality treatment for primary gastrointestinal non-Hodgkin's lymphoma: The Milan Cancer Institute experience. Ann Oncol. 1993;4:831–837. doi: 10.1093/oxfordjournals.annonc.a058388. [DOI] [PubMed] [Google Scholar]
- 9.Merkle EM, Bender GN, Brambs HJ. Imaging findings in pancreatic lymphoma: Differential aspects. AJR Am J Roentgenol. 2000;174:671–675. doi: 10.2214/ajr.174.3.1740671. [DOI] [PubMed] [Google Scholar]
- 10.Rose JF, Jie T, Usera P, Ong ES. Pancreaticoduodenectomy for primary pancreatic lymphoma. Gastrointest Cancer Res. 2012;5:32–34. [PMC free article] [PubMed] [Google Scholar]