Abstract
Vascular pathologies are associated with changes in the presence and expression of morphologically distinct vascular smooth muscle cells. In particular, in complex human vascular lesions and models of disease in pigs and rodents, an intimal smooth muscle cell (iSMC) which exhibits a stable epithelioid or rhomboid phenotype in culture is often found to be present in high numbers, and may represent the reemergence of a distinct developmental vascular smooth muscle cell phenotype. The CYP450-oxylipin - soluble epoxide hydrolase (sEH) pathway is currently of great interest in targeting for cardiovascular disease. sEH inhibitors limit the development of hypertension, diabetes, atherosclerosis and aneurysm formation in animal models. We have investigated the expression of CYP450-oxylipin-sEH pathway enzymes and their metabolites in paired intimal (iSMC) and medial (mSMC) cells isolated from rat aorta. iSMC basally released significantly larger amounts of epoxy-oxylipin CYP450 products from eicosapentaenoic acid > docosahexaenoic acid > arachidonic acid > linoleic acid, and expressed higher levels of CYP2C12, CYP2B1, but not CYP2J mRNA compared to mSMC. When stimulated with the pro-inflammatory TLR4 ligand LPS, epoxy-oxylipin production did not change greatly in iSMC. In contrast, LPS induced epoxy-oxylipin products in mSMC and induced CYP2J4. iSMC and mSMC express sEH which metabolizes primary epoxy-oxylipins to their dihydroxy-counterparts. The sEH inhibitors TPPU or AUDA inhibited LPS-induced NFκB activation and iNOS induction in mSMC, but had no effect on NFκB nuclear localization or inducible nitric oxide synthase in iSMC; effects which were recapitulated in part by addition of authentic epoxy-oxylipins. iSMCs are a rich source but not a sensor of anti-inflammatory epoxy-oxylipins. Complex lesions that contain high levels of iSMCs may be more resistant to the protective effects of sEH inhibitors.
Keywords: Smooth muscle cell, LPS, Oxylipin, Epoxygenase, Soluble epoxide hydrolase
Abbreviations: AA, arachidonic acid; DHA, docosahexaenoic acid; DHET, dihydroxy eicosatrienoic acid; DHOME, dihydroxy-octadecenoic acid; DiHDPA, dihydroxy-docosapentaenoic acid; EET, epoxyeicosatrienoic acid; EPA, eicosapentaenoic acid; EPOME, epoxy-octadecenoic acid; HETE, hydroxyeicosatetraenoic acid; iSMC, intimal smooth muscle cell; LA, linoleic acid; mSMC, medial smooth muscle cell; PPAR, peroxisome proliferator activated receptor; sEH, soluble epoxide hydrolase; SMC, smooth muscle cell
Highlights
-
•
We examined oxylipin production in different SMC phenotypes.
-
•
Intimal SMC produced more oxylipins than medial SMC.
-
•
CYPs were differentially expressed and regulated by LPS in intimal and medial SMC.
-
•
sEH inhibitors reduce inflammation in medial but not intimal SMC.
-
•
Intimal SMC are a source but not sensor of epoxy-oxylipins.
1. Introduction
Vascular smooth muscle cells (SMC) exhibit both plasticity and heterogeneity in culture and in vascular pathologies such as atherosclerosis or aneurysm [1–5]. In particular vascular disease is associated with stable phenotypically distinct populations of SMC that can be cultured for further analysis. The most commonly utilized SMC phenotype used in culture is the “adult” medial spindle shaped (m)SMC that grows typically with “hill and valley” morphology. However, diseased vessels also contain epithelial, rhomboid or “π” phenotype SMC often associated with neointimal thickening and remodeling. These intimal iSMC phenotypes can be isolated from rat aorta [1,5–7], pig coronary artery and human atherosclerotic lesions [3,8,9]. iSMC phenotypes differ from adult medial SMC not only in morphology, but also in terms of proliferative ability and their expression of S100A, PDGF-B, PDGF α-receptor, α1 (I) collagen, CYP1AI, elastin, osteopontin, plasminogen activator [6–8,10,11], along with cyclo-oxygenase-1 and COX-2, prostanoid production and peroxisome-proliferator activated receptor (PPAR)-γ expression [12,13].
In addition to COX pathways, lipoxygenase and CYP450 enzymes are also capable of metabolizing arachidonic acid and related polyunsaturated fatty acids (linoleic acid (LA), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA)) to series of biologically active mediators [14,15]. The roles of both CYP450 lipid metabolizing pathways, and potential beneficial effects of n3-PUFAs DHA and EPA in vascular biology, are of current intense interest [16–20]. Feeding DHA and/or EPA to humans [21] or rats [22] increases their respective epoxy-oxylipin products in circulation. Whether iSMC and mSMC differentially metabolize or use these products is not known. A number of CYPs can metabolize fatty acids into series of oxylipin mediators by a combination of either epoxygenases, lipoxygenase-like or ω- and ω-1-hydroxylase activities [15]. Using AA as an example, both epoxyeicosatrienoic acids (EETs) and hydroxyeicosatetraenoic acids (HETEs) can be formed from these activities [15]. Although approximately one fifth of the 57 putative CYPs in man have shown lipid metabolizing activities [16], the major contributors to epoxygenase activity are thought to be the CYP2 enzymes, in particular members of the CYP2J and CYP2C subfamilies [15,23–26].
Once formed, epoxygenase products are rapidly removed [15,18]. Soluble epoxide hydrolase (sEH; encoded by the gene ephx2) appears to be key in the metabolism of these oxylipins [18,27], and sEH-inhibitors (sEH-I) have been developed which limit the breakdown of oxylipins to their more soluble dihydroxy-counterparts. sEH-I use in mouse models, or mice with genetic disruption of sEH exhibit reduced neointima formation after injury [28], reduced atherosclerosis, and aneurysm formation, reduced hypertension [29] and reduced indices of type 2 diabetes [30], inflammatory cell recruitment [31] and pain [32]. The aim of this study was to assess i) the formation and ii) the roles of oxylipins derived from CYP450 enzymes in regulating inflammatory responses in paired mSMC and iSMC.
2. Materials and methods
2.1. Materials
AUDA (12-[[(tricyclo[3.3.1.13,7]dec-1-ylamino)carbonyl]amino]-dodecanoic acid), TPPU (N-[1-(1-oxopropyl)-4-piperidinyl]-N′-[4-(trifluoromethoxy)phenyl)]-urea), and authentic oxylipins (EETs, DHEQ, and HDPA) were from Cayman Chemical Company (Cambridge Bioscience, Cambridge, UK). SKF525A (α-phenyl-α-propyl-2-(diethylamino)ethyl ester-benzeneacetic acid) was from Biomol (Affiniti Research Products, Exeter, UK). Rabbit anti-p65 was from Santa Cruz (Heidelberg, Germany). Unless stated, all other reagents were from Sigma–Aldrich (Poole, Dorset, UK).
2.2. Cell and tissue culture
iSMC (WKY12-22) and mSMC (WKY3m-22) (Supplemental Figure 1) were isolated and cultured in DMEM supplemented with antibiotic/antimycotic mix, and 10% FBS; 37 °C; 5% CO2; 95% air, as previously described [12]. Since FBS interferes with lipid substrate composition and the release and detection of eicosanoids (M. Edin, unpublished observations), all experiments were performed with DMEM supplemented with antibiotic/antimycotic mix, without FBS.
2.3. Real-time qRT-PCR
CYP2J3 and sEH mRNA was measured by the Taqman qRT-PCR ddCt method. mRNA for other CYPs and sEH were measured using the Sybr Green ddCT method. Targets were normalized to 18S expression. RNA was extracted using the Thermo Scientific RNA extraction kit and 1 μg of total RNA was used to generate cDNA using Superscript II (Invitrogen) according to manufacturer's instructions. Sybr green qPCR was performed using Premix Ex Taq II mastermix (Takara) using a Chromo-4 machine and Opticon software. Genomic sequences were obtained from the UCSC Genome Browser website (http://genome.ucsc.edu/cgi-bin/hgGateway) and primers for rat CYP2J4, CYP2J10, CYP2B1, CYP2C11, CYP2C12, CYP2C22, CYP2C23, and CYP2C24 (Supplemental Table 1) were designed from NCBI's Primer Blast website (http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi? LINK_LOC=BlastHome).
2.4. Inducible nitric oxide synthase activity, cell viability, immunoassays and oxylipin measurements
iNOS activity was measured by the accumulated formation of nitrite in the medium by the Greiss reaction as previously described [33]. In these experiments cell viability by MTT assays was also routinely performed as previously described [34]; and there were no significant changes between treatments groups. Immunofluorescence for p65 was performed as previously described using primary antibody dilutions of 1:100 [34]. LC/MS/MS analysis of oxylipin products in culture supernatants was as previously described [35].
3. Results
3.1. Unstimulated iSMC produce larger amounts of epoxy-oxylipins than mSMC
The epoxygenase-sEH products of AA 5,6-DHET, 14,15-DHET (Fig. 1A and B); LA: 9,10-, and 12,13-epoxy-octadecenoic acid (EpOME), and their respective sEH products 9,10-DHOME, and 12,13-DHOME; DHA: 19,20-dihydroxy-docosapentaenoic acid (DiHDPA); and EPA (17,18-DHEQ) (Fig. 1C and D) were released by mSMC and iSMC over 48 h. 17,18-DHEQ was the most abundant epoxygenase product detected under basal culture conditions in both iSMC and mSMC cultures. iSMC secreted 2–3 fold more of EPA, DHA and AA derived oxylipins than mSMC (Fig. 1), whereas EPOME production from LA (the most minor product formed in both cell types) was higher in mSMC compared to iSMC.
Fig. 1.
Basal and LPS stimulated epoxy-oxylipin production iSMC and mSMC. Endogenous 5,6- and 14,15-DHETs (A and B), 9,10, 12-13-EPOME and DHOME, 17,18-DHEQ, and 19,20-DiHDPA (C and D) release (pg/ml) from untreated, and LPS (1 μg/ml) treated iSMC (A and C) and mSMC (B and D) over 48 h. For comparison, panels A and B and C and D have been shown with the same scale. Panel D is also shown with an expanded scale so significant differences can more clearly be seen. * indicates p < 0.05 by Wilcoxon signed rank test between control and LPS treatment. (E) EET and DHET production (pg/ml) from untreated intimal (i)SMC and medial (m)SMC in response to the addition of arachidonic acid (10 mM; 30 min), to show capacity each cell type to produce EETs. (F) Change in EET production capacity in iSMC and mSMC after TLR-4 ligand activation (LPS, 1 μg/ml; 24 h). After 24 h, medium from untreated or LPS treated cells was removed and fresh medium containing arachidonic acid added (10 mM; 30 min). The level of each EET produced in response to AA from the LPS treated cells was compared to the levels produced from AA in untreated cells to give total combined fold EET difference. * indicates p < 0.05 by one-sample t-test between control and LPS treatment. Data represent the mean ± SEM from n = 4 experiments.
3.2. mSMC but not iSMC epoxy-oxylipin production is induced by TLR-4 activation
A number of epoxy-oxylipins are anti-inflammatory and are induced during inflammation [16]. In response to TLR-4 activation, total epoxy-oxylipin production did not dramatically change in iSMC, though there were small but significant increases in the minor products (9,10-EPOME, 12,13-EPOME and 5,6-DHET; Fig. 1A and C). In contrast, in mSMC, 5,6-, 14,15-DHET, 17,18-DHEQ, and 19,20-DiHDPA were all significantly elevated in response to TLR-4 activation with LPS (Fig. 1B and D).
AA and LA lipoxygenase products (5-, 8-, 11-, 12-, 15 and 19-HETE, and 9- and 13-HODE respectively), were also detected from unstimulated iSMC and mSMC (Supplemental Figure 2), and with the exception of 12-HETE, could all be induced by LPS. Lipoxygenase product formation was higher in mSMC compared to iSMC both basally and in response to LPS (Supplemental Figure 2).
To test at which level epoxy-oxylipin production is regulated, iSMC and mSMC in serum free culture were treated with AA (10 mM; 30 min) to examine the maximum capacity of each cell type to produce epoxygenase products independently of substrate formation by PLA2. AA alone induced a similar release of 5,6-, 8,9-, 11,12-, and 14,15-EET in both iSMC and mSMC (Fig. 1E). DHETs were produced in much lower amounts (Fig. 1E); as would be expected from using these high levels of AA over only 30 min. In contrast to this identical basal capacity, TLR-4 activation reduced this capacity in iSMC, whereas the capacity in mSMC increased 1.5 fold, suggesting LPS induces CYP-epoxygenase enzyme activity in mSMC while reduces them in iSMC (Fig. 1F).
3.3. iSMC and mSMC differentially express lipid metabolizing CYPs and sEH basally and after TLR-4 activation
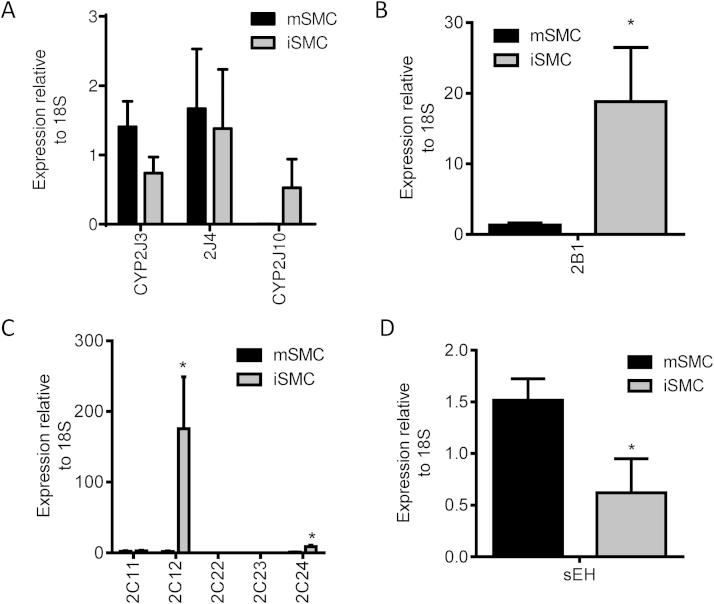
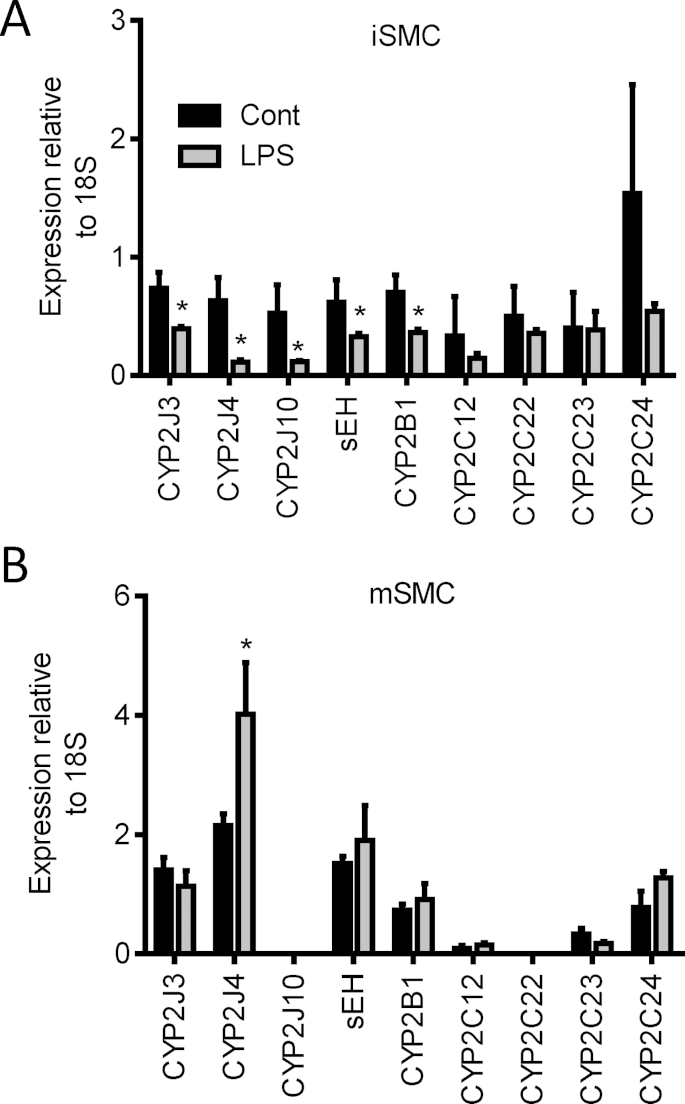
mSMC and iSMC both expressed CYP2J3, CYP2J4, CYP2J10, CYP2B1, CYP2C11, CYP2C12, CYP2C24 and sEH (ephx2) mRNA, but not CYP2C22 or CYP2C23 mRNA (Fig. 2). There was no difference in CYP2J or CYP2C11 expression at the mRNA level (relative to 18S), however iSMC expressed relatively more CYP2B1, CYP2C11 and CYP2C24 mRNA, whereas mSMC expressed significantly higher levels of sEH (Fig. 2D). In iSMC, CYP2J3, CYP2J4 and CYP2J10 mRNA levels were suppressed after LPS treatment mirroring the decrease in total synthetic capacity (Fig. 3A). In contrast, in mSMC CYP2J4 mRNA was induced and CYPJ3, CYP2B1, CYP2C isoforms and sEH were unaltered (Fig. 3B).
Fig. 2.
Basal expression of CYPs and sEH expression in iSMC and mSMC. qRT-PCR analysis of rat CYP2J family (A), CYP2B1 (B), CYP2C family (C) and sEH (D) in iSMC and mSMC. Data show relative expression compared to 18S as mean ± SEM from n = 3 separate experiments. * indicates p < 0.05 by Wilcoxon signed rank test between iSMC and mSMC.
Fig. 3.
Regulation of CYP2J and sEH expression in iSMC and mSMC in response to LPS. Changes in CYP2J, CYP2C family, CYP2B1 and sEH mRNA expression in iSMC (A) and mSMC (B) in response to LPS treatment (1 μg/ml; 24 h). Data show relative expression compared to 18S as mean ± SEM from n = 3 separate experiments. * indicates p < 0.05 by Wilcoxon signed rank test between untreated and LPS treatment for each cell type individually.
3.4. sEH inhibitors reduce TLR-4 induced iNOS and NF-κB in mSMC but not iSMC
Treatment with the sEH-I TPPU (1 μM) significantly reduced TLR-4 ligand LPS induced iNOS activity in mSMC, but not iSMC (Fig. 4A). Conversely, inhibiting basal epoxygenase activity using SKF525A (10 μM; 72 h) induced iNOS activity in mSMC but not iSMC (Fig. 4B). In addition, a distinct sEH-I, AUDA (10 μM) or addition of 11,12-EET or 14,15-EET (300 nM) similarly inhibited LPS-induced iNOS activity (Fig. 4C). As TLR-4 induced iNOS requires NF-κB activation, we examined the effects of sEH-Is on the nuclear localization of the p65 subunit. iSMC had constitutively high levels of p65 expressed in the nucleus, which were not greatly altered by LPS, TPPU or AUDA (Fig. 4D). In contrast, in mSMC p65 was predominantly cytoplasmic under standard culture conditions. LPS induced a distinct nuclear trans-localization of p65 in mSMC, which was abolished when cells were pretreated with either AUDA or TPPU (Fig. 4D).
Fig. 4.
Epoxy-oxylipins are anti-inflammatory in mSMC but not iSMC. Inducible nitric oxide synthase activity (iNOS) measured by accumulated nitrite formation (μM) in: (A) iSMC and mSMC treated with or without LPS (1 μg/ml; 72 h), in the presence or absence of the sEH inhibitor TPPU (1 μM; given as a 1 h pretreatment before addition of LPS); (B) iSMC and mSMC treated with or without the epoxygenase inhibitor SKF525A (10 μM; 72 h); and (C) mSMC treated with or without LPS (1 μg/ml; 72 h), in the presence or absence of the sEH inhibitor AUDA (10 μM), 11,12-EET (300 nM) or 14,15-EET (300 nM). (D) Effect of sEH inhibitors on LPS-induced NF-κB p65 nuclear localization. iSMC (left hand panels) or mSMC (right hand panels) were treated with LPS (1 μg/ml) in the presence or absence of TPPU (1 μM) or AUDA (10 μM) for 2 h and p65 nuclear localization examined. iSMC show constitutive p65 nuclear localization which is not affected by AUDA or TPPU. LPS induces p65 nuclear localization in mSMC (top right panels; indicated by white arrows), which is inhibited by either AUDA or TPPU co-incubation. Immunofluorescent micrographs are representative of n = 3 experiments. As a negative control, in some experiments primary antibody was omitted (2° Ab control) which showed no specific staining.
4. Discussion
Restenotic and complex vascular lesions are associated with a heterogeneous population of vascular smooth muscle cells [1,2]. Although vascular smooth muscle cells show great plasticity i.e. the phenotypic switch between contractile and synthetic phenotypes classically seen with mSMC, and potential to form macrophage-like cells [36], distinct phenotypic stable populations also emerge which have a distinct epitheliod/rhomboid morphology when grown in culture [1,4,6,8,9]. These iSMC may represent a developmental phenotype that expands during disease progression [1–5]. Functionally these iSMC in vitro are highly proliferative, even in low serum concentrations and are more inflammatory [1,4–6,8,9,13], highlighted by our finding that iSMC exhibit constitutive p65 nuclear localization. As such iSMC are considered a pathological phenotype in restenosis, and contribute to the pro-inflammatory milieu of atherosclerotic lesions.
We used a targeted lipidomic approach to identify the profile of oxylipins in iSMC and mSMC. iSMC were a rich source of oxylipins which are known to have anti-inflammatory activities [18,20,27,32,37]. Interestingly, iSMC unlike mSMC were not sensitive to the anti-inflammatory actions of sEH inhibitors. This lack of sensitivity of endogenous anti-inflammatory oxylipins may add to the explanation of the pro-inflammatory phenotype of these cells. Moreover, since sEH inhibitors are being tested experimentally and clinically, the emergence and contribution of iSMC to a lesion may limit the effectiveness of sEH inhibitors. iSMC do express relatively lower levels of sEH mRNA; however, this is unlikely to explain the lack of sensitivity of sEH-inhibitors as the EH metabolites are clearly still the major products. A synthetic role combined with a lack of sensitivity for iSMC is not uncommon, as similar findings have also been described for iNOS, in that intimal cells are high expressers and producers, but lack sensitivity to the actions of NO itself [38].
There is a great interest in identifying markers that may differentiate iSMC and mSMC, and although no single oxylipin metabolite distinguishes the two, here at least in vitro CYP2C12, CYP2B1 and potentially CYP2J10 may help to distinguish iSMC from mSMC. In the liver, inflammation tends to results in a widespread suppression of CYPs [39]. In contrast, in human monocytes, macrophages and endothelial cells, TLR-4 activation results in the induction of CYP2J2, but not CYP2C8 or CYP2C9 [37,40]. Interestingly, CYP regulation in iSMC in response to LPS resembles a response similar to the liver, in that a widespread suppression of CYPs were observed. In contrast, in mSMC, LPS induced total CYP capacity and the mRNA for at least one CYP, CYP2J4. Unlike humans who have just one CYP2J family member (CYP2J2), rats have multiple CYP2J enzymes. CYP2J4, with respect of sensitivity to LPS, may be most similar to human CYP2J2.
EPA and DHA are considered potential key constituents underlying the cardiovascular health benefits of diets such as the Mediterranean diet. Of the products measured, the AA products, 5,6- and 14,15,DHET; the LA products: 9,10-EPOME and 12,13-EPOME (and 9,10-DHOME and 12,13-DHOME); the DHA product 19,20-DiHDPA; and the EPA product 17,18-DHEQ were all detectable. The EPA product 17,18-DHEQ was the most abundant; a finding consistent with measurements from fresh rat aorta (Supplemental Figure 3), and circulating levels of oxylipins in human healthy volunteers [41]. The enrichment of human or rodent diets with DHA or EPA leads to increases in respective DHA and EPA epoxy-oxylipin products [21,22]. Vascular metabolism of EPA and DHA into epoxy-oxylipins could therefore contribute to the purported benefits of these lifestyle modifications.
Since the receptor targets of these oxylipin products remain elusive [42], and we cannot selectively remove these mediators, we are still greatly limited in our ability to dissect out the roles of these endogenously produced epoxy-oxylipin mediators. We and others have suggested PPARs as anti-inflammatory receptor targets for EETs [43]. We previously reported iSMC and mSMC contain all PPARs [12], however in our hands, the concomitant use of PPARα (GW6471), β/δ (GSK0660) or -γ (GW9662) antagonists did not affect the inhibitory actions of EETs on iNOS (SJT and DBB; unpublished observations). iSMC and mSMC are derived from rats of an identical genetic background [6], and have been grown and treated in identical cell culture conditions. The fact that iSMC produce but do not respond to epoxy-lipins, whereas mSMC produce and respond to epoxy-oxylipins may make these two cell populations extremely useful tools for identifying oxylipin receptor targets.
In conclusion, pathological iSMC represent a source but not sensor of epoxy-oxylipin mediators. Although the contribution of iSMC to differing vascular pathologies is still being debated, their presence in larger amounts and in complex lesions may limit the anti-inflammatory and protective effects of sEH inhibitors in highly developed pre-existing lesions. Understanding the signaling (or lack thereof) of oxylipins in iSMC may help to reveal new receptor targets and help explain the benefits of diets rich in ω−3 DHA and EPA.
Acknowledgment
This work was supported by funding from the British Heart Foundation (PG/11/39/28890 to DBB and DWG), and in part, by funds from the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences Z01 ES025034 to DCZ and R01 ES002710 to BDH.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.bbrc.2015.06.012.
Transparency document related to this article can be found online at http://dx.doi.org/10.1016/j.bbrc.2015.06.012.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Transparency document
References
- 1.Hao H., Gabbiani G., Bochaton-Piallat M.L. Arterial smooth muscle cell heterogeneity: implications for atherosclerosis and restenosis development. Arterioscler. Thromb. Vasc. Biol. 2003;23:1510–1520. doi: 10.1161/01.ATV.0000090130.85752.ED. [DOI] [PubMed] [Google Scholar]
- 2.Shanahan C.M., Weissberg P.L. Smooth muscle cell phenotypes in atherosclerotic lesions. Curr. Opin. Lipidol. 1999;10:507–513. doi: 10.1097/00041433-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Hao H., Gabbiani G., Camenzind E., Bacchetta M., Virmani R., Bochaton-Piallat M.L. Phenotypic modulation of intima and media smooth muscle cells in fatal cases of coronary artery lesion. Arteriosclerosis Thromb. Vasc. Biol. 2006;26:326–332. doi: 10.1161/01.ATV.0000199393.74656.4c. [DOI] [PubMed] [Google Scholar]
- 4.Majesky M.W., Giachelli C.M., Reidy M.A., Schwartz S.M. Rat carotid neointimal smooth muscle cells reexpress a developmentally regulated mRNA phenotype during repair of arterial injury. Circ. Res. 1992;71:759–768. doi: 10.1161/01.res.71.4.759. [DOI] [PubMed] [Google Scholar]
- 5.Villaschi S., Nicosia R.F., Smith M.R. Isolation of a morphologically and functionally distinct smooth-muscle cell-type from the intimal aspect of the normal rat aorta – evidence for smooth-muscle cell heterogeneity. In Vitro Cell. Dev. Biol. Anim. 1994;30A:589–595. doi: 10.1007/BF02631257. [DOI] [PubMed] [Google Scholar]
- 6.Lemire J.M., Covin C.W., White S., Giachelli C.M., Schwartz S.M. Characterization of cloned aortic smooth muscle cells from young rats. Am. J. Pathol. 1994;144:1068–1081. [PMC free article] [PubMed] [Google Scholar]
- 7.Myit S., Delafontaine P., Bochaton-Piallat M.L., Giraud S., Gabbiani G., Brink M. Different growth properties of neointimal and medial smooth muscle cells in response to growth factors. J. Vasc. Res. 2003;40:97–104. doi: 10.1159/000070706. [DOI] [PubMed] [Google Scholar]
- 8.Brisset A.C., Hao H., Camenzind E., Bacchetta M., Geinoz A., Sanchez J.C., Chaponnier C., Gabbiani G., Bochaton-Piallat M.L. Intimal smooth muscle cells of porcine and human coronary artery express S100A4, a marker of the rhomboid phenotype in vitro. Circ. Res. 2007;100:1055–1062. doi: 10.1161/01.RES.0000262654.84810.6c. [DOI] [PubMed] [Google Scholar]
- 9.Hao H., Ropraz P., Verin V., Camenzind E., Geinoz A., Pepper M.S., Gabbiani G., Bochaton-Piallat M.L. Heterogeneity of smooth muscle cell populations cultured from pig coronary artery. Arteriosclerosis Thromb. Vasc. Biol. 2002;22:1093–1099. doi: 10.1161/01.atv.0000022407.91111.e4. [DOI] [PubMed] [Google Scholar]
- 10.Majesky M.W., Schwartz S.M. Smooth muscle diversity in arterial wound repair. Toxicol. Pathol. 1990;18:554–559. [PubMed] [Google Scholar]
- 11.Neuville P., Geinoz A., Benzonana G., Redard M., Gabbiani F., Ropraz P., Gabbiani G. Cellular retinol-binding protein-1 is expressed by distinct subsets of rat arterial smooth muscle cells in vitro and in vivo. Am. J. Pathol. 1997;150:509–521. [PMC free article] [PubMed] [Google Scholar]
- 12.Bishop-Bailey D., Hla T., Warner T.D. Intimal smooth muscle cells as a target for peroxisome proliferator-activated receptor-gamma ligand therapy. Circ. Res. 2002;91:210–217. doi: 10.1161/01.res.0000029080.15742.85. [DOI] [PubMed] [Google Scholar]
- 13.Bishop-Bailey D., Warner T.D. PPARgamma ligands induce prostaglandin production in vascular smooth muscle cells: indomethacin acts as a peroxisome proliferator-activated receptor-gamma antagonist. FASEB J. 2003;17:1925–1927. doi: 10.1096/fj.02-1075fje. [DOI] [PubMed] [Google Scholar]
- 14.Bishop-Bailey D., Wray J. Peroxisome proliferator-activated receptors: a critical review on endogenous pathways for ligand generation. Prostagl. Other Lipid Mediat. 2003;71:1–22. doi: 10.1016/s0090-6980(03)00003-0. [DOI] [PubMed] [Google Scholar]
- 15.Zeldin D.C. Epoxygenase pathways of arachidonic acid metabolism. J. Biol. Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 16.Bishop-Bailey D., Thomson S., Askari A., Faulkner A., Wheeler-Jones C. Lipid-metabolizing CYPs in the regulation and dysregulation of metabolism. Annu. Rev. Nutr. 2014;34:261–279. doi: 10.1146/annurev-nutr-071813-105747. [DOI] [PubMed] [Google Scholar]
- 17.Westphal C., Konkel A., Schunck W.H. CYP-eicosanoids–a new link between omega-3 fatty acids and cardiac disease? Prostagl. Other Lipid Mediat. 2011;96:99–108. doi: 10.1016/j.prostaglandins.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Imig J.D., Hammock B.D. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Askari A., Thomson S.J., Edin M.L., Zeldin D.C., Bishop-Bailey D. Roles of the epoxygenase CYP2J2 in the endothelium. Prostagl. Other Lipid Mediat. 2013;107:56–63. doi: 10.1016/j.prostaglandins.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming I. The pharmacology of the cytochrome P450 epoxygenase/soluble epoxide hydrolase axis in the vasculature and cardiovascular disease, Pharmacol. Rev. 2014;66:1106–1140. doi: 10.1124/pr.113.007781. [DOI] [PubMed] [Google Scholar]
- 21.Fischer R., Konkel A., Mehling H., Blossey K., Gapelyuk A., Wessel N., von Schacky C., Dechend R., Muller D.N., Rothe M., Luft F.C., Weylandt K., Schunck W.H. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J. Lipid Res. 2014;55:1150–1164. doi: 10.1194/jlr.M047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold C., Markovic M., Blossey K., Wallukat G., Fischer R., Dechend R., Konkel A., von Schacky C., Luft F.C., Muller D.N., Rothe M., Schunck W.H. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J. Biol. Chem. 2010;285:32720–32733. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.W.B. Campbell, I. Fleming, Epoxyeicosatrienoic acids and endothelium-dependent responses, Pflugers Arch. 459 881–895. [DOI] [PMC free article] [PubMed]
- 24.Capdevila J.H., Falck J.R., Harris R.C. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J. Lipid Res. 2000;41:163–181. [PubMed] [Google Scholar]
- 25.Spiecker M., Liao J.K. Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch. Biochem. Biophys. 2005;433:413–420. doi: 10.1016/j.abb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Askari A., Thomson S.J., Edin M.L., Zeldin D.C., Bishop-Bailey D. Roles of the epoxygenase CYP2J2 in the endothelium. Prostagl. Other Lipid Mediat. 2013;107:56–63. doi: 10.1016/j.prostaglandins.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris T.R., Hammock B.D. Soluble epoxide hydrolase: gene structure, expression and deletion. Gene. 2013;526:61–74. doi: 10.1016/j.gene.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.M. Revermann, M. Schloss, E. Barbosa-Sicard, A. Mieth, S. Liebner, C. Morisseau, G. Geisslinger, R.T. Schermuly, I. Fleming, B.D. Hammock, R.P. Brandes, Soluble epoxide hydrolase deficiency attenuates neointima formation in the femoral cuff model of hyperlipidemic mice, Arterioscler. Thromb. Vasc. Biol. 30 909–914. [DOI] [PMC free article] [PubMed]
- 29.C.R. Lee, J.D. Imig, M.L. Edin, J. Foley, L.M. DeGraff, J.A. Bradbury, J.P. Graves, F.B. Lih, J. Clark, P. Myers, A.L. Perrow, A.N. Lepp, M.A. Kannon, O.K. Ronnekleiv, N.J. Alkayed, J.R. Falck, K.B. Tomer, D.C. Zeldin, Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice, FASEB J. 24 3770–3781. [DOI] [PMC free article] [PubMed]
- 30.P. Luo, H.H. Chang, Y. Zhou, S. Zhang, S.H. Hwang, C. Morisseau, C.Y. Wang, E.W. Inscho, B.D. Hammock, M.H. Wang, Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis, J. Pharmacol. Exp. Ther. 334 430–438. [DOI] [PMC free article] [PubMed]
- 31.Deng Y., Edin M.L., Theken K.N., Schuck R.N., Flake G.P., Kannon M.A., DeGraff L.M., Lih F.B., Foley J., Bradbury J.A., Graves J.P., Tomer K.B., Falck J.R., Zeldin D.C., Lee C.R. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J. 2011;25:703–713. doi: 10.1096/fj.10-171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang G., Kodani S., Hammock B.D. Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Prog. Lipid Res. 2014;53:108–123. doi: 10.1016/j.plipres.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bishop-Bailey D., Larkin S.W., Warner T.D., Chen G., Mitchell J.A. Characterization of the induction of nitric oxide synthase and cyclo-oxygenase in rat aorta in organ culture. Br. J. Pharmacol. 1997;121:125–133. doi: 10.1038/sj.bjp.0701100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piqueras L., Sanz M.J., Perretti M., Morcillo E., Norling L., Mitchell J.A., Li Y., Bishop-Bailey D. Activation of PPAR{beta}/{delta} inhibits leukocyte recruitment, cell adhesion molecule expression, and chemokine release. J. Leukoc. Biol. 2009;86:115–122. doi: 10.1189/jlb.0508284. [DOI] [PubMed] [Google Scholar]
- 35.Y. Deng, M.L. Edin, K.N. Theken, R.N. Schuck, G.P. Flake, M.A. Kannon, L.M. DeGraff, F.B. Lih, J. Foley, J.A. Bradbury, J.P. Graves, K.B. Tomer, J.R. Falck, D.C. Zeldin, C.R. Lee, Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice, FASEB J. 25 703–713. [DOI] [PMC free article] [PubMed]
- 36.Feil S., Fehrenbacher B., Lukowski R., Essmann F., Schulze-Osthoff K., Schaller M., Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ. Res. 2014;115:662–667. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 37.Askari A.A., Thomson S., Edin M.L., Lih F.B., Zeldin D.C., Bishop-Bailey D. Basal and inducible anti-inflammatory epoxygenase activity in endothelial cells. Biochem. Biophys. Res. Commun. 2014;446:633–637. doi: 10.1016/j.bbrc.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan Z., Hansson G.K. Overexpression of inducible nitric oxide synthase by neointimal smooth muscle cells. Circ. Res. 1998;82:21–29. doi: 10.1161/01.res.82.1.21. [DOI] [PubMed] [Google Scholar]
- 39.Renton K.W. Regulation of drug metabolism and disposition during inflammation and infection. Expert Opin. Drug Metab. Toxicol. 2005;1:629–640. doi: 10.1517/17425255.1.4.629. [DOI] [PubMed] [Google Scholar]
- 40.Bystrom J., Thomson S.J., Johansson J., Edin M.L., Zeldin D.C., Gilroy D.W., Smith A.M., Bishop-Bailey D. Inducible CYP2J2 and its product 11,12-EET promotes bacterial phagocytosis: a role for CYP2J2 deficiency in the pathogenesis of Crohn's disease? PLoS One. 2013;8:e75107. doi: 10.1371/journal.pone.0075107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuchardt J.P., Schmidt S., Kressel G., Dong H., Willenberg I., Hammock B.D., Hahn A., Schebb N.H. Comparison of free serum oxylipin concentrations in hyper- vs. normolipidemic men. Prostagl. Leukot. Essent. Fat. Acids. 2013;89:19–29. doi: 10.1016/j.plefa.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomson S.J., Askari A., Bishop-Bailey D. Anti-inflammatory effects of epoxyeicosatrienoic acids. Int. J. Vasc. Med. 2012;2012:605101. doi: 10.1155/2012/605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wray J.A., Sugden M.C., Zeldin D.C., Greenwood G.K., Samsuddin S., Miller-Degraff L., Bradbury J.A., Holness M.J., Warner T.D., Bishop-Bailey D. The epoxygenases CYP2J2 activates the nuclear receptor PPARalpha in vitro and in vivo, PLoS One. 2009;4:e7421. doi: 10.1371/journal.pone.0007421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.