Abstract
Settings: Tuberculosis (TB) is an ongoing public health challenge in Fiji. Clinical case detection and management are critical for effective TB control. Most TB cases in Fiji are hospitalised for the intensive phase of treatment.
Objectives: To describe the demographic and clinical characteristics, comorbidities and final treatment outcomes of TB patients hospitalised for the intensive phase of treatment in Fiji.
Design: A retrospective, descriptive study of all TB cases hospitalised during the intensive phase over a 3-year period (2010–2012).
Results: A total of 395 TB hospitalised cases were included, of whom 61% were sputum smear-positive. The largest proportions of cases were among young adults (15–34 years) and the unemployed, respectively 43% and 71%. Diabetes (13%) and smoking (22%) were common comorbidities. Final anti-tuberculosis treatment outcomes were available for 96% of cases; 81% were cured or completed treatment. Default was more common in those with current employment. Death was the final treatment outcome in 4%, and was more common (11%) in the oldest group aged 355 years (OR 5.7, 95%CI 1.9–17).
Conclusion: This study provides original and comprehensive descriptive data on TB cases in Fiji and identifies characteristics associated with poor treatment outcomes.
Keywords: tuberculosis, comorbidities, diabetes, Fiji, treatment outcomes
Abstract
Contexte :La tuberculose (TB) constitue un défi de santé publique persistant aux Fidji, et la détection des cas ainsi que leur prise en charge sont cruciales pour lutter efficacement contre la TB. La majorité des cas de TB aux Fidji sont hospitalisés pendant la phase intensive du traitement.
Objectif :Décrire les caractéristiques démographiques et cliniques, la comorbidité et le résultat final du traitement des patients tuberculeux hospitalisés pendant la phase intensive aux Fidji.
Méthodes : Etude rétrospective descriptive de tous les cas de TB hospitalisés en phase intensive sur une période de 3 ans (2010–2012).
Résultats : Au total, 395 cas de TB hospitalisés ont été inclus et 61% d'entre eux avaient une TB à frottis de crachats positif. La majorité était de jeunes adultes (15–34 ans ; 43%) et sans emploi (71%). Le diabète (13%) et le fait de fumer (22%) étaient des comorbidités courantes. Les résultats finaux du traitement étaient disponibles pour 96% des cas et 81% ont été guéris ou ont achevé leur traitement. L'abandon du traitement était plus fréquent parmi ceux qui avaient un emploi. Les malades décédés (4%) étaient plus souvent les plus âgés (⩾55 ans) (11%, OR 5,7 ; IC95% 1,9–17).
Conclusion : Cette étude fournit des données descriptives originales et complètes de la TB aux Fidji et identifie les caractéristiques associées à un mauvais résultat du traitement.
Abstract
Marco de Referencia: La tuberculosis (TB) representa un problema actual de salud pública en Fiji y la detección y el tratamiento de los casos son primordiales para un control eficaz de la enfermedad. En Fiji, la mayoría de pacientes con TB se hospitaliza durante la fase intensiva del tratamiento.
Objetivo: Describir las características demográficas y clínicas, las enfermedades concomitantes y los desenlaces terapéuticos definitivos de los pacientes hospitalizados con diagnóstico de TB, durante la fase intensiva del tratamiento en Fiji.
Método: Se llevó a cabo un estudio descriptivo retrospectivo de todos los casos de TB hospitalizados durante la fase inicial del tratamiento, en un período de 3 años (del 2010 al 2012).
Resultados: Se incluyeron en el estudio 395 casos de TB hospitalizados, de los cuales el 61% presentó baciloscopia positiva del esputo. En su mayoría, se trató de adultos jóvenes entre 15 y 34 años de edad (43%) y desempleados (71%). Las morbilidades asociadas con mayor frecuencia fueron la diabetes (13%) y el tabaquismo (22%). Se conoció el desenlace terapéutico definitivo en el 96% de los casos y de ellos el 81% alcanzó la curación o completó el tratamiento. El abandono fue más frecuente en los pacientes con un empleo actual. El fallecimiento fue el desenlace en 4% de los casos, más frecuente en el grupo de mayor edad, a partir de los 55 años (OR 5,7; IC95% 1,9–17).
Conclusión: El presente estudio aporta datos descriptivos detallados de los casos de TB en Fiji y define las características que se asocian con los desenlaces terapéuticos desfavorables.
Tuberculosis (TB) is a major cause of worldwide morbidity and mortality among infectious diseases. There were an estimated 8.6 million new cases and 1.3 million deaths due to TB worldwide in 2012,1 the majority of which occurred in resource-limited settings. Fiji is a low TB burden, resource-limited setting, with an incidence rate of 24 cases per 100 000 population reported to the World Health Organization (WHO) in 2012.1 However, a sharp increase in the numbers of TB cases registered and reported by the National TB Programme (NTP) of Fiji was noted, from 132 new/relapse cases in 2005 to over 200 per year from 2010. A total of 614 new/relapse cases were reported to the WHO from 2010 to end 2012.1,2
The recent increase in numbers likely reflects in part recent improvements in case detection.1,2 Case detection is an ongoing challenge in Fiji as a resource-limited country with particular issues of access to care. People with suspected TB are referred to the nearest TB treatment centre for further investigation and management. There are only three such centres in Fiji, covering a country of around 100 permanently inhabited islands. It is important in a low-burden setting such as Fiji to maintain clinical awareness and to identify at-risk population groups. In addition, diabetes mellitus (DM) is highly prevalent in Fijian adults,3 and is potentially an important comorbidity. Diabetes has been linked to a higher TB incidence rate and poorer outcomes in other settings.4,5
No data about TB patients in Fiji have been previously published, and data from elsewhere in the Oceania region are limited. As awareness about the risk of TB among diabetic patients and clinicians is limited, screening is rarely performed. We aimed to describe clinical and demographic features of TB patients hospitalised for the intensive phase of treatment in Fiji and their treatment outcomes.
METHODOLOGY
Study design
This was a retrospective cross-sectional study involving a review of the clinical files of TB patients hospitalised for the intensive phase of treatment and of the TB register.
Study setting
General
Fiji is an island nation in the South Pacific Ocean. The country occupies an archipelago of about 322 islands, of which 106 are permanently inhabited. The population of Fiji is approximately 837 271. The two major islands, Viti Levu and Vanua Levu, account for 87% of the population. The Ministry of Health divides Fiji into three health divisions for administrative purposes: the Central and Eastern Division, the Western Division and the Northern Division. In the public health sector there are three main hospitals, 17 sub-divisional hospitals, 78 health centres and 101 nursing stations. There are also a number of private hospitals.
National Tuberculosis Programme
Fiji's NTP was established in 1951. Short-course anti-tuberculosis chemotherapy was introduced in 1997 with the application of the DOTS strategy. There are three TB treatment centres, in the Central, Western and Northern divisions. The diagnosis and decision to treat for TB is made by a clinical specialist in TB care. The decision to commence anti-tuberculosis treatment as an in- or out-patient is also made by the clinician. The aim is to complete the intensive phase for all sputum smear-positive TB cases as an in-patient. For sputum smear-negative pulmonary TB (PTB) and extra-pulmonary TB (EPTB), the decision to provide in-patient care is made on clinical grounds, such as severity of disease.
In-patient TB cases are also screened for DM using a random blood glucose test; if the result is above the normal range (>6.1 mmol/l), the patient is advised to undergo a fasting blood glucose test (FBG). Diabetes is diagnosed if FBG is >7 mmol/l. Any patient with a new or established diagnosis of DM or hypertension (systolic blood pressure ⩾140 mmHg or diastolic blood pressure ⩾90 mmHg) is managed accordingly.
Study population
All TB cases hospitalised for the intensive phase of treatment at each of the three anti-tuberculosis treatment centres from January 2010 to December 2012 were included in the study.
Data collection
Data were collected from the clinical files of all in-patient TB cases listed in the TB register over the 3-year period using a structured proforma. In Fiji, it is mandatory to register all TB cases commenced on anti-tuberculosis treatment. A TB register is maintained in all three treatment centres, and is managed by a NTP medical officer or nurse coordinator. Data from clinical files were validated where possible against those available in the TB register, such as age, TB type and treatment outcomes.
Data variables collected included TB registration number, age, sex, symptoms, weight, occupation, type of TB disease, sputum smear result and presence of co-morbidities such as DM, hypertension or smoking. Treatment outcomes were recorded according to standardised NTP classifications, based on WHO definitions. Names were not included in the data collection file.
Data analysis
Data were double entered, cleaned for accuracy and analysed using EpiData, version 3.1 (EpiData Association, Odense, Denmark). Characteristics and treatment outcomes of the study population were analysed as proportions. For the purposes of identifying characteristics associated with poor outcomes, poor outcome was defined as default, death or treatment failure. The χ2 test was used to assess differences in proportions between groups, with differences at the 5% level being regarded as significant.
Ethics approval
Ethics approval was obtained from the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France, and the Fiji National Health Research Ethics Committee, Suva, Fiji.
RESULTS
Over a 3-year period (2010–2012), 462 in-patient TB cases were admitted to any one of the three TB treatment centres for the intensive phase of treatment. This represents 75% of the total case-load of 614 new/relapse TB cases registered with the NTP and reported to the WHO over the same period.1 The clinical files of 395 (90%) of all TB cases hospitalised for the intensive phase were evaluated; those of the remaining 67 (10%) in-patient cases could not be located. Those files that were available provided complete data for at least 94% of the cases for all the variables evaluated (Table 1). The most common type of TB in this cohort was sputum smear-positive PTB, representing 61% of cases. Common forms of EPTB included pleural effusion, spinal TB and TB meningitis.
TABLE 1.
Clinical and demographic characteristics of TB patients hospitalised during the intensive phase by type of TB in Fiji, 2010–2012
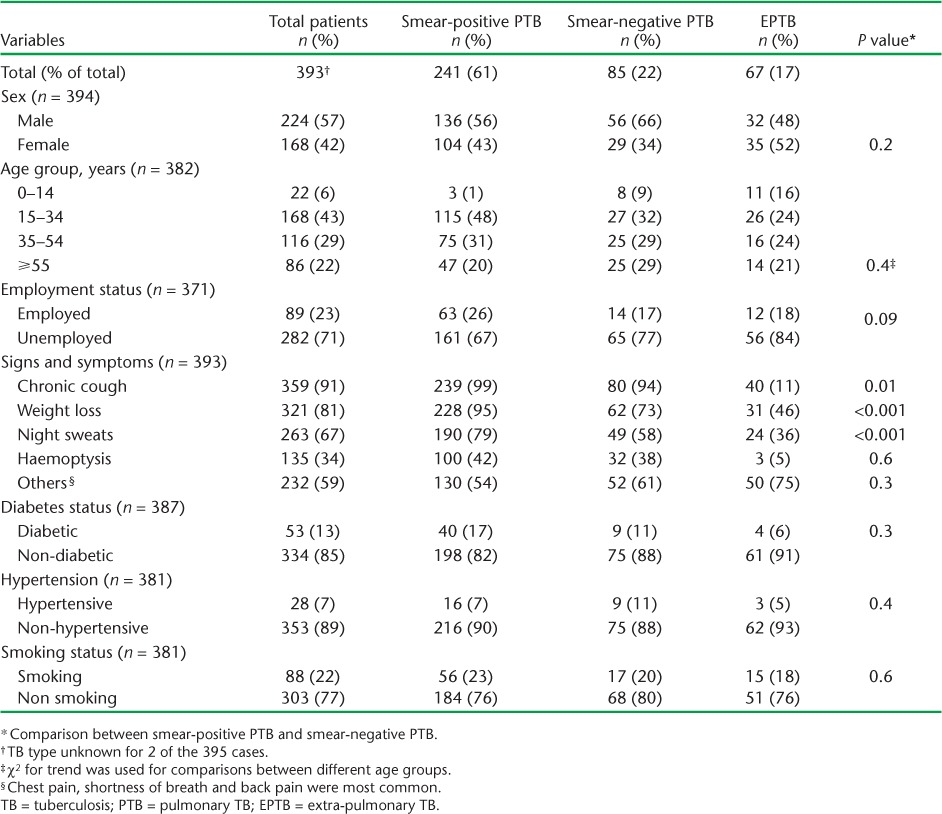
Table 1 lists the clinical and demographic characteristics of the TB patients hospitalised for the intensive phase by TB type. The largest proportions represented were young adults (15–34 years) and the unemployed, while DM and smoking were common co-morbidities. Those with sputum smear-positive PTB were more likely to present with chronic cough, weight loss and night sweats than those with sputum smear-negative PTB or EPTB.
Outcome refers to status at end of anti-tuberculosis treatment per standardised NTP reporting and not status at the time of discharge from hospital. Of the cohort of 395 TB cases hospitalised during the intensive phase, 322 (83%) had a known favourable treatment outcome, i.e., cured or completed treatment, while 55 (14%) had a poor outcome (Table 2). Death was the final treatment outcome in 4% overall, and in 3% of sputum smear-positive PTB cases. Mortality was higher among the unemployed than in the employed; however, the difference was not statistically significant. Death was more common in those with DM or hypertension than in those without, but the difference was not statistically significant. However, mortality was significantly higher in the oldest group aged ⩾55 years than among those aged <55 years (odds ratio [OR] 5.7, 95% confidence interval [CI] 1.9–17, P < 0.001). In addition, the default rate was higher in employed than in unemployed patients (OR 2.21, 95%CI 1.01–4.8, P = 0.045).
TABLE 2.
Final treatment outcomes and characteristics of TB patients hospitalised during the intensive phase, Fiji, 2010–2012
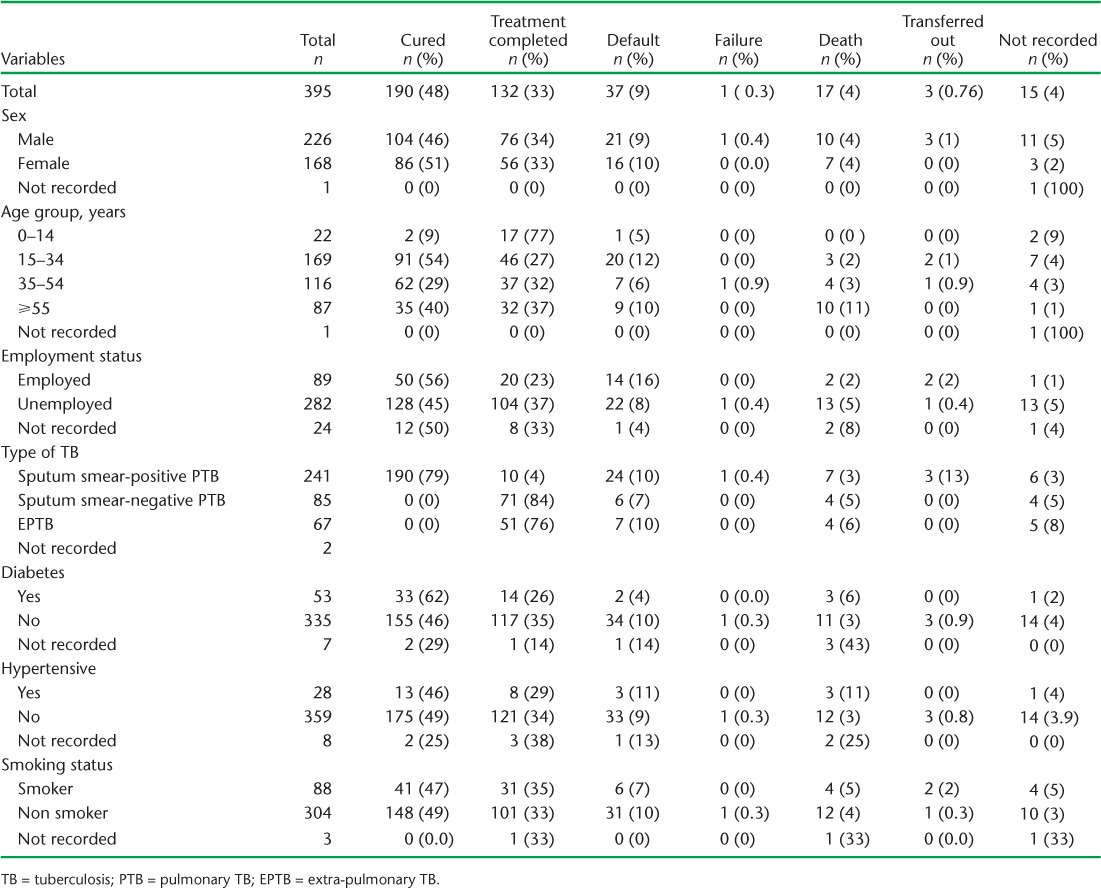
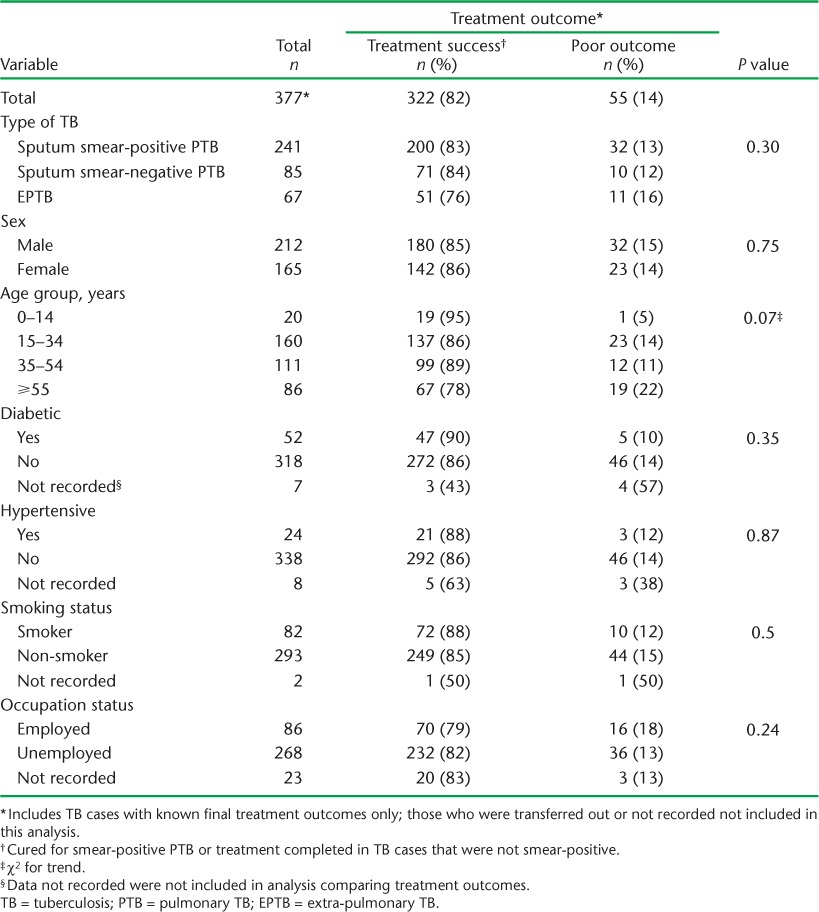
Table 3 presents findings from an analysis of characteristics associated with poor treatment outcome. It should be noted that there was no significant difference in outcomes associated with TB type. The presence of the comorbidities DM, hypertension and smoking was not associated with poorer treatment outcomes.
TABLE 3.
Patient characteristics associated with known treatment outcomes
DISCUSSION
This study provides original descriptive data on the profile of TB patients hospitalised for the intensive phase in Fiji over a recent 3-year period. The study population reported represents around two thirds of the total TB caseload registered in Fiji at the time. The most common type of TB in this cohort was smear-positive PTB, representing 61% of cases. This is higher than reported for smear-positive PTB (50% of the national caseload) to the WHO.1 Similarly, our finding of a mortality rate of 3% for sputum smear-positive PTB cases is higher than the rate (>1%) most recently reported to the WHO.1 This study population is skewed to the more severe cases and a higher proportion of sputum smear-positive PTB cases due to the admission criteria combined with clinical judgement that determines hospitalisation for the intensive phase of treatment.
Clinical features typically associated with PTB were significantly more common in smear-positive PTB cases than in smear-negative cases, but the differences would not necessarily be able to improve the clinical diagnosis, as they were also common in smear-negative disease. Comorbidities, particularly DM and smoking, both of which have been associated with an increased risk of TB and poorer outcomes,4–7 were common in our study but were not significantly associated with poor treatment outcomes. A recent systematic review reported that DM was associated with a significantly increased risk of treatment failure, death and relapse.4 The lack of an association in our study may reflect the small numbers of cases and inaccurate reporting.
The overall treatment outcomes of the TB cases were satisfactory. Treatment outcomes did not differ by TB type; the largest group with poor outcomes were young adults, and employment was associated with a higher rate of default. The finding that older people (⩾55 years) are more likely to die and have smear-negative disease has been noted in previous studies.8,9
A strength of the study is that data variables were documented in the in-patient clinical file in most cases and data such as the TB types and treatment outcomes could be validated in the TB register. However, the study has a number of important limitations. As the study was retrospective, we could not validate the accuracy of the data recorded. As mentioned, the study represents features of TB patients hospitalised for the intensive phase, but not necessarily those of the total TB caseload in Fiji, as it was influenced by a policy to prioritise admission of sputum smear-positive cases and the most severe cases requiring hospitalisation. A number of patient folders in the register could not be located. Finally, although human immunodeficiency virus (HIV) infection is an important comorbidity that strongly affects outcome for TB cases,10,11 no HIV data were available for analysis in this study. In Fiji, HIV prevalence among TB cases who are tested is reported at <5%.1
In conclusion, this study provides original data on the clinical and demographic characteristics of TB patients in Fiji as well as identification of at-risk groups and comorbidity data. These findings will be useful in informing policies to improve TB control in Fiji.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT). The training was run in Fiji by the College of Medicine, Nursing and Health Sciences, Fiji National University, Suva, Fiji and the International Union Against Tuberculosis and Lung Disease (The Union), Paris, France.
Additional support for running the course was provided by the Public Health Division of the Secretariat of the Pacific Community (SPC), Nouméa, New Caledonia; Centre for International Child Health (CICH), the University of Melbourne, Melbourne, VIC, Australia; School of Population Health, University of Queensland, St Lucia, QLD, Australia; Regional Public Health, Hutt Valley District Health Board, Lower Hutt, New Zealand; and the National TB Programme, Fiji Ministry of Health, Suva, Fiji. Funding for the course was provided by the Global Fund to Fight AIDS, TB and Malaria (Geneva, Switzerland), with co-funding by The Union; the Special Programme for Research and Training in Tropical Diseases (TDR, World Health Organization, Geneva, Switzerland); Public Health Division of the SPC, Nouméa, New Caledonia; CICH, the University of Melbourne, Parkville, VIC, Australia; and the School of Population Health, University of Queensland, Herston, QLD, Australia.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report, 2013. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.11. [Google Scholar]
- 2.World Health Organization. External Review of the National TB Programme — Republic of Fiji: 21st November–2nd December 2011. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 3.Brian G, Ramke J, Maher L, Page A, Szetu J. The prevalence of diabetes among adults aged 40 years and over in Fiji. N Z Med J. 2010;123:68–75. [PubMed] [Google Scholar]
- 4.Baker M A, Harries A D, Jeon C Y et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeon C Y, Murray M B. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLOS MED. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrara G, Murray M, Winthrop K et al. Risk factors associated with pulmonary tuberculosis: smoking, diabetes and anti-TNFα drugs. Curr Opin Pulm Med. 2012;18:233–240. doi: 10.1097/MCP.0b013e328351f9d6. [DOI] [PubMed] [Google Scholar]
- 7.Jee S H, Golub J E, Jo J, Park I S, Ohrr H, Samet J M. Smoking and risk of tuberculosis incidence, mortality, and recurrence in South Korean men and women. Am J Epidemiol. 2009;170:1478–1485. doi: 10.1093/aje/kwp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C S, Chen H C, Yang C J et al. The impact of age on the demographic, clinical, radiographic characteristics and treatment outcomes of pulmonary tuberculosis patients in Taiwan. Infection. 2008;36:335–340. doi: 10.1007/s15010-008-7199-8. [DOI] [PubMed] [Google Scholar]
- 9.Pratt R H, Winston C A, Kammerer J S, Armstrong L R. Tuberculosis in older adults in the United States, 1993–2008. J Am Geriatr Soc. 2011;59:851–857. doi: 10.1111/j.1532-5415.2011.03369.x. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez M, Bartholomay P, Arakaki-Sanchez D et al. Outcomes of TB treatment by HIV status in national recording systems in Brazil, 2003–2008. PLOS ONE. 2012;7:e33129. doi: 10.1371/journal.pone.0033129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harries A D, Zachariah R, Corbett E L et al. The HIV-associated tuberculosis epidemic — when will we act? Lancet. 2010;375:1906–1919. doi: 10.1016/S0140-6736(10)60409-6. [DOI] [PubMed] [Google Scholar]


