Abstract
Settings: Three tuberculosis (TB) treatment centres under the Fiji National Tuberculosis Programme.
Objectives: To determine the prevalence of diabetes mellitus (DM) among TB patients for the period 2010–2012, and to evaluate sputum smear conversion and anti-tuberculosis treatment outcomes, comparing patients with and without DM.
Design: A retrospective descriptive study using routinely collected data from the TB register and in-patient folders.
Results: Of 577 TB patients identified, information on DM was available for 567 (98%), of whom 68 (12%) had DM. Smear status at 2 months was available for 254 (82%) patients with sputum smear-positive pulmonary TB. The sputum smear conversion rate (from positive to negative) was equivalent in TB patients with and without DM (78% vs. 80%, P = 0.66). Anti-tuberculosis treatment outcome information was available for 462 patients; the difference in outcome comparing successfully treated patients with those unsuccessfully treated was not statistically significant (91% in TB patients with DM vs. 84% in TB patients without DM, P = 0.06).
Conclusion: DM is common among TB patients in Fiji. Sputum smear conversion rates were not different in TB patients with and without DM; no difference in treatment success between the two groups was observed.
Keywords: tuberculosis, diabetes mellitus, treatment outcomes, Fiji
Abstract
Contexte : Trois centres de traitement de la tuberculose (TB) dans le cadre du Programme national de Lutte contre la TB aux Fidji.
Objectif : Déterminer la prévalence du diabète (DM) parmi les patients tuberculeux entre 2010 et 2012 et d'évaluer la conversion des frottis de crachats et les résultats du traitement de la TB en comparant les patients avec et sans DM.
Schéma : Etude rétrospective descriptive basée sur les données recueillies en routine à partir des registres de la TB et des dossiers des patients hospitalisés.
Résultats : De 577 patients tuberculeux identifiés, des informations relatives au DM étaient disponibles pour 567 d'entre eux (98%) ; parmi ceuxci, 68 (12%) étaient diabétiques. Le résultat du frottis à 2 mois était disponible pour 254 (82%) patients atteints de TB pulmonaire à frottis positif. Le taux de conversion du frottis (de positif à négatif) était identique chez tous les patients tuberculeux avec ou sans DM (78% contre 80% ; P = 0,66). Le résultat du traitement anti-tuberculeux était disponible pour 462 patients ; il n'y avait pas non plus de différence significative en termes de succès du traitement (91% chez les patients avec DM contre 84% chez les patients sans DM ; P = 0,06).
Conclusion : Le DM est fréquent chez les patients tuberculeux aux Fidji. Le taux de conversion du frottis ne s'est cependant pas avéré différent chez les patients avec et sans DM, et il n'y a pas non plus eu de différence significative en termes de succès du traitement entre les deux groupes.
Abstract
Marco de referencia: Tres centros de tratamiento de la tuberculosis (TB) del Programa Nacional contra la Tuberculosis de Fiji.
Objetivo: Determinar la prevalencia de diabetes (DM) en los pacientes con diagnóstico de TB entre el 2010 y el 2012 y comparar la tasa de conversión de la baciloscopia del esputo y los desenlaces terapéuticos entre los pacientes con y sin diagnóstico concomitante de DM.
Método: Se llevó a cabo un estudio descriptivo retrospectivo a partir de los datos recogidos sistemáticamente en el registro de TB y en las historias clínicas de los pacientes hospitalizados.
Resultados: Se encontraron 577 pacientes con diagnóstico de TB, de los cuales 567 contaban con información sobre la DM (98%) y en 68 casos se registró DM concomitante (12%). Se contó con resultados de la baciloscopia a los 2 meses de 254 pacientes con TB pulmonar y baciloscopia inicial positiva (82%). La tasa de conversión del esputo fue equivalente entre los pacientes con TB y DM o TB exclusivamente (78% contra 80%; P = 0,66). Se obtuvieron datos sobre el desenlace del tratamiento antituberculoso de 462 pacientes; no se observó una diferencia significativa cuando se compararon los desenlaces favorables y desfavorables (éxito terapéutico de 91% en los pacientes con DM concomitante comparado con 84% en los pacientes sin DM; P = 0,06).
Conclusión: La DM es una enfermedad frecuente en los pacientes con diagnóstico de TB en Fiji. Las tasas de conversión de la baciloscopia del esputo fueron equivalentes en los pacientes con DM concomitante o sin ella y no se observó una diferencia en la tasa de éxito terapéutico entre ambos grupos.
Fiji is a low tuberculosis (TB) burden country, with a TB incidence rate of 24 cases per 100 000 population and a high treatment success rate of >90% (2012 data).1 However, diabetes mellitus (DM) is very common in Fiji, and its prevalence is thought to be increasing. The STEPwise approach to Surveillance (STEPs) study conducted in 2002 reported a population prevalence of 16% for DM.2 A more recent estimate, using a different methodology, gave a DM prevalence among adults in Fiji of 10.9%, with impaired glucose tolerance in a further 11.0%.3 This remarkably high prevalence of DM is a matter of concern for TB control in Fiji. Published data from other settings have concluded that people with DM have a significantly increased risk of developing TB and worse treatment outcomes, with a higher risk of mortality and treatment failure during anti-tuberculosis treatment than among patients without DM.4–6 In response to this evidence, the Fiji National Tuberculosis Programme (NTP) introduced random blood glucose (RBG) testing to screen for DM in all registered TB patients in 2011.7
A recent local study reported DM in 14% of TB patients in Fiji.8 It has been noted by local clinical and public health staff that sputum smear-positive TB patients with DM take longer to convert to smear-negative than TB patients without DM, and that they thus require longer periods of hospitalisation. However, this observation has not been verified empirically, and there are no published data from Fiji reporting sputum smear conversion or treatment response in TB patients with and without DM.
In the present study, we aimed to evaluate treatment outcomes and sputum smear conversion in TB patients with DM and compare these indicators in TB patients without DM.
METHODS
Study design
This was a retrospective descriptive study using routinely collected data from the TB register and in-patient folders from three TB treatment hospitals in Fiji.
Setting
Fiji is an island nation, located in the Pacific Ocean, classified as an upper middle-income economy.9 It comprises 332 islands, of which 110 are inhabited. The population of 837 231 comprises 56.8% i-Taukei (indigenous Fijians) and 37.5% Fijians of Indian descent; the remaining 5.7% are from other ethnic backgrounds.10
The Fiji NTP is largely funded by the government, and TB diagnosis and treatment are provided free of charge. Three government hospitals provide TB services for the entire country; the private sector is not involved in TB control. All patients diagnosed with TB are registered as TB cases and treated at one of the three TB treatment centres. The majority of TB patients are admitted for the 2-month intensive phase of treatment, during which they receive anti-tuberculosis treatment and clinical follow-up. Clinical evaluation on admission routinely includes a full medical history and RBG screening if the patient is not known to have DM. If RBG is ⩾6.1 mmol/l, fasting blood glucose (FBG) is tested. If the FBG is >7 mmol/l,8 patients are diagnosed as having DM and referred to a physician for further assessment and diagnosis. Patients with TB and DM are treated according to national TB and DM guidelines.7,11
Patients with sputum smear-positive pulmonary TB (PTB) undergo a repeat sputum smear test 2 months after starting treatment. Those patients with a positive sputum smear result receive another month of intensive phase treatment (i.e., four anti-tuberculosis drugs), while those with a negative result move to the continuation phase (i.e., two anti-tuberculosis drugs). The 2-month smear result is therefore an important clinical indicator. After completion of a course of anti-tuberculosis treatment, treatment outcomes for all TB patients are recorded in the TB register according to standardised, internationally accepted definitions.7
Study population
The study population consisted of 577 TB patients registered with the NTP during a 3-year period from 1 January 2010 to 31 December 2012. The study population comprised 94% of all TB cases notified during the study period.
Data variables and data collection
Data were collected using a data collection form designed for the study. Data variables comprised TB register number, age, sex, ethnicity, type and category of TB, DM status, year of DM diagnosis and whether DM was already known or newly diagnosed after TB diagnosis. Outcome variables included sputum smear status at 2 months and treatment outcome following a full course of anti-tuberculosis treatment.
Analysis and statistics
All data were double entered into EpiData version 3.1 (EpiData Association, Odense, Denmark) and validated prior to analysis. Data were analysed using EpiData Analysis version 2.2.2.178. We calculated frequencies to compare TB patients with and without DM and used the χ2 test to determine the difference between proportions. The level of significance was set at 5%.
Ethics approval
Ethics approval was obtained from the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France, and the Fiji National Health Research and Ethics Review Committee, Suva, Fiji.
RESULTS
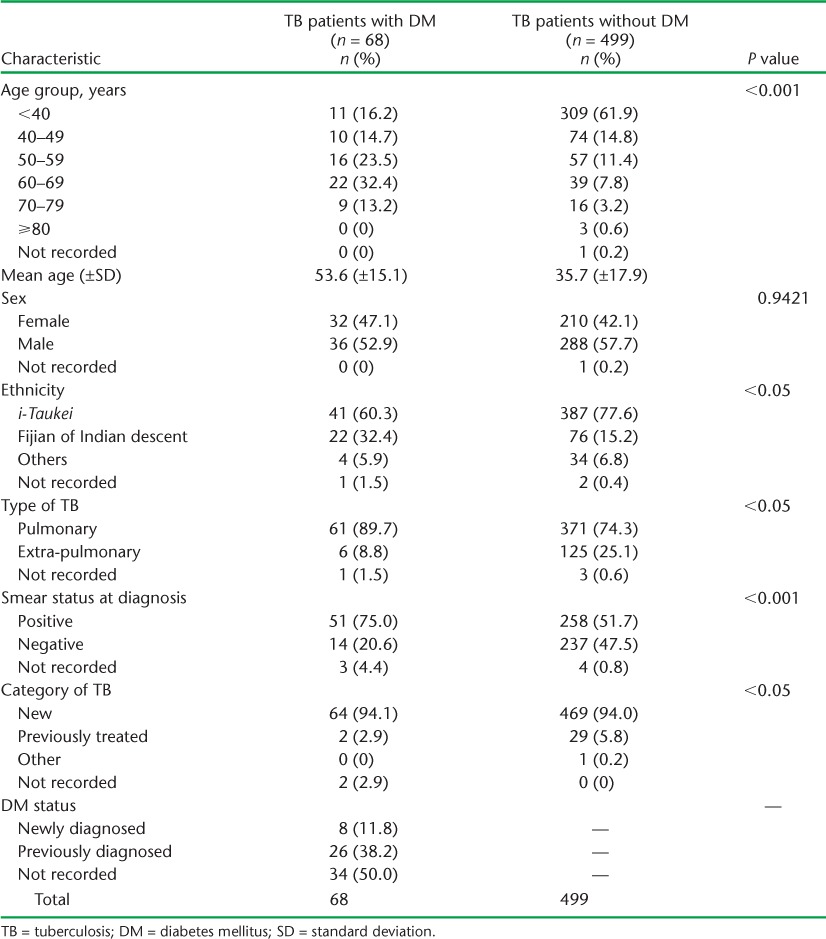
Of 614 TB patients registered during the study period, medical case notes were available for 577 (94%). Information on DM status was available for 567 (98%), of whom 68 (12%) had a documented diagnosis of DM. The demographic and clinical characteristics of the patients are given in Table 1. TB patients with a diagnosis of DM were older (mean age 53.6 vs. 35.7 years, P < 0.001), more likely to be of Fijians of Indian ethnicity (32.4% vs. 15.2%, P < 0.05) and more likely to have pulmonary sputum smear-positive TB (75% vs. 51.7%, P < 0.001) than TB patients without DM. Of the TB patients with a DM diagnosis, 11.8% were newly diagnosed due to the policy of screening TB patients for DM as part of routine clinical care. A further 38.2% of TB patients were already aware of their DM status; this information was not documented for almost half of the TB patients (50%).
TABLE 1.
Demographic and clinical characteristics of TB patients with and without DM, Fiji, 2010–2012
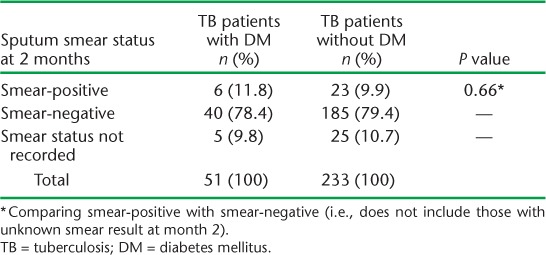
Sputum smear-positive PTB was noted in 309 patients, of whom 254 (82%) had smear status available at 2 months (Table 2). The sputum smear conversion rate, from positive to negative, was similar in TB patients with and without DM (78.4% vs. 79.4%, P = 0.66), and respectively 11.8% and 9.7% of patients remained sputum smear-positive in the two groups. Information on sputum smear conversion at 3 or 5 months was not available.
TABLE 2.
Smear status of sputum smear-positive pulmonary TB patients with and without DM at 2 months, Fiji, 2010–2012
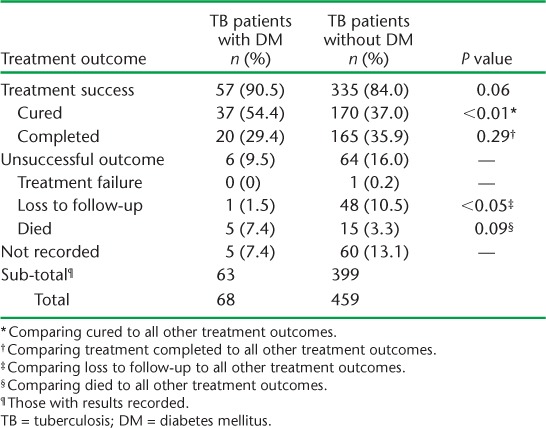
Information on anti-tuberculosis treatment outcome was available for 462 patients (Table 3). There were no significant differences in treatment outcome when comparing successfully treated patients with those with an unsuccessful treatment outcome; 90.5% of TB patients with DM had a successful treatment outcome (the sum of cured and completed treatment) compared to 84% of TB patients without DM (P = 0.06). In terms of mortality, 7.4% of TB patients with DM died compared to 3.3% of TB patients without DM; the difference was not statistically significant (P = 0.09). We did, however, note a difference in the cure rate when comparing patients with and without DM. In TB patients with DM, 54.4% were cured, compared to 37% of TB patients without DM; this result was statistically significant (P < 0.01). We also noted a significant difference in the proportion of TB patients with and without DM who were lost to follow-up (1 [1.5%] TB patient with DM vs. 48 [10.5%] TB patients without, P < 0.05); however, overall numbers were small.
TABLE 3.
Treatment outcomes for TB patients with and without DM, Fiji, 2010–2012
DISCUSSION
According to our study findings, 11.8% of TB patients had DM, a slightly higher rate than in the general population, which has an estimated DM prevalence of 10.9%.3 However, both of these figures are lower than the results of the STEPs survey, which reported a population DM prevalence of 16% among adults.2 Other studies in the Pacific and elsewhere have shown a higher prevalence of DM among TB patients than in the general population.12–14 In an unpublished study from Kiribati, where the prevalence of both diseases is relatively high, the prevalence of DM in TB patients was twice that of the general population (37% vs. 19%, P ⩽ 0.001).
Other studies from outside the Pacific Islands region have also reported a higher prevalence of DM among TB patients than in the general population. In Tanzania, the prevalence of DM among TB patients was 16.7% compared to 9.4% in a group of matched controls.12 In a similar study in China, where all TB patients were tested for DM, 6.7% of TB patients had DM compared to 4.3% in the community (P < 0.05),15 while in Indonesia 13.2% of TB patients had DM compared to 3.2% in the community (odds ratio [OR] 4.7, 95% confidence interval [CI] 2.7–8.1).14 In our study, the prevalence of DM among TB patients was only marginally higher than in the general community; this may be a valid finding or it may be due to incomplete screening of TB patients for DM, which was formally introduced as a policy by the Fiji NTP in 2011.7 This may also be due to the fact that the TB patient population in our study included children, who have a lower prevalence of DM than adults.
We noted a higher proportion of sputum smear-positive PTB in patients with concomitant DM. Other studies do not report this consistently; some have noted that sputum smear-positive PTB is more common in TB patients with DM,16,17 while others report no difference in type of TB at diagnosis.18,19 Studies that have assessed radiological differences between TB patients with and without DM have noted that cavities are more common in TB patients with DM; this may also influence sputum smear status.20,21 While data may be conflicting, there is some evidence to suggest that DM influences the clinical presentation of TB. This has implications for infection control and ongoing transmission of TB in the community, as sputum smear-positive TB patients are infectious and can transmit TB to others.
We observed no significant differences in sputum smear conversion at 2 months among sputum smear-positive TB patients with and without DM, nor were there differences in rates of treatment success between the two groups. Generally speaking, a delayed sputum smear conversion rate may be influenced by the initial bacterial load in the patient, and this clinical indicator was not assessed in our study. In a more thorough prospective assessment of TB and DM, data should be collected on sputum smear grade at diagnosis in order to be certain about sputum smear conversion at 2 months and the relationship between DM status and delayed sputum smear conversion.
In this study, TB treatment outcomes, including death, were not different in TB patients with and without DM. However, we noted that an outcome of ‘cured’ was more frequent among patients with DM. A systematic review has reported that TB patients with DM have a two-fold increased risk of anti-tuberculosis treatment failure and death (relative risk [RR] 1.69, 95%CI 1.36–2.12) and a four-fold increased risk of relapse (RR 3.89, 95%CI 2.43–6.23).6 Higher treatment failure rates have also been observed in TB patients with DM in China (17% vs. 2%, P < 0.01),22 Maryland, USA (OR 6.9, 95%CI 1.1–38.0, P = 0.039)23 and in southern Mexico (adjusted OR 2.93, 95%CI 1.18–7.23).24 We are not sure why the cure rate was higher in TB patients with DM in our study, as these patients were also more likely to have sputum smear-positive TB. One possible reason may be that TB patients with concomitant DM are more closely linked to the health system due to their chronic disease, and are therefore more likely to be followed up and undergo sputum microscopy at the end of anti-tuberculosis treatment, thereby increasing their chance of an outcome of cure. It is also not clear why TB patients with DM were less likely to be lost to follow-up, but this may be for reasons similar to the above. The relatively small numbers and few deaths may have affected our findings regarding treatment outcome; an analysis of TB data over several years could therefore be useful.
Our study has some limitations. The Fiji NTP did not prescribe routine screening of TB patients for DM before 2011; the prevalence of DM among TB patients may therefore have been underestimated. A recent assessment of DM screening practices in Fiji noted that, in 2011, 62% of TB patients had their DM status recorded in the patient notes; the study concluded that improvements in screening practices were required.8 It is also estimated that approximately 50% of DM in the Pacific Islands region is undiagnosed; we may therefore expect the proportion of TB patients with DM to be higher than observed in our study.3 Studies conducted in India have reported that many TB patients had had undiagnosed DM.16,25 In a recent prospective study in Kiribati, where all TB patients aged ⩾18 years were tested for DM at the time of TB diagnosis, 55% were newly diagnosed with DM and had not known their DM status before attending the TB clinic.
We did not collect information on sputum smear status at diagnosis and other potentially confounding factors such as smoking and comorbidities, cause and time of death of those who died during anti-tuberculosis treatment, and other clinical information such as chest X-ray results. A comprehensive, prospective multi-country study may provide useful information on the presentation of TB patients with and without DM and the clinical course of anti-tuberculosis treatment in the two groups. Finally, a number of variables were not recorded in our data set and this may have affected our findings. The issue of incomplete recording of health data is a common one,26,27 and highlights the importance of accurate and complete recording and reporting by health programme staff involved in DM and TB programmes.
CONCLUSION
DM is a common condition among TB patients in Fiji: 12% of all TB patients had concomitant DM. TB patients with DM are more likely to present with sputum smear-positive PTB than those without. Health care workers caring for people with DM should be aware that TB is a common respiratory infection in this population. When comparing TB patients with and without DM, we noted no differences in sputum smear conversion at 2 months or treatment success, indicating that, at present, the presence of DM does not adversely affect TB outcomes. However, a larger prospective study could provide more definitive results; there is therefore scope for prospective studies on the association between TB and DM in the Pacific to improve health care for persons suffering from both diseases.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT). The training was run in Fiji by the College of Medicine, Nursing and Health Sciences, Fiji National University, Suva, Fiji, and the International Union Against Tuberculosis and Lung Disease, Paris, France.
Additional support for running the course was provided by the Public Health Division of the Secretariat of the Pacific Community, Nouméa, New Caledonia; Centre for International Child Health, the University of Melbourne, Melbourne, VIC, Australia; School of Population Health, University of Queensland, Brisbane, QLD, Australia; Regional Public Health, Hutt Valley District Health Board, Lower Hutt, New Zealand; the National TB Programme, Fiji Ministry of Health, Suva, Fiji.
Funding for the course was provided by the Global Fund to Fight AIDS, TB and Malaria, Geneva, Switzerland, with co-funding from The Union; the Special Programme for Research and Training in Tropical Diseases; Public Health Division of the Secretariat of the Pacific Community, Nouméa, New Caledonia; Centre for International Child Health, the University of Melbourne, Melbourne, VIC, Australia; School of Population Health, University of Queensland, Brisbane, QLD, Australia.
Footnotes
Conflict of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report 2013. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.11. [Google Scholar]
- 2.Ministry of Health Fiji, World Health Organization. Fiji non-communicable diseases (NCD) STEPS survey 2002. Suva, Fiji: Ministry of Health Fiji, World Health Organization; 2004. [Google Scholar]
- 3.International Diabetes Federation. Diabetes Atlas Update 2012. Copenhagen, Denmark: International Diabetes Federation; 2012. http://www.idf.org/diabetesatlas/5e/Update2012. Accessed July 2014. [Google Scholar]
- 4.Jeon C, Murray M. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLOS MED. 2008;5:1091–1101. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevenson C, Critchley J, Forouhi N et al. Diabetes and the risk of tuberculosis: a neglected threat to public health? Chronic Illness. 2007;3:228–245. doi: 10.1177/1742395307081502. [DOI] [PubMed] [Google Scholar]
- 6.Baker M, Harries A, Jeon C et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:1–15. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiji National Tuberculosis Programme. Technical guide for tuberculosis control in Fiji. Suva, Fiji: Fiji NTP; 2011. [Google Scholar]
- 8.Gounder S, Harries A. Screening tuberculosis patients for diabetes mellitus in Fiji: notes from the field. Public Health Action. 2012;2:145–147. doi: 10.5588/pha.12.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The World Bank. Data: Fiji. Washington DC, USA: World Bank; 2014. http://data.worldbank.org/country/fiji. Accessed July 2014. [Google Scholar]
- 10.Fiji Bureau of Statistics. Fiji statistics at a glance. Suva, Fiji: Fiji Bureau of Statistics; 2013. http://www.statsfiji.gov.fj/. Accessed July 2014. [Google Scholar]
- 11.Fiji Ministry of Health. Diabetes management guidelines. Suva, Fiji: Fiji MoH; 2012. [Google Scholar]
- 12.Faurholt-Jepsen D, Range N, PrayGod G et al. Diabetes is a risk factor for pulmonary tuberculosis: A case-control study from Mwanza, Tanzania. PLOS ONE. 2011;6:e24215. doi: 10.1371/journal.pone.0024215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Yang C, Chen H et al. Impact of type 2 diabetes on manifestations and treatment outcome of pulmonary tuberculosis. Epidemiol Infect. 2009;137:203–210. doi: 10.1017/S0950268808000782. [DOI] [PubMed] [Google Scholar]
- 14.Alisjahbana B, Van Crevel R, Sahiratmadja E et al. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int J Tuberc Lung Dis. 2006;10:696–700. [PubMed] [Google Scholar]
- 15.Wang Q, Han X, Ma A et al. Screening and intervention of diabetes mellitus in patients with pulmonary tuberculosis in poverty zones in China: rationale and study design. Diabetes Res Clin Pract. 2011;96:385–391. doi: 10.1016/j.diabres.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Vishwanathan V, Kumpatla S, Aravindalochanan V et al. Prevalence of diabetes and pre-diabetes associated risk factors among tuberculosis patients in India. PLOS ONE. 2012;7:e41367. doi: 10.1371/journal.pone.0041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanna A, Lohya S, Sharath B, Harries A. Characteristics and treatment response in patients with tuberculosis and diabetes mellitus in New Delhi, India. International Journal of Tuberculosis and Lung Disease. 2013;3:S48–S50. doi: 10.5588/pha.13.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alladin B, Mack S, Singh A et al. Tuberculosis and diabetes in Guyana. Int J Infect Dis. 2011;15:e818–e821. doi: 10.1016/j.ijid.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Alisjahbana B, Sahiratmadja E, Nelwan E et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Commentary. Clin Infect Dis. 2007;45:428–438. doi: 10.1086/519841. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Guzman C, Torres-Cruz A, Villarreal-Velarde H, Salazar-Lezama M, Var-gas M. Atypical radiological images of pulmonary tuberculosis in 192 diabetic patients: a comparative study. Int J Tuberc Lung Dis. 2001;5:455–461. [PubMed] [Google Scholar]
- 21.Shaikh M, Singla R, Khan N, Sharif N, Saigh M. Does diabetes alter the radiological presentation of pulmonary tuberculosis. Saudi Med J. 2003;24:278–281. [PubMed] [Google Scholar]
- 22.Chang J, Dou H, Yen C et al. Effect of type 2 diabetes mellitus on the clinical severity and treatment outcome in patients with pulmonary tuberculosis: a potential role in the emergence of multidrug resistance. J Formosan Med Assoc. 2011;110:372–381. doi: 10.1016/S0929-6646(11)60055-7. [DOI] [PubMed] [Google Scholar]
- 23.Dooley K, Tang T, Golub J, Dorman S, Cronin W. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg. 2009;80:634–639. [PMC free article] [PubMed] [Google Scholar]
- 24.Jimenez-Corona M, Cruz-Hervert L, Garcia-Garcia L et al. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax. 2013;68:214–220. doi: 10.1136/thoraxjnl-2012-201756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nair S, Kumari A, Subramonianpillai J et al. High prevalence of undiagnosed diabetes among tuberculosis patients in peripheral health facilities in Kerala. Public Health Action. 2013;3:38–42. doi: 10.5588/pha.13.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunbar R, Lawrence K, Verver S E et al. Accuracy and completeness of recording of confirmed tuberculosis in two South African communities. Int J Tuberc Lung Dis. 2011;15:337–343. [PubMed] [Google Scholar]
- 27.Ronveaux O, Rickert D, Hadler S et al. The immunization data quality audit: verifying the quality and consistency of immunization monitoring systems. Bull World Health Organ. 2005;83:503–510. [PMC free article] [PubMed] [Google Scholar]


