Abstract
The reasoning that neural reflexes maintain homeostasis in other body organs, and that the immune system is innervated, prompted a search for neural circuits that regulate innate and adaptive immunity. This elucidated the inflammatory reflex, a prototypical reflex circuit that maintains immunological homeostasis. Molecular products of infection or injury activate sensory neurons traveling to the brainstem in the vagus nerve. The arrival of these incoming signals generates action potentials that travel from the brainstem to the spleen and other organs. This culminates in T cell release of acetylcholine, which interacts with α7 nicotinic acetylcholine receptors (α7 nAChR) on immunocompetent cells to inhibit cytokine release in macrophages. Herein is reviewed the neurophysiological basis of reflexes that provide stability to the immune system, the neural- and receptor-dependent mechanisms, and the potential opportunities for developing novel therapeutic devices and drugs that target neural pathways to treat inflammatory diseases.
Keywords: inflammatory reflex, innate immunity, vagus nerve
INTRODUCTION
Understanding immunity has emerged as a major focus for science in the twenty-first century. Effective immunity has made it possible for vertebrates to flourish and survive in a world teeming with microbes, pathogens, and other threats. There are two major types of immunity: innate and adaptive. Innate immunity, the most evolutionarily ancient defense network, is operative in even the earliest multicellular organisms. It forms the front line, recognizing molecular products of infection and injury through receptors encoded in the germ line. Adaptive immunity emerged more recently in evolutionary time, capitalizing on mechanisms for antigen-specific responses and immunological memory in T and B lymphocytes. The protection conferred by immunity has come with a cost, however, because innate and adaptive immune mechanisms are also intrinsic to the development of diseases that can impair organ function and produce severe disabilities, even death. Today, vertebrate life requires that the immune response to microbes and pathogens is maintained within a carefully balanced response range between dual threats: insufficient immunity, which would enable the pathogens to prevail, and excessive immunity, which can kill or impair the host, directly. An important question arises: How is homeostasis maintained in the immune system?
The search for answers has provided some key insights. Humoral factors, including glucocorticoid hormones and anti-inflammatory cytokines, protect the host by inhibiting the release and toxicity of potentially damaging inflammatory mediators (1). Cellular mechanisms, including regulatory T cells and alternatively activated macrophages, can also prevent damage to host tissues by suppressing excessive immune responses. These humoral and cellular pathways utilize intercellular communication by cytokines and other secreted regulatory molecules, and by direct cell-to-cell contact as cells move and traffic between tissues, to limit damage to host tissues during immune responses. Further consideration reveals that these humoral and cellular mechanisms are inherently limited in several aspects. First, their response time to a rapidly changing environment is relatively slow because of the reliance on the production of mediators, cell trafficking, and the distribution of mediators and cells by the circulatory system. Second, these systems are inefficient for integrating biological responses to numerous stimulating inputs across a widely distributed network of immune tissues. Third, as these regulatory systems rely on the circulatory system to operate systemically, they inherently lack the means to exert efficient regulatory responses in a regional- or tissue-specific fashion. Thus, humoral and cellular counter-regulatory mechanisms do not provide all the essential features required to fully maintain immunological homeostasis in vivo.
As reviewed here, major advances in twentieth-century neuroscience and immunology revealed that neuronal circuits maintain homeostasis during immune responses. Neural signaling is rapid, directional, regional, and capable of integrating divergent stimulating and inhibiting inputs from diffuse networks, features ideally suited to monitor and modulate the milieu interieur. In other organ systems, including the cardiovascular, pulmonary, and gastrointestinal systems, well-characterized neural circuits and specific neurotransmitter mechanisms maintain homeostasis of organ responses to changes in the internal and external environment. Until recently, however, it was dogmatic that the immune system is self-regulating through humoral and cellular pathways that are essentially autonomous. Immune functions were presumed to be independent from neuronal control, their effector mechanisms operating outside of neurological mechanisms that establish homeostasis in other organs. The current advanced status for understanding how neural reflexes regulate immune functions arose by applying the neurophysiological methods used to delineate homeostatic mechanisms in other organs to immunological studies. This established new principles that, like other complex organ systems, immune homeostasis is maintained in vivo by the nervous system.
Homeostatic control of immune responses by neural reflex circuits occurs in a time frame that operates extremely fast relative to humoral and cell-trafficking mechanisms. Afferent reflex arcs sense pathogenic molecules, cytokines, and other products of infection and cell injury, thereby activating action potentials that travel rapidly, specifically, and directionally. These incoming neural signals to brainstem nuclei stimulate efferent action potentials that travel to the principal organs of the immune system, including the spleen, lymph nodes, and reticuloendothelial organs. Arrival of action potentials in these innervated immune tissues culminates in release of neurotransmitters that interact with specific receptors expressed by monocytes, macrophages, lymphocytes, and other cells of the innate and adaptive immune systems. Neurotransmitter receptor-mediated intracellular signal transduction modulates immune cellular function by specific molecular mechanisms, resulting in exquisite, timely, and regional control of immunity and in establishment of immune homeostasis.
BASIC PRINCIPLES OF NEURAL REFLEXES
A neuron, the elementary signaling unit of the nervous system, is composed of a cell body, containing the nucleus, and an axon that can extend for significant distances to communicate with another cell. The axon terminates at the nerve synapse, the site where vesicles containing neurotransmitters are released into the synaptic cleft, the narrow space adjacent to the innervated cell. Neuronal action potentials, a rapid sequential increase and decrease of electrical membrane potential generated by specialized voltage-gated ion channels, propagate to the end of the axon. The arrival of the action potential at the synaptic terminus initiates both fusion of vesicles with the cell membrane and neurotransmitter release into the synaptic cleft. Neurons can communicate with other neurons when the axon terminates on a dendrite, shorter projections arising near the cell body of the recipient or innervated neuron. Specific neurons communicate with specific cells in a directional fashion, because neuronal action potentials travel in one direction and axons terminate on specific responding cells, but not with other cells. The principles of unidirectional signaling and specific, targeted innervation by axons determine the direction of information flow in neural circuits.
Neurons can be categorized into one of three major classes by their function. Sensory neurons respond to stimulation from the external or internal environment. They express receptors that are activated by touch, pain, temperature, oxygen tension, vibration, pressure, and chemical and other changes in the environment. Sensory-receptor activation generates action potentials that propagate toward the central nervous system. Motor neurons transmit information from the central nervous system to the periphery. Interneurons function as relay stations because they receive signals from sensory nerves and project outgoing signals via their axons to the dendrites on motor neurons. Early twentieth-century neuroscientists were split in their views about whether the basis for information transfer between neurons was electrical or chemical. By electrically stimulating the vagus nerve of an innervated frog heart prepared in media bath, Otto Loewi (2) provided the necessary proof to establish the chemical basis of synaptic transmission. Observing that this intervention significantly slowed the heart rate, he then transferred media conditioned by the electrically stimulated nerve to a bath containing a second, denervated heart; addition of the conditioned media caused the noninnervated heart to slow. Loewi termed the unknown chemical agent of neurotransmission Vagusstoff, thereafter identified by Henry Dale (3) as acetylcholine, which he isolated as an active natural product from ox spleen. Dale established a nomenclature to describe neurons on the basis of the chemical nature of their neurotransmitters, terming neurons that utilized catecholamines as adrenergic and those that utilized acetylcholine as cholinergic. Decades of subsequent work firmly established that specific adrenergic and cholinergic neurons occupy crucial roles in maintaining physiological homeostasis in all organ systems.
PRINCIPLES FOR REFLEX-BASED HOMEOSTASIS
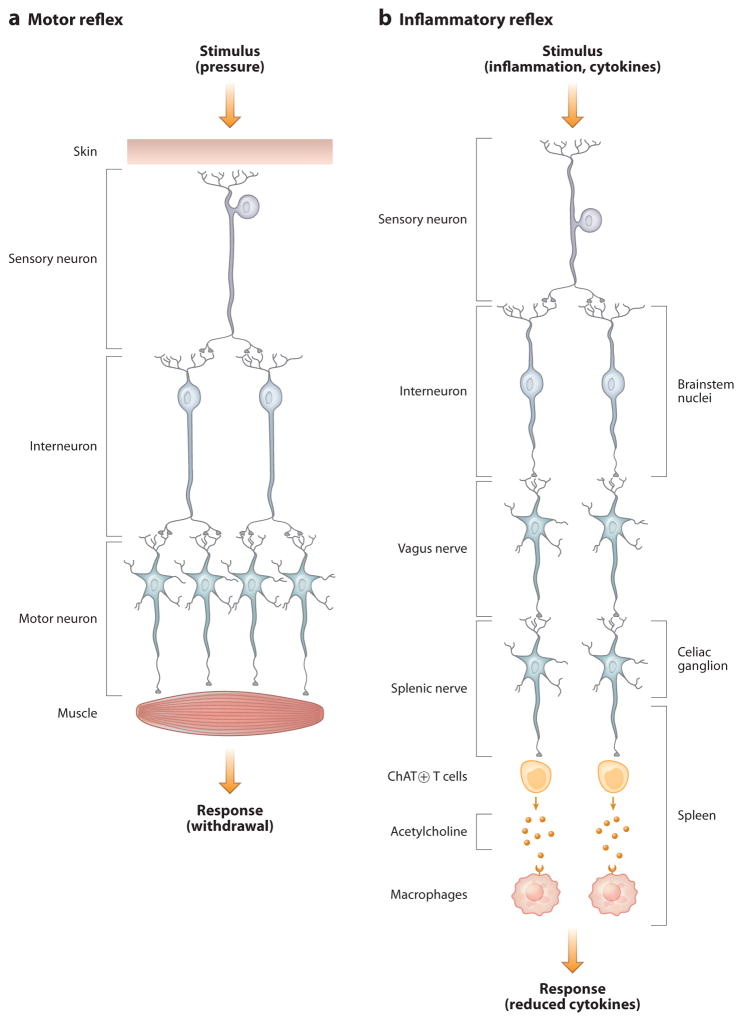
Landmark studies by Sherrington in the early twentieth century revealed that simple neural circuits provide the mechanistic basis for reflex action (4). His studies utilized the patellar tendon reflex; tapping the patella elicits extension of the ipsilateral leg. Tapping activates stretch receptors in sensory neurons that produce action potentials that travel centrally. The arrival of incoming action potentials in the spinal cord activates motor neurons that produce contraction of the corresponding extensor muscle groups (Figure 1). Unexpectedly, Sherrington also discovered that action potentials arising in sensory neurons simultaneously activated spinal cord interneurons that transmitted signals that inhibited corresponding flexor muscle groups. Realizing the importance of inhibitory neurons in maintaining homeostasis, he proposed that inhibitory neuronal components in a reflex circuit confer stability, particularly in systems capable of receiving multiple, simultaneous, and sometimes conflicting inputs. The inhibitory or dampening action provided by interneurons in a reflex circuit stabilizes the response to conflicting and widely ranging inputs, in this case preventing simultaneous flexion and extension of opposing muscle groups. Sherrington’s classic treatise, The Integrative Action of the Nervous System, taught that simple neural circuits coordinate the responses to diverse environmental changes to maintain homeostatic stability in the milieu interieur (4). The net output of motor neurons that provide regulatory constraints to innervated organs is mediated by the sum of exciting and inhibitory impulses. These principles of integrated neural reflex responses are the major mechanism for achieving organ homeostasis and modulating organ output within a safe functional range.
Figure 1.
Cellular basis of neural reflex circuits. (a) A simple motor reflex is composed of a sensory neuron that is activated by a stimulus (in this example, pressure). Sensory axons project to dendrites of interneurons, which in turn relay excitatory or inhibitory signals to corresponding muscle groups that produce withdrawal. (b) The inflammatory reflex is composed of sensory neurons traveling in the vagus nerve that are activated by products of infection (e.g., LPS) or inflammation (e.g., IL-1β). These project to brainstem nuclei, where interneurons relay the signals to the motor nuclei of the vagus nerve. The efferent signals travel in the vagus nerve to the celiac ganglion, where they interact with the cell bodies that give rise to axons that project in the splenic nerve. Action potentials lead to the release of norepinephrine, which activates acetylcholine release by a subset of T lymphocytes. Acetylcholine interacts with α7 nicotinic acetylcholine receptor (α7 nAChR) expressed in red pulp and marginal-zone macrophages to inhibit cytokine release.
For obvious reasons, the early work to elucidate neural regulation of organ homeostasis focused on relatively accessible organs, including the heart, lungs, kidneys, and gastrointestinal tract. The cardiovascular system provided dual advantages as an experimental study target because it is readily accessible and its principal experimental end points (heart rate and blood pressure) respond rapidly to manipulation of neural signals. Consideration of heart rate provides an ideal model for understanding how integrated reflexes maintain homeostasis. The vagus nerve, the major cholinergic nerve to the heart, provides a tonic inhibitory influence on the heart rate; therefore, stimulating the vagus nerve decreases heart rate. By contrast, stimulation of the adrenergic nerves to the heart increases the heart rate. The reflex circuit begins with sensory input transmitted in the vagus nerve to the brainstem, which “informs” about the status of heart rate. The nervous system establishes the target heart rate or “set point.” When sensory input indicates that heart rate has deviated from the set point, interneurons in brainstem nuclei activate cholinergic or adrenergic signals that slow or increase heart rate, correspondingly. These reflex responses maintain homeostasis at the heart-rate set point. Similar principles underlie the baroreflex, a homeostatic mechanism to maintain blood pressure. Baroreceptor neurons in the carotid sinuses and aortic arch detect changes in blood pressure and transmit afferent action potentials to the nucleus of the solitary tract in the brainstem. The arrival of these neural signals modulates the activity of interneurons that project to nuclei extending adrenergic and cholinergic nerves to the heart. The net effect is either a decrease or increase in cardiac contractility and heart rate, which moves blood pressure back toward the targeted blood pressure set point.
THE INFLAMMATORY REFLEX
In the mid-1990s, a surreptitious observation led to the discovery that innate immunity, like other organ systems, is regulated by a reflex circuit of the vagus nerve (5). It occurred during the course of mechanistic studies of CNI-1493, a tetravalent quanylhydrazone inhibitor of tumor necrosis factor (TNF) synthesis. Intracerebral administration of CNI-1493 significantly inhibited systemic TNF release during endotoxemia, but this suppression of TNF release could not be explained as a systemic, pharmacological effect of CNI-1493 because serum TNF production was inhibited by extremely small quantities of intracerebral CNI-1493, even with amounts that were orders of magnitude less than required to suppress TNF release in isolated macrophages (6, 7). Surprised, we considered that low levels of intracerebral CNI-1493 may have activated motor or efferent neurons that in turn inhibited TNF in the peripheral immune system. This intriguing possibility was experimentally pursued by cutting the vagus nerve as it exited the brainstem en route to the reticuloendothelial organs, including the liver and spleen. Selectively cutting the vagus nerve completely prevented the TNF-inhibiting action of intracerebral CNI-1493. These striking results meant that signals transmitted by the vagus nerve controlled the systemic release of TNF, a pivotal, and potentially toxic, cytokine product of the innate immune response.
Subsequent studies to delineate the mechanism of vagus neural signaling in regulating innate immune responses were aided by focusing on TNF (8). Although the results have been replicated for many other cytokines, the major advantage provided by TNF as an end point for these early studies was that peak levels occur within 60 to 90 min after acute exposure to endotoxin, a relatively short time frame that could be monitored in nerve-stimulation experiments. Selective mapping of the efferent vagus nerve path that inhibits the release of systemic TNF revealed that 90% of the TNF released into the bloodstream during the first 90 min of endotoxemia is produced in the spleen by marginal-zone macrophages and red-pulp macrophages (9). Vagus nerve action potentials control this response by descending from the brainstem to the celiac ganglion, where they activate signals in the splenic nerve, the sole nerve to the spleen. Electrical stimulation of either the vagus nerve or the splenic nerve drives action potentials into the spleen and inhibits TNF synthesis in marginal-zone and red-pulp macrophages (Figure 1).
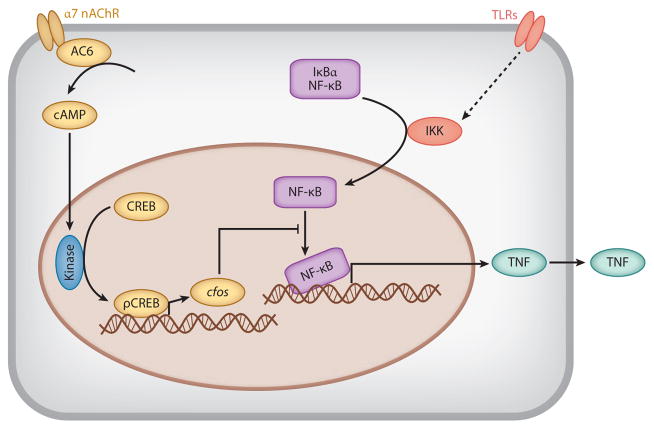
Studies in isolated spleen macrophages revealed that cholinergic agonists, which pharmacologically mimic the effects of acetylcholine, significantly inhibit the production of TNF and other cytokines; gene-knockout studies then established that vagus nerve–mediated inhibitory signaling culminates on a specific acetylcholine receptor, namely the α7 nicotinic acetylcholine receptor (α7 nAChR) (10). Deficiency of α7 nAChR in genetically engineered mice, or inhibiting this receptor with pharmacologic agents, impairs vagus nerve control of TNF. This first established that α7 nAChR is an anti-inflammatory target, and, as predicted by this theory, administration of highly selective α7 agonists significantly inhibited the production of TNF and other cytokines by macrophages and monocytes. This anti-inflammatory activity of α7 nAChR is not limited to the context of endotoxemia because α7 agonists significantly inhibit cytokine release in macrophages activated by exposure to a range of pathogenic products, including ligands to Toll-like receptor (TLR)2, TLR3, TLR4, TLR7, and TLR9 (Figure 2) (11). The molecular signaling mechanism of α7 inhibition of TNF occurs through a physical interaction between α7 and adenylate cyclase 6, which generates increased levels of intracellular cAMP. This activates phosphorylation of CREB, which increases expression of cfos, a member of the immediate early gene family of transcription factors. Activation of cfos inhibits NF-κB activity, effectively shutting down the transcription of cytokines. This efferent neural path that utilizes action potentials in the vagus nerve and culminates on α7-mediated inhibition of cytokines in spleen macrophages has been termed the cholinergic anti-inflammatory pathway.
Figure 2.
Mechanism of inhibition of cytokine release mediated by α7 nicotinic acetylcholine receptor (α7 nAChR).
The inflammatory reflex is the neural circuit composed of afferent and efferent neurons that travel in the vagus nerve to regulate immunity (Figure 1). In contrast to the relatively detailed understanding of the efferent arc (the cholinergic anti-inflammatory pathway), the mechanisms underlying the afferent, sensory arc are just beginning to emerge. Seminal studies by Watkins and her colleagues first revealed that sensory signals propagated in the vagus nerve mediate the fever response to innate immune activators (12, 13). They injected either endotoxin or IL-1β into the abdominal cavity of rodents and observed the expected hyperthermic response. However, when they cut the vagus nerve prior to the injections, they observed that the pyrogenic response did not occur. They had discovered an unanticipated proximal step leading to fever: Endotoxin and IL-1β in the abdomen activate sensory neurons in the vagus nerve, which in turn provides the neural signals to brainstem nuclei that initiates the physiological redistribution of blood flow and other responses culminating in hyperthermia. Neurons express TLRs as well as receptors for IL-1β and other cytokines, and although more work is needed to understand fully the molecular mechanisms underlying sensory receptor–mediated generation of action potentials in response to immunological mediators, the neurophysiological integrity of the inflammatory reflex has been established. In further work, Niijima administered IL-1β into the liver and recorded afferent action potentials ascending to the brainstem in the vagus nerve and efferent action potentials descending in the vagus nerve to the celiac ganglion and splenic nerve (14, 15). Together with the evidence that descending action potentials in vagus nerve to spleen inhibit cytokine release, these results establish the neurophysiological and neurotransmitter-dependent mechanism of the inflammatory reflex.
The inflammatory reflex can be thought of as a prototypical reflex circuit capable of maintaining a balanced, homeostatic immune response. Molecular mediators of innate immunity activate afferent signals in the vagus nerve that travel to the brainstem. Interneurons in the nucleus tractus solatarius, in turn, regulate the motor nuclei of the vagus nerve. Action potentials in neurons arising in these nuclei descend in the vagus nerve to the celiac ganglion, which serves as a relay station to propagate the signals to the splenic nerve and into the spleen. The signaling pathway culminates in the spleen with the release of acetylcholine, the signaling molecule required to complete the cytokine-inhibiting circuit by binding to α7 nAChR expressed on macrophages. This prototypical circuit, which monitors and modulates innate immune responses in a rapid and specific time frame, can be activated or inhibited by numerous inputs from diverse body regions. By analogy to the neural control of heart rate, vagus nerve signals tonically suppress the activity of the innate immune response. When the vagus nerve or splenic nerve is cut, or the molecular components of the cholinergic anti-inflammatory pathway are experimentally knocked out or pharmacologically inhibited, the result is a hypersensitive or uninhibited immune response to invasive and infectious stimuli. Indeed, preclinical work in animal models, and recent and ongoing clinical studies, indicate that insufficient activity in the inflammatory reflex predisposes the organism to damaging consequences mediated by excessive innate immune responses.
NEURONS REGULATE INNATE IMMUNITY IN CAENORHABDITIS ELEGANS
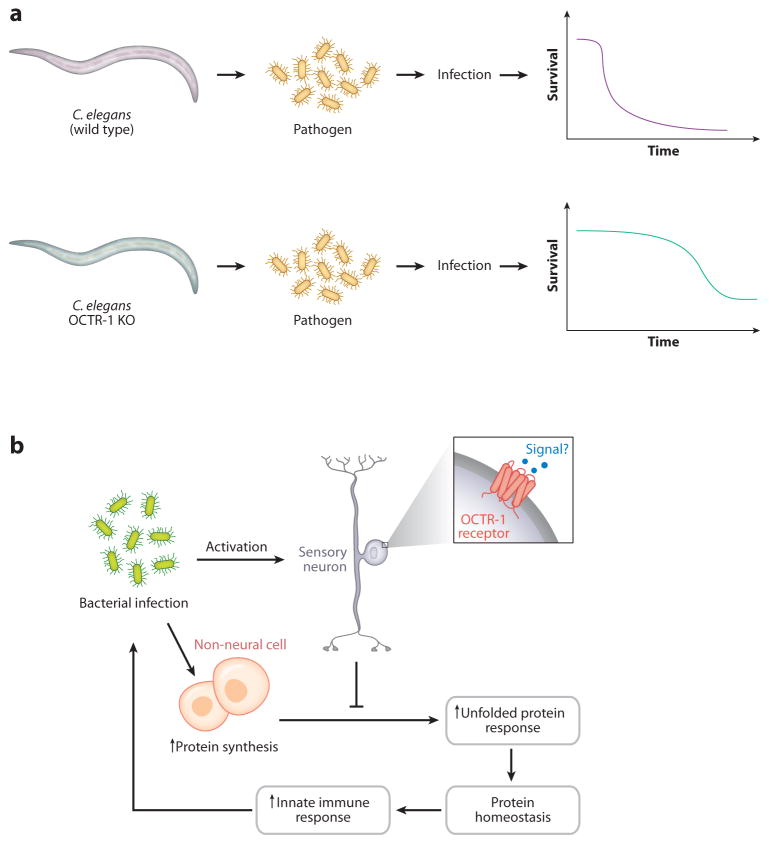
As summarized above, studies of the inflammatory reflex in mammals produced a fundamental map of the components of the neural circuit that evolved to maintain innate immune homeostasis. It remained unclear, however, whether the neural reflex regulation of innate immune responses evolved as a relatively modern event or dated back to early periods in the evolution of immunity. Aballay and colleagues recently addressed this question by studying Caenorhabditis elegans, a nematode worm that possesses both a primitive innate immune system and an evolutionarily ancient nervous system (16, 17). As one of the simplest animals, C. elegans has a nervous system composed of 302 neurons and 56 glial cells that regulate the physiological response to nutrients and environmental stressors and produce signaling peptides that are related to mammalian TGF-β, insulin, and neurotransmitters, including acetylcholine. Soil bacteria are the worm’s major nutrient source, but certain microbes are pathogenic to C. elegans, including Pseudomonas aeruginosa. Pathogen invasion of C. elegans activates expression of innate immune response genes and leads to specific behavioral changes including pathogen avoidance and migration away from the site of pathogen odors. Aballay observed that genetically deleting npr-1, a gene that encodes a G protein–coupled receptor related to mammalian neuropeptide Y receptors, enhanced the worm’s susceptibility to pathogen-mediated killing by P. aeruginosa. Genetic ablation of neurons expressing npr-1, which include AQR, PQR, and URX, significantly increased the worm’s ability to survive pathogen invasion because this neuronal family mediated signals that suppress immunity-related genes expressed in tissues in direct contact with pathogens. ASH and ASI sensory neurons also express OCTR-1, an octopamine receptor for the catecholamine-like neurotransmitter in C. elegans (Figure 3). Signals arising in ASH and ASI neurons inhibit the unfolded protein response in nonneuronal cells by downregulating the expression of pqn/abu. The absence of these inhibitory signals on the unfolded protein response during innate immunity significantly improves survivability during infection. Together, the results from C. elegans indicate that regulation of innate immunity by neurons is an evolutionarily ancient principle, one that dates back to the origins of the immune and nervous systems. Neuronal circuits in the simplest of animals integrate conflicting and multiple inputs mediated by the presence of pathogens and corresponding responses in the internal environment. The activity of the neural circuitry produces efferent responses that reflexively regulate the output of the innate immune system by targeting the expression and activity of specific genes implicated in host defense and stress responses, an essential feature for maintaining homeostasis and survival.
Figure 3.
Neurons regulate innate immunity in Caenorhabditis elegans. (a) Wild-type C. elegans infected with bacterial pathogens die, but OCTR-1 knockout animals are significantly protected from lethal infection. (b) The mechanism depends on the role of OCTR-1-expressing neurons to inhibit innate gene expression required for the unfolded protein response.
There is a tendency to view the nervous system from a top-down perspective, like a process controller capable of overseeing and directing organ function and the behavior of the organism. In reality, the nervous system does not generate output as an independent operator. Rather, neural circuits operate from the bottom up, beginning with complex streams of sensory information reporting the status of the body’s internal and external environment. This incoming information is relayed through interneurons in evolutionarily ancient brainstem and hindbrain nuclei, which maintain homeostasis by integrating the incoming data and producing either inhibitory or stimulatory signals in motor neurons that descend back to the innervated organs. The nervous system is uniquely hardwired to monitor the presence of the molecular products of pathogens, inflammation, and injury as well as the physiological state of the cardiovascular and other fundamental systems. The principles underlying the inflammatory reflex and its ancient evolutionary origins support the prediction that other individual neural reflex circuits will be discovered to control other specific immune responses. Neural reflexes can be a key mechanism for closing the loop between immunologically and physiologically relevant changes in the environment and the need to maintain homeostatic, stable immune responses.
INNERVATION OF ACETYLCHOLINE-PRODUCING T LYMPHOCYTES
The detailed mapping of the inflammatory reflex recently produced another major surprise, one with significant implications for understanding how neural circuits integrate signals during innate and adaptive immunity. As reviewed above, initial work established that α7 nAChR is required for the functional integrity of the vagus nerve–dependent regulation of systemic TNF, which terminates in the spleen (9, 10, 18). α7 nAChR knockout mice, rendered genetically deficient in this key receptor, produce excessive quantities of TNF, a response that is refractory to vagus nerve signals. Together with pharmacological evidence using selective α7 nAChR agonists and antagonists, these and other results indicated that acetylcholine signaling through α7 nAChR is integral to the cholinergic anti-inflammatory pathway regulation of cytokine release by macrophages and other innate immune cells. Neuroanatomy studies indicated that the vagus nerve, which utilizes acetylcholine as the principal neurotransmitter, terminates in the celiac ganglion and that neurons residing in the celiac ganglion project axons in the splenic nerve to the spleen (19, 20). But these results created an extremely important conundrum because splenic neurons arising in the celiac ganglion are adrenergic, not cholinergic. Thus, while splenic neurons are integral to the cholinergic anti-inflammatory pathway, norepinephrine released by splenic neurons cannot signal through α7 nAChR, the receptor required to complete the neural circuit involving the vagus nerve, the splenic nerve, and the regulation of TNF synthesis (9). Therefore, at this point in mapping the reflex mechanism from the vagus nerve to the α7 nAChR–expressing, TNF-producing cells in the spleen, it became crucial to answer another question: What is the identity of acetylcholine-producing cells in the spleen?
To answer this, we began by replicating prior work to simply establish that neurons in the spleen do not express the enzymes necessary to catalyze acetylcholine biosynthesis. Stimulation of the vagus nerve significantly increased spleen acetylcholine levels within minutes, as measured by implanting microdialysis catheters into the spleen (21). As the spleen is innervated solely by the adrenergic splenic nerve, it was then necessary to hypothesize that some non-neuronal cell released acetylcholine in response to action potentials descending in the vagus nerve and relayed into the spleen via the splenic nerve. Adrenergic splenic neurons enter the spleen and travel with splenic arteries, where they branch in white-pulp regions that are rich in T and B lymphocytes. Electron microscopy of spleen sections reveal neuronal axon terminations with synapse-like structures adjacent to lymphocytes in the white pulp. Accordingly, we reasoned that lymphocytes may function as relay stations that receive adrenergic signals from splenic neurons and become activated to produce acetylcholine to inhibit innate immune responses (21).
Support for this possibility came from Kawashima, who had shown that lymphocytes are capable of producing acetylcholine (22). This emboldened us to explore whether these cells may be integral to the cholinergic anti-inflammatory pathway. Beginning with studies of nude mice, which possess functional B lymphocytes but are deficient in T lymphocytes, we observed that stimulating the vagus nerve failed to suppress TNF. Reconstituting T cells in nude mice by adoptive transfer, however, re-established the ability of the vagus nerve to inhibit TNF (21). The inescapable conclusion from these results was that T cells are necessary for a complete, functional cholinergic anti-inflammatory pathway. To study the identity of these acetylcholine-producing T cells, we utilized a transgenic mouse strain expressing GFP under the control of promoter elements that regulate the expression of choline O-acetyltransferase, the enzyme that catalyzes the biosynthesis of acetylcholine. Of the total T cell population in these mice, only 2.5% of the T cells expressed GFP, indicating that only a relatively small subset of the total T cell population can synthesize acetylcholine. Immunohistochemistry revealed that many of these GFP-positive cells in the spleen resided in close proximity to adrenergic splenic nerve endings. As expected, GFP-positive T cells harvested from these animals could be stimulated to release acetylcholine by exposure to norepinephrine. Adoptive transfer of purified acetylcholine-producing T cells into nude mice restored the functional integrity of the cholinergic anti-inflammatory pathway (21). These and other results revealed that a subset of T cells, capable of producing acetylcholine, is necessary and sufficient for the functional integrity of the cholinergic anti-inflammatory pathway mediated by action potentials arising in the vagus nerve, transmitted via the splenic nerve into the spleen, leading to the release of acetylcholine by the T lymphocytes, and terminating on α7 nAChR signaling that suppresses cytokine release by spleen macrophages (Figure 1).
Further characterization of the acetylcholine-producing T cell subset is expected to be the focus of additional work. It is likely that acetylcholine-producing T cells modulate innate immunity in lymph nodes and Peyer’s patches because immunohistochemistry reveals the presence of these cells in those tissues, which are also the target of adrenergic and cholinergic neurons (21). T lymphocytes express β2 adrenergic receptors, and it is plausible that this G protein–coupled receptor regulates acetylcholine release by a T cell subset (23). Acetylcholine release in the spleen can extensively perfuse the extracellular milieu of that organ, resulting in signal transduction via α7 nAChR that inhibits TNF in red-pulp and marginal-zone macrophages at sites distal to the splenic nerve termini. Although only 4.4% of total CD4-positive cells express acetylcholine, the acetylcholine-producing cells are predominantly CD44high CD62Llow. Within the total memory CD4+CD44highCD62Llow population, 10.5% express acetylcholine, and activation enhances the production of acetylcholine by these T cells. Together, these findings have important implications for understanding both reflex control of innate immune responses and the role of acetylcholine-producing T cells during adaptive immunity. Adoptively transferring these acetylcholine-producing T cells to recipient animals inhibits innate immune responses, raising the intriguing possibility that these cells can be targeted to suppress damaging inflammation.
IMPLICATIONS FOR TREATING DISEASE
Key discoveries in the fields of cytokine and basic molecular biology, including the ability to generate specific neutralizing monoclonal antibodies and the identification of specific cytokines that mediate tissue injury, revolutionized the treatment of autoinflammatory and autoimmune diseases affecting significant patient cohorts. These anti-inflammatory agents are distributed by the circulatory system and function systemically to modify the activity of specific cytokines or cellular targets. Although these therapies have been beneficial to many, they are not a panacea. Many agents are ineffective in a significant percentage of patients, or their use is limited by serious safety issues attributable to immunosuppression and other toxicities. Additional anti-inflammatory treatment approaches are clearly needed. During the course of mapping the inflammatory reflex, the therapeutic potential became readily apparent (5). Extensive preclinical studies have activated the cholinergic anti-inflammatory pathway using specific vagus or splenic nerve–stimulating devices. Pharmacological agents that target signal transduction through α7 nAChR, a new anti-inflammatory drug target, have also been studied. These results indicate that it should be possible to develop novel therapeutic strategies. Preclinical studies thus far reveal that targeted therapies to increase the activity of the inflammatory reflex can modulate innate immune responses without abolishing them or producing significant immunosuppression, as perhaps is expected by considering that there should be no evolutionary pressure to preserve a reflex system that would subject the host to immunosuppression. As reviewed below and summarized in Table 1, two major treatment options have been widely studied in experimental disease models: electrical stimulation using electrodes and pharmacological agonists of α7 nAChR. These results are discussed in the context of possible frameworks for future clinical trials.
Table 1.
Experimental disease models responding to the cholinergic anti-inflammatory pathwaya
| Model | Mode of cholinergic stimulation | Result |
|---|---|---|
| Endotoxemia | Vagus nerve stimulation α7 nAChR agonist |
Attenuation of shock and decreased TNF levels Improved survival and reduced TNF levels |
| Sepsis | Vagus nerve stimulation α7 nAChR agonist Nicotine |
Improved survival and reduced HMGB1 levels Improved survival and reduced HMGB1 levels Improved survival and reduced TNF, IL-1β, and IL-6 levels Exaggerated inflammation in vagotomized animals |
| Colitis | α7 nAChR agonist | Decreased colitis severity |
| Pancreatitis | α7 nAChR agonist | Decreased pancreatitis severity |
| Hemorrhagic shock | Vagus nerve stimulation | Prolonged survival, milder hypotension, reduced TNF levels, and inhibited NF-κB activity |
| Intracerebral hemorrhage | Muscarine (intracerebral administration) | Reduced CNS damage |
| Ischemia-reperfusion injury | Vagus nerve stimulation Physostigmine |
Attenuation of shock and tissue damage and reduced cytokine release Reduced inflammation |
| Myocardial ischemic reperfusion | Vagus nerve stimulation | Reduced lethality and arrhythmias and decreased free radical formation |
| Renal ischemic reperfusion | α7 nAChR agonist Nicotine |
Improved renal function Reduced tubular damage |
| Post–cardiac arrest | α7 nAChR agonist | Decreased neuroinflammation |
| Artery occlusion | Vagus nerve stimulation | Improved survival |
| Carrageenan-induced inflammation | Vagus nerve stimulation | Reduced cell infiltration and edema |
| Arthritis | Vagus nerve stimulation α7 nAChR agonist |
Decreased arthritis severity Decreased arthritis severity and reduced TNF release |
Abbreviations: CNS, central nervous system; α7 nARChr, α7 nicotinic acetylcholine receptor; TNF, tumor necrosis factor.
Endotoxemia
Administration of endotoxin is among the most widely used models for studying the development of therapeutic strategies to inhibit the damaging consequences of uncontrolled innate immunity. Electrical stimulation of the vagus nerve improves survival and selectively reduces systemic and tissue levels of TNF and other proinflammatory cytokines (8, 24). The requirement for α7 nAChR–dependent signaling was also established in this model because vagus nerve stimulation failed to reduce serum TNF levels during endotoxemia in α7 nAChR gene–deficient mice (10). Vagotomy in wild-type mice exposed to toxic doses of endotoxin significantly worsened the severity of disease (8). Protection has been achieved using pharmacological agents that target α7 nAChR, including nicotine and other more selective α7 nAChR agonists (25). Administration of GTS-21 in endotoxemic mice attenuated the release of TNF, macrophage inflammatory protein 2, and keratinocyte-derived cytokine and inhibited neutrophil migration by a cytokine-independent mechanism, suggesting that these agents may confer protection against tissue damage by suppressing the migration of inflammatory cells into tissues (26). A clinical study demonstrated that nicotine administration to human subjects attenuated the inflammatory responses to intravenous endotoxin (27). Other approaches have been to utilize centrally activating agents that target brain circuits to stimulate the cholinergic anti-inflammatory pathway. Agonists of M1 cholinergic receptors, which are expressed in cholinergic brain neurons, can stimulate increased activity in the vagus nerve and inhibit systemic TNF release by this mechanism (28). Indeed, harking back to CNI-1493, the tetravalent guanylhydrazone molecule that led to the discovery of the inflammatory reflex, this molecule later proved to be an agonist of the M1 receptor (6). Thus, intracerebral CNI-1493 stimulates muscarinic brain networks to significantly increase vagus nerve activity and confer significant protection against endotoxin-mediated damage (6).
Sepsis
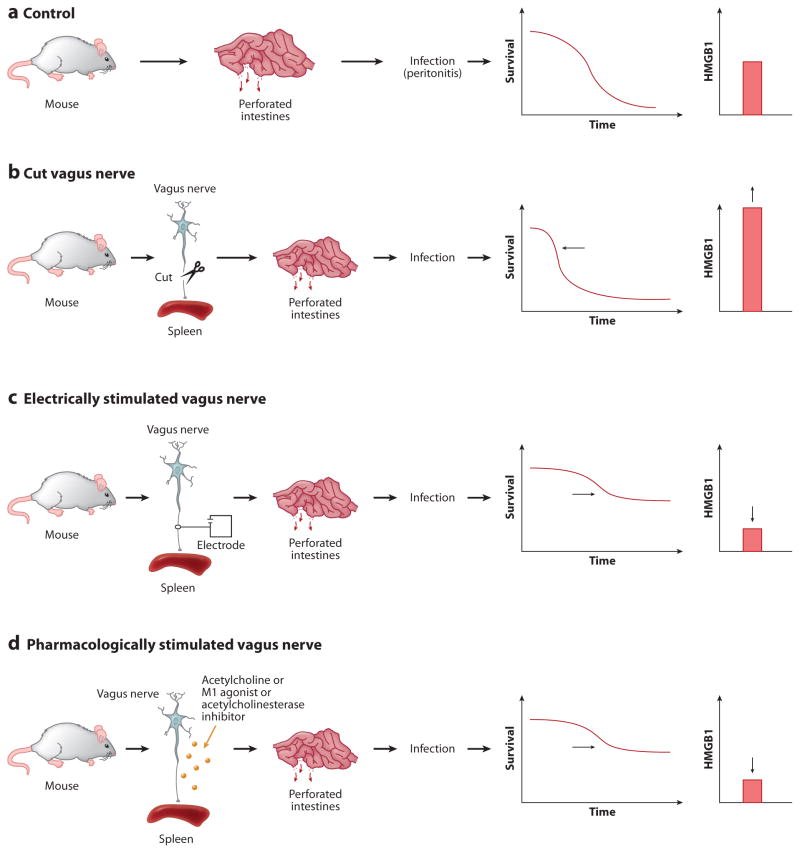
Sepsis is a syndrome of organ damage mediated by a prolonged and uncontrolled cytokine response to infection. Clinical evidence suggests that vagus nerve activity during sepsis is decreased, a finding with important implications for pathogenesis. These studies utilized measures of heart rate variability as a surrogate indicator of vagus nerve activity. The mortality rate in subjects with sepsis and decreased vagus nerve activity at hospital admission was 64%, as compared with 0% in subjects with higher vagus nerve activity (p <0.0001) (29). Although this study was not designed to prove causality of diminished vagus activity in lethal outcomes, the functional importance for intrinsic anti-inflammatory activity mediated by vagus nerve signaling is strongly supported by experimental evidence that vagotomy in animal sepsis models increases mortality and worsens tissue damage secondary to uncontrolled cytokine release (30, 31). Moreover, experimentally activating the cholinergic anti-inflammatory pathway in preclinical studies of sepsis by vagus nerve stimulation or administration of selective or universal α7 nAChR agonists improves survival, reduces proinflammatory cytokine levels (including HMGB1, implicated as a necessary and sufficient mediator of lethal sepsis in rodent models), and prevents the development of disseminated intravascular coagulation (24, 25, 30, 32, 33) (Figure 4).
Figure 4.
Vagus nerve regulates HMGB1 release and lethality during sepsis. (a) Control mice subjected to lethal peritonitis secondary to a perforated cecum overexpress HMGB1, which mediates lethal organ injury. (b) Cutting the vagus, which normally maintains immunological homeostasis by transmitting signals that inhibit HMGB1 release, leads to significantly higher levels of HMGB1 (and other cytokines, not shown) and higher mortality rates. (c) Electrically stimulating the vagus nerve enhances the inhibitory signals and suppresses HMGB1, which improves survival. (d) The vagus nerve can also be stimulated pharmacologically using agents that target the cholinergic brain networks to increase vagus nerve activity. Vagus nerve activity is increased by CNI-1493, selective M1 receptor agonists, and centrally acting acetylcholinesterase inhibitors. These effectively increase the inhibitory vagus nerve signals that suppress HMGB1 levels and improve survival.
Targeting the activation of brain cholinergic networks may also represent a therapeutic strategy in sepsis because intravenous administration of the orexigenic hormone ghrelin significantly reduces TNF and IL-6 levels in sepsis through central activation of the vagus nerve (34). Similar results have been observed following systemic administration of oxytocin, another pharmacological strategy that activates increased signaling by central vagus neurons (35). Administration of centrally acting acetylcholinesterase inhibitors also stimulates vagus nerve signaling and reduces cytokine release during experimental sepsis (36–38).
Arthritis
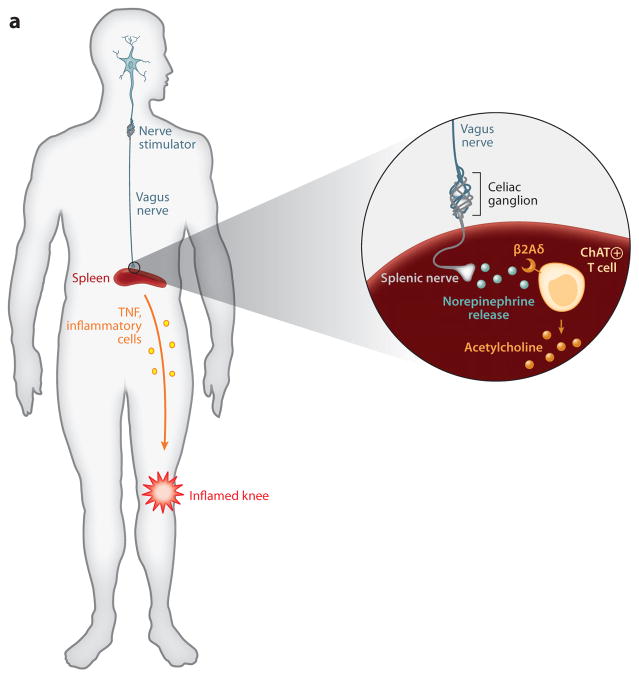
Vagus nerve activity is significantly impaired in most rheumatoid arthritis patients, as revealed by measurement of heart rate variability to quantify vagus nerve tone, and it is plausible that diminished endogenous cholinergic anti-inflammatory pathway activity contributes to the progression of inflammation (39–41). Experiments in collagen-induced arthritis in α7 nAChR–deficient mice demonstrated that clinical arthritis scores and synovial inflammation were significantly increased as compared to wild-type controls (42, 43). Systemic treatment in murine collagen-induced arthritis with selective α7 nAChR agonists ameliorated arthritis activity, and vagotomy exacerbated the disease (42, 44, 45). The therapeutic effects of the α7 nAChR–selective agonist were more efficacious than was nicotine, although it remains to be elucidated whether the beneficial effects were mediated by inhibiting cytokine release or by a direct effect on synovial tissue cells that express α7 nAChR (46). Vagus nerve signaling to the spleen may “reprogram” inflammatory cells and attenuate their ability to migrate to joints affected with synovitis (Figure 5). The vagus nerve does not innervate synovial tissues in musculoskeletal joints, but non-neuronal acetylcholine-expressing cells, including mononuclear cells and fibroblasts that express choline acetyltransferase, are found in the inflamed joints (47). In experimental models of localized soft-tissue inflammation induced by carrageenan-induced paw swelling, vagus nerve stimulation reduced edema formation and attenuated local cytokine production (7). Vagus nerve stimulation significantly attenuated inflammation in a subcutaneous air pouch because recruitment of granulocytes and activation of endothelial cells were both significantly inhibited (48). Indeed, the vagus nerve does not innervate the paw, or the subcutaneous air pouch, so the observed results are likely attributable to downregulation of cytokine release and a redirection of leukocyte trafficking through the spleen and other organs. It is also plausible that vagus nerve regulation of a subset of T cells that express acetylcholine might also participate in the anti-inflammatory mechanisms.
Figure 5.
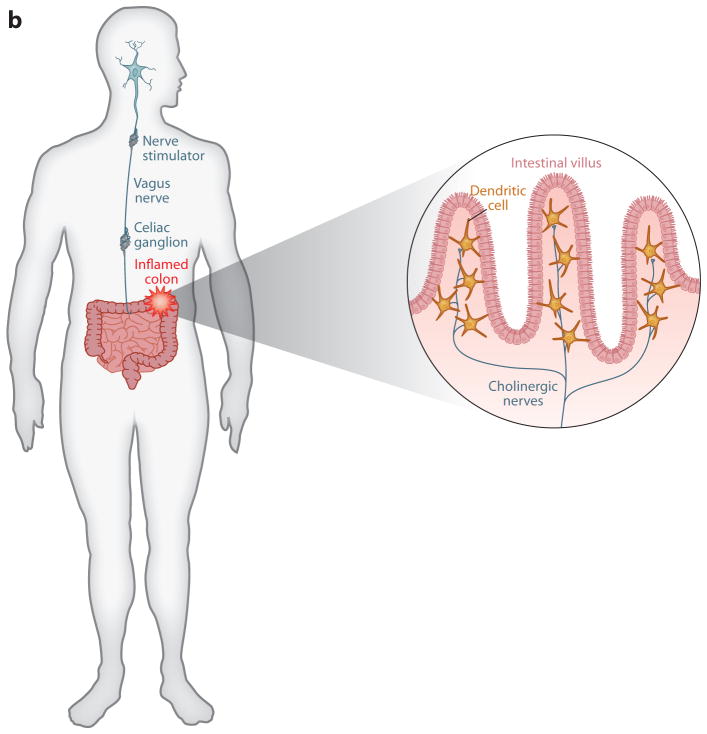
Implanted vagus nerve stimulators in arthritis and inflammatory bowel disease. (a) Vagus nerve–stimulating prototypes are being tested in the clinic to determine whether electrically stimulating the vagus nerve in humans will confer protection against cytokine-mediated inflammatory tissue damage. The approach is based on principles established in animal models that the vagus nerve regulates the activity of splenic neurons, which culminate in norepinephrine release in the spleen. Norepinephrine interacts with β2 adrenergic receptors expressed by T cells that, in turn, release acetylcholine, which inhibits cytokine release by signaling through α7 nAChR. At least two major effects of this neural mechanism prevent tissue injury: decreased release of TNF and other proinflammatory cytokines and decreased expression of adhesion molecules, HLA-DR, and other activation markers on monocytes as they transit the spleen en route to the joints or site of inflammation. (b) Vagus nerve stimulation in patients with inflammatory bowel disease may function through dual mechanisms, including the spleen pathway noted in panel a, and also by directly modulating inhibitory cholinergic neural signals to the intestine. Cytokine-producing cells that express α7 nAChR reside in the intestinal villi, where they lie in close proximity to acetylcholine-producing neurons that are regulated by vagus nerve signals.
Inflammatory Bowel Disease
Previously unexplained clinical observations dating back nearly 50 years revealed that cigarette smoke provides significant benefit to some ulcerative colitis patients (49). Furthermore, the incidence of ulcerative colitis is significantly less among smokers (50), which is consistent with the hypotheses that nicotine interaction with α7 nAChR can inhibit immune responses underlying inflammatory bowel disease and that agonists of α7 nAChR may be therapeutically useful (51, 52). Vagus nerve function is impaired in patients with ulcerative colitis, raising the possibility that diminished signaling in this important regulatory path predisposes patients to develop excessive inflammation and tissue injury (53). The vagus nerve innervates the celiac ganglion, which is the origin of cholinergic neurons that innervate the bowel (Figure 5). Administration of nicotine or specific α7 nAChR agonists is highly protective in models of experimental colitis (54, 55). Oral administration of nicotine decreased proinflammatory mediator levels in colonic mucosa during experimental colitis. Predictably, administration of α7 nAChR antagonists, or cervical vagotomy, enhances the severity of tissue damage and increases the release of proinflammatory mediators. Other cholinergic receptors may also contribute to pathogenesis, given that experimental colitis is significantly enhanced in α5 nAChR–deficient mice (56). Because the bowel is directly innervated by cholinergic neurons and because dendritic cells and other cytokine-producing cells of hematopoietic origin reside in the region of cholinergic nerve endings, cholinergic signals regulated by action potentials arising in the vagus nerve may suppress cytokine release and inflammation locally.
Pancreatitis
Acute pancreatitis, which is associated with a high risk of systemic inflammation, multiorgan failure, and lethality, leads to locally and systemically increased proinflammatory cytokine levels (57, 58). Vagotomy during acute pancreatitis severely aggravated the clinical course (59), whereas activation of the cholinergic anti-inflammatory pathway using GTS-21 as an α7 nAChR agonist alleviated the disease, attenuated pulmonary inflammation, and improved survival (59). The administration of α7 nAChR agonists was effective even when the vagus nerve had been cut, indicating that a pharmacological approach to therapy may be useful even when the basal activity of the vagus nerve during the disease is insufficient to suppress cytokine production.
Hemorrhagic Shock
Shock, a syndrome of inadequate organ perfusion and tissue ischemia, significantly complicates severe hemorrhage following trauma. Hemorrhagic shock is associated with excessive production of proinflammatory cytokines during reperfusion that contribute to the progression of organ failure. Vagus nerve stimulation in rodents during lethal hemorrhagic shock decreased TNF release, conferred significant protection against the development of hypotension, and prolonged survival (60). The protective effects of vagus nerve stimulation were reversed after administration of α7 nAChR antagonists, indicating that signal transduction via nicotinic receptors is required for the vagus nerve to control the cytokine storm and provide protection against tissue injury.
Intracerebral Hemorrhage
Inflammation following intracerebral hemorrhage contributes to worsened tissue damage, neuronal loss, and subsequent disability. The intracerebroventricular administration of muscarine, a pharmacological method to increase vagus nerve activity, improved neurologic outcome, reduced brain edema, and decreased levels of inflammatory mediators in the brain and spleen (61). Central muscarine injection was ineffective at reducing cerebral edema in splenectomized animals, raising the interesting possibility that the protective effects were mediated through a vagus nerve–spleen pathway, rather than through a direct interaction with the brain.
Ischemia-Reperfusion Injury
There is no effective treatment to prevent reperfusion injury, the tissue damage that occurs when blood flow is restored to ischemic tissue. Experimental models indicate that ischemia-reperfusion injury is mediated by proinflammatory factors released from injured and necrotic cells, including HMGB1, other cytokines, complement components, proteases, superoxide anion radicals, and products of white blood cell degranulation. Therapeutic administration of physostigmine attenuated brain inflammation, endothelial injury, and superoxide anion radical generation in a model of forebrain ischemic reperfusion (62). Resuscitated patients who survive cardiac arrest are at increased risk for developing inflammation in the brain as well as neuronal damage (63–65). One consequence of this process is a significant decline in central cholinergic signaling that may reduce the capacity of the cholinergic anti-inflammatory pathway to control inflammation. Postischemic treatment with selective α7 nAChR agonists provided significant protection against ischemia-generated cell death and inflammation within a clinically relevant time frame (66).
Direct vagus nerve stimulation has been a successful strategy to attenuate damage in several models of ischemia-reperfusion injury (6, 67–71). The incidence of severe arrhythmias, release of free radicals, and lethality were all reduced after electrical vagus nerve stimulation in myocardial ischemic reperfusion in rats (68). Treatment with α7 nAChR agonists in ischemia-reperfusion injury in rats improved renal function, reduced renal tubular damage and renal TNF, and decreased neutrophil infiltration to the kidneys (69). Nicotine failed to ameliorate renal injury in α7 nAChR gene–deficient mice (70). Administration of selective nicotine agonists may also be a potential strategy to prevent renal failure caused by ischemia and reperfusion during renal transplantation and major cardiovascular surgery. Vagus nerve stimulation also conferred protection against the development of shock after splanchnic artery reperfusion injury and inhibited release of TNF and other cytokines (71).
FUTURE PERSPECTIVES
As reviewed here, the initial focus in this new field has been on the principle that reflexes maintain immunological homeostasis. This included mapping the inflammatory reflex and identifying the neural and molecular mechanisms of this circuit. The direct therapeutic implications for targeting the efferent pathway of the reflex using devices and drugs have been explored. Ongoing study addresses the mechanisms by which the nervous system senses the presence of immunomodulatory agents in the periphery at the proximal stage of activating the protective reflex circuit. Indeed, extremely interesting findings from clinical studies using fMRI in rheumatoid arthritis patients recently revealed that the onset of inflammation in peripheral joints produces distinct changes in the activity of brain signaling networks associated with pain and sickness syndrome (72). Administration of anti-TNF antibodies significantly reversed the activity of these brain networks in an extremely short time frame. The brain responses occurred so quickly, well in advance of any clinical improvement or signs of attenuated inflammation in the peripheral tissues, that TNF appears to be directly involved in establishing neural signaling patterns in human brain during disease. Moreover, similar results were recapitulated in an animal model of arthritis using TNF-expressing transgenic mice (73). Administration of anti-TNF to these arthritic animals modified the activity of the sickness-syndrome brain networks within a rapid time frame that preceded evidence of reduced inflammatory burden in the peripheral tissues. Administration of anti-TNF antibodies into the cerebrospinal fluid significantly attenuated the inflammatory response in peripheral joints during collagen-induced arthritis (73, 74). Neurons express receptors for TNF, IL-1β, and other cytokines, which may activate neuronal signaling responses that can contribute to the afferent or sensory arc of the inflammatory reflex. Cytokines can provide sensory information to the nervous system about the status of the body environment during infection and injury (75–77). Cytokine input to the nervous system is processed and interpreted in relation to immunological set points established in the brainstem. In this way, the molecular products of the immune response are monitored by the nervous system as a proximal mechanistic step for maintaining homeostasis.
It is useful to consider the inflammatory reflex as a prototypical circuit that has been mapped in detail. The approaches developed during the generation of this map should lead to the identification of additional reflex circuits that regulate other aspects of immunity. For instance, the sensory arc of the inflammatory reflex has a hardwired capacity to activate other neuronal circuits targeting immunocompetent tissues. Adrenergic, peptidergic, glutaminergic, and other classes of neurons expressing neurotransmitters can specifically modulate the function of immune cells. Lymphocytes, monocytes, dendritic cells, and other immunocompetent cells express a host of receptors for neurotransmitters, and the functional role of these receptors in transducing discrete neuronal signals has been proposed. Prior to elucidation of the reflex principles reviewed here, the approach to understanding immune homeostasis was driven primarily by models of humoral and cellular signaling. Now, ongoing studies can reveal neurotransmitter receptor mechanisms as novel targets of rapidly controllable, regionally specific, directional, and integrated neuronal reflex circuits in the network that coordinates immune responses on the basis of integrative reflex actions. One can now imagine that devices designed to modulate these neural circuits may replace pharmacological agents in the treatment of inflammatory disease.
Acknowledgments
Research in the authors’ laboratories is supported by the Karolinska University Hospital (U.A.), the Karolinska Institutet (U.A.), the Swedish Research Council (U.A.), and the National Institutes of General Medical Sciences (K.J.T.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Ulf Andersson, Email: Ulf.Andersson@ki.se.
Kevin J. Tracey, Email: kjtracey@nshs.edu.
LITERATURE CITED
- 1.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–82. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Loewi O. Über homorale Übertragbarkeit der Herznervenwirkung. Pflügers Archiv. 1921;189:239–42. [Google Scholar]
- 3.Dale HH, Dudley HW. The presence of histamine and acetylcholine in the spleen of the ox and the horse. J Physiol. 1929;68:97–123. doi: 10.1113/jphysiol.1929.sp002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherrington C. The Integrative Action of the Nervous System. New Haven, CT: Yale Univ. Press; 1906. [Google Scholar]
- 5.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–59. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 6.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781–88. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borovikova LV, Ivanova S, Nardi D, Zhang M, Yang H, et al. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci. 2000;85:141–47. doi: 10.1016/S1566-0702(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 8.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 9.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci USA. 2008;105:11008–13. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–88. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 11.Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdés-Ferrer SI, et al. The selective α7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med. 2009;15:195–202. doi: 10.2119/molmed.2009.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, et al. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;183:27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- 13.Milligan ED, McGorry MM, Fleshner M, Gaykema RP, Goehler LE, et al. Subdiaphragmatic vagotomy does not prevent fever following intracerebroventricular prostaglandin E2: further evidence for the importance of vagal afferents in immune-to-brain communication. Brain Res. 1997;766:240–43. doi: 10.1016/s0006-8993(97)00705-1. [DOI] [PubMed] [Google Scholar]
- 14.Niijima A. The afferent discharges from sensors for interleukin 1βin the hepatoportal system in the anesthetized rat. J Auton Nerv Syst. 1996;61:287–91. doi: 10.1016/s0165-1838(96)00098-7. [DOI] [PubMed] [Google Scholar]
- 15.Niijima A, Hori T, Aou S, Oomura Y. The effects of interleukin-1β on the activity of adrenal, splenic and renal sympathetic nerves in the rat. J Auton Nerv Syst. 1991;36:183–92. doi: 10.1016/0165-1838(91)90042-2. [DOI] [PubMed] [Google Scholar]
- 16.Aballay A. Neural regulation of immunity: role of NPR-1 in pathogen avoidance and regulation of innate immunity. Cell Cycle. 2009;8:966–69. doi: 10.4161/cc.8.7.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun J, Singh V, Kajino-Sakamoto R, Aballay A. Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science. 2011;332:729–32. doi: 10.1126/science.1203411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–28. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powley TL, Prechtl JC, Fox EA, Berthoud HR. Anatomical considerations for surgery of the rat abdominal vagus: distribution, paraganglia and regeneration. J Auton Nerv Syst. 1983;9:79–97. doi: 10.1016/0165-1838(83)90133-9. [DOI] [PubMed] [Google Scholar]
- 20.Berthoud HR, Powley TL. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J Auton Nerv Syst. 1993;42:153–69. doi: 10.1016/0165-1838(93)90046-w. [DOI] [PubMed] [Google Scholar]
- 21.Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawashima K, Fujii T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. 2003;74:675–96. doi: 10.1016/j.lfs.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Vida G, Peña G, Kanashiro A, Del Rocio Thompson-Bonilla M, Palange D, et al. β2-adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J. 2011;25:4476–85. doi: 10.1096/fj.11-191007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35:2762–68. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 25.Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, et al. Selective α7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 2007;35:1139–44. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 26.Giebelen IA, van Westerloo DJ, La Rosa GJ, de Vos AF, van der Poll T. Stimulation of α7 cholinergic receptors inhibits lipopolysaccharide-induced neutrophil recruitment by a tumor necrosis factor α-independent mechanism. Shock. 2007;27:443–47. doi: 10.1097/01.shk.0000245016.78493.bb. [DOI] [PubMed] [Google Scholar]
- 27.Wittebole X, Hahm S, Coyle SM, Kumar A, Calvano SE, Lowry SF. Nicotine exposure alters in vivo human responses to endotoxin. Clin Exp Immunol. 2007;147:28–34. doi: 10.1111/j.1365-2249.2006.03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci USA. 2006;103:5219–23. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pontet J, Contreras P, Curbelo A, Medina J, Noveri S, et al. Heart rate variability as early marker of multiple organ dysfunction syndrome in septic patients. J Crit Care. 2003;18:156–63. doi: 10.1016/j.jcrc.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 30.van Westerloo DJ, Giebelen IA, Florquin S, Daalhuisen J, Bruno MJ, et al. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis. 2005;191:2138–48. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 31.Kessler W, Traeger T, Westerholt A, Neher F, Mikulcak M, et al. The vagal nerve as a link between the nervous and immune system in the instance of polymicrobial sepsis. Langenbecks Arch Surg. 2006;391:83–87. doi: 10.1007/s00423-006-0031-y. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Liao H, Ochani M, Justiniani M, Lin X, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–21. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 33.Czura CJ, Schultz A, Kaipel M, Khadem A, Huston JM, et al. Vagus nerve stimulation regulates hemostasis in swine. Shock. 2010;33:608–13. doi: 10.1097/SHK.0b013e3181cc0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu R, Dong W, Cui X, Zhou M, Simms HH, et al. Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Ann Surg. 2007;245:480–86. doi: 10.1097/01.sla.0000251614.42290.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clodi M, Vila G, Geyeregger R, Riedl M, Stulnig TM, et al. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. Am J Physiol Endocrinol Metab. 2008;295:E686–91. doi: 10.1152/ajpendo.90263.2008. [DOI] [PubMed] [Google Scholar]
- 36.Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009;23:41–45. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofer S, Eisenbach C, Lukic IK, Schneider L, Bode K, et al. Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Crit Care Med. 2008;36:404–8. doi: 10.1097/01.CCM.0B013E31816208B3. [DOI] [PubMed] [Google Scholar]
- 38.Fodale V, Santamaria LB. Cholinesterase inhibitors improve survival in experimental sepsis: a new way to activate the cholinergic anti-inflammatory pathway. Crit Care Med. 2008;36:622–23. doi: 10.1097/CCM.0B013E31816297CE. [DOI] [PubMed] [Google Scholar]
- 39.Evrengül H, Dursunoglu D, Cobankara V, Polat B, Seleci D, et al. Heart rate variability in patients with rheumatoid arthritis. Rheumatol Int. 2004;24:198–202. doi: 10.1007/s00296-003-0357-5. [DOI] [PubMed] [Google Scholar]
- 40.Kamal A. Assessment of autonomic function in patients with rheumatoid arthritis using spectral analysis and approximate entropy method. Neurosciences. 2007;12:136–39. [PubMed] [Google Scholar]
- 41.Stojanovich L, Milovanovich B, de Luka SR, Popovich-Kuzmanovich D, Bisenich V, et al. Cardiovascular autonomic dysfunction in systemic lupus, rheumatoid arthritis, primary Sjögren syndrome and other autoimmune diseases. Lupus. 2007;16:181–85. doi: 10.1177/0961203306076223. [DOI] [PubMed] [Google Scholar]
- 42.van Maanen MA, Lebre MC, van der Poll T, La Rosa GJ, Elbaum D, et al. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009;60:114–22. doi: 10.1002/art.24177. [DOI] [PubMed] [Google Scholar]
- 43.van Maanen MA, Stoof SP, Larosa GJ, Vervoordeldonk MJ, Tak PP. Role of the cholinergic nervous system in rheumatoid arthritis: aggravation of arthritis in nicotinic acetylcholine receptor α7 subunit gene knockout mice. Ann Rheum Dis. 2010;69:1717–23. doi: 10.1136/ard.2009.118554. [DOI] [PubMed] [Google Scholar]
- 44.van Maanen MA, Stoof SP, van der Zanden EP, de Jonge WJ, Janssen RA, et al. The α7 nicotinic acetylcholine receptor on fibroblast-like synoviocytes and in synovial tissue from rheumatoid arthritis patients: a possible role for a key neurotransmitter in synovial inflammation. Arthritis Rheum. 2009;60:1272–81. doi: 10.1002/art.24470. [DOI] [PubMed] [Google Scholar]
- 45.Li T, Zuo X, Zhou Y, Wang Y, Zhuang H, et al. The vagus nerve and nicotinic receptors involve inhibition of HMGB1 release and early pro-inflammatory cytokines function in collagen-induced arthritis. J Clin Immunol. 2010;30:213–20. doi: 10.1007/s10875-009-9346-0. [DOI] [PubMed] [Google Scholar]
- 46.Waldburger JM, Boyle DL, Pavlov VA, Tracey KJ, Firestein GS. Acetylcholine regulation of synoviocyte cytokine expression by the α7 nicotinic receptor. Arthritis Rheum. 2008;58:3439–49. doi: 10.1002/art.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grimsholm O, Rantapää-Dahlqvist S, Dalén T, Forsgren S. Unexpected finding of a marked non-neuronal cholinergic system in human knee joint synovial tissue. Neurosci Lett. 2008;442:128–33. doi: 10.1016/j.neulet.2008.06.082. [DOI] [PubMed] [Google Scholar]
- 48.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–23. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolf JM, Lashner BA. Inflammatory bowel disease: sorting out the treatment options. Clevel Clin J Med. 2002;69:621–31. doi: 10.3949/ccjm.69.8.621. [DOI] [PubMed] [Google Scholar]
- 50.Birrenbach T, Böcker U. Inflammatory bowel disease and smoking: a review of epidemiology, pathophysiology, and therapeutic implications. Inflamm Bowel Dis. 2004;10:848–59. doi: 10.1097/00054725-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 51.Scott DA, Martin M. Exploitation of the nicotinic anti-inflammatory pathway for the treatment of epithelial inflammatory diseases. World J Gastroenterol. 2006;12:7451–59. doi: 10.3748/wjg.v12.i46.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonaz B. The cholinergic anti-inflammatory pathway and the gastrointestinal tract. Gastroenterology. 2007;133:1370–73. doi: 10.1053/j.gastro.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 53.Lindgren S, Stewenius J, Sjölund K, Lilja B, Sundkvist G. Autonomic vagal nerve dysfunction in patients with ulcerative colitis. Scand J Gastroenterol. 1993;28:638–42. doi: 10.3109/00365529309096103. [DOI] [PubMed] [Google Scholar]
- 54.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–30. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Bai A, Guo Y, Lu N. The effect of the cholinergic anti-inflammatory pathway on experimental colitis. Scand J Immunol. 2007;66:538–45. doi: 10.1111/j.1365-3083.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- 56.Orr-Urtreger A, Kedmi M, Rosner S, Karmeli F, Rachmilewitz D. Increased severity of experimental colitis in α5 nicotinic acetylcholine receptor subunit-deficient mice. NeuroReport. 2005;16:1123–27. doi: 10.1097/00001756-200507130-00018. [DOI] [PubMed] [Google Scholar]
- 57.Bhatia M, Zhi L, Zhang H, Ng SW, Moore PK. Role of substance P in hydrogen sulfide-induced pulmonary inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2006;291:L896–904. doi: 10.1152/ajplung.00053.2006. [DOI] [PubMed] [Google Scholar]
- 58.Rakonczay Z, Jr, Hegyi P, Takács T, McCarroll J, Saluja AK. The role of NF-κB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259–67. doi: 10.1136/gut.2007.124115. [DOI] [PubMed] [Google Scholar]
- 59.van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–30. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 60.Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, et al. Efferent vagal fibre stimulation blunts nuclear factor-κB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189–94. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- 61.Lee ST, Chu K, Jung KH, Kang KM, Kim JH, et al. Cholinergic anti-inflammatory pathway in intracerebral hemorrhage. Brain Res. 2010;1309:164–71. doi: 10.1016/j.brainres.2009.10.076. [DOI] [PubMed] [Google Scholar]
- 62.Kutsuna S, Tsuruta R, Fujita M, Todani M, Yagi T, et al. Cholinergic agonist physostigmine suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in rats with forebrain ischemia/reperfusion. Brain Res. 2010;1313:242–49. doi: 10.1016/j.brainres.2009.11.077. [DOI] [PubMed] [Google Scholar]
- 63.Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–65. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 64.Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–18. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, et al. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28:927–38. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- 66.Norman GJ, Morris JS, Karelina K, Weil ZM, Zhang N, et al. Cardiopulmonary arrest and resuscitation disrupts cholinergic anti-inflammatory processes: a role for cholinergic α7 nicotinic receptors. J Neurosci. 2011;31:3446–52. doi: 10.1523/JNEUROSCI.4558-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, et al. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36:1231–36. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 68.Mioni C, Bazzani C, Giuliani D, Altavilla D, Leone S, et al. Activation of an efferent cholinergic pathway produces strong protection against myocardial ischemia/reperfusion injury in rats. Crit Care Med. 2005;33:2621–28. doi: 10.1097/01.ccm.0000186762.05301.13. [DOI] [PubMed] [Google Scholar]
- 69.Yeboah MM, Xue X, Duan B, Ochani M, Tracey KJ, et al. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 2008;74:62–69. doi: 10.1038/ki.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sadis C, Teske G, Stokman G, Kubjak C, Claessen N, et al. Nicotine protects kidney from renal ischemia/reperfusion injury through the cholinergic anti-inflammatory pathway. PLoS ONE. 2007;2:e469. doi: 10.1371/journal.pone.0000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Altavilla D, Guarini S, Bitto A, Mioni C, Giuliani D, et al. Activation of the cholinergic anti-inflammatory pathway reduces NF-κB activation, blunts TNF-α production, and protects against splanchic artery occlusion shock. Shock. 2006;25:500–6. doi: 10.1097/01.shk.0000209539.91553.82. [DOI] [PubMed] [Google Scholar]
- 72.Hess A, Axmann R, Rech J, Finzel S, Heindl C, et al. Blockade of TNF-α rapidly inhibits pain responses in the central nervous system. Proc Natl Acad Sci USA. 2011;108:3731–36. doi: 10.1073/pnas.1011774108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boettger MK, Hensellek S, Richter F, Gajda M, Stöckigt R, et al. Antinociceptive effects of tumor necrosis factor αneutralization in a rat model of antigen-induced arthritis: evidence of a neuronal target. Arthritis Rheum. 2008;58:2368–78. doi: 10.1002/art.23608. [DOI] [PubMed] [Google Scholar]
- 74.Boettger MK, Weber K, Grossmann D, Gajda M, Bauer R, et al. Spinal tumor necrosis factor α neutralization reduces peripheral inflammation and hyperalgesia and suppresses autonomic responses in experimental arthritis: a role for spinal tumor necrosis factor α during induction and maintenance of peripheral inflammation. Arthritis Rheum. 2010;62:1308–18. doi: 10.1002/art.27380. [DOI] [PubMed] [Google Scholar]
- 75.Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, et al. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull. 1997;43:357–64. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 76.Hosoi T, Okuma Y, Matsuda T, Nomura Y. Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion. Auton Neurosci. 2005;120:104–7. doi: 10.1016/j.autneu.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 77.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102:5856–61. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]