Abstract
Pneumatosis intestinalis is a characteristic imaging phenomenon indicating the presence of gas in the bowel wall. The link between pneumatosis intestinalis and various kinds of autoimmune diseases has been reported anecdotally, while information regarding the cases with antineutrophil cytoplasmic antibodies (ANCA)–associated vasculitis complicated by concurrent pneumatosis intestinalis is lacking. In this report, we describe our serendipitous experience with one such case of pneumatosis intestinalis in a patient with ANCA-associated glomerulonephritis. We also discuss several therapeutic concerns that arose in the current case, which had an impact on the pathogenesis of the disease.
Keywords: ANCA, pneumatosis intestinalis, steroid, α-glucosidase inhibitor, rapidly progressive glomerulonephritis
Introduction
Pneumatosis intestinalis is a characteristic imaging phenomenon indicating the presence of gas in the bowel wall.1,2 It represents a wide spectrum of conditions, ranging from the innocuous to the fatal.1–3 In this report, we describe our serendipitous experience with a case of pneumatosis intestinalis in a patient with a recent diagnosis of antineutrophil cytoplasmic antibodies (ANCA)–associated glomerulonephritis. Several concerns regarding the pathogenesis of the disease in this patient are also discussed.
Case Report
A 75-year-old male was admitted to our hospital in the middle of February 2013 due to rapid deterioration of his renal function, general fatigue, and myalgia in his lower extremities. Although he had no history of renal disease, his serum creatinine (sCr) level was 0.9 mg/dL at the end of January 2013 and increased to 1.2 mg/dL at the beginning of February 2013. Four years prior to admission, the patient was found to have rectal carcinoma, which was treated successfully with surgical removal combined with transient postoperative chemotherapy. He had smoked for more than 20 years until 54 years of age, while he denied any history of drug abuse. The laboratory data obtained on admission are summarized in Table 1.
Table 1.
The laboratory data on admission.
| White blood cell | 8800/µl | (3900–9800) |
| Hemoglobin | 11.3 g/dl | (13.5–17.6) |
| Platelet count | 24.5 × 104/µl | (13.0–36.9) |
| Blood urea nitrogen | 28 mg/dl | (8–20) |
| Serum creatinine | 1.13 mg/dl | (0.63–1.03) |
| Total protein | 6.6 g/dl | (6.9–8.4) |
| Serum albumin | 2.7 g/dl | (3.9–5.1) |
| Sodium | 142 mmol/l | (136–148) |
| Potassium | 3.7 mmol/l | (3.6–5.0) |
| Chloride | 107 mmol/l | (96–108) |
| Calcium | 8.6 mg/dl | (8.8–10.1) |
| Phosphorus | 3.2 mg/dl | (2.4–4.6) |
| Aspartate aminotransferase | 24 U/l | (11–30) |
| Alanine aminotransferase | 26 U/l | (4–30) |
| C-reactive protein | 10.23 mg/dl | (0–0.14) |
| IgG | 1211 mg/dl | (870–1700) |
| IgA | 583 mg/dl | (110–410) |
| IgM | 153 mg/dl | (33–160) |
| C3 | 108 mg/dl | (86–160) |
| C4 | 34 mg/dl | (17–45) |
| FDP | 18.8 µg/ml | (0–5) |
| fibrinogen | 519 mg/dl | (129–271) |
| FBS | 97 mg/dl | (70–120) |
| HbA1c | 5.00% | (4.6–6.2) |
Note: The reference ranges for each parameter used at our institute are indicated in the parentheses.
Abbreviations: Ig, immunoglobulin; FDP, fibrinogen degradation product; FBS, fasting blood sugar; HbA1c, hemoglobin A1c.
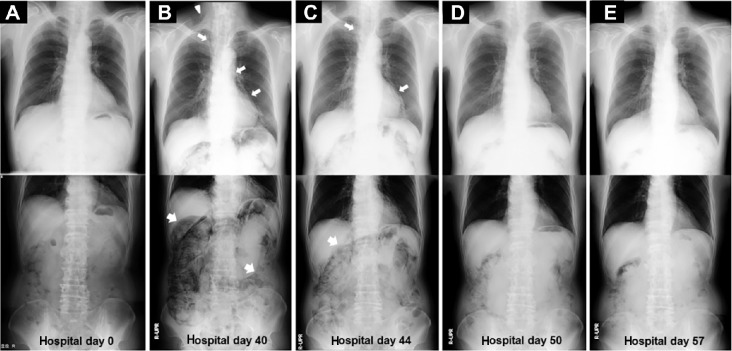
An increase in the titer of antimyeloperoxidase–ANCA (MPO-ANCA) above 300 U/mL, but no increase in antiproteinase 3–ANCA (PR3-ANCA) or antiglomerular basement membrane (GBM) antibodies, was also found, while tests for the presence of antinuclear antibodies, hepatitis B virus surface antigens (HBsAg), anti-HBsAg antibodies, and hepatitis C virus antibodies were all negative. No abnormal findings were detectable in either the chest or abdominal X-rays (Fig. 1A). A urinalysis revealed a urine protein level of 3+ and a red blood cell count of 30–49/high power field (HPF), and the creatinine clearance was 79.5 mL/min.
Figure 1.
The serial chest and abdominal X-ray findings during the observation period. No abnormal findings were detectable on admission (A). On hospital day 40 (B), streaky lucencies over the mediastinum (narrow arrows) that extended into the neck (arrowhead) and remarkable gaseous distention of the bowel (wide arrows) were demonstrated by a routine chest and abdominal X-ray, respectively. These radiological findings, with slight improvement, were noted on hospital day 44 (C), followed by further improvements on hospital day 50 (D) and 57 (E) despite the resumption of dietary intake on hospital day 49.
A renal biopsy performed 4 days after admission contained five cores of renal parenchyma with 23 glomeruli, 2 of which were globally sclerotic. Although vasculitic changes were absent in the blood vessels, cellular crescent formation was seen in the rest of the glomeruli, some of which exhibited a break in the GBM associated with fibrin extravasation (Fig. 2). An immunofluorescence analysis demonstrated a lack of immunoglobu-lin and complement deposition within the glomeruli. Based on the laboratory and pathological findings, the patient was diagnosed to have MPO-ANCA–associated glomerulonephritis, and oral prednisolone (PSL) at a dose of 40 mg/day was started. Despite the improvement of his general status and the successful relief from myalgia, his renal function gradually worsened, and intravenous pulse therapy with methylprednisolone at 500 mg/day was given for three consecutive days from the 22nd hospital day, when his sCr had increased up to 1.92 mg/dL.
Figure 2.
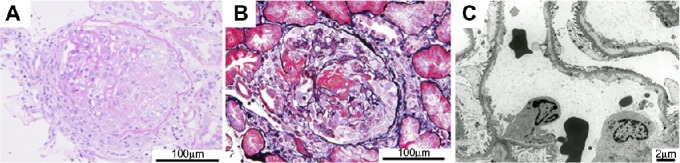
Photomicrographs of the renal biopsy specimen. A light micrograph showed cellular crescent formation in several glomeruli (A). Periodic acid–Schiff stain), some of which were associated with segmental fibrinoid necrosis (B). Periodic acid methenamine stain). Immunohistochemical staining demonstrated the lack of immunoglobulin and complement deposition, and electron microscopy failed to show the presence of deposits along the capillary walls (C). The scale bars are indicated in each panel.
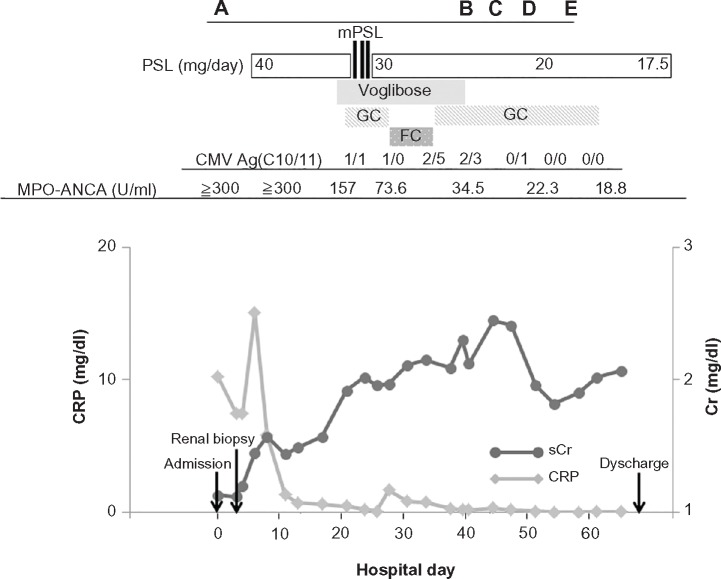
At almost the same time, the cytomegalovirus (CMV) antigenemia assay with monoclonal antibodies C10/C11 revealed that the patient had a CMV infection. He was subjected to preemptive treatment with ganciclovir, which was continued until the CMV antigen assays became negative. The peak titer of CMV antigenemia (CMV Ag) was 2/5 when the treatment had been terminated for 1 week, because we gave priority to treatment with oral famciclovir for a concurrent herpes zoster infection that developed on the 27th hospital day (Fig. 3). A laboratory analysis revealed that the patient had postprandial hyperglycemia (290 mg/dL) 12 days after the initiation of oral PSL, leading to a diagnosis of steroid-induced diabetes. Then, voglibose (single dose of 0.3 mg, given three times a day just before each meal) treatment was commenced on hospital day 18, resulting in good glycemic control.
Figure 3.
The clinical course of the current patient. A prompt decrease in the serum C-reactive protein level was confirmed just after the initiation of oral prednisolone, while the sCr levels began to decrease approximately 7 weeks after the initiation of corticosteroids treatment. Ganciclovir treatment for the CMV infection was transiently switched to oral famciclovir, when overt herpes zoster (characterized by a vesicular rash with a dermatomal distribution) developed. The time points corresponding to each panel in Figure 1 are demonstrated at the top.
Abbreviations: GC, ganciclovir; FV, famciclovir.
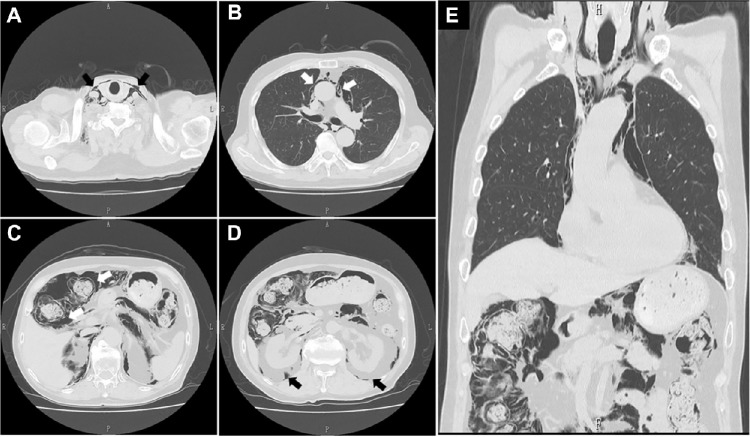
A routine chest X-ray performed 5 weeks after he had started oral PSL suggested the presence of right cervical subcutaneous emphysema and pneumomediastinum, while an abdominal X-ray showed remarkable gas collections along the bowel wall (Fig. 1B). Although the patient did not have any respiratory or abdominal symptoms, subsequent chest and abdominal computed tomography (CT) scans demonstrated intramural air in the bowel, gas collection in the mes-entery, and an abnormal air density in the mediastinum, as well as in the cervical subcutaneous regions (Figs. 4A–E). Voglibose was ceased, and fasting was imposed when a diagnosis of pneumatosis intestinalis was made. Subsequently, fluid supplementation and oxygen inhalation were started, while a reduced dose PSL of 20 mg/day was continued. Thereafter, the radiological indications of the disease were gradually improved and were no longer present on the 57th hospital day, despite the resumption of normal dietary intake (Figs. 1C–E). His sCr levels were finally maintained around 1.9 mg/dL with 2-h postprandial blood glucose levels of 150–180 mg/dL when he was treated at the outpatient clinic with PSL at a dose of 15 mg/day, with a MPO-ANCA titer of 16.5–21.0 U/mL.
Figure 4.
CT scans of the chest and abdomen. Abnormal air accumulations characterized by radiolucent areas (black or white arrows in each panel) were observed in the cervical regions (A), mediastinum (B), intramural parts of the ascending colon (C) and mesentery, along with the retroperitoneum (D). A coronal reconstructed image (E) failed to show free air.
Discussion
The combination of a rapid deterioration of the renal function and crescentic glomerulonephritis mediated by ANCA is a well-described clinical entity.4,5 The standard treatments for ANCA-associated vasculitis, which are based on the results of a series of clinical trials conducted by the European Vasculitis Study Group, consist of corticosteroids combined with oral or intravenous cyclophosphamide6; however, there are some differences in the characteristics of Japanese ANCA-associated vasculitis patients compared with those in Europe. For instance, microscopic polyangiitis and MPO-ANCA are more common in Japan, while granulomatosis with polyangiitis and PR3-ANCA are more common in the United Kingdom.7–9 Meanwhile, it has been shown that the average age of such Japanese patients is high and the most frequent cause of death is infectious complications.9 Thus, the Japanese clinical practice guideline for the disease has emphasized the need for reduced immunosuppressive treatment, such as a reduced dose of PSL with or without an immunosuppressant, and the concurrent application of cyclophosphamide has not necessarily been recommended.8,9 Consequently, the monotherapeutic protocol with corticosteroids applied in the current patient may not be surprising.
Despite the clinical benefit of corticosteroids on the overall management of ANCA-associated vasculitis,5–8 numerous adverse effects, including opportunistic infections and metabolic disturbances, have been attributed to the agents.10 This was also the case in the current patient, since the treatment with PSL seemed to result in the improvement of renal function, but led to the concurrent development of CMV infection, herpes zoster infection, and impaired glycemic control, characterized by postprandial hyperglycemia. One may argue that the clinical and therapeutic manifestations of our patient are too common to describe in the literature. However, the clinical significance of the present report should be evaluated carefully in terms of the fact that he was subsequently complicated with cervical subcutaneous emphysema, pneumomediastinum, and air accumulation in the bowel wall, leading to a diagnosis of pneumatosis intestinalis.
Pneumomediastinum and cervical subcutaneous emphysema have been associated with a wide range of structural lung diseases, including asthma, emphysema, bronchiectasis, and interstitial lung disease,11 while the simultaneous occurrence of pneumomediastinum and pneumatosis intestinalis with or without subcutaneous emphysema is not exceptional.2,3,12–15 Such a combination may result from alveolar rupture based on the pressure gradient between the alveoli and the lung interstitium, which leads air to pass along the vascular channels in the mediastinum, tracking caudally through the retroperitoneum and then to the mesentery of the bowel.2,3 However, our patient did not manifest any pulmonary symptoms such as cough, chest pain, or discomfort during the observation period, and thus, descending gas that originated from the lungs was considered to be a less likely cause in the current case. Rather, it appears that retroperi-toneal air that had arisen from gas accumulated within the bowel wall escaped upward into the mediastinum and cervical subcutaneous area in the present patient, as has been described anecdotally.14,15
Numerous clinical conditions (Table 2) have been regarded to be underlying diseases associated with pneumatosis intestinalis.1–3 A vasculitis-based pathogenesis within the intestinal territory has been proposed as an etiological background for the development of the disease among select subjects with systemic lupus erythematosus.12 A gastrointestinal tract involvement resulting from a vascular injury can be observed in patients with ANCA-associated vasculitis as well16; however, the lack of systemic disease manifestations in our patient led us to attribute the patient’s renal abnormalities to the renal-limited phenotype and to conclude that the clinical impact of a MPO-ANCA–mediated mechanism on the development of pneumatosis intestinalis might be marginal. Otherwise, the paucity of data regarding the occurrence of pneumatosis intestinalis in patients with ANCA-associated vasculitis may imply that such a vascular injury does not play a role as a predisposing factor equivalent to that of lupus, although the case of systemic vasculitis associated with PR3-ANCA, but not with MPO-ANCA, in a patient accompanied by the disease has been shown anecdotally.17
Table 2.
Diseases and conditions associated with pneumatosis intestinalis.
| Gastrointestinal | Vascular | Drugs |
|---|---|---|
| Pyloric obstruction | Ischemia | Corticosteroids |
| Bowel obstruction | Infarction | Chemotherapeutic agents |
| Peptic ulcer disease | Mesenteric vascular disease | Lactulose |
| Necrotizing enterocolitis | Sorbitol | |
| Crohn disease | Autoimmune and systemic | Glucosidase inhibitor |
| Ulcerative colitis | Lupus enteritis | Chloral hydrate |
| Diverticulitis | Dermatomyositis | |
| Polyarteritis nodosa | Trauma | |
| Pulmonary | Scleroderma | Surgery, anastomosis or bypass |
| Asthma | Endoscopy | |
| COPD | Infectious | Penetrating or blunt injury |
| Cystic fibrosis | HIV and AIDs | Enema studies, barium or other |
| Pulmonary fibrosis | Cytomegalovirus | Mechanical ventilation |
| Rotavirus | ||
| Malignancies | Adenovirus | Idiopathic |
| Gastrointestinal carcinoma | Candida albicans | |
| Leukemia or lymphoma | Mycobacterium tuberculosis |
Abbreviations: COPD, chronic obstructive pulmonary disease; HIV and AIDs, human immunodeficiency virus and acquired immunodeficiency syndrome.
We feel it is reasonable to consider that certain medications and comorbidities may have played a role in the development of pneumatosis intestinalis in our patient. The common candidates are corticosteroids, which have been suggested to deplete the lymphoid tissue within Payer’s patches, leading to disturbances in the mucosal integrity which permits the dispersion of gas into the noninflamed bowel wall.2,18 Alternatively, or in addition, alpha-glucosidase inhibitors (AGIs) such as acarbose, voglibose, and miglitol, which delay the absorption of carbohydrates from the gastrointestinal tract by inhibiting AGI, thereby limiting postprandial plasma glucose excursions,19 have also been reported to be associated with pneumatosis intestinalis.3,20 An increase in intraluminal pressure due to excessive gas production based on the bacterial fermentation of undigested carbohydrates in the bowel might result in the development of pneumatosis intestinalis.20 Otherwise, concurrent opportunistic infections with CMV may have also predisposed our patient to the disease. Indeed, a presumable causal relationship between pneumatosis intestinalis and CMV infection has been mentioned anecdot-ally,21–23 and the primary cytopathogenic effect of CMV on the bowel mucosa or vasculitic reaction mediated by CMV infection may be implicated in the disease process.23 Finally, we are of the opinion that the development of the disease in our patient could be attributed to the additive or synergistic effects of multiple factors, including the use of corticosteroids and voglibose, as well as CMV infection. The rapid radiological improvement, along with the disappearance of CMV antigenemia in the current patient after the cessation of voglibose and the dose reduction of PSL supports this assumption, although it is difficult to determine the precise contributions of each factor.
Despite the accumulation of anecdotal or systemic studies disclosing the nature of pneumatosis intestinalis,2,3,24 we feel that an early and accurate diagnosis, as well as awareness of the disease, remains a challenge for physicians. The current report apparently emphasizes the pitfalls of managing patients with ANCA-associated glomerulonephritis who are treated with corticosteroids. The validity of the treatment with AGIs for the steroid-induced diabetes, which is occasionally reported in subjects with ANCA-associated vasculitis,10 may also need to be evaluated carefully, even if there are only few subjects who may benefit from such agents.25 No specific surgical approach may be required in asymptomatic cases, while there are some subsets of patients who require urgent surgical intervention due to concurrent abdominal sepsis, perforation, or peritonitis.2,3,18,26 The establishment of an optimal diagnostic and therapeutic policy for the disease is clearly mandatory since the origin of the gas is occasionally unclear, and the patient’s symptoms can be volatile, presenting a diagnostic dilemma for the surgeon in charge.3
Footnotes
ACADEMIC EDITOR: Athavale Nandkishor, Associate Editor
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 544 words, excluding any confidential comments to the academic editor.
FUNDING: This study was supported in part by a Grant-in-Aid for Research on Advanced Chronic Kidney Disease, Practical Research Project for Renal Diseases from Japan Agency for Medical Research and development, AMED. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Drafted the manuscript: SN, TA. Made contributions to the acquisition of the clinical data: ST, MO, AM, HY. Provided a detailed review of the contents and structure of the manuscript, resulting in significant changes to the original document: SM, EK, DN. All authors have read and approved the final manuscript.
REFERENCES
- 1.Feczko PJ, Mezwa DG, Farah MC, White BD. Clinical significance of pneumatosis of the bowel wall. Radiographics. 1992;12(6):1069–78. doi: 10.1148/radiographics.12.6.1439012. [DOI] [PubMed] [Google Scholar]
- 2.Pear BL. Pneumatosis intestinalis: a review. Radiology. 1998;207(1):13–9. doi: 10.1148/radiology.207.1.9530294. [DOI] [PubMed] [Google Scholar]
- 3.Khalil PN, Huber-Wagner S, Ladurner R, et al. Natural history, clinical pattern, and surgical considerations of pneumatosis intestinalis. Eur J Med Res. 2009;14(6):231–9. doi: 10.1186/2047-783X-14-6-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akimoto T, Ando Y, Ito C, Muto S, Kusano E, Asano Y. Effect of plasmapheresis as initial monotherapy in a case of anti-neutrophil cytoplasmic autoantibody positive crescentic glomerulonephritis. ASAIO J. 1999;45(5):509–13. doi: 10.1097/00002480-199909000-00026. [DOI] [PubMed] [Google Scholar]
- 5.Koyama A, Yamagata K, Makino H, et al. Japan RPGN Registry Group A nationwide survey of rapidly progressive glomerulonephritis in Japan: etiology, prognosis and treatment diversity. Clin Exp Nephrol. 2009;13(6):633–50. doi: 10.1007/s10157-009-0201-7. [DOI] [PubMed] [Google Scholar]
- 6.Ntatsaki E, Mooney J, Watts RA. ANCA vasculitis: time for a change in treatment paradigm? Not yet. Rheumatology (Oxford) 2011;50(6):1019–24. doi: 10.1093/rheumatology/ker002. [DOI] [PubMed] [Google Scholar]
- 7.Imai T, Takeda S, Kawaguchi K, et al. Delayed development of pulmonary hemorrhage in a patient with positive circulating anti-neutrophil cytoplasmic antibody: a clinical dilemma. Case Rep Nephrol Urol. 2013;3(2):121–7. doi: 10.1159/000355509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozaki S, Atsumi T, Hayashi T, et al. Severity-based treatment for Japanese patients with MPO-ANCA-associated vasculitis: the JMAAV study. Mod Rheumatol. 2012;22(3):394–404. doi: 10.1007/s10165-011-0525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamagata K, Usui J, Saito C, et al. ANCA-associated systemic vasculitis in Japan: clinical features and prognostic changes. Clin Exp Nephrol. 2012;16(4):580–8. doi: 10.1007/s10157-012-0598-2. [DOI] [PubMed] [Google Scholar]
- 10.Turnbull J, Harper L. Adverse effects of therapy for ANCA-associated vasculitis. Best Pract Res Clin Rheumatol. 2009;23(3):391–401. doi: 10.1016/j.berh.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Iyer VN, Joshi AY, Ryu JH. Spontaneous pneumomediastinum: analysis of 62 consecutive adult patients. Mayo Clin Proc. 2009;84(5):417–21. doi: 10.1016/S0025-6196(11)60560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizoguchi F, Nanki T, Miyasaka N. Pneumatosis cystoides intestinalis following lupus enteritis and peritonitis. Intern Med. 2008;47(13):1267–71. doi: 10.2169/internalmedicine.47.0748. [DOI] [PubMed] [Google Scholar]
- 13.Han BG, Lee JM, Yang JW, Kim MS, Choi SO. Pneumatosis intestinalis associated with immunesuppressive agents in a case of minimal change disease. Yonsei Med J. 2002;43(5):686–9. doi: 10.3349/ymj.2002.43.5.686. [DOI] [PubMed] [Google Scholar]
- 14.Saito M, Tanikawa A, Nakasute K, Tanaka M, Nishikawa T. Additive contribution of multiple factors in the development of pneumatosis intestinalis: a case report and review of the literature. Clin Rheumatol. 2007;26(4):601–3. doi: 10.1007/s10067-005-0179-9. [DOI] [PubMed] [Google Scholar]
- 15.Honne K, Maruyama A, Onishi S, Nagashima T, Minota S. Simultaneous pneumatosis cystoides intestinalis and pneumomediastinum in a patient with systemic sclerosis. J Rheumatol. 2010;37(10):2194–5. doi: 10.3899/jrheum.100254. [DOI] [PubMed] [Google Scholar]
- 16.Pagnoux C, Mahr A, Cohen P, Guillevin L. Presentation and outcome of gastrointestinal involvement in systemic necrotizing vasculitides: analysis of 62 patients with polyarteritis nodosa, microscopic polyangiitis, Wegener granulomatosis, Churg-Strauss syndrome, or rheumatoid arthritis-associated vasculitis. Medicine (Baltimore) 2005;84(2):115–28. doi: 10.1097/01.md.0000158825.87055.0b. [DOI] [PubMed] [Google Scholar]
- 17.Omoto T, Tedoriya T, Ishikawa N, Miyauchi T, Nagano N, Oi M. Granulomatous endocarditis in a patient with Wegener’s granulomatosis. J Cardiol Cases. 2011;4(2):e98–100. doi: 10.1016/j.jccase.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heng Y, Schuffler MD, Haggitt RC, Rohrmann CA. Pneumatosis intestinalis: a review. Am J Gastroenterol. 1995;90(10):1747–58. [PubMed] [Google Scholar]
- 19.Joubert PH, Venter HL, Foukaridis GN. The effect of miglitol and acarbose after an oral glucose load: a novel hypoglycaemic mechanism? Br J Clin Pharmacol. 1990;30(3):391–6. doi: 10.1111/j.1365-2125.1990.tb03789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kojima K, Tsujimoto T, Fujii H, et al. Pneumatosis cystoides intestinalis induced by the α-glucosidase inhibitor miglitol. Intern Med. 2010;49(15):1545–8. doi: 10.2169/internalmedicine.49.3634. [DOI] [PubMed] [Google Scholar]
- 21.van Son WJ, van der Jagt EJ, van der Woude FJ, et al. Pneumatosis intestinalis in patients after cadaveric kidney transplantation. Possible relationship with an active cytomegalovirus infection. Transplantation. 1984;38(5):506–10. doi: 10.1097/00007890-198411000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Mannes GP, de Boer WJ, van der Jagt EJ, Meinesz AF, Meuzelaar JJ, van der Bij W. Pneumatosis intestinalis and active cytomegaloviral infection after lung transplantation. Groningen Lung Transplant Group. Chest. 1994;105(3):929–30. doi: 10.1378/chest.105.3.929. [DOI] [PubMed] [Google Scholar]
- 23.Gelman SF, Brandt LJ. Pneumatosis intestinalis and AIDS: a case report and review of the literature. Am J Gastroenterol. 1998;93(4):646–50. doi: 10.1111/j.1572-0241.1998.183_b.x. [DOI] [PubMed] [Google Scholar]
- 24.Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. AJR Am J Roentgenol. 2007;188(6):1604–13. doi: 10.2214/AJR.06.1309. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka M, Endo K, Suzuki T, Maruyama Y, Kondo A. Treatment for steroid-induced diabetes with alpha-glucosidase inhibitor, voglibose. Eur J Neurol. 1998;5(3):315. doi: 10.1046/j.1468-1331.1998.530315.x. [DOI] [PubMed] [Google Scholar]
- 26.Sagara A, Kitagawa K, Furuichi K, et al. Three cases of pneumatosis intestinalis presenting in autoimmune diseases. Mod Rheumatol. 2012;22(4):610–5. doi: 10.1007/s10165-011-0551-3. [DOI] [PubMed] [Google Scholar]