Abstract
Parallel advances in neuroscience and immunology established the anatomical and cellular basis for bidirectional interactions between the nervous and immune systems. Like other physiological systems, the immune system—and the development of immunity—is modulated by neural reflexes. A prototypical example is the inflammatory reflex, comprised of an afferent arm that senses inflammation and an efferent arm, the cholinergic anti-inflammatory pathway, that inhibits innate immune responses. This mechanism is dependent on the α7 subunit of the nicotinic acetylcholine receptor, which inhibits NF-κB nuclear translocation and suppresses cytokine release by monocytes and macrophages. Here we summarize evidence showing that innate immunity is reflexive. Future advances will come from applying an integrative physiology approach that utilizes methods adapted from neuroscience and immunology.
Reflex Control of Immunity
Immunity can be innate, occurring when molecular products derived from pathogens or injured cells activate cytokine production from monocytes and macrophages, or adaptive, the specific and long-lasting response of lymphocytes based on prior exposure to antigen. Cytokines released during innate immunity mediate inflammation, producing the cardinal signs of swelling, pain, erythema, and fever. This response is usually short-lived, and inflammation resolves. In some cases, however, cytokine production can become excessive, and rather than resolving, inflammation persists or even spreads, causing damage in adjacent tissues. This is the basis for the “cytokine theory of disease,” the paradigm that cytokines are necessary and sufficient for disease pathogenesis (Tracey, 2007). This is axiomatic for modern therapeutic approaches to rheumatoid arthritis, inflammatory bowel disease, and other inflammatory diseases that are significantly ameliorated by treatment with drugs that selectively target cytokines (e.g., anti-TNF and anti-IL-1β therapies) to the benefit of millions of patients.
Recent advances in fundamental neuroscience have provided an answer to the critical question raised by the cytokine theory of disease. If cytokines mediate pathophysiological damage, do neural reflexes regulate cytokine production to maintain homeostasis? The answer stems from studies addressing the role of neural networks, which revealed that signals originating in the brain, and conveyed as action potentials transmitted in the vagus nerve, regulate cytokine production by the innate immune system (Tracey, 2009). This neural circuit, termed the cholinergic anti-inflammatory pathway, is mediated by the vagus nerve and the α7 subunit of the nicotinic acetylcholine receptor expressed on cytokine-producing cells (Wang et al., 2003). Activation of this pathway by electrical stimulation of the vagus nerve or administration of α7 selective drugs is effective in ameliorating inflammation and improving survival in experimental models of sepsis (Borovikova et al., 2000; Pavlov et al., 2007; Wang et al., 2004), hemorrhagic shock (Guarini et al., 2003), pancreatitis (van Westerloo et al., 2006), postoperative ileus (The et al., 2007), and endothelial cell activation (Saeed et al., 2005).
Nicotinic receptors are ligand-gated ion channels comprising a family of hetero- or homopentameric structures derived from the products of 17 genes (Millar, 2003). In brain neurons, α7 is a homopentameric calcium channel expressed predominantly in presynaptic nerve terminals where it modulates neurotransmitter release, and in postsynaptic neurons where it induces excitatory impulses. Signaling through α7 in the central nervous system is associated with neuronal plasticity and cell survival (Berg and Conroy, 2002; Drisdel and Green, 2000). In macrophages, signaling through α7 attenuates TNF production through a mechanism dependent upon inhibition of NF-κB nuclear translocation and activation of Jak-STAT pathways (Borovikova et al., 2000; de Jonge et al., 2005; Parrish et al., 2008).
Experiments addressing the anatomical basis of the cholinergic anti-inflammatory pathway indicated that the spleen is the target organ of the vagus nerve for controlling TNF production (Huston et al., 2006). Further studies identified macrophages as the cell source of spleen TNF and showed that the splenic nerve is required for vagus nerve stimulation to suppress systemic TNF levels. A two-neuron system comprised of the vagus nerve and the splenic nerve, via the celiac ganglion, conveys signals from the brain to the immune cells residing in the spleen (Rosas-Ballina et al., 2008).
The requirement for a functional splenic nerve in the cholinergic anti-inflammatory pathway is noteworthy because it is primarily composed of catecholaminergic nerve fibers (Klein et al., 1982). In fact, ablation of catecholamines by treatment with reserpine abrogates the suppressive effect of vagus nerve stimulation (Rosas-Ballina et al., 2008). It is clear that α7 signal transduction is required for the control of cytokine release by monocytes and macrophages in vitro and that the cholinergic anti-inflammatory pathway requires α7 signaling to control cytokine production in vivo. It is possible that acetylcholine released by the vagus nerve signals through α7, which is expressed in the celiac ganglion (Lips et al., 2006), but this cannot explain the requirement for α7 expression to control cytokine production in vitro. An alternative explanation is based on the decades old observations that the spleen contains acetylcholine and that electrical stimulation of the splenic nerve mediates acetylcholine release in spleen (Dale and Dudley, 1929; Leaders and Dayrit, 1965). Since the spleen lacks cholinergic nerve fibers (Bellinger et al., 1993), acetylcholine in spleen may be derived from lymphocytes and other immune cells that synthesize and release acetylcholine located in the vicinity of nerve endings (Grando et al., 1993; Kawashima and Fujii, 2004; Wessler and Kirkpatrick, 2001). This may explain how electrical stimulation of the vagus nerve can induce acetylcholine release in spleen, which in turn regulates cytokine release through α7 expressed on responding cells.
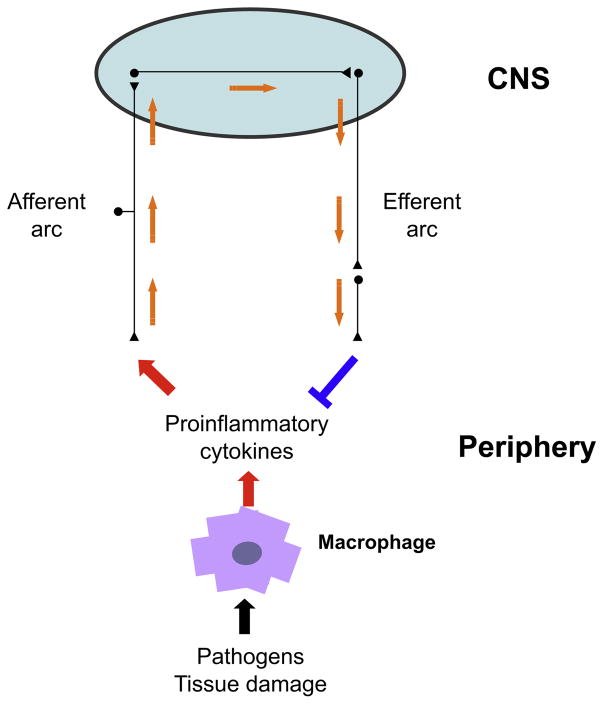
The sensory arm of the inflammatory reflex can be activated by the presence of IL-1β in peripheral tissues. Specific IL-1β binding sites have been revealed on glomus cells adjacent to the vagus nerve (Goehler et al., 1997). IL-1β binding and an intact vagus nerve are both required for the development of fever following intraperitoneal administration of low quantities of IL-1β (Maier et al., 1998). Administration of larger amounts of IL-1β bypasses this mechanism and directly activates the fever response by directly binding to hypothalamic neurons (Hansen et al., 2001). The sensory arm of the inflammatory reflex represents a crucial early detection system that activates a conserved set of neurophysiological responses, including fever, anorexia, and behavioral withdrawal characteristic of sickness behavior. It can also activate opposing effector responses that suppress ongoing inflammation through at least two anti-inflammatory routes: (1) via activation of the hypothalamic pituitary axis and increasing corticosteroid hormone levels (Butts and Sternberg, 2008) and (2) via activation of the cholinergic anti-inflammatory pathway (Figure 1).
Figure 1. The Inflammatory Reflex.
Control systems orchestrated by the autonomic nervous system (e.g., heart rate control) integrate input signals and deliver responses that modify bodily function according to changing physiologic demands. Similarly, cytokines produced by immune cells in response to endogenous and exogenous stimuli activate afferent neurons of the vagus nerve that conveys this information to the brain where signal integration occurs. A response is elicited through the cholinergic anti-inflammatory pathway, the efferent arc of this inflammatory reflex, which modifies immune function and maintains homeostasis. The cholinergic anti-inflammatory pathway conveys signals from the brain to the spleen via the vagus nerve and the splenic nerve and is dependent on the α7 subunit of the nicotinic acetylcholine receptor.
Innervation of Lymphoid Organs
Bone marrow, thymus, lymph nodes, and spleen are all innervated by fibers of the autonomic nervous system that utilize a range of neurotransmitters. This is the anatomical and functional basis of interactions between nerves and immune cells (reviewed in Mignini et al., 2003). Accumulating evidence indicates that innervation of lymphoid organs is not a static phenomenon. Rather, the number of nerve endings and their distribution within immune organs undergoes a dynamic process of remodeling that is modulated in part by immune cell function. For example, subcutaneous injection of antigen is associated with increased nerve ending density in the medulla of the draining lymph node, particularly at the declining phase of antibody production. This augmented nerve fiber density occurs specifically in the parenchyma of the medulla, while nerve fibers associated with the lymph node’s vasculature remain unchanged (Novotny et al., 1994). Mouse spleen injected with tuberculin shows increased numbers of nerve ending branches and richer nerve varicosities (Yang et al., 1998). SCID mice, which lack functional B and T cells, have increased numbers of nerve endings around central arteries and reduced numbers in the white pulp parenchyma of spleen. Adoptive transfer of T cells restores the normal pattern through a mechanism dependent in part on IL-3 release, indicating that immune cells modulate neural network formation (Kannan-Hayashi et al., 2008). Nude mice, which lack functional T cells, have a higher density of nerve fibers in spleen, and adoptive transfer of T cells into these mice reduces spleen catecholaminergic load (Besedovsky et al., 1987). In addition, coculture studies indicate that neurons selectively establish and maintain contacts with immune cells and that cytokines such as IL-3 and IL-6, and GM-CSF and NGF derived from immune cells promote neurite expansion (Kannan et al., 1994, 1996, 2000). It is plausible that other factors, including integrins and adhesion molecules, contribute to establishing and maintaining nerve and immune cell interactions. Together, these and other results implicate the immune system in modulating its own neural input.
Nerve remodeling in immune organs has been observed in both physiologic and pathological conditions. Splenic nerve endings increase in number in the hilar region of the spleen and decrease in spleen zones opposite to the hilus in a model of rat adjuvant-induced arthritis. As disease progresses, nerve endings sprout with different distribution patterns, including increased nerve fibers in red pulp (Lorton et al., 2009). A similar reduction in spleen nerve fibers was observed in a model of collagen-induced arthritis in mice, which was accompanied by changes in spleen cytokine release (Straub et al., 2008). On the one hand, nerves convey information to immune cells and modulate their responses (for examples see Antonica et al., 1996; Besedovsky et al., 1979; Esquifino et al., 2001); on the other, immune cell activity reshapes the number and anatomical distribution of nerve endings and modulates their neurotransmitter content. The nervous system thus maintains a reciprocal and functional interaction with immune cells residing in lymphoid organs through dynamic remodeling of nerve endings.
Nerve Plasticity in Lymphoid Organs
Plasticity in neuroscience is the ability of neural synapses to modify connection strength depending upon experience. It is plausible that nerve endings in lymphoid organs, which contract and expand, in part in response to signals originating from target cells, can be thought of as “plastic.” Then, what is the relevant “experience” or “behavior” driving neural plasticity in lymphoid organs? Is nerve plasticity in lymphoid organs a manifestation of sensitization and desensitization phenomena intrinsic to the inflammatory reflex?
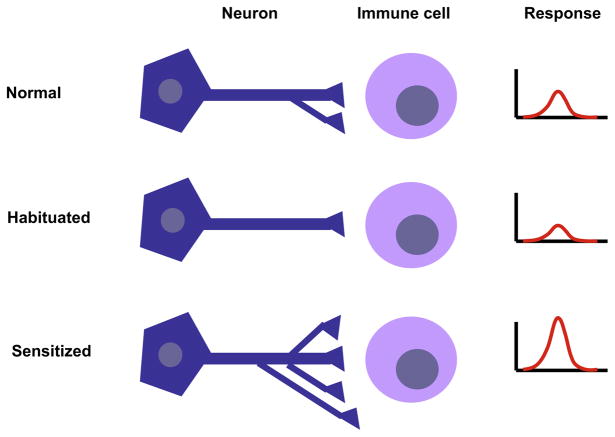
Neuroscience has investigated the molecular mechanism of nonassociative learning and its functional consequence in short-and long-term sensitization. When a noxious stimulus is applied to the tail of Aplysia, a gill-withdrawal reflex is produced. This response becomes greater when the stimulus is repeated and short-term sensitization lasts for minutes. Repeatedly stimulating at regular intervals produces long-term sensitization, which lasts for days (Castellucci et al., 1986). This elementary form of implicit memory requires new protein synthesis and the growth of new synapses (Bailey and Chen, 1983; Castellucci et al., 1989). We propose that it should be possible to study the effect of stimulating single neurons, once or repeatedly, and measure the function of immune cell activity. Nerve stimulation (e.g., vagus nerve stimulation) could be coupled with recording of immune cell function to make observations about the development of neuroimmune plasticity (Figure 2).
Figure 2. Experimental Model for Studying Neuroimmune Plasticity.
Transmission of neural signals to immune cells in lymphoid organs is a dynamic process involving remodeling of nerve fibers. Cells of the immune system are likely exposed to constant fluctuation in neurotransmitter concentration in their microenvironment as a result of second-to-second changes in nerve firing rate. Studying the effects of nerve firing frequency on immune cell function using electrophysiology together with assessment of immune function could reveal habituation and sensitization phenomena underlying nerve-to-immune cell interactions. The figure depicts a hypothetical two-cell culture system showing different activity states of a neuroimmune synapse. The number and frequency of synapses and the magnitude of the immune response would vary depending on whether the system is at rest, habituated, or under sensitization.
An obvious impediment to implementing this technique is that immune cells, in contrast to neurons and muscle, are not studied as excitable cells. Immune cells do express ion channels that modulate membrane potential and calcium signaling. Antigenic stimulation of T cells through the TCR/CD3 complex releases Ca2+ from the endoplasmic reticulum (ER). Increased intracellular calcium is then followed by transcription factor NF-AT activation, a process mediated by calcineurin. Release of Ca2+ from ER is not sufficient to initiate gene transcription, and long-lasting elevated Ca2+ concentrations maintained by ion channels expressed on the T cell plasma membrane are required for full activation of the signaling cascade (Krasznai, 2005; Panyi et al., 2004). For example, the Ca2+ release-activated Ca2+ (CRAC) channel and the voltage-gated Kv1.3 channel are involved in T cell activation and proliferation, and their expression depends on state of activation and differentiation (Chandy et al., 1984; DeCoursey et al., 1984; Nagy et al., 1995; Panyi et al., 2004). Drugs that selectively block channel function in T cells are effective in improving the clinical course in experimental models of multiple sclerosis and delayed type hypersensitivity (Beeton et al., 2001; Matheu et al., 2008). Moreover, neurotransmitters can affect membrane potential and ion channel function in lymphocytes. Acetylcholine exerts depolarizing and K+ channel blocking effects in lymphocytes and reduces the proliferative response to PHA (Gaspar et al., 1996); and β adrenergic stimulation modulates voltage-dependent K+ conductance in CD8 lymphocytes (Soliven and Nelson, 1990). Thus, it is reasonable to hypothesize that neurotransmitter release elicited by nerve stimulation can translate into measurable changes in membrane potential, calcium signaling, and altered immune cell function.
A similar approach could be used to study the long-term effect of neurotransmitters on macrophage membrane potential, calcium signaling, and cytokine production. Macrophages obtained from calcium-activated potassium channel KCa3.1 knockout mice show decreased activation, and administration of selective blockers diminishes macrophage migration to aortic plaques in a mouse model of atherosclerosis (Toyama et al., 2008). Furthermore, patch-clamp analysis shows that transient membrane depolarization in macrophages is accompanied by increased IL-6 transcription when stimulated with ATP (Hanley et al., 2004), which is released from catecholaminergic nerve endings (Sneddon and Westfall, 1984). We have found that macrophages incubated with acetylcholine for 60 min retain an immunosuppressed phenotype for as long as 2 days, such that subsequent stimulation with endotoxin even 48 hr after exposure to acetylcholine is associated with persistently low TNF production (Huston et al., 2007). The in vivo correlate of this observation is that mice continue to show an attenuated endotoxin-induced TNF response 48 hr after the vagus nerve is electrically stimulated (Huston et al., 2007). Studying the effect of nerve pulse frequency and neurotransmitter release on macrophage cytokine production should also be utilized to address whether macrophages develop neural-induced sensitization or desensitization.
Neural Control of Immune Memory
It has been suggested that afferent fibers can “sense” antigen in collaboration with dendritic cells and contribute to the early development of adaptive immunity. Rats sensitized with hapten administered to the abdomen develop an inflammatory response in the ear when the same hapten is later administered to the ear. This response requires antigen presentation to T cells in the abdominal lymph node and the establishment of immunological memory. Denervation of either the abdominal tissue or the ear prevents the inflammatory response in the ear, but does not alter the development of immune memory to antigen (Beresford et al., 2004). This indicates that immune memory occurs without neural input but that neural networks are required for mobilization of T cells from the abdominal lymph node to the challenged area. It is possible that in the contact sensitivity model, antigen exposure creates immunologic memory that is specific to the antigen and neural memory that is required to recall prior antigen exposure (Shepherd et al., 2005). It is plausible that immune cells communicate with afferent neurons via cytokines or other mediators (Goehler et al., 1998; Uceyler et al., 2009; Weinreich and Undem, 1987) to establish neuroimmune connections encoding previous inflammatory and antigenic experience. Understanding nerve plasticity at the site where immune responses occur, perhaps by studying the effect of immune cell activity on sensory neurons in vitro, could reveal neural networks that establish and maintain immunological memory.
Concluding Remarks
The immune system is no longer regarded as autonomous. It is a distributed system that exhibits complex behavior orchestrated by neural networks and reflexes. Certain, if not all, aspects of innate and adaptive immunity require neural systems to manifest the full range of coordinated, physiological, and immunological responses that underlie homeostasis. The cholinergic anti-inflammatory pathway is but an example of a well-characterized neural circuit that controls inflammation through a highly integrated physiological system. Electrical stimulation of the vagus nerve, a new therapeutic approach to attenuate inflammation, takes advantage of the neuroanatomical circuitry to deliver anti-inflammatory signals to discrete cells in specific organs. Advances in this field will undoubtedly reveal other neural networks that coordinate immunity by enhancing or suppressing immune responses via specific molecular mechanisms. We believe that the time has come for studying the interdependent activities of neural and immune cells using an integrative physiologic approach with technical methods adopted from neuroscience. Selective in vivo and in vitro stimulation of nerves or neurons in conjunction with electrophysiological measurement of immune cell activity and vice versa will provide new insights into the cellular and molecular basis of the interplay between nerves and immune cells and its contribution to physiological homeostasis during infection and injury.
Acknowledgments
We thank Isabel Wong, Peder Oloffson, and Valentin Pavlov for helpful comments.
References
- Antonica A, Ayroldi E, Magni F, Paolocci N. J Neuroimmunol. 1996;64:115–122. doi: 10.1016/0165-5728(95)00157-3. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Chen M. Science. 1983;220:91–93. doi: 10.1126/science.6828885. [DOI] [PubMed] [Google Scholar]
- Beeton C, Wulff H, Barbaria J, Clot-Faybesse O, Pennington M, Bernard D, Cahalan MD, Chandy KG, Beraud E. Proc Natl Acad Sci USA. 2001;98:13942–13947. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DL, Lorton D, Hamill RW, Felten SY, Felten DL. Brain Behav Immun. 1993;7:191–204. doi: 10.1006/brbi.1993.1021. [DOI] [PubMed] [Google Scholar]
- Beresford L, Orange O, Bell EB, Miyan JA. Immunology. 2004;111:118–125. doi: 10.1111/j.1365-2567.2003.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DK, Conroy WG. J Neurobiol. 2002;53:512–523. doi: 10.1002/neu.10116. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A, Sorkin E, Da Prada M, Keller HH. Cell Immunol. 1979;48:346–355. doi: 10.1016/0008-8749(79)90129-1. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A, Sorkin E, Burri R, Honegger CG, Schlumpf M, Lichtensteiger W. Brain Behav Immun. 1987;1:185–193. doi: 10.1016/0889-1591(87)90020-1. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Butts CL, Sternberg EM. Cell Immunol. 2008;252:7–15. doi: 10.1016/j.cellimm.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci VF, Frost WN, Goelet P, Montarolo PG, Schacher S, Morgan JA, Blumenfeld H, Kandel ER. J Physiol (Paris) 1986;81:349–357. [PubMed] [Google Scholar]
- Castellucci VF, Blumenfeld H, Goelet P, Kandel ER. J Neurobiol. 1989;20:1–9. doi: 10.1002/neu.480200102. [DOI] [PubMed] [Google Scholar]
- Chandy KG, DeCoursey TE, Cahalan MD, McLaughlin C, Gupta S. J Exp Med. 1984;160:369–385. doi: 10.1084/jem.160.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale HH, Dudley HW. J Physiol. 1929;68:97–123. doi: 10.1113/jphysiol.1929.sp002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Nature. 1984;307:465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- Drisdel RC, Green WN. J Neurosci. 2000;20:133–139. doi: 10.1523/JNEUROSCI.20-01-00133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquifino AI, Castrillon PO, Chacon F, Cutrera R, Cardinali DP. Brain Res. 2001;888:227–234. doi: 10.1016/s0006-8993(00)03060-2. [DOI] [PubMed] [Google Scholar]
- Gaspar R, Jr, Varga Z, Bene L, Marcheselli F, Pieri C, Damjanovich S. Biochem Biophys Res Commun. 1996;226:303–308. doi: 10.1006/bbrc.1996.1351. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, Maier SF, Watkins LR. Brain Res Bull. 1997;43:357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Hammack SE, Maier SF, Watkins LR. Brain Res. 1998;804:306–310. doi: 10.1016/s0006-8993(98)00685-4. [DOI] [PubMed] [Google Scholar]
- Grando SA, Kist DA, Qi M, Dahl MV. J Invest Dermatol. 1993;101:32–36. doi: 10.1111/1523-1747.ep12358588. [DOI] [PubMed] [Google Scholar]
- Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, Marini H, Squadrito G, Minutoli L, Bertolini A, Marini R, et al. Circulation. 2003;107:1189–1194. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- Hanley PJ, Musset B, Renigunta V, Limberg SH, Dalpke AH, Sus R, Heeg KM, Preisig-Muller R, Daut J. Proc Natl Acad Sci USA. 2004;101:9479–9484. doi: 10.1073/pnas.0400733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MK, O’Connor KA, Goehler LE, Watkins LR, Maier SF. Am J Physiol Regul Integr Comp Physiol. 2001;280:R929–R934. doi: 10.1152/ajpregu.2001.280.4.R929. [DOI] [PubMed] [Google Scholar]
- Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, et al. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, Rosas-Ballina M, Ashok M, Goldstein RS, Chavan S, Pavlov VA, et al. Crit Care Med. 2007;35:2762–2768. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- Kannan Y, Stead RH, Goldsmith CH, Bienenstock J. J Neurosci Res. 1994;37:374–383. doi: 10.1002/jnr.490370309. [DOI] [PubMed] [Google Scholar]
- Kannan Y, Bienenstock J, Ohta M, Stanisz AM, Stead RH. J Immunol. 1996;157:313–320. [PubMed] [Google Scholar]
- Kannan Y, Moriyama M, Sugano T, Yamate J, Kuwamura M, Kagaya A, Kiso Y. Neuroimmunomodulation. 2000;8:132–141. doi: 10.1159/000054273. [DOI] [PubMed] [Google Scholar]
- Kannan-Hayashi Y, Okamura K, Hattori S, Kuwamura M, Higuchi E, Terayama H, Moriyama M, Mukamoto M, Okada M, Ohsugi Y, Nakamura Y. J Immunol. 2008;180:4227–4234. doi: 10.4049/jimmunol.180.6.4227. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. Front Biosci. 2004;9:2063–2085. doi: 10.2741/1390. [DOI] [PubMed] [Google Scholar]
- Klein RL, Wilson SP, Dzielak DJ, Yang WH, Viveros OH. Neuroscience. 1982;7:2255–2261. doi: 10.1016/0306-4522(82)90135-x. [DOI] [PubMed] [Google Scholar]
- Krasznai Z. Arch Immunol Ther Exp (Warsz) 2005;53:127–135. [PubMed] [Google Scholar]
- Leaders FE, Dayrit C. J Pharmacol Exp Ther. 1965;147:145–152. [PubMed] [Google Scholar]
- Lips KS, Konig P, Schatzle K, Pfeil U, Krasteva G, Spies M, Haberberger RV, Grando SA, Kummer W. J Mol Neurosci. 2006;30:15–16. doi: 10.1385/JMN:30:1:15. [DOI] [PubMed] [Google Scholar]
- Lorton D, Lubahn C, Sweeney S, Major A, Lindquist CA, Schaller J, Washington C, Bellinger DL. Brain Behav Immun. 2009;23:276–285. doi: 10.1016/j.bbi.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Maier SF, Goehler LE, Fleshner M, Watkins LR. Ann NY Acad Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- Matheu MP, Beeton C, Garcia A, Chi V, Rangaraju S, Safrina O, Monaghan K, Uemura MI, Li D, Pal S, et al. Immunity. 2008;29:602–614. doi: 10.1016/j.immuni.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignini F, Streccioni V, Amenta F. Auton Autacoid Pharmacol. 2003;23:1–25. doi: 10.1046/j.1474-8673.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- Millar NS. Biochem Soc Trans. 2003;31:869–874. doi: 10.1042/bst0310869. [DOI] [PubMed] [Google Scholar]
- Nagy P, Panyi G, Jenei A, Bene L, Gaspar R, Jr, Matko J, Damjanovich S. Immunol Lett. 1995;44:91–95. doi: 10.1016/0165-2478(94)00198-z. [DOI] [PubMed] [Google Scholar]
- Novotny GE, Heuer T, Schottelndreier A, Fleisgarten C. Anat Rec. 1994;238:213–224. doi: 10.1002/ar.1092380208. [DOI] [PubMed] [Google Scholar]
- Panyi G, Varga Z, Gaspar R. Immunol Lett. 2004;92:55–66. doi: 10.1016/j.imlet.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, Hudson L, Lin X, Patel N, Johnson SM, et al. Mol Med. 2008;14:567–574. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR, Rosas-Ballina M, Czura CJ, et al. Crit Care Med. 2007;35:1139–1144. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Proc Natl Acad Sci USA. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. J Exp Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AJ, Downing JE, Miyan JA. Immunology. 2005;116:145–163. doi: 10.1111/j.1365-2567.2005.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P, Westfall DP. J Physiol. 1984;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliven B, Nelson DJ. J Membr Biol. 1990;117:263–274. doi: 10.1007/BF01868456. [DOI] [PubMed] [Google Scholar]
- Straub RH, Rauch L, Fassold A, Lowin T, Pongratz G. Arthritis Rheum. 2008;58:3450–3460. doi: 10.1002/art.24030. [DOI] [PubMed] [Google Scholar]
- The FO, Boeckxstaens GE, Snoek SA, Cash JL, Bennink R, Larosa GJ, van den Wijngaard RM, Greaves DR, de Jonge WJ. Gastroenterology. 2007;133:1219–1228. doi: 10.1053/j.gastro.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Toyama K, Wulff H, Chandy KG, Azam P, Raman G, Saito T, Fujiwara Y, Mattson DL, Das S, Melvin JE, et al. J Clin Invest. 2008;118:3025–3037. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uceyler N, Schafers M, Sommer C. Exp Brain Res. 2009;196:67–78. doi: 10.1007/s00221-009-1755-z. [DOI] [PubMed] [Google Scholar]
- van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ, van der Poll T. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, et al. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- Weinreich D, Undem BJ. J Clin Invest. 1987;79:1529–1532. doi: 10.1172/JCI112984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler IK, Kirkpatrick CJ. Pulm Pharmacol Ther. 2001;14:423–434. doi: 10.1006/pupt.2001.0313. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang L, Huang CS, Ju G. Neuroimmunomodulation. 1998;5:53–60. doi: 10.1159/000026326. [DOI] [PubMed] [Google Scholar]