The central nervous system (CNS) interacts with the periphery to provide feedback regulation during inflammation. The brain and spinal cord sense danger signals released by pathogens outside the CNS and activate wide-ranging defense mechanisms, such as fever and the hypothalamic–pituitary–adrenal axis. Under conditions of autoimmunity the CNS can also modulate somatic responses. For instance, p38 MAP kinase inhibition in the CNS reduces inflammation and bone destruction in rat adjuvant arthritis (1). The spinal mechanisms of this phenomenon have been well delineated (1,2) but the efferent neurologic connections remain poorly characterized.
Direct stimulation of the vagus nerve suppresses systemic production of cytokines implicated in arthritis, such as tumor necrosis factor, interleukin-1, and interleukin-6, via a cholinergic antiinflammatory pathway (3). We therefore postulated that spinal p38 engages this pathway. To test this possibility, we used power spectral analysis of heart rate variability to quantify vagal output after intrathecal administration of a p38 inhibitor. The high-frequency power spectral component of heart rate variability (HFP) is a widely used parameter of parasympathetic activity that directly correlates with activation of the cholinergic antiinflammatory pathway (4,5).
All animals were handled according to US Department of Agriculture guidelines, and procedures were reviewed and approved by the institutional animal subjects committee. Rats were anesthetized with isoflurane and placed in a supine dorsal recumbent position. Unipolar platinum electrodes were placed onto the anterior chest wall and attached to an amplifier (ECG 100C; Biopac Systems, Santa Barbara, CA). Data were recorded for 70 minutes at a sampling rate of 1 kHz. Power spectral analysis of heart rate variability was calculated using Acqknowledge software (Biopac Systems). After performing fast Fourier transform, the HFP was calculated using a frequency range adapted to the physiologic setting in rats (0.6–1 Hz), to quantify cholinergic outflow.
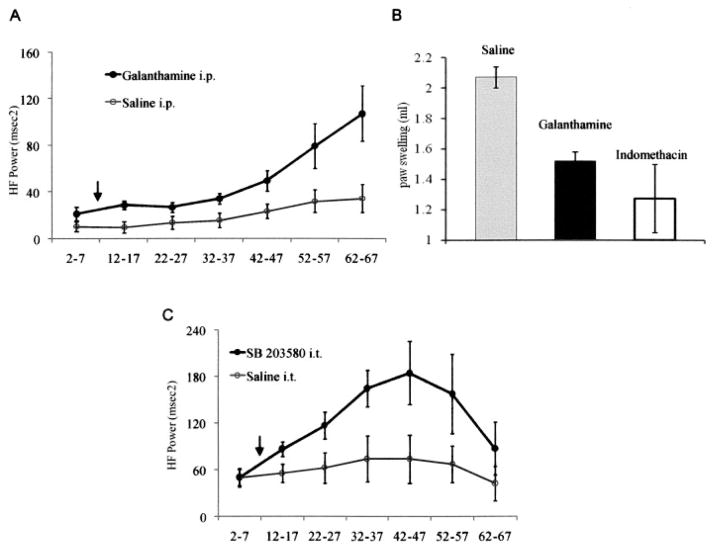
We initially used a cholinesterase inhibitor, galanthamine, to verify that we could detect cholinergic changes in HFP. Vagal efferent fibers augment HFP by releasing acetylcholine, which binds to muscarinic receptors expressed by cardiac pacemaker cells. Acetylcholine is then rapidly converted into inactive metabolites by cholinesterases that can be inhibited with galanthamine. Galanthamine modulates this process by inhibiting acetylcholine esterase and through a central excitatory effect on vagal outflow (5). As shown in Figure 1A, rats treated with galanthamine exhibited significantly increased HFP. The effect of galanthamine on HFP was comparable with the level of vagal stimulation that produces antiinflammatory effects in septic shock models (5). This degree of pharmacologic or electrical vagal stimulation can reduce paw inflammation (6). Galanthamine can mimic the antiinflammatory effects of the vagus nerve, presumably through a combination of increased acetylcholine release and decreased esterase function (3,7).
Figure 1.
A, Systemic administration of the cholinesterase inhibitor galanthamine increases the cholinergic antiinflammatory pathway. The high-frequency component of heart rate variability (HFP), a measure of cholinergic vagal activity, was recorded for 5-minute intervals every 10 minutes at the indicated time points (minutes). Galanthamine (2 mg/kg) or saline was injected intraperitoneally (IP) after baseline measurements (arrow). Galanthamine significantly increased HFP compared with saline (P < 0.0001 by 2-way analysis of variance [ANOVA]), confirming increased cholinergic drive. Values are the mean ± SEM (n = 5 rats per group). B, Galanthamine decreases paw swelling in the carrageenan paw edema model. Galanthamine (5 mg/kg) or saline was injected IP, and 30 minutes later, 0.1 ml of 1% carrageenan was injected into the left footpad as previously described (6). Carrageenan-induced swelling was measured 3 hours later, by water displacement plethysmometry. Edema was significantly decreased by galanthamine (P < 0.001 versus saline treatment, by Student’s unpaired t-test). The effect of indomethacin (2 mg/kg orally) is shown as a positive control. Values are the mean ± SEM of 3 independent experiments (n = 6 rats per group). C, Intrathecal (IT) administration of SB203580 increases cholinergic activity measured by HFP in rats. SB203580 (8 μg) or the equivalent volume of saline was given intrathecally (arrow). Intrathecal administration of SB203580 significantly increased HFP up to 3-fold above baseline (P < 0.001 versus saline treatment, by 2 way ANOVA). Values are the mean ± SEM (n = 5 rats per group). The experiments were performed 3 times; results shown are from 1 representative experiment.
We next evaluated the antiinflammatory effect of galanthamine. Figure 1B shows that the compound significantly reduced swelling in the rat carrageenan paw edema model (6). This observation supports the notion of a potential link between increases in HFP and peripheral antiinflammatory effects, although the relationship between the intensity of cholinergic stimulation in inflamed paws and that in normal cardiac tissue is not known.
We then investigated whether p38 within the CNS controls the vagal cholinergic outflow, using the same HFP measurement protocol. Isoflurane-anesthetized Lewis rats (200–250 gm) were implanted with an intrathecal catheter as previously described (2). After a 6-day recovery period, HFP was measured before and after injection of 8 μg of SB203580 through the intrathecal catheter. Intrathecal SB203580 caused a 3-fold rise in HFP that was not observed with intrathecal saline (P < 0.001 by 2-way analysis of variance) (Figure 1C). The increase in cholinergic outflow was noted within 5 minutes and persisted for at least 1 hour after injection. The concentration of SB203580 used has been shown to reduce arthritis after intrathecal administration in the rat adjuvant arthritis model (1). It is a small fraction of the doses typically required to suppress arthritis when given systemically (8), suggesting that the effect is mediated by the CNS. Additional mechanistic studies are needed to determine whether the intensity of acetylcholine receptor stimulation in the synovium correlates with HFP.
In conclusion, we found that a very small dose of a p38 inhibitor delivered locally to the intrathecal space increased HFP in the rat. This novel observation demonstrates that MAP kinase signaling within the CNS can potentially activate the cholinergic antiinflammatory pathway. Because vagal stimulation of similar intensity can block inflammation during septic shock (5), this pathway could contribute to the suppression of synovitis after spinal p38 blockade (1). While the peripheral cholinergic mechanisms are still being explored, some studies implicate the alpha7 cholinergic receptor. For instance, acetylcholine can reduce inflammation by stimulating alpha7 receptors on macrophages (7) and can decrease the production of cytokines and chemokines by synoviocytes in vitro (9). If this receptor is confirmed as a key link between the CNS and peripheral inflammation, then selective agonists could have therapeutic utility. Since HFP is a recognized marker of parasympathetic activity in humans (4), our findings using this model suggest that HFP could represent a useful measure in clinical studies of therapies aimed at modulating inflammation via the CNS.
Acknowledgments
Supported by grants from the Arthritis Foundation and The American College of Rheumatology Research and Education Foundation Within Our Reach program. Dr. Waldburger’s work was supported by the Swiss National Science Foundation. Dr. Tracey’s work was supported by the National Institute of General Medical Sciences. Dr. Firestein’s work was supported by Procter & Gamble. Dr. Tracey is cofounder of Critical Therapeutics, Inc. He holds a patent on the use of cholinergic agents to treat inflammation.
Footnotes
AUTHOR CONTRIBUTIONS
Dr. Firestein had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Waldburger, Boyle, Sorkin, Tracey, Firestein.
Acquisition of data. Waldburger, Edgar, Levine, Pavlov.
Analysis and interpretation of data. Waldburger, Boyle, Levine, Firestein.
Manuscript preparation. Waldburger, Pavlov, Sorkin, Tracey, Firestein.
Statistical analysis. Waldburger.
Contributor Information
Jean-Marc Waldburger, University of California, San Diego La Jolla, CA.
David L. Boyle, University of California, San Diego La Jolla, CA.
Meghan Edgar, University of California, San Diego La Jolla, CA.
Linda S. Sorkin, University of California, San Diego La Jolla, CA.
Yaakov A. Levine, Feinstein Institute for Medical Research Manhasset, NY.
Valentin A. Pavlov, Feinstein Institute for Medical Research Manhasset, NY.
Kevin Tracey, Feinstein Institute for Medical Research Manhasset, NY.
Gary S. Firestein, University of California, San Diego La Jolla, CA.
References
- 1.Boyle DL, Jones TL, Hammaker D, Svensson CI, Rosengren S, Albani S, et al. Regulation of peripheral inflammation by spinal p38 MAP kinase in rats. PLoS Med. 2006;3:e338. doi: 10.1371/journal.pmed.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle DL, Moore J, Yang L, Sorkin LS, Firestein GS. Spinal adenosine receptor activation inhibits inflammation and joint destruction in rat adjuvant-induced arthritis. Arthritis Rheum. 2002;46:3076–82. doi: 10.1002/art.10595. [DOI] [PubMed] [Google Scholar]
- 3.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 4.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 5.Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci U S A. 2006;103:5219–23. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borovikova LV, Ivanova S, Nardi D, Zhang M, Yang H, Ombrellino M, et al. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci. 2000;85:141–7. doi: 10.1016/S1566-0702(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 8.Badger AM, Griswold DE, Kapadia R, Blake S, Swift BA, Hoffman SJ, et al. Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthritis Rheum. 2000;43:175–83. doi: 10.1002/1529-0131(200001)43:1<175::AID-ANR22>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Waldburger JM, Boyle DL, Edgar M, Pavlov VA, Tracey KJ, Firestein GS. Regulation of synoviocyte cytokine expression by acetylcholine and the α7 nicotinic receptor. Arthritis Rheum. doi: 10.1002/art.23987. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]