Abstract
The body’s first defense against invading pathogens or tissue injury is the innate immune system. Since excessive immune responses can be damaging, anti-inflammatory mechanisms function to control the pro-inflammatory response and prevent injury. The cholinergic anti-inflammatory pathway is a neural mechanism that suppresses the innate inflammatory response. Knowledge concerning innervation of the immune system offers a unique opportunity to explore previously unrecognized techniques to treat disease. It also enables consideration of the neurological basis of complementary and alternative medical therapies, such as meditation and acupuncture. This evolving area of research has implications for the pathogenesis of chronic inflammatory conditions including inflammatory bowel disease, rheumatoid arthritis, type 2 diabetes, and other conditions of excessive cytokine release.
Keywords: cytokine, TNF, inflammation, cholinergic anti-inflammatory pathway, inflammatory reflex
Introduction
Inflammation, defined as the development of pain, swelling, erythema, and warmth in response to an injury or infection, plays an important role in warding off invasion.1 The immune system is the first line of defense against invading pathogens and proper functioning of this system is essential for survival.2 A central feature of the immune response is the production and release of cytokines: proteins produced by immune cells to orchestrate inflammation. Invasion or injury activates a discrete, localized inflammatory response with release of cytokines including tumor necrosis factor (TNF), interleukin (IL)-1, IL-6, high mobility group B1 (HMGB1), among others, that produce a precise, and tightly controlled response.3,4 In the vast majority of cases, the innate immune system successfully thwarts pathogenic threats and restores homeostasis.3 Nonetheless, in some cases, the inflammatory response can become excessive or persistent, and mediate dysfunction and damage to normal tissue. Cytokine overproduction has been implicated in the pathogenesis of rheumatoid arthritis, Crohn’s disease/inflammatory bowel disease, atherosclerosis, type 2 diabetes, Alzheimer’s disease, multiple sclerosis, endotoxemia, and severe sepsis.5,6
It has recently become known that the immune system can be significantly regulated by the vagus nerve.1,2,7 Stimulation of this nerve releases acetylcholine which inhibits the release of pro-inflammatory cytokines by macrophages and other cytokine-producing cells to dampen inflammation. Elucidation of the neuroanatomy of this pathway has enabled investigation into drugs, therapeutic techniques such as electrical stimulation, and even complementary and alternative medical therapies to activate the pathway to decrease inflammation. Herein, we discuss the role of cytokines in the immune response, describe the inflammatory reflex and the cholinergic anti-inflammatory pathway, consider previous clinical trial results in the context of the inflammatory reflex, and present the potential relationship between the inflammatory reflex and the field of complementary and alternative medicine.
The Critical Balance between Pro-and Anti-inflammatory Cytokines
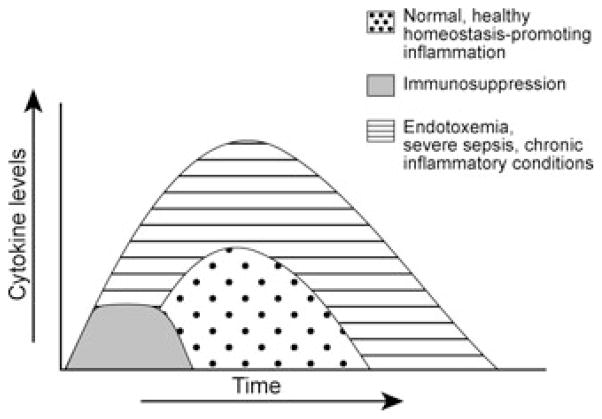
Cytokines play a central role in the immune response. The cytokine response to infection or injury, when functioning properly, is a well-orchestrated, redundant system3 that is successful in eradicating invading pathogenic organisms and in restoring homeostasis. If the magnitude or duration of the immune response is excessive, it mediates serious complications as depicted in Figure 1.3 Pro- and anti-inflammatory cytokines are complementary classes of inflammatory mediators that function in concert to ensure an appropriate immune response. Pro-inflammatory mediators include TNF, IL-1β, IL-6, IL-18, and HMGB1 (among others); anti-inflammatory mediators include IL-10, TGF-β, TNF-binding protein, in addition to a variety of hormones and local effectors.2,3,8
FIGURE 1.
The cytokine response to infection or injury. Abnormal duration or intensity of inflammation results in disease (depression, fever, anorexia, pain, edema, rheumatoid arthritis, inflammatory bowel disease, tissue damage, shock, organ failure, or death) (Modified from Tracey et al.2 and Tracey.7)
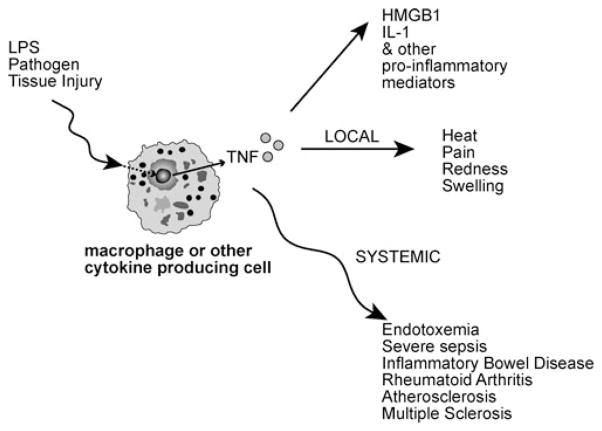
As illustrated in Figure 2, TNF and other cytokines are synthesized by macrophages and other cells in response to endotoxin (lipopolysaccharide; LPS), invading pathogens, or tissue injury.8 TNF, an early mediator of inflammation,8 is a necessary and sufficient mediator of both local and systemic inflammation.8–10 Local increases in TNF result in the signs and symptoms of inflammation.8 Increasing levels of TNF during the inflammatory process stimulate the production and release of additional pro-inflammatory mediators such as IL-1, IL-18, HMGB1 as well as eicosanoids, nitric oxide, and reactive oxygen species.8,11
FIGURE 2.
Local and systemic effects of tumor necrosis factor (TNF) in response to endotoxin (LPS), invading pathogens, or tissue injury.
TNF and other pro-inflammatory cytokines and soluble mediators clearly play an integral role in limiting the spread of infection, promoting coagulation to localize the invading pathogen, and stimulating growth in tissues damaged by the invading pathogen.2 Nonetheless, an excessive immune response can result in high TNF levels1 that cause significant morbidity and mortality. For example, TNF has been implicated in the pathogenesis of endotoxemia, shock, and other inflammatory diseases including inflammatory bowel disease, rheumatoid arthritis, atherosclerosis, multiple sclerosis, and type 2 diabetes as indicated in Table 1.1,2,5,12,13 Control of TNF, and other potentially damaging cytokines, is imperative for health.14–21
TABLE 1.
| Inflammatory condition | Exposure/Incidence | Reference |
|---|---|---|
| Severe sepsis |
|
Stone, R. (1994) |
|
Angus, D.C. and Wax, R.S. (2001) | |
| Rheumatoid arthritis |
|
National Institute of Health |
| Crohn’s disease/Inflammatory bowel disease |
|
Crohn’s and Colitis Foundation of America |
| Type 2 diabetes |
|
Diabetes in America, 2nd edition. (National Institute of Health) |
| Alzheimer’s disease |
|
Hebert, L.E. (2003) |
| Multiple sclerosis (MS) |
|
National Multiple Sclerosis Society |
| Atherosclerosis |
|
American Heart Association |
The resolution of an immune response is mediated by anti-inflammatory cytokines including IL-10, transforming growth factor-β (TGF-β),22 and hormones (adrenal corticosteroids, adrenaline, α-melanocyte stimulating hormone). In concert with local effectors such as spermine, prostaglandin E2, fetuin, heat shock proteins, and acute phase proteins, these factors interact to inhibit macrophage activation and down regulate the synthesis of pro-inflammatory cytokines.3,23 Thus, inhibition of cytokine production is an essential component of a healthful immune response.
Link between the Central Nervous System and the Immune System
The importance of regulating TNF and other pro-inflammatory cytokines during an immune response perhaps suggests that cytokine release should be controlled at multiple levels. Indeed, it has long been known that the central nervous system (CNS) is activated by inflammatory stimuli.5 In 1957, Wexler and colleagues reported that endotoxin activates pituitary-dependent adrenal responses.24 It later became clear that endotoxin and other products of inflammation also stimulate afferent neural signals in the vagus nerve that increase acute-phase responses, induce fever, and upregulate the expression of IL-1β in the brain.25–28
Afferent vagus nerve signals are transmitted to the medullary reticular formation, locus ceruleus, hypothalamus, and dorsal vagal complex, ultimately leading to an increase in adrenocorticotropin hormone (ACTH) from the anterior pituitary gland.5 These afferent signals stimulate an increase in systemic glucocorticoid levels which can inhibit pro-inflammatory cytokine release by the immune system.29–31 Inflammation can also enhance the release of melanocyte-stimulating hormone: a potent anti-inflammatory protein that inhibits the synthesis of cytokines.32 Ascending sensory fibers of the vagus nerve that synapse in the nucleus tractus solitarius (NTS) of the upper medulla can also activate nerve signals to inhibit cytokine release.33
The Inflammatory Reflex
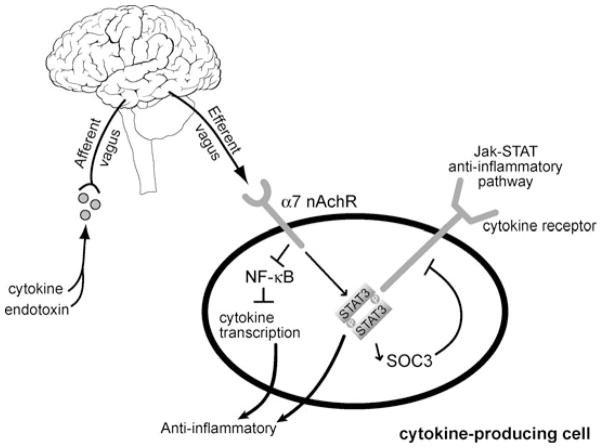
Like other reflex arcs, the inflammatory reflex is comprised of a sensory or afferent arm (described above) and a motor (efferent) arm that results in a discrete, rapid, and opposing reaction.5 As illustrated in Figure 3, the cholinergic anti-inflammatory pathway (i.e., the efferent arm of the inflammatory reflex), inhibits inflammation.3,5,34 Stimulation of the efferent vagus nerve leads to the release of acetylcholine (ACh) in organs of the reticuloendothelial system, including spleen, liver, and gastrointestinal tract.5,25 ACh can bind to the α7 nicotinic ACh receptor (α7nAChR) expressed on the surface of activated macrophages (and other cytokine-producing cells)3,36 whereby cytokine synthesis and release is prevented by inhibiting NF-κB (i.e. decreasing transcription activity of the transcription factor NF-κB subunit p65) and by stimulating the JAK-STAT anti-inflammatory pathway.3,35–39 The culmination of this neurological control of inflammation via ACh is the suppression of pro-inflammatory cytokines, and prevention of tissue damage.3
FIGURE 3.
The inflammatory reflex. Activation of the vagus nerve results in the release of acetylcholine which inhibits NF-κB and stimulates the STAT3-SOC3 anti-inflammatory pathway via α7 nicotinic receptors (nAChR) on activated macrophages and other cytokine-producing cells. Ultimately, synthesis of TNF, HMGB1, and other pro-inflammatory cytokines implicated in inflammatory conditions is inhibited. (Modified from Tracey,3 Tracey,7 and Metz & Tracey.39)
Elucidation of the hard wiring of this reflex has opened the door for studies of modulating vagus nerve activity to suppress inflammation. Studies have included both pharmacologic manipulation of the inflammatory reflex and direct electrical stimulation of the vagus nerve.40,41
Complementary and Alternative Medical Therapies and the Inflammatory Reflex
Complementary and alternative medical therapies, used for centuries worldwide, have been touted as treatment modalities for a variety of systemic conditions, but there has been a dearth of scientific evidence for physiological mechanisms.42 Given recent advances in our understanding of the molecular pathways involved in inflammation, it is now possible to monitor the activity of the inflammatory reflex.3 Results from clinical trials evaluating the impact of two of these therapies on heart rate variability have been reported.
Acupuncture
In a study by Ouyang et al.43 performed at the University of Texas, electroacupuncture (a modified form of acupuncture) was found to significantly increase vagus nerve activity as compared to baseline levels measured by spectral analysis of heart rate variability in female hound dogs. Electrical acupuncture has also been evaluated in healthy human subjects. Hsu et al.44 studied the effect of electrical acupuncture on a variety of biological responses, including heart rate variability. This study included 10 healthy subjects who underwent electrical acupuncture on acupoint BL15—the bladder meridian. Both the normalized high frequency power component of heart rate variability (determined via spectral analysis of the electrocardiogram) and the normalized low frequency power components were significantly increased and decreased, respectively (P < 0.05).
In a related study, heart rate variability in 39 human subjects receiving acupuncture at the Neiguan (P6) point was compared to 38 subjects receiving sham acupuncture, and 34 subjects receiving no acupuncture.45 Results revealed that normalized high-frequency power (used as the index of vagal modulation) after acupuncture increased significantly in the Neiguan group, but not in the sham acupuncture or no acupuncture groups. The RR interval (the interval between R waves in the electrocardiogram) was significantly increased in both acupuncture groups, but was not significantly altered in the group not receiving acupuncture. The authors concluded that acupuncture can increase vagus nerve activity. These results were confirmed in a subsequent study reported by Sakai and colleagues who studied the relationship between acupuncture manipulation, and sympathetic and parasympathetic autonomic function.46 They found that acupuncture manipulation resulted in significant decreases in heart rate, a significant decrease in the low frequency components of heart rate variability, and a significant positive correlation between the high frequency component of heart rate variability and the number of acupuncture sensations. These and other studies have been interpreted to suggest that acupuncture may enhance vagus nerve activity.
Meditation
Heart rate variability has also been employed to study the mechanism of action of transcendental meditation (TM). For example, in a randomized, placebo-controlled 16-week trial evaluating the efficacy of TM on the metabolic syndrome and heart disease, it was found that subjects in the TM group experienced significant changes in heart rate variability (normalized high-frequency power) as compared to the control group (P = 0.07).47 The author concluded that TM may be capable of modulating physiological responses and may be a novel therapeutic target for the treatment of coronary heart disease. Another study measured heart rate variability in subjects during Zen meditation. Kubota et al.48 examined frontal midline theta rhythm (Fm theta), a distinct theta activity of electroencephalogram (EEG) reflecting mental concentration and meditative state or relief from anxiety, during Zen meditation in 24 subjects. In the 12 subjects in which Fm theta activities were identified, a recently developed technique of heart rate variability to evaluate cardiac sympathetic and parasympathetic functions separately was applied during periods of meditation and control periods. Results suggested that a close relationship between cardiac and autonomic function and activity of medial frontal neural hard wiring existed in this group of subjects since both sympathetic and parasympathetic indices were increased during Fm theta periods relative to control cycles.
Testable Hypotheses in Other Clinical Settings
Based on these and other clinical trial results, it has been hypothesized that such therapies (among others) may be capable of impacting the cholinergic anti-inflammatory pathway to modulate inflammation. To date, however, the role of complementary and alternative medical therapies in Western medicine remains unclear. According to a consensus statement from the NIH, “preclinical studies have documented acupuncture’s effects, but they have not been able to fully explain how acupuncture works within the framework of a Western system of medicine.”49 Not only have acupuncture and meditation been advocated for the treatment of a myriad of inflammatory conditions, but behavioral modification, hypnosis, biofeedback, and cognitive and relaxation therapies have also been demonstrated to stimulate vagus nerve activity.50–56 This research lays a solid foundation for future studies to determine whether these therapies are capable of activating the cholinergic inflammatory pathway. Such clinical trials may be considered for inflammatory conditions including diabetes, artherosclerosis, and arthritis, among others as indicated in Table 1.
Pharmacological approaches can also target this pathway. For example, administration of nicotine confers a significant benefit in a subset of patients with ulcerative colitis,57,58 and protects against the development of osteoarthritis.59 It is currently postulated that nicotine inhibits cytokine production through its binding to an α7 nACh receptor.7 Importantly, cholinergic and anticholinergic drugs are widely utilized in intensive care units in patients with sepsis and organ failure in which high cytokine levels and decreased vagus nerve activity co-occur.42,60–63
Surgical transaction of the vagus nerve was historically performed for treatment of peptic ulcer disease. To date, there is no evidence that cytokine responses in these patients are dysregulated. It is possible that compensatory mechanisms restore cytokine control in cases where the vagus nerve is lacking. Splenectomized patients may also provide a population to study compensatory mechanisms over cytokine control to address issues of sensitization, desensitization, or tolerance within the cholinergic anti-inflammatory pathway. Exercise is widely advocated for a variety of health conditions. It also decreases levels of cytokines, protects against cardiovascular disease and other debilitating disorders such as type 2 diabetes, increases activity of the vagus nerve, and protects against development of atherosclerosis.64–70 Conversely, obesity is associated with increased cytokine levels and decreased vagus nerve activity. A combination of weight loss and increased exercise enhance vagus nerve activity and decrease cytokine levels potentially via modulation of the cholinergic anti-inflammatory pathway. These unanswered questions present testable hypotheses that may impact the cholinergic anti-inflammatory pathway and influence health and longevity.
Conclusion
A balance exists between pro- and anti-inflammatory mechanisms to protect the host against invading pathogens and maintain homeostasis. In addition to the well-described soluble mediators of inflammation, an additional level of control of inflammation is afforded via the nervous system. Stimulation of the inflammatory reflex, and in particular the efferent arm of the reflex (referred to as the cholinergic anti-inflammatory pathway) results in a decreased production of TNF, and ultimately, an inhibitory effect on inflammation.
The fact that the central nervous system and the immune system are intimately linked offers a unique opportunity to explore the effects of therapeutic agents or experimental techniques on efferent vagus nerve signaling. Since heart rate variability affords a direct means of assessing vagus nerve activity during complementary and alternative medical therapies, it is now feasible to design clinical trials to evaluate the mechanism of action of various therapies on the inflammatory reflex using testable clinical endpoints linked to vagus nerve activity, inflammatory responses, and disease severity. Evaluating the effect of complementary and alternative medical therapies (including meditation and acupuncture) on the immune system via the impact on the cholinergic anti-inflammatory pathway is an evolving area of research. It will be interesting to observe the developments in this field over the next several years.
Footnotes
Conflicts of Interest
KJT is consultant to SetPoint Medical, Inc.
References
- 1.Libert C. A nervous connection. Nature. 2003;421:328–329. doi: 10.1038/421328a. [DOI] [PubMed] [Google Scholar]
- 2.Tracey KJ, Czura CJ, Ivanova S. Mind over immunity. FASEB J. 2001;15(9):1575–1576. doi: 10.1096/fj.01-0148hyp. [DOI] [PubMed] [Google Scholar]
- 3.Tracey KJ. Fat meets the cholinergic anti-inflammatory pathway. J Exp Med. 2005;202:1017–1021. doi: 10.1084/jem.20051760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 5.Tracey JK. The inflammatory reflex. Nature. 2002;20:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 6.Popa C. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48:751–762. doi: 10.1194/jlr.R600021-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 9.Tracey KJ, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteremia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 10.Tracey KJ, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 12.Huston JM, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 14.Stone R. Search for sepsis drugs goes on despite past failures. Science. 1994;64:365–367. doi: 10.1126/science.8153620. [DOI] [PubMed] [Google Scholar]
- 15.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 16.National Institute of Health. http://www.niams.nih.gov/hi/topics/arthritis/rahandout.htm#ra_3.
- 17.Crohn’s and Colitis Foundation of America. http://www.ccfa.org/info/about/crohns.
- 18.Kenny SJ, Aubert RE, Geiss LS. Diabetes in American. Chapter 4: Prevalence and incidence of non-insulin-dependent diabetes. (2) http://diabetes.niddk.nih.gov/dm/pubs/america/contents.htm.
- 19.Hebert LE, et al. Alzheimer disease in the U.S. population: prevalence estimates using the 2000 census. Arch Neurology. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 20.National Multiple Sclerosis Society. http://www.nationalmssociety.org/Who%20gets%20MS.asp.
- 21.American Heart Association. http://www.americanheart.org/presenter.jhtml?identifier=4478.
- 22.Tsunawaki S, et al. Deactivation of macrophages by transforming growth factor-β. Nature. 1988;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, et al. Spermine inhibition of monocyte activation and inflammation. Mol Med. 1999;5:595–605. [PMC free article] [PubMed] [Google Scholar]
- 24.Wexler BC, et al. Effects of a bacterial polysaccharide (Piromen) on the pituitary-adrenal axis: adrenal ascorbic acid, cholesterol and histologic alterations. Endocrinol. 1957;61:300–308. doi: 10.1210/endo-61-3-300. [DOI] [PubMed] [Google Scholar]
- 25.Fleshner M, et al. Thermogenic and corticosterone responses to intravenous cytokines (IL-1 beta and THF-alpha) are attenuated by a subdiaphragmatic vagotomy. J Neuroimmunol. 1998;86:134–141. doi: 10.1016/s0165-5728(98)00026-5. [DOI] [PubMed] [Google Scholar]
- 26.Maier SF, et al. The role of the vagus nerve in cytokine-to-brain communication. Ann NY Acad Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- 27.Watkins LR, et al. Blockade of interleukin-1-induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;183:27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- 28.Scheinman RI, et al. Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 29.Sternberg EM, et al. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci USA. 1989;86:2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watkins LR, Maier SF. Implications of the immune-to-brain communication for sickness and pain. Proc Natl Acad Sci USA. 1999;96:7710–7713. doi: 10.1073/pnas.96.14.7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sternberg EM. Neural-immune interactions in health and disease. J Clin Invest. 1997;100:2641–2647. doi: 10.1172/JCI119807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiao H, et al. Alpha-melanocyte-stimulating hormone reduces endotoxin-induced liver inflammation. J Clin Invest. 1996;97:2038–2044. doi: 10.1172/JCI118639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernik TR, et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 35.Yoshikawa H, et al. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-κB phosphorylation and nuclear factor-κB transcriptional activity through nicotinic acetylcholine receptor α7. Clin Exp Immunol. 2006;146(1):116–123. doi: 10.1111/j.1365-2249.2006.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 38.de Jonge WJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 39.Metz CN, Tracey KJ. It takes nerve to dampen inflammation. Nat Immunol. 2005;6:756–757. doi: 10.1038/ni0805-756. [DOI] [PubMed] [Google Scholar]
- 40.Arai I, et al. Possible involvement of non-steroidal anti-inflammatory drugs in vagal mediated gastric acid secretion in rats. Jpn J Pharmacol. 1985;37:91–99. doi: 10.1254/jjp.37.91. [DOI] [PubMed] [Google Scholar]
- 41.Schachter SC, et al. Vagus nerve stimulation: where are we? Curr Opin Neurol. 2002;15:201–206. doi: 10.1097/00019052-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt HB, et al. Autonomic dysfunction in the ICU patient. Curr Opin Crit Care. 2001;7:314–322. doi: 10.1097/00075198-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Ouyang H, et al. Electroacupuncture accelerates gastric emptying in association with changes in vagal activity. Am J Gastrointest Liver Physiol. 2002;282:390–396. doi: 10.1152/ajpgi.00272.2001. [DOI] [PubMed] [Google Scholar]
- 44.Hsu CC, et al. Effects of electroacupuncture on acupoint BL15 evaluated in terms of heart rate variability, pulse rate variability and skin conductance response. Am J Chin Med. 2006;34:23–36. doi: 10.1142/S0192415X06003606. [DOI] [PubMed] [Google Scholar]
- 45.Huang ST, et al. Increase in the vagal modulation by acupunture at Neiguan point in the healthy subjects. Am J Chin Med. 2005;33:157–164. doi: 10.1142/S0192415X0500276X. [DOI] [PubMed] [Google Scholar]
- 46.Sakai S, et al. Specific acupuncture sensation correlates with EEGs and autonomic changes in human subjects. Auton Neurosci. 2007;133:158–169. doi: 10.1016/j.autneu.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Paul-Labrodor M, et al. Effects of a randomized controlled trial of transcendental meditation on components of the metabolic syndrome in subjects with coronary heart disease. Arch Int Med. 2006;166:1218–1224. doi: 10.1001/archinte.166.11.1218. [DOI] [PubMed] [Google Scholar]
- 48.Kubato Y, et al. Frontal midline theta rhythm is correlated with cardiac autonomic activities during the performance of an attention demanding meditation procedure. Brain Res Cogn Brain Res. 2001;11:281–287. doi: 10.1016/s0926-6410(00)00086-0. [DOI] [PubMed] [Google Scholar]
- 49.National Center for Complementary and Alternative Medicine, Nation Institute of Health. NCCAM publication D003: Acupuncture. 2004 http://nccam.nih.gov/health/acupuncture/
- 50.Nolan RP, et al. Heart rate variability biofeedback as a behavioral neurocardiac intervention to enhance vagal heart rate control. Am Heart J. 2005;149:1137.e1–1137.e7. doi: 10.1016/j.ahj.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Palomba D, et al. Biofeedback-assisted heart rate modification after myocardial infarction. J Psychosom Res. 1982;26:469–473. doi: 10.1016/0022-3999(82)90086-1. [DOI] [PubMed] [Google Scholar]
- 52.Cowan MJ, et al. Power spectral analysis of heart rate variability after biofeedback training. J Electrocardiol. 1990;23(suppl):85–94. doi: 10.1016/0022-0736(90)90081-c. [DOI] [PubMed] [Google Scholar]
- 53.Sakakibara M, et al. Effect of relaxation training on cardiac parasympathetic tone. Psychophysiol. 1994;31:223–228. doi: 10.1111/j.1469-8986.1994.tb02210.x. [DOI] [PubMed] [Google Scholar]
- 54.Terahongkum S, Pickler RH. Relationships among heart rate variability and hypertension. J Vasc Nurs. 2004;22:78–82. doi: 10.1016/j.jvn.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Peng CK, et al. Heart rate dynamics during three forms of meditation. Int J Cardiol. 2004;95:19–27. doi: 10.1016/j.ijcard.2003.02.006. [DOI] [PubMed] [Google Scholar]
- 56.van Dixhoorn J, White A. Relaxation therapy for rehabilitation and prevention in ischaemic heart disease: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2005;12:193–202. doi: 10.1097/00149831-200506000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Pullan RD, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- 58.Sandborn WJ, et al. Transdermal nicotine for mildly to moderately active ulcerative colitis. A randomized double-blind, placebo-controlled trial. Ann Intern Med. 1997;126:364–371. doi: 10.7326/0003-4819-126-5-199703010-00004. [DOI] [PubMed] [Google Scholar]
- 59.Felson DT, et al. Does smoking protect against osteoarthritis? Arthritis Rheum. 1989;32:166–172. doi: 10.1002/anr.1780320209. [DOI] [PubMed] [Google Scholar]
- 60.Barnaby D, et al. Heart rate variability in emergency department patients with sepsis. Acad Emerg Med. 9:661–670. doi: 10.1111/j.1553-2712.2002.tb02143.x. [DOI] [PubMed] [Google Scholar]
- 61.Pontet J, et al. Heart rate variability as early marker of multiple organ dysfunction syndrome in septic patients. J Crit Care. 2003;18:156–163. doi: 10.1016/j.jcrc.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt H, et al. Autonomic dysfunction predicts mortality in patients with multiple organ dysfunction syndrome of different age groups. Crit Care Med. 2005;33:1994–2002. doi: 10.1097/01.ccm.0000178181.91250.99. [DOI] [PubMed] [Google Scholar]
- 63.Korach M, et al. Cardiac variability in critically ill adults: influence of sepsis. Crit Care Med. 2001;29:1380–1385. doi: 10.1097/00003246-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 64.Jae SY, et al. Effects of lifestyle modifications on C-reactive protein: contribution of weight loss and improved aerobic capacity. Metabolism. 2006;55:825–831. doi: 10.1016/j.metabol.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Goldhammer E, et al. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol. 2005;100:93–99. doi: 10.1016/j.ijcard.2004.08.073. [DOI] [PubMed] [Google Scholar]
- 66.Obisesan TO, et al. C-reactive protein genotypes affect baseline, but not exercise training-induced changes in C-reactive protein levels. Arterioscler Thromb Vasc Biol. 2004;24:1874–1879. doi: 10.1161/01.ATV.0000140060.13203.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barbeau P, et al. Hemostatic and inflammatory markers in obese youths: effects of exercise and adiposity. J Pediatr. 2002;141:415–420. doi: 10.1067/mpd.2002.127497. [DOI] [PubMed] [Google Scholar]
- 68.Mattusch F, et al. Reduction of the plasma concentration of C-reactive protein following nine months of endurance training. Int H Sports Med. 2000;21:21–24. doi: 10.1055/s-2000-8852. [DOI] [PubMed] [Google Scholar]
- 69.Lanza GA, et al. Relation of heart rate variability to serum levels of C-reactive protein in patients with unstable angina pectoris. Am J Cardiol. 2006;97:1702–1706. doi: 10.1016/j.amjcard.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 70.Peterson AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]