Abstract
The ability of human embryonic stem cells (hESCs) and their derivatives to differentiate and contribute to tissue repair has enormous potential to treat various debilitating diseases. However, improving the in vivo viability and function of the transplanted cells, a key determinant of translating cell-based therapies to the clinic, remains a daunting task. Here, we develop a hybrid biomaterial consisting of hyaluronic acid (HA) grafted with 6-aminocaproic acid moieties (HA-6ACA) to improve cell delivery and their subsequent in vivo function using skeletal muscle as a model system. Our findings show that the biomimetic material-assisted delivery of hESC-derived myogenic progenitor cells into cardiotoxin-injured skeletal muscles of NOD/SCID mice significantly promotes survival and engraftment of transplanted cells in a dose-dependent manner. The donor cells were found to contribute to the regeneration of damaged muscle fibers and to the satellite cell (muscle specific stem cells) compartment. Such biomimetic cell delivery vehicles that are cost-effective and easy-to-synthesize could play a key role in improving the outcomes of other stem cell-based therapies.
Keywords: human embryonic stem cells, myogenesis, biomaterials, hyaluronic acid, stem cell transplantation
Although transplantation of stem cells has been touted as a promising strategy for treating impaired skeletal muscles, the therapeutic potential of such an approach has been hampered by poor to modest survival, low retention, and lack of integration of the transplanted cells with the host tissue.1–4 Thus, there is a strong interest in developing delivery strategies that can improve the survival, continued differentiation, and contribution of the transplanted cells to muscle tissue repair.5 Here, we describe the development of a hybrid biomaterial containing hyaluronic acid (HA) grafted with 6-aminocaproic acid molecules (6ACA), hereafter termed as HA-6ACA, and demonstrate that HA-6ACA-assisted administration of hESCderived cells significantly improves the survival and engraftment of transplanted cells within an injured skeletal muscle tissue.
HA, a nonsulfated linear glycosaminoglycan, is a well-studied biomaterial for cell and drug delivery, soft-tissue repair, and tissue engineering.6–14 HA is a key extracellular matrix molecule found in the interstitial matrix of skeletal muscle, and has been implicated in mediating interactions with various proteins and growth factors, cell migration, cell signaling, matrix reorganization, and regeneration.15–21 HA also interacts with cells through the CD44 receptor, which is expressed in most cells.22–25 Several studies have suggested that HA does not exhibit strong binding to basic fibroblast growth factor (bFGF);26,27 but can weakly interact with bFGF through positively charged regions. We hypothesize that biomaterials that can regulate bFGF signaling could have an added advantage as cell delivery vehicles given that bFGF signaling plays a key role in skeletal muscle tissue homeostasis and function by maintaining a balance between proliferation and differentiation of myogenic progenitor cells.28,29 Hence, we endowed the HA molecules with 6ACA moieties to improve their interactions with bFGF. Previously, we have shown that incorporating 6ACA moieties onto hydrogels can impart them with unique features such as healing,30 biomineralization,31 and increased protein adsorption and cell–matrix interaction.32
The synthesis scheme of HA-6ACA is shown in Figure 1. Subsequent characterization using 1H NMR and FTIR spectra (see Figure S1A, B in the Supporting Information) showed successful grafting (~60–70%) of 6ACA moieties onto the HA backbone. To investigate the effect of 6ACA incorporation on the ability of HA to interact with bFGF, we carried out molecular docking studies and enzyme-linked immunosorbent assay (ELISA) measurements. Our docking calculations yielded hundreds of low-energy configurations of bFGF-bound HA- 6ACA and HA that were further categorized into clusters of closely resembling configurations (Figure S2A). The lowest energy configuration in the most populated cluster is generally considered as the putative binding mode, and its corresponding energy value the binding free energy.33 Because HA-6ACA exhibits two highly populated clusters, both binding modes are considered equally likely. Hence, HA-6ACA, which exhibits binding free energies of −5.6 kcal mol−1 or −5.5 kcal mol−1 for the two modes, binds more strongly to bFGF than HA, which exhibits a binding free energy of −5.2 kcal mol−1 (Figure 2). To probe the molecular basis for the observed higher affinity of HA-6ACA to bFGF, we compared the lowest-energy bFGF-bound configuration of the HA-6ACA and HA molecules. We find that the terminal carboxyl group of dangling side chain of 6ACA acts as a flexible arm that wraps around the positively charged region of bFGF, allowing it to interact easily with positively charged amino acids on the surface of bFGF. The fact that HA-6ACA exhibits multiple binding modes suggests a high degree of flexibility in how HA-6ACA can bind to bFGF, making its binding even more favorable from an entropic perspective.
Figure 1.
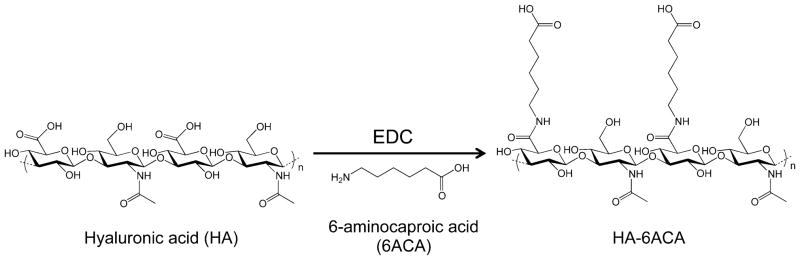
Reaction scheme for the synthesis of HA-6ACA.
Figure 2.
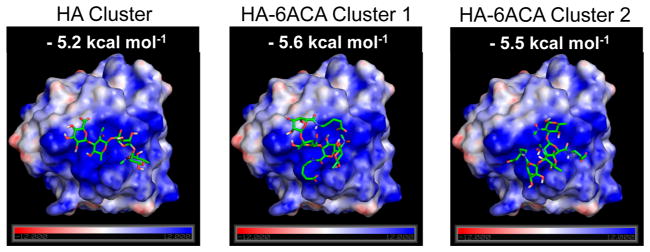
Lowest-energy configuration from the most populated binding cluster(s) of HA and HA-6ACA. This represents the most likely configuration assumed by the ligand when interacting with bFGF. The computed binding energies for the molecules are also provided.
These results from our computational analyses were further verified by ELISA. In order to immobilize the HA-6ACA molecules for the ELISA measurements, we used semiinterpenetrating networks (semi-iPNs). The semi-IPNs were created by incorporating the linear HA or HA-6ACA molecules into chemically cross-linked polyethylene glycol diacrylate (PEGDA) hydrogels, which are known to be antifouling.34 We use PEGDA with a molecular weight of 508 Da to ensure a tight network with minimal swelling in an aqueous milieu. The amounts of adsorbed bFGF onto both semi-iPN HA-PEGDA and semi-iPN HA-6ACA-PEGDA were also compared against those on PEGDA hydrogels. As shown in Figure S3A, the amount of bFGF adsorbed by semi-iPN containing HA-6ACA molecules was significantly higher than that on corresponding semi-iPN containing HA molecules, corroborating our computational findings. No significant differences in bFGF measurements were found between semi-iPN HA-PEGDA and PEGDA networks. The positive measurements observed here could be attributed to the absorption of bFGF solution into the polymer network.35 The same semi-iPN experimental setup was used to determine the interaction of HA and HA-6ACA molecules with various ECM proteins relevant to skeletal muscle tissues–collagen type I, collagen type IV, and laminin. The results in Figure S3B–D show higher levels of interactions between the ECM proteins and HA-6ACA-PEGDA semi-iPN hydrogels compared to its HA-PEGDA and PEGDA counterparts. This is in accordance with our previous report that demonstrates the ability of 6ACA to promote nonspecific adsorption of proteins from their extracellular milieu.32
Hyaluronan molecules exhibit rapid turnover in vivo and chemical modifications of HA have been used to retard their degradation kinetics.36 To determine the effect of 6ACA incorporation on the degradation of HA, we used a modified uronic acid assay, which shows approximately 45% less degradation of HA-6ACA compared to HA in the presence of hyaluronidase, whereas no detectable degradation products were observed in the controls–HA and HA-6ACA incubated in PBS without hyaluronidase (Figure S4). The slower degradation of HA-6ACA suggests that the terminal carboxyl groups of the pendant side chains hinder the accessibility of hyaluronidase enzymes to their binding sites on HA backbone. The slow degradation of HA-6ACA along with their ability to interact with growth factors and ECM proteins could have multiple effects such as modulating signaling and prolonging the half-life of growth factors by inhibiting their proteolytic cleavage.32
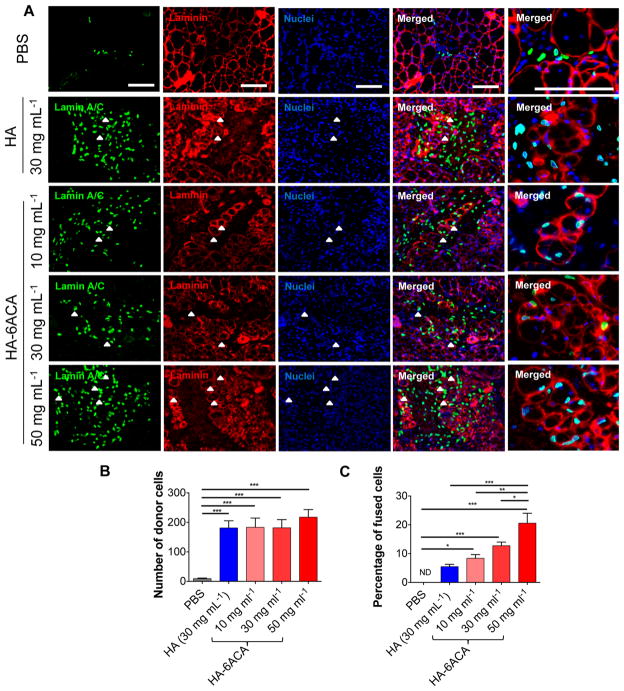
We next determined the effect of HA-6ACA in promoting in vivo survival and function of transplanted hESC-derived progenitor cells. Previously, we have shown that hESC-derived PDGFRA+ cells can undergo myogenic differentiation in vitro and in vivo. 37,38 However, when implanted in vivo, only a few cells were found to be viable post-transplantation with a majority of the survived donor cells residing within the interstitial space.37 To assess the potential of HA-6ACA to support the survival and function of transplanted cells, hESCderived myogenically committed cells were injected using HA- 6ACA molecules of varying concentrations (10, 30, and 50 mg mL−1) into cardiotoxin injured tibialis anterior (TA) muscles of NOD/SCID mice. The HA-6ACA-assisted survival and engraftment of donor cells 14 days post-transplantation were compared against the same cell population injected using saline solution or 30 mg mL−1 HA.
The TA muscles were harvested and characterized to evaluate the survival and in vivo engraftment efficiency of the transplanted donor cells. The muscle sections were stained for human-specific lamin A/C, laminin, and nuclei (Figure 3A). The positive staining for lamin A/C indicates the presence of transplanted hESC-derived cells. Interestingly, the use of HA- 6ACA dramatically improved the survival and engraftment of transplanted donor cells. Quantification of histological sections showed a striking ~200 fold increase in donor cells within the host tissue when they were transplanted using either HA or HA-6ACA compared to that of saline groups (Figure 3B). The HA-6ACA-assisted in vivo survival of transplanted donor cells was found to be independent of their concentration. A similar increase in cell survival was also observed in the presence of 30 mg mL−1 HA. In addition to supporting viability of transplanted cells, both HA-6ACA and HA also promoted the dissemination of transplanted cells within the host tissue. In contrast to HA-6ACA and HA, only a few donor cells were found to be viable when saline was used as a delivery vehicle and the majority of the donor cells were found within the interstitial space nearby the host muscle fibers akin to previous reports.37
Figure 3.
In vivo survival and engraftment of transplanted hESC-derived myogenic progenitor cells. (A) Immunofluorescence staining for humanspecific lamin A/C (green), laminin (red), and nuclei (blue). Scale bar = 100 μm. Quantitative estimation of (B) the total donor cells and (C) the contribution of donor cells to the host myofibers 14 days post-transplantation.
Although similar levels of cell viability were observed between HA and HA-6ACA, HA-6ACA molecules had a significant effect in determining the contribution of donor cells toward regeneration of damaged muscles. Histological analyses of donor cell contribution to the regenerating myofibers showed a remarkably higher number of fused donor cells when the cells were injected with 50 mg mL−1 HA-6ACA (Figure 3A, C and Figure S5). The contribution of donor cells to tissue repair was found to be dependent on the concentration of HA- 6ACA (Figure 3C). When the cells were injected with 30 mg mL−1 HA, only few cells were found to integrate with the host muscle fibers, whereas in the case of saline assisted cell transplantation no donor cells were fused with the host muscle fibers. Even though cell transplantation using 30 mg mL−1 HA exhibited some contribution of the donor cells toward the host tissue, the total number of cells integrated with the host myofibers was significantly lower than that observed when the cells were delivered using 10 mg mL−1 HA-6ACA, the lowest concentration of HA-6ACA used.
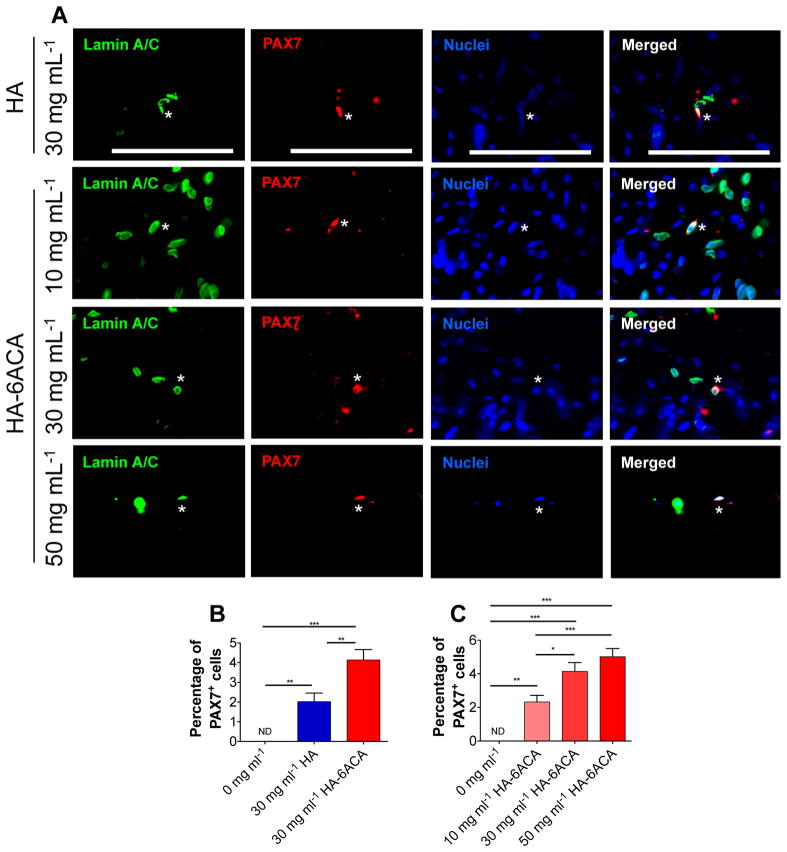
We also investigated the donor cell contribution to the satellite cell compartment by staining serial muscle sections with human-specific lamin A/C, PAX7 (a satellite cell marker), and nuclei. Our results show that although only a small percentage of the transplanted cells contributed to satellite cell compartment, the donor cell-derived satellite cells were only found in cells administered using either HA or HA-6ACA (Figure 4). Similar to the contribution of donor cells to the host myofibers, the donor cell-derived satellite cells exhibited a monotonic increase with HA-6ACA concentration. The cells administered using 30 mg mL−1 of HA had the lowest number of donor cell-derived satellite cells, whereas cells injected using 50 mg mL−1 HA-6ACA showed the highest contribution to the satellite cell compartment.
Figure 4.
Biomimetic material-assisted contribution of donor cells to the satellite cell compartment: (A) Immunofluorescence staining for humanspecific lamin A/C (green), PAX7 (red) for satellite cells, and nuclei (blue). Scale bar =100 μm. (B, C) Quantification of the donor cell-derived satellite cells.
Our results clearly demonstrate the beneficial effect of HA- 6ACA in promoting the survival and engraftment of transplanted hESC-derived myogenic progenitor cells. The observed beneficial effect of HA-based biomaterials (HA and HA-6ACA) on improving the survival of transplanted cells could be attributed to various biological functions of HA. HA molecules have been shown to interact with cells through cell surface hyaluronic acid receptor, CD44.39,40 As reported previously, a majority of HUES9 derived mesoderm progenitor cells are positive for CD44 cell surface markers.23,24,41–43 So we anticipate that the biological functions of HA molecules along with their plausible interactions with the cells through CD44 could be contributing to the enhanced cell survival. Among HA and HA-6ACA, the latter was found to have a better effect on the contribution of donor cells to the myofibers and satellite cell pool. We speculate that this could possibly be due to enhanced interaction between HA-6ACA and growth factors such as bFGF. The enhanced interaction of HA-6ACA molecules with bFGF along with its slower degradation could facilitate sequestration of bFGF away from cell surface and subsequent modulation of bFGF signaling. Previous studies have shown that continuous and high levels of bFGF in cultures inhibit myogenic differentiation of progenitor cells while suppressing bFGF signaling (or low levels of bFGF) promotes in vitro myogenesis of myoblasts.28,29 In native tissue, bFGF is regulated by heparin and heparan sulfate proteoglycans,26 and previously we have shown that synthetic analogues of heparin in culture conditions can promote myogenic differentiation of C2C12 cells to myotubes through bFGF signaling.44
In summary, this study demonstrates that HA-6ACA-assisted delivery of hESC-derived myogenic progenitor cells significantly improves the in vivo survival, differentiation, and integration of transplanted donor cells to the host tissues. Such biomimetic material-based cell delivery vehicles that are cost-effective and easy-to-synthesize might be able to augment the therapeutic outcomes of stem cell-based therapy and accelerate their use from the bench to the bedside.
Supplementary Material
Acknowledgments
Funding
This work was funded by the California Institute of Regenerative Medicine (RN2–00945).
The authors acknowledge Karl E. Marquez for experimental assistance with FACS. We also acknowledge the Developmental Studies Hybridoma Bank for providing the PAX7 antibody generated by Kawakami Lab. The antibodies are developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Footnotes
Notes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
The following file is available free of charge on the ACS Publications website at DOI: 10.1021/ab500021a: Experimental details and characterization data (PDF)
References
- 1.Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142(5):1257–67. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13(5):642–8. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 3.Darabi R, Gehlbach K, Bachoo RM, Kamath S, Osawa M, Kamm KE, Kyba M, Perlingeiro RC. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat Med. 2008;14(2):134–43. doi: 10.1038/nm1705. [DOI] [PubMed] [Google Scholar]
- 4.Goudenege S, Lebel C, Huot NB, Dufour C, Fujii I, Gekas J, Rousseau J, Tremblay JP. Myoblasts derived from normal hESCs and dystrophic hiPSCs efficiently fuse with existing muscle fibers following transplantation. Mol Ther. 2012;20(11):2153–67. doi: 10.1038/mt.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentzinger CF, Wang YX, von Maltzahn J, Rudnicki MA. The emerging biology of muscle stem cells: implications for cell-based therapies. BioEssays. 2013;35(3):231–41. doi: 10.1002/bies.201200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solchaga LA, Gao J, Dennis JE, Awadallah A, Lundberg M, Caplan AI, Goldberg VM. Treatment of osteochondral defects with autologous bone marrow in a hyaluronan-based delivery vehicle. Tissue Eng. 2002;8(2):333–47. doi: 10.1089/107632702753725085. [DOI] [PubMed] [Google Scholar]
- 7.Rossi CA, Flaibani M, Blaauw B, Pozzobon M, Figallo E, Reggiani C, Vitiello L, Elvassore N, De Coppi P. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J. 2011;25(7):2296–304. doi: 10.1096/fj.10-174755. [DOI] [PubMed] [Google Scholar]
- 8.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23(12):H41–56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104(27):11298–303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shu XZ, Ahmad S, Liu Y, Prestwich GD. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J Biomed Mater Res, Part A. 2006;79(4):902–12. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 11.Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng, Part A. 2009;15(2):243–54. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Y, Bernshaw NJ, Lu ZR, Kopecek J, Prestwich GD. Targeted delivery of doxorubicin by HPMA copolymer-hyaluronan bioconjugates. Pharm Res. 2002;19(4):396–402. doi: 10.1023/a:1015170907274. [DOI] [PubMed] [Google Scholar]
- 13.Luo Y, Prestwich GD. Synthesis and selective cytotoxicity of a hyaluronic acid-antitumor bioconjugate. Bioconjugate Chem. 1999;10(5):755–63. doi: 10.1021/bc9900338. [DOI] [PubMed] [Google Scholar]
- 14.Luo Y, Ziebell MR, Prestwich GD. A hyaluronic acid-taxol antitumor bioconjugate targeted to cancer cells. Biomacromolecules. 2000;1(2):208–18. doi: 10.1021/bm000283n. [DOI] [PubMed] [Google Scholar]
- 15.Cheung WF, Cruz TF, Turley EA. Receptor for hyaluronan-mediated motility (RHAMM), a hyaladherin that regulates cell responses to growth factors. Biochem Soc Trans. 1999;27(2):135–141. doi: 10.1042/bst0270135. [DOI] [PubMed] [Google Scholar]
- 16.Tammi RH, Passi AG, Rilla K, Karousou E, Vigetti D, Makkonen K, Tammi MI. Transcriptional and post-translational regulation of hyaluronan synthesis. FEBS J. 2011;278(9):1419–28. doi: 10.1111/j.1742-4658.2011.08070.x. [DOI] [PubMed] [Google Scholar]
- 17.Hunt LC, Gorman C, Kintakas C, McCulloch DR, Mackie EJ, White JD. Hyaluronan synthesis and myogenesis: a requirement for hyaluronan synthesis during myogenic differentiation independent of pericellular matrix formation. J Biol Chem. 2013;288(18):13006–21. doi: 10.1074/jbc.M113.453209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calve S, Isaac J, Gumucio JP, Mendias CL. Hyaluronic acid, HAS1, and HAS2 are significantly upregulated during muscle hypertrophy. Am J Physiol Cell Physiol. 2012;303(5):C577–88. doi: 10.1152/ajpcell.00057.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4(7):528–39. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 20.Prestwich GD. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J Controlled Release. 2011;155(2):193–9. doi: 10.1016/j.jconrel.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purcell BP, Elser JA, Mu A, Margulies KB, Burdick JA. Synergistic effects of SDF-1alpha chemokine and hyaluronic acid release from degradable hydrogels on directing bone marrow derived cell homing to the myocardium. Biomaterials. 2012;33(31):7849–57. doi: 10.1016/j.biomaterials.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, Wagers AJ. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119(4):543–54. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Darabi R, Arpke RW, Irion S, Dimos JT, Grskovic M, Kyba M, Perlingeiro RC. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell. 2012;10(5):610–9. doi: 10.1016/j.stem.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng J, Adkin CF, Xu SW, Muntoni F, Morgan JE. Contribution of human muscle-derived cells to skeletal muscle regeneration in dystrophic host mice. PLoS One. 2011;6(3):e17454. doi: 10.1371/journal.pone.0017454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24(4):928–35. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 26.Bashkin P, Doctrow S, Klagsbrun M, Svahn CM, Folkman J, Vlodavsky I. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin-like molecules. Biochemistry. 1989;28(4):1737–43. doi: 10.1021/bi00430a047. [DOI] [PubMed] [Google Scholar]
- 27.Malkowski A, Sobolewski K, Jaworski S, Bankowski E. FGF binding by extracellular matrix components of Wharton’s jelly. Acta Biochim Pol. 2007;54(2):357–63. [PubMed] [Google Scholar]
- 28.Clegg CH, Linkhart TA, Olwin BB, Hauschka SD. Growth factor control of skeletal muscle differentiation: commitment to terminal differentiation occurs in G1 phase and is repressed by fibroblast growth factor. J Cell Biol. 1987;105(2):949–56. doi: 10.1083/jcb.105.2.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen RE, Rankin LL. Regulation of satellite cells during skeletal muscle growth and development. Proc Soc Exp Biol Med. 1990;194(2):81–6. doi: 10.3181/00379727-194-43060. [DOI] [PubMed] [Google Scholar]
- 30.Phadke A, Zhang C, Arman B, Hsu CC, Mashelkar RA, Lele AK, Tauber MJ, Arya G, Varghese S. Rapid self-healing hydrogels. Proc Natl Acad Sci U S A. 2012;109(12):4383–8. doi: 10.1073/pnas.1201122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phadke A, Shih YR, Varghese S. Mineralized synthetic matrices as an instructive microenvironment for osteogenic differentiation of human mesenchymal stem cells. Macromol Biosci. 2012;12(8):1022–32. doi: 10.1002/mabi.201100289. [DOI] [PubMed] [Google Scholar]
- 32.Ayala R, Zhang C, Yang D, Hwang Y, Aung A, Shroff SS, Arce FT, Lal R, Arya G, Varghese S. Engineering the cell-material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials. 2011;32(15):3700–11. doi: 10.1016/j.biomaterials.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Yang D, Arya G. Structure and binding of the H4 histone tail and the effects of lysine 16 acetylation. Phy Chem Chem Phys. 2011;13(7):2911–21. doi: 10.1039/c0cp01487g. [DOI] [PubMed] [Google Scholar]
- 34.Ekblad T, Bergstrom G, Ederth T, Conlan SL, Mutton R, Clare AS, Wang S, Liu Y, Zhao Q, D’Souza F, Donnelly GT, Willemsen PR, Pettitt ME, Callow ME, Callow JA, Liedberg B. Poly(ethylene glycol)-containing hydrogel surfaces for antifouling applications in marine and freshwater environments. Biomacromolecules. 2008;9(10):2775–83. doi: 10.1021/bm800547m. [DOI] [PubMed] [Google Scholar]
- 35.Zieris A, Prokoph S, Welzel PB, Grimmer M, Levental KR, Panyanuwat W, Freudenberg U, Werner C. Analytical approaches to uptake and release of hydrogel-associated FGF-2. J Mater Sci Mater Med. 2010;21(3):915–23. doi: 10.1007/s10856-009-3913-z. [DOI] [PubMed] [Google Scholar]
- 36.Sahoo S, Chung C, Khetan S, Burdick JA. Hydrolytically degradable hyaluronic acid hydrogels with controlled temporal structures. Biomacromolecules. 2008;9(4):1088–92. doi: 10.1021/bm800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang Y, Suk S, Lin S, Tierney M, Du B, Seo T, Mitchell A, Sacco A, Varghese S. Directed in vitro myogenesis of human embryonic stem cells and their in vivo engraftment. PLoS One. 2013;8(8):e72023. doi: 10.1371/journal.pone.0072023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang Y, Suk S, Shih YR, Seo T, Du B, Xie Y, Li Z, Varghese S. WNT3A promotes myogenesis of human embryonic stem cells and enhances in vivo engraftment. Sci Rep. 2014;4:5916. doi: 10.1038/srep05916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peach RJ, Hollenbaugh D, Stamenkovic I, Aruffo A. Identification of hyaluronic acid binding sites in the extracellular domain of CD44. J Cell Biol. 1993;122(1):257–64. doi: 10.1083/jcb.122.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahrens T, Assmann V, Fieber C, Termeer C, Herrlich P, Hofmann M, Simon JC. CD44 is the principal mediator of hyaluronic-acid-induced melanoma cell proliferation. J Invest Dermatol. 2001;116(1):93–101. doi: 10.1046/j.1523-1747.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- 41.Mahmood A, Harkness L, Schroder HD, Abdallah BM, Kassem M. Enhanced differentiation of human embryonic stem cells to mesenchymal progenitors by inhibition of TGF-beta/activin/nodal signaling using SB-431542. J Bone Miner Res. 2010;25(6):1216–33. doi: 10.1002/jbmr.34. [DOI] [PubMed] [Google Scholar]
- 42.de Peppo GM, Svensson S, Lenneras M, Synnergren J, Stenberg J, Strehl R, Hyllner J, Thomsen P, Karlsson C. Human embryonic mesodermal progenitors highly resemble human mesenchymal stem cells and display high potential for tissue engineering applications. Tissue Eng, Part A. 2010;16(7):2161–82. doi: 10.1089/ten.TEA.2009.0629. [DOI] [PubMed] [Google Scholar]
- 43.Lian Q, Lye E, Suan Yeo K, Khia Way Tan E, Salto-Tellez M, Liu TM, Palanisamy N, El Oakley RM, Lee EH, Lim B, Lim SK. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells. 2007;25(2):425–36. doi: 10.1634/stemcells.2006-0420. [DOI] [PubMed] [Google Scholar]
- 44.Sangaj N, Kyriakakis P, Yang D, Chang CW, Arya G, Varghese S. Heparin mimicking polymer promotes myogenic differentiation of muscle progenitor cells. Biomacromolecules. 2010;11(12):3294–300. doi: 10.1021/bm101041f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.