Abstract
Accurate co-ordination of accommodation and convergence is necessary to view near objects and develop fine motor co-ordination. We used a remote haploscopic videorefraction paradigm to measure longitudinal changes in simultaneous ocular accommodation and vergence to targets at different depths, and to all combinations of blur, binocular disparity, and change-in-size (“proximity”) cues. Infants were followed longitudinally and compared to older children and young adults, with the prediction that sensitivity to different cues would change during development. Mean infant responses to the most naturalistic condition were similar to those of adults from 6-7 weeks (accommodation) and 8-9 weeks (vergence). Proximity cues influenced responses most in infants less than 14 weeks of age, but sensitivity declined thereafter. Between 12-28 weeks of age infants were equally responsive to all three cues, while in older children and adults manipulation of disparity resulted in the greatest changes in response. Despite rapid development of visual acuity (thus increasing availability of blur cues), responses to blur were stable throughout development. Our results suggest that during much of infancy, vergence and accommodation responses are not dependent on the development of specific depth cues, but make use of any cues available to drive appropriate changes in response.
Introduction
Accommodation and convergence are necessary to focus and align both eyes of an infant on an object of interest. This allows accurate feedback of visual information necessary for the development of perception and motor co-ordination at different distances. Inaccuracies of vergence and accommodation result in double vision (diplopia), loss of binocular vision, and blur, so it is important for the developing infant to acquire these skills as a basic foundation for visuo-motor co-ordination.
Infants have been reported to be proficient at converging and accommodating by four months of age (Bharadwaj & Candy, 2008; Thorn, Gwiazda, Cruz, Bauer, & Held, 1994; Turner, Horwood, Houston, & Riddell, 2002), but how they use different visual cues to learn these responses in their very early weeks, or how they adapt to the changes in the balance of visual inputs that occur in later infancy, is less clear. This is of importance in terms of visual development and also for anyone planning infant research using visual stimuli. Since so many studies use visual images to investigate infant development, picking an experimental target that contains elements that maximise accurate fixation is important not only to vision scientists but also to scientists studying other aspects of development.
More specifically for those involved with atypical children, defects of vergence and accommodation can lead to strabismus, permanent loss of stereovision and failure to grow out of infantile refractive error (emmetropize) (e.g. Ingram, Gill, & Goldacre, 1994; Scott & Kutschke, 2005; Tychsen, 2005). These outcomes are more common in children with atypical general development(McClelland, Parkes, Hill, Jackson, & Saunders, 2006; Sobrado, Suarez, Garcia-Sanchez, & Uson, 1999; Woodhouse et al., 1996) or prematurity (Buysse, Casteels, Dieltiens, Eggermont, & Missotten, 1994; Gallo & Lennerstrand, 1991; Holmstrom, Rydberg, & Larsson, 2006) but the early predictive value of atypical developmental trajectories is hindered by incomplete research on the typical development of these systems that are so necessary for the development of fine motor co-ordination.
There are several cues available to determine a change in distance of an isolated object moving in depth, but the main ones are disparity in the position of the target image on the two retinae, the blur associated with the image, and the change in size of the image on the retina. In adults, blur is considered a major cue for accommodation, and has also been shown to drive vergence via the accommodative convergence (AC/A) linkage. Likewise, binocular disparity is a major cue for vergence eye movements, and has also been shown to drive accommodation via the convergence accommodation (CA/C) linkage. Other “proximity” cues such as change in size or looming, overlay of contours, perspective and luminance also contribute to both vergence and accommodation (henceforward abbreviated to proximity cues) (for overview see Ciuffreda & Kenyon (1985)). In adults, proximity cues may contribute to perception of target distance with lesser change in actual vergence position (Wismeijer, Erkelens, van Ee, & Wexler, 2010; Wismeijer, van Ee, & Erkelens, 2008).
The complex interactions between these cues and the parallel systems of vergence and accommodation have been studied extensively in adults (Fukushima, Torii, Ukai, Wolffsohn, & Gilmartin, 2009; Hung, Ciuffreda, & Rosenfield, 1996; Maxwell & Schor, 2004; Schor, Alexander, Cormack, & Stevenson, 1992). The literature more rarely considers development of these systems in infants at a time when anatomy, physiology and perception are changing rapidly and information about target distance from different external sources is changing in availability and/or accuracy.
The ability to differentiate between clear and blurred images develops rapidly over the first two years of life as visual acuity and contrast sensitivity improve (Boothe, Dobson, & Teller, 1985), and refractive errors, especially hyperopia (long sight) and astigmatism, reduce (Ehrlich et al., 1997; Gwiazda, Thorn, Bauer, & Held, 1993; Mayer, Hansen, Moore, Kim, & Fulton, 2001). Infants therefore will be getting more focused retinal images as emmetropisation occurs, and the visual pathways are better able to transmit and utilise more detailed information about this image. Increased depth of focus of the infant eye may mitigate the effects of anatomical optical factors somewhat (Green, Powers, & Banks, 1980), but acuity and refraction do not necessarily have parallel developmental trajectories. Accommodation is unresponsive to target distance in the neonatal period, with most infants maintaining focus at a near distance, and becomes increasingly responsive to target distance after the first month of life (Banks, 1980; Bharadwaj & Candy, 2008; Braddick, Atkinson, French, & Howland, 1979; Brookman, 1983; Currie & Manny, 1997; Hainline, Riddell, Grose Fifer, & Abramov, 1992), but it is not clear whether improvements in accommodation responses are due to increasingly available blur signals associated with developing visual acuity, or concurrent improvements in disparity detection making convergence more accurate which then has an indirect effect on accommodation, as it does in adults (Horwood & Riddell, 2008).
Published research, (Bharadwaj & Candy, 2008; Horwood & Riddell, 2008; Judge, 1996; Wismeijer et al., 2010) suggests that in older children and naïve adults, where multiple cues are available, binocular cues (disparity) dominate as a drive to both vergence and accommodation, with blur, and especially proximity cues playing lesser roles (Wismeijer & Erkelens, 2009). However, adult-like binocular vision is not present in new-born infants. Only gross motor vergence is present and active within the first two months of age (Horwood, 1993; Riddell, Horwood, Houston, & Turner, 1999; Thorn, Gwiazda, Cruz, Bauer, & Held, 1992), possibly driven by gross motor fusion or by two separate monocular foveations (Hainline & Riddell, 1996) (for review see chapter by Aslin (1993)). Sensitivity to binocular correlation (Braddick, Atkinson, Julesz, & Kropfl, 1980) and fusible targets lying in front of or behind the fixation plane (stereopsis) appears more abruptly at 12-16 weeks (Birch, Shimojo, & Held, 1985; Fox, Aslin, Shea, & Dumais, 1980; Petrig, Julesz, Kropfl, Baumgartner, & Anliker, 1981). If stereopsis helps drive vergence, its onset might be expected to result in a step change in the precision of vergence control at this time. If vergence does not depend on stereopsis, then such a step change is less likely. Another complication for the co-ordination of the developing accommodation and vergence systems is that inter-pupillary-distance (IPD) also increases steadily with skull growth during childhood, resulting in a gradual change in the amount of angular vergence that needs to be associated with accommodation if the two systems are to work in parallel.
Proximity cues are a combination of dynamic cues such as looming, motion parallax and motion in depth, as well as static cues such as relative size, overlap, perspective, shading and texture gradients, and perceived position in space (Schor et al., 1992). Infants are known to be responsive to moving targets in their first month (Teller & Palmer, 1996; Volkmann & Dobson, 1976) but directional sensitivity which would be necessary to drive appropriate near responses emerges later than subcortical motion responses such as OKN, and has only been demonstrated from 7 weeks of age (for review see Braddick et al (2003)), although still well before stereopsis emerges. Direction-specific responses to optic flow have been demonstrated from 4-8 weeks (Náñez & Yonas, 1994), but appear to continue to develop much later (Gilmore, Hou, Pettet, & Norcia, 2007). Early vergence and neonatal ocular misalignments may also involve this motion detection system (Horwood & Riddell, 2004). Before the emergence of fine visual acuity and stereopsis, motion cues could help to drive early accommodation and vergence by signalling changes in position in depth. It is also possible, however, that use of higher proximity cues to depth which involve learning, such as “pictorial cues” involving relative size, perspective or overlay of contours which appear to emerge between 5 and 7 months of age (Yonas, Elieff, & Arterberry, 2002) might only emerge after multi-sensory experience of the environment in the first months of life. If this was the case the total proximity response might show gradual, learned, improvements in infancy.
In this study, we document the development of the use of the main cues to vergence and accommodation (blur, disparity and the proximity cues of size and looming) over the first six months of life. We compared infants with a group of children between 5-9 years of age and a further group of naïve adults, with the prediction that the main cues to accommodation and vergence would change both during infancy, and between infancy and later life. Although previous studies have studied vergence or accommodation to a range of cues in infancy (Currie & Manny, 1997; Thorn et al., 1992) or vergence and accommodation to blur and/or disparity (the AC/A and CA/C ratios) (Bharadwaj & Candy, 2008; Bharadwaj & Candy, 2009; Bobier, Howland, Thompson, Giunta, & Peck, 1995; Turner et al., 2002) none have attempted to study simultaneous vergence and accommodation development to all combinations of blur, disparity and proximity cues over the first six months of life. As the characteristics, quality and reliability of individual cues change throughout infancy, flexibility could carry a developmental advantage by allowing adaptation to environmental and developmental changes. We therefore predicted that infants would be more flexible in their cue use, responding to any combination of cues during infancy. In later life, once all neural and anatomical systems have matured, one cue, such as that provided by the very accurate disparity detection system, might eventually become the dominant cue to response to changes in target depth.
This is a large, unique and complex dataset upon which gives valuable insights into typical infant visual development.
Methods
The study adhered to the tenets of the Declaration of Helsinki and was given permission to proceed by University of Reading and UK National Health Service Ethics Committees. Adults and parents of children under six years of age gave fully informed consent. Parents of children older than six years gave fully informed consent while the children themselves gave age-appropriate assent.
We used a remote haploscopic device to present targets at five fixation distances while a PlusoptiX SO4 PowerRefII photorefractor collected continuous recordings of eye position and accommodative response (Figure 1).
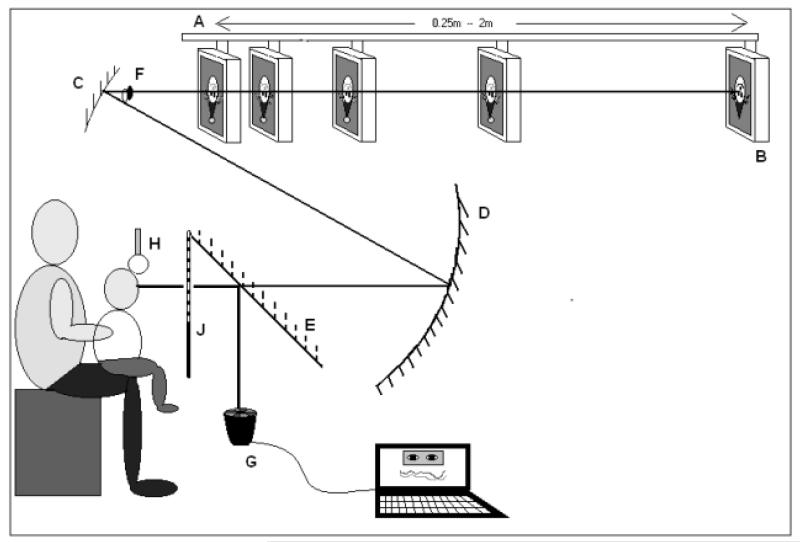
Figure 1.
The remote haploscopic videorefractor. A: motorised beam; B: target monitor; C: upper concave mirror; D: lower concave mirror; E: hot mirror; F: image of participant’s eye where occlusion takes place; G: PlusoptiX SO4 PowerRef II; H: headrest; J: black cloth screen that could be raised to occlude the target when required.
Participants viewed a target placed at five fixation distances between 25cm and 2m in the same pseudo-random order of 33cm (where it subtended approximately 18°), 2m (where it subtended 3°), 25cm, 1m, 50cm representing response demand of 4, 3, 2, 1 and 0.5 diopters (D) and meter angles (MA). The method and calibration procedures have been published previously (Horwood & Riddell, 2008) and are described in more detail in Supplemental Materials 1. For the participant the illuminated target appears straight ahead in wide black tunnel, so there were minimal attentional distractors for infants.
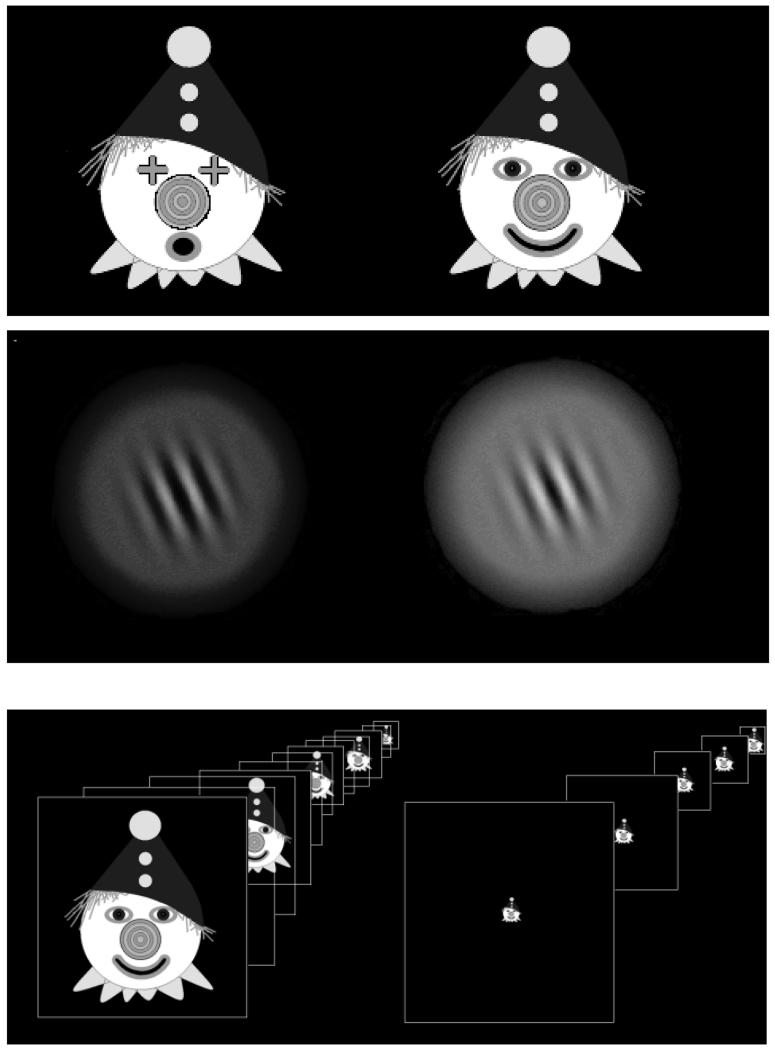
Blur cues were manipulated by using two alternative targets (Figure 2), one designed to stimulate accommodation by providing fine spatial detail (down to one pixel (<1 min arc) at 33cm) for participants with different levels of acuity, and the other a similarly sized image of a Gabor patch taken from Allard & Faubert (2006) merged with a diffuse target against a black background using image processing software which minimized spatial detail while still retaining binocular fusional elements (for details see Supplemental Materials 1). This target had a grating resolution of 1.58cycles/deg at 2m and 0.27cycles/deg at 33cm when unscaled, and 1.58 cycles /deg at all distances when scaled. These values were chosen to provide a compromise between optimum opening of the accommodative loop (Tsuetaki & Schor, 1987) and both retention of some cognitive interest in the target and providing a sufficient stimulus for accurate fusion. Each target had two versions which alternated at 1Hz. The clown had a smile and oval eyes or a circular mouth and cross-shaped eyes, and the Gabor alternated between green and yellow versions (chosen for being towards the middle of the visible spectrum and matched for luminance). All targets were matched for subjective size. See Supplemental Materials 1 for validation of use of this target in comparison to possible alternatives of lower spatial frequency.
Figure 2.
Targets. Two targets were designed to maximize or minimize blur cues respectively. The brightly coloured clown target (top figure) and alternating green/yellow Gabor target (middle figure) (note: conversion to black and white for this illustration has resulted in loss of picture quality and apparent size difference not present in the real targets). When proximity/looming cues were included, the same target was used at every distance and was visible during target motion so that size and luminance change information (looming) was available (left bottom figure). The cues were minimized by scaling the target for each fixation distance and obscuring the monitor as it moved between fixation positions by raising a black cloth screen between the participant and the target (right bottom figure). The white square target outline is for illustration purposes only and was not part of the actual target).
Size change/looming proximity cues were presented by allowing participants to view targets moving between positions, so target motion and size change were visible. The proximity cues were minimized by obscuring the target as it moved between positions and scaling the target size for each distance. Binocular disparity was present when both eyes could see the target, and was eliminated by occlusion of the image seen by one eye by occluding half of the upper mirror (C in Figure 1). The mirror arrangement meant that when half the mirror was occluded only one eye could see the target, but data could be still collected from each eye.
Eight target conditions were created to provide all possible combinations of blur (clown or Gabor), disparity (occluded or not) and proximity/looming (scaled and screened or not) cues. This allowed us to assess not only the effect of single cues, as is common in most open loop experiments where all but the experimental cue are controlled, but also the effect of removing each cue individually, leaving the other two cues intact. Two cues are commonly available in clinical situations e.g. in strabismus, when monocular suppression removes disparity, but blur and proximity remain available; blur cues may be degraded in non-strabismic refractive error, while leaving disparity and proximity cues largely intact. A cue that is a major contributor to target distance for an individual participant would be expected to produce maximum reduction in responses when removed from the target, and also a maximum increase in response from baseline when presented in isolation, so providing the opportunity for obtaining convergent evidence of each cue use across conditions.
A testing order was devised to maximize data collection in what was necessarily a long testing session for very young infants. Pilot studies suggested that infants were more difficult to test in the most impoverished conditions, and once co-operation was lost, it was often impossible to continue the session, so full counterbalancing of all eight cues risked excessive data loss. We therefore presented the all cue condition first (BlDiPr – blur, disparity, proximity) in order to determine baseline, naturalistic responses and attract maximum attention. We then tested a block of conditions with one cue removed (BlPr – occluded, looming clown, DiPr – binocular, looming Gabor, and BlDi - binocular, scaled clown), presented in a counterbalanced order between infants. If attention permitted, we progressed to a block of single cue conditions (Bl – occluded, scaled clown, Di – binocular, scaled, Gabor, and Pr – occluded, unscaled Gabor), again counterbalanced between infants. We next presented a “minimum-cue” Min condition (occluded, scaled, Gabor) to assess the effect of any residual cues that we could not totally eliminate, such as motion parallax if the infant moved his/her head. Finally, to assess whether reduction in response in the impoverished conditions was due simply to fatigue rather than the direct result of cue reduction, we re-presented the all-cue BlDiPr condition. This testing order was designed to ensure that even if all eight target conditions were not testable, we could at least obtain the full first block, with each cue removed in turn. Two testing sessions per visit were attempted, with the eight target conditions presented in a different counterbalanced order within each block, and where this was possible data from both sessions was averaged. In between these sessions, time was given for feeding, rest and clinical testing, including Mohindra retinoscopy (Mohindra, 1977), by an experienced orthoptist (AH) to exclude visual abnormality. A session where a full set of data were collected under ideal conditions took approximately 40 minutes, so was not possible with the youngest infants.
Vignettes of 25 continuous and stable data points for accommodation and eye position representative of one second of steady fixation at each target position were selected for analysis from each cue condition for each participant. Data were only scored if the infant had been observed to be looking steadily at the target for at least two seconds. Averaged data from these vignettes were used to calculate vergence (in MA) and accommodation (in D) responses at each fixation distance, making corrections for individual angle lambda, IPD and a systematic error in comparison to dynamic retinoscopy found during earlier calibration studies (Horwood & Riddell, 2008) (see Supplemental Materials 1 for more details). Data from the 25cm (4MA & 4D demand) target were discarded due to excessive pupillary constriction preventing collection of sufficient data. Accommodation and vergence responses were plotted against target demand for every target condition. This was used to provide measures of response gain and linear fit (r2). Since targets were presented in a pseudo-random order with alternation between near and far targets, response gain = 1 indicated an overall appropriate response to change in demand, while a fitted r2 value = 1 indicated highly reliable and linear responses.
Participants
45 infants (22 females and 23 males) were recruited at four weeks of age from the School of Psychology Infant Database. All were typically developing and, at the time of writing, all have reached the age of at least 3 years without developing strabismus, significant refractive error or other ocular pathology. All were born within 3 weeks of their due date and ages were corrected for gestational age.
We aimed to test infants every two weeks from six weeks to 20 weeks of age (to obtain at least two visits before, during and after the period when stereopsis is thought to develop (Birch, Gwiazda, & Held, 1982; Birch & Stager, 1985), and again at 26 weeks.
86.6% of the infants completed at least five visits (mean 6.4 visits), including at least one before 9 weeks of age, one between 12-16 weeks, one between 16-20 weeks and one at 26 weeks (±2 weeks). The data were analyzed at two-weekly intervals and data from each infant was used only once in each age bin.
We compared our infant data with that from a group of 27 visually typical children between five and nine years of age. Data from 32 naive emmetropic (non-glasses wearing) adults aged between 18 & 24 years of age were also used for comparison using the same selection criteria. Data on these two older groups have been published in detail previously (Horwood & Riddell, 2008).
As expected, fewer runs of the different conditions were possible in the younger infants, who needed frequent breaks between testing, but at 6-7 weeks 57% of infants provided data for all four of the basic set of BlDiPr, BlPr, BlDi, and DiPr cues. By 14-15 weeks 90% of infants performed the basic cue condition trials and 52% were able to provide data for all eight cue conditions. At 24-28 weeks 86% of infants provided data for all cue conditions. The use of a “gold standard” cycloplegic refraction to determine refractive error was not possible at each visit due to recruitment and ethical constraints. Refractive error was estimated from the maximum hyperopic spherical refraction (MHR) recorded at any stage during the whole session, a measure which has been previously shown to correlate highly with cycloplegic refraction (Horwood & Riddell, 2009) (see Supplemental Materials 1 for validation). Any infant who showed any evidence of hyperopia over 2.0D by this method or Mohindra retinoscopy at the age of 24-26 weeks was referred for cycloplegic refraction at the local hospital, and if this was found to be genuine, the infant was not included in this dataset, even if in earlier infancy this error had not been apparent.
Most infants would be expected to show some hyperopia in infancy, but the focus of this study was on infants with only very low errors so that comparison with typical older groups could be made. Data were completely excluded from 9 additional infants showing evidence of manifest hyperopia greater than +2.00 MSE on more than two visits, 7 of whom were still >+2.00D hyperopic at 26 weeks. If hyperopia of over +2.00D was only found on one or two runs within up to two visits, the infant was not excluded from the study but the data from that whole session was excluded. For example, 15 infants showed mild hyperopia between 2.00D and 4.00D on one or two individual lab runs in their first weeks, but which subsequently disappeared.
We chose these strict criteria of absence of hyperopia, when low levels may be considered typical, for two reasons. Firstly, it has been shown that the accommodation of hyperopic infants and children is very different from that of emmetropes (Horwood & Riddell, 2011; Tarczy-Hornoch, 2012), so including hyperopic infants would make comparisons with the older non-hyperopic control groups problematical, particularly because the blur, and so the accommodative demand, would not be similar in all age groups; and secondly we were interested in studying infants with optimal potential for typical development without the additional factor of significant refractive error. We hope to publish the comparative data between this group and those with more hyperopia in the future.
Data were also excluded if more than one of the four possible data points from a testing run was missing, if they exceeded the linear operating range of the PlusoptixSO4 (+5.0D /−7.0D) or if both accommodation and vergence responses were erratic (r2 of the fitted response slope ≤0.7) which could have been due to inattention or truly erratic responses. 5.5% of the total number of runs were excluded before the main analysis due to these doubly erratic responses (which ranged from 3% in the BlDiPr condition to 37% in the least engaging Min condition). Of the single cue targets, 35.7% of the Bl runs, 9.2% of the Di runs and 16% of the Pr runs were excluded for this reason. It was noticeable that while the majority of these exclusions were made for the younger infants, for the Bl target they could be necessary at any age.
1.7% of runs were excluded because more than one out of the four data points was missing or out of range. This was usually due to an excessive “all or nothing” response at 33cm (1.14% of all data points) (see Figure 3) taking the near response beyond the operating range of the photorefractor, but occasionally due to small pupils or an insufficiently long vignette being captured. We were able to perform analysis on 2739 runs that fulfilled our attention and accuracy criteria.
Figure 3.
Typical example of an “all or nothing” accommodation response from an infant (15% of the infant data). The vergence response is linear but accommodation is non-linear (see also Supplemental Materials 3).
Analysis
Initial data scoring and analysis was carried out using Excel and SPSSv16 software. Statistical analysis used mixed ANOVA with target (eight cue conditions) and response (vergence and accommodation) as within-subjects factors and fixation distance as the independent variable. There were few infants for whom a complete set of longitudinal data was available for all 8 target conditions on every visit between 7 and 26 weeks. As a result, comparisons across the different age groups were carried out using age group in (2-week bins) as a (more conservative) between-subjects factor. Where assumptions of sphericity were violated, the more conservative Greenhouse-Geisser statistics are quoted. Post hoc testing used Bonferroni correction for multiple comparisons. An alpha level of .05 was used for all statistical tests. Because of the large number of statistical comparisons possible, we only report the relevant post hoc findings. For example, between-cue differences were universal and so are not quoted, but age-related trends and interactions between age and target, or age and response allowed us to examine developmental differences.
Results
Refraction and attention
We first tested to determine whether there were differences in refractive error across age. Although we had included only emmetropic or minimally hyperopic infants, a one-way ANOVA of maximal hyperopic refraction (MHR) across the different age groups still showed a significant effect of age on refraction (F(10,220)=3.38, p=.0004). Post-hoc testing demonstrated that the 12-15 week infants were somewhat more hyperopic than the youngest infants and also than the older control groups. Unless dilating eyedrops are used, infants of 6-7 weeks often appear short-sighted because of neonatal fixed focus at around 50cm (Banks, 1980), so MHR is likely to be a less reliable measure of refraction in these very youngest infants than when older (Horwood & Riddell, 2009). MHR was added as a covariate in the ANOVAs, and was found to be a significant covariate for most analyses of accommodation, but not vergence, gain. To account for this, main effect and interaction statistics are quoted with MHR partialled out.
The infant’s eyes were visible in real time on the Plusoptix screen. We used an auditory cue (buzzer/squeaker) mounted at the far end of the apparatus, in the line of sight but not visible, to attract attention. Once gained, attention during the run was assessed by the tester while infants viewed targets and each attempted run was scored on a 5 point Likert scale(1= totally calm and attentive throughout, 5= totally inattentive to the target). Only runs scoring 3 and less (3 = at least two seconds at each fixation distance when the infant was observed to be looking at the target despite mild fussiness) were analysed off-line. It seemed easier to achieve attention to moving, and particularly, binocular targets. Attention was significantly better in the 3-cue vs 1-cue (1.1 vs 1.36; z=−4.421, p<.0001), 2-cue vs 1-cue (z=−4.13, p<.0001) and 1-cue vs “zero”-cue (z=−4.06, p<.001) conditions although surprisingly there were no attention differences between targets within the 1 or 2 cue blocks once attention was gained. We did not collect data on how easy it had been to gain attention (as opposed to retaining it once achieved), which in hindsight would have been useful. The most noticeable difference was at the end of testing when, after the sequence of single-cue targets, attention often immediately improved when the all-cue BrDiPr was re-presented. At the analysis stage, it was easy to detect and exclude sections of data when the infant had not been fixating the target, either because the photorefractor had not been able to detect data at all, or had recorded large fixation position errors in both eyes.
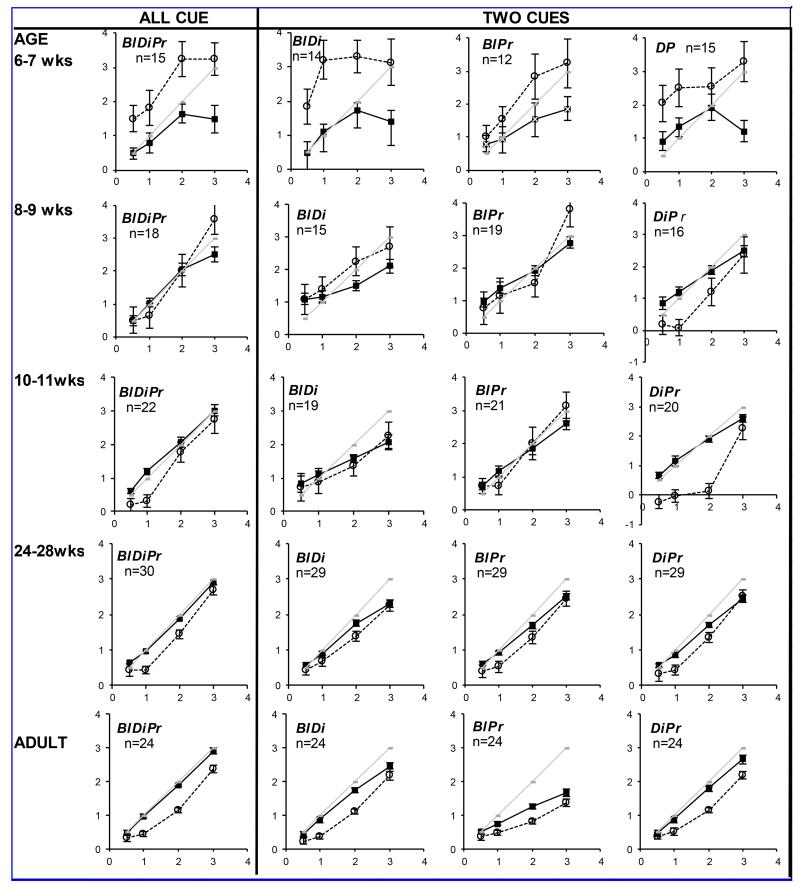
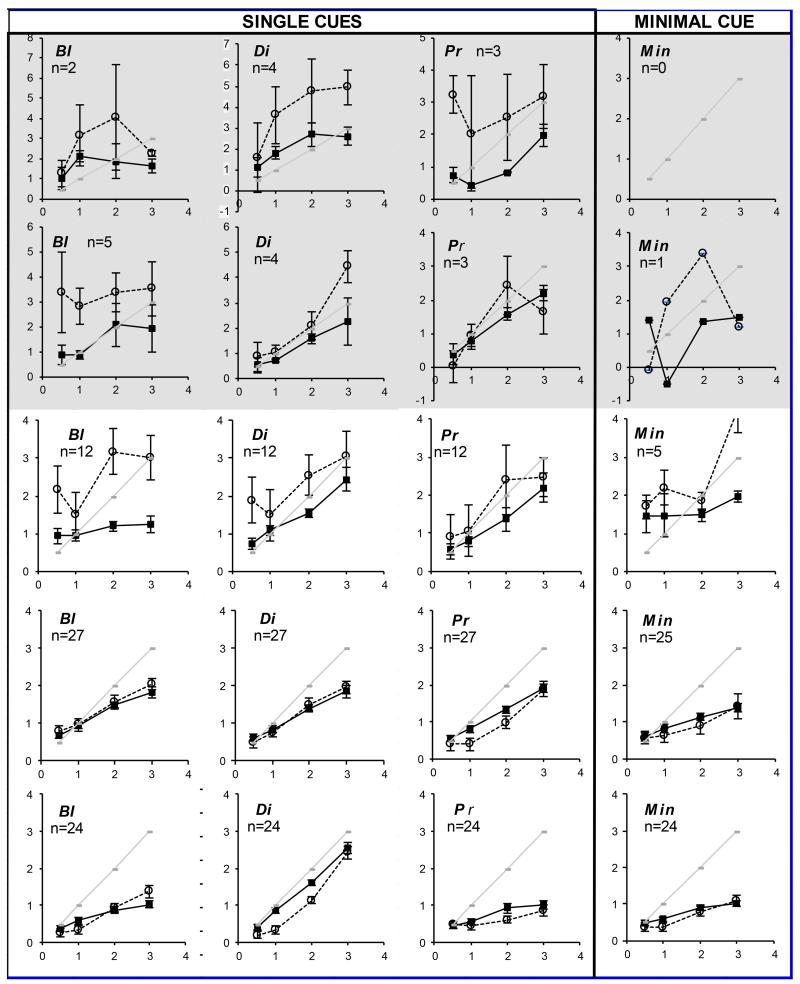
Examples of mean responses to each target across the age range tested are shown in Figure 4 (for full dataset in 2-weekly bins between 6-20 weeks, then at 24-28 weeks, 5-9 yrs and adults see Supplemental Materials 2). Immature response slopes are typically flatter, with under-convergence for near, over- accommodation in the distance, and under-accommodation for near. These flatter response slopes persisted later into development in the more impoverished target conditions, suggesting that the youngest infants needed targets containing more cues to be able to respond appropriately. 15% of infants showed “all or nothing” accommodation responses (quadratic fit >0.9 and linear fit <0.8) (see Figure 3) occurring with concurrent linear vergence responses (linear fit >0.9). Linear vergence concurrent with more erratic accommodation was common (see examples in Supplemental Materials 3).
Figures 4A and 4B.
(Axes scales are in the equivalent units of D for accommodation and MA for vergence) Response (y-axis) to target demand (x-axis) to all cue conditions in infants of 6-7wks, 8-9wks,10-11wks, 24-28wks and in adults. Solid black lines and points = vergence. Dotted lines and open points = accommodation. Pale gray line = ideal response for demand. Youngest infant responses are shown towards the top of the figure. Chart headings: Bl = blur (detail) available; Di=disparity available (binocular stimulus); Pr = looming, size cues available. Figure 4A(left page) Naturalistic target (BlDiPr) and two cue targets (BlDi, BlPr and DiPr).Figure 4B (right page); single cue targets (Bl, Di, Pr) and minimal cue (Min) target. Error bars denote standard error (SE). Grey shaded charts indicate small numbers and/or changed y-axis scale. Numbers for which testing was successful at each age and condition are indicated on each chart. (These data represent main findings and for full dataset see Supplemental Materials 2).
Some of the “all or nothing” accommodation responses at 3D demand indicated that accommodation increased beyond the linear operating range of the PlusoptixSO4 in the very youngest infants and so were discarded and response gains calculated from the remaining three points. Thus mean accommodation gain data in these youngest infants represents a slight under-estimation of true mean gains. The prevalence of these “all or nothing” response did not differ between cue conditions.
Naturalistic Responses to All Cue (BDP) Target
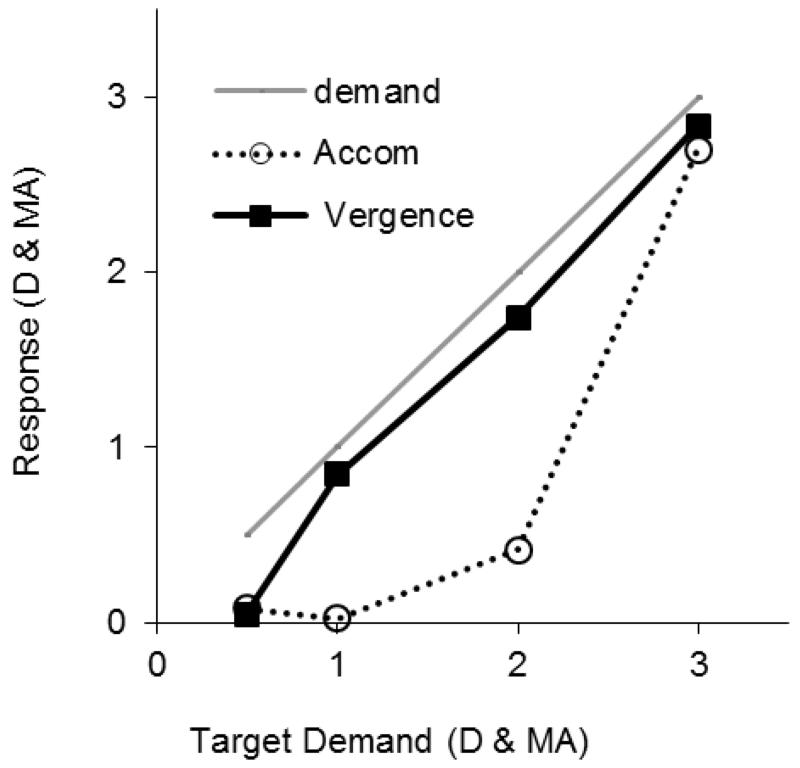
Even at 6-7 weeks of age, most infants could change accommodation and vergence by an appropriate amount in response to changes in target demand, although over-accommodation at all target distances was typical at first. Mean vergence gain to the all-cue, naturalistic BlDiPr target was 0.8 (95%CI ±0.17) at 6-7 weeks. Although ANOVA of vergence gain showed an overall significant effect of age group (F(10,228)=4.74, p<.0001), beyond 8-9 weeks there were no significant between-age-group differences (F(9,214)=0.79, p=.62) and vergence gain was not significantly different from that of the adults 0.94(95%CI ±0.11) vs 0.95(95%CI ±0.06), (t(14.82)=0.5, p=.48.
When the significant effect of MHR on accommodation gain (F(1,136) 22.13, p<.0001) was partialled out, mean accommodation gain did not differ significantly between any of the age groups (F(10,217)=1.52, p=.13). Accommodation was less accurate for target demand for both near and distant fixation throughout infancy and variability in the accommodation data was slower to reduce (see error bars in Figure 4) than vergence. For the very youngest infants of 6-7 weeks, four of the 15 (28%) infants showed the flat, unresponsive, myopic behavior found by others in infants of this age (Banks, 1980; Hainline et al., 1992) with gains of <0.3, while 5 other infants (33%) showed excessive “all or nothing” changes to near targets with gains of >1.5 (despite appropriate vergence) so averaging hides a wide distribution of behaviour that was not found in infants beyond this age.
Responses according to cue condition
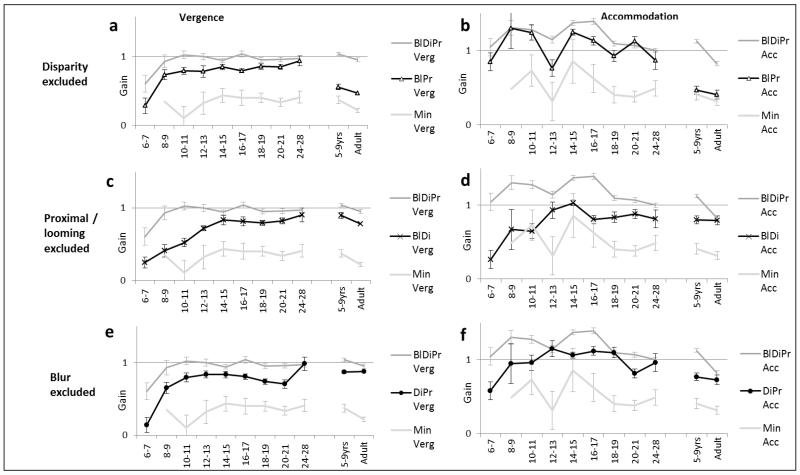
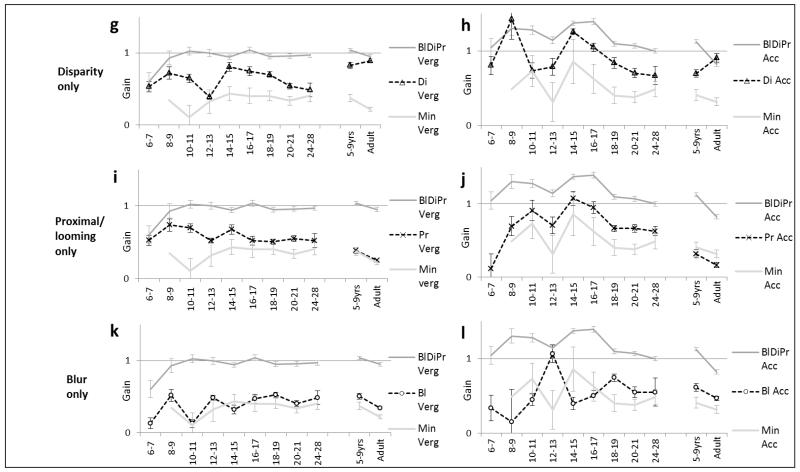
Figure 5 illustrates vergence and accommodation response gains of the six different two- and single-cue conditions in comparison to the all-cue (BlDiPr) and minimal (Min) cue conditions. 5a and 5b show the effects of excluding a cue, such as would occur naturally in situations such as monocular activity, ocular suppression or extreme refractive error. 5c and 5d show the effect of presenting a single cue, as is common in most laboratory studies of responses to any cue. We were particularly interested in developmental trends in the differences between cues and so conducted ANOVAs and post-hoc tests to assess differences between both vergence and accommodation across age and between conditions.
Figures 5.
a-l. Response gains (error bars ±SE) to the different cues throughout development showing weighting in comparison to all-cue naturalistic (dark grey) and minimal cue(light grey) baseline conditions. Key abbreviations: “Bl”= blur; “Di”=disparity; “Pr”=proximity (looming) available in a target condition. Vergence responses on the left and simultaneous accommodation on the right of each page. Figs. a-e: response gains if a single cue is excluded (a-b – disparity excluded; c-d - proximity/looming excluded; e-f – blur excluded). Figs g-l: response gains to single cue conditions (g-h – disparity; i-j – proximity/looming; k-l - blur). Overall vergence response gains were less variable than accommodation gains, with especially wide variability of accommodation in early infancy.
Removing a cue from the naturalistic BlDiPr condition reduced all three two-cue response gains. Excluding proximity cues had a large effect in reducing vergence responses in early infancy but not in older children and adults. In comparison, excluding disparity had a lesser effect than excluding proximity in early infancy, but much more in older children and adults.
There was a highly significant linear trend for response gain to reduce with reduction in the number of available cues (vergence F(1,1251)=61.47,p<0.00001; accommodation F(1,1251)=94.73, p<0.00001), so not only was it less possible to collect data for more impoverished cues (see numbers testable in Figure 4) and data were more erratic, but even when the infants did attend to the target, they generally responded less well to fewer cues.
We examined the effect of cue condition and age on the gain of the accommodation and vergence responses using an ANOVA with type of response (accommodation and vergence), cue condition and age as factors. All three main effects were significant; response (F(1,137)=16.61, p<.0001), cue(F(5.4,740)=47.87, p<.0001) and age (F(9,137)=2.27, p=.021). There were significant two-way interactions for gain between accommodation and vergence response and both cue (response*cue: F(7,959)=3.166, p=.043) and age (response*age: F(9,137)=3.45, p=.0007) and a significant three-way interaction between response*cue*age (F(63,959)=3.166, p=<.00001). Thus, despite number of cues alone having an effect, the interactions suggest that there were differences in the accommodation and vergence gain between cue conditions, within the 3, 2, 1 and minimal cue blocks and over development.
Responses were then analysed according to manipulation of the three cues of disparity, proximity and blur separately and the results for manipulation of each cue are given below.
Disparity
We predicted that disparity might a less precise cue for infants less than 12 weeks of age when only more gross binocularity without stereopsis is demonstrable. Even the youngest infants could respond to the disparity-only (Di) target (binocular, scaled, Gabor), with vergence response slopes of even the 6-9 week infants significantly greater than zero (slope= 0.63; F(1,28)=14.09, p=.001). We were unable to make comparisons to responses to the Min target as none of the youngest infants would attend to it. There was a significant linear trend for vergence gain to increase across age (linear trend F(1,166)=24.02, p=<.00001) from 0.53 at 6-7 weeks to 0.85 at 5-9 years, and 0.88 in the adults.
In the case of accommodation, response gains between 6-9 weeks were also significantly greater than zero (gain=1.11; F(1,28)=13.38, p=.001). The disparity-driven accommodation gains did not show significant developmental increases in responses, averaging 0.89 across all ages with no significant trend across age (p=.18).
If disparity becomes increasingly important, we predicted that excluding it by occlusion (BlDiPr vs BlPr conditions) would have a greater effect in reducing response gains in older than younger participants. As predicted this manipulation reduced vergence gain significantly less in infants aged between 8-28 weeks than in older children and adults (t(172)=9.03, p=<.0001). Across the pooled infant groups, vergence gain was reduced from the BlDiPr mean of 0.97 to a BlPr mean of 0.82 (by 13%) but in the pooled group of older children and adults gain dropped from 0.99 to 0.51 (48%). Effects were similar for accommodation, with a significant reduction in accommodation gain on occlusion between the infants and the older children and adults (t(173)=4.07, p=<.0001), from BlDiPr 1.2 to BlPr 1.12 (7%) in infancy compared to BlDiPr 0.97 to BlPr 0.43 (55%) in the older groups. There were no significant between-age-group differences in infants between 8-28 weeks for either vergence or accommodation gain, and no detectable alteration of developmental trajectory at 12-16 weeks when stereopsis would be emerging.
Proximity/ Size/Looming
Below the age of 12-16 weeks of age, blur cues are less available and disparity detection is immature (pre-stereoptic) so proximity cues might be relatively more useful to the youngest infants. Vergence and accommodation gains to the single cue Pr target (occluded, looming Gabor) were significantly better in early infancy and decreased with age. Vergence gains reduced steadily from around 0.9 between 10-17 weeks of age to 0.2 in the adults (F(9,160)=5.446, p<.00001; linear trend F(1,160)=38.77, p<.00001). Accommodation gains of near 1.0 in infants between 10 and 17 weeks of age steadily reduced to 0.16 in the adults (quadratic trend p=.00007).Vergence gains to the Pr target in the older children and adults were not significantly better than the minimal cue Min condition (t(56)=0.15, p=.8).
Excluding proximity cues by scaling and obscuring target movement (BlDiPr vs BlDi) allowed us a further way of examining the influence of proximity, as we did in the case of disparity above. This reduced response gains more in early infancy than later, and much more in infants than the older groups (infant vs adult difference; vergence t(53.8)=2.01, p=.05, accommodation t(55.34)=3.05, p=.003). This effect was greater than found when excluding either blur or disparity cues. Between 8 and 12 weeks of age the BlDiPr vergence gain of 0.97 was reduced to 0.46 when proximity cues were excluded in the BlDi condition)(53% reduction) and the accommodation gain of 1.29 reduced to 0.65 (50% reduction). In comparison the vergence gain reduced from 0.99 to 0.83 (16% reduction) and the accommodation gain reduced from 0.97 to 0.78 (20% reduction) respectively in the older children and adults. Together these results confirmed our prediction that proximity cues are influential in infancy but much less so in later life.
Blur
From the literature it could be predicted that sensitivity and responses to blur might increase as acuity improves, although our adult results generally show both vergence and accommodation responses to blur to be weaker than those to disparity (Horwood & Riddell, 2008). At all ages, responses to the Bl target were more frequently discarded due to low r2 for both accommodation and vergence than the other two cues. When the acceptable data was analysed vergence gains to the blur-only Bl target (occluded, scaled clown) improved only slightly with age (F (9,168) =2.242, p=.022; quadratic trend (F (1,168) =3.916, p=.049)), from 0.34 at 6-7 weeks to around 0.5 in most infants after 10-11 weeks and at 5-9 years. Differences in vergence gain between the Min and Bl conditions across all age groups were very small (mean gain difference 0.06 (18%)).
Accommodation gains to this detailed cue were also modest over the age ranges tested (mean 0.53), and only 14% better than those to the blurred Min condition, with no significant linear or quadratic trends (p>.18 in all contrasts). This was the only cue where the estimate of refractive error was not a significant covariate for accommodation gain (F (1,168) =1.79, p=.18), also suggesting any changes in refractive blur did not influence accommodation gain.
When we investigated changes in gain resulting from minimizing detail cues (BlDiPr vs DiPr), this resulted in a small reduction in vergence at all ages and the size of this effect lessened with age (linear trend F(1,200)= 15.49, p<.00001). Minimizing blur cues reduced accommodation by less than 0.5D at 33cm at any age, with no suggestion of any developmental trend (p=.37). BlDiPr vs DiPr vergence response gains (thus retaining disparity and proximity cues) at 8-28 weeks of age reduced from 0.97 to 0.79 (18% reduction) and accommodation response gains reduced from 1.20 to 1.01 (16% reduction) compared with 0.99 to 0.87 (12%) and 0.97 to 0.74 (24%) respectively in the pooled older groups. This suggests that overall presence or absence of blur cues makes relatively little difference to vergence or accommodation and that increasing availability of blur cues that accompany developing acuity do not result in greater or more precise use of blur as a cue to target position with increasing age.
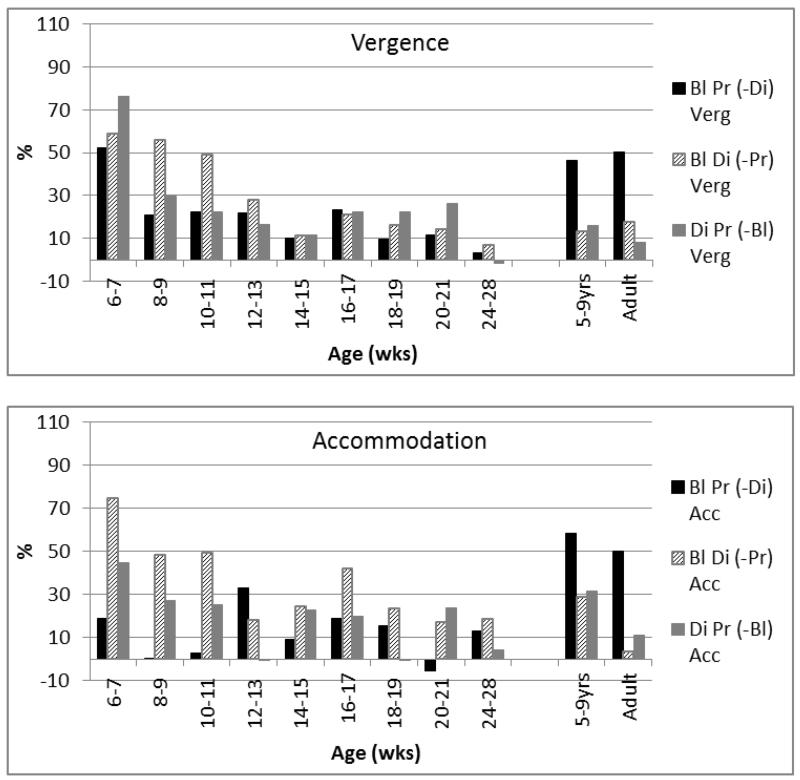
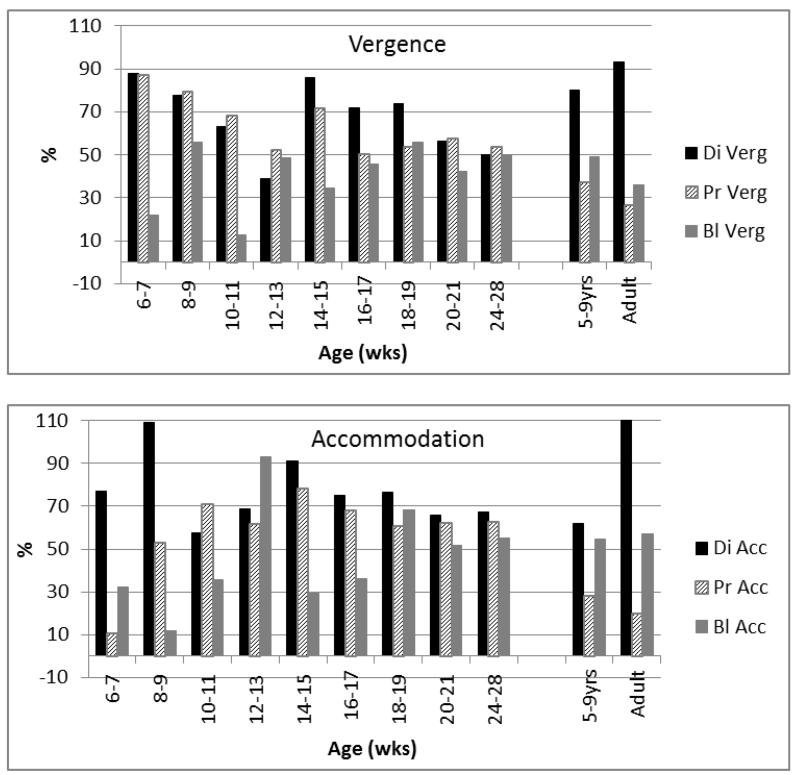
Figures 6 & 7 illustrate the relative weightings of the individual cues. Figure 6 shows the percentage of the BrDiPr response slope that is lost when a single cue is excluded, and Figure 7 illustrates how much of this all-cue response can be driven by an isolated single cue.
Figure 6.
Proportion of the all-cue BlDiPr responses eliminated by excluding a single cue. Note the gradually reducing effect of minimising proximity/looming cues(hatched bars), and the increased effect of eliminating disparity in the 5-9 yr children and adults (black bars).
Figure 7.
Proportion of the BlDiPr responses able to be driven by the different single cues. Note the gradual reduction of weighting of proximity looming cues on vergence responses (hatched bars) and the increased weighting of disparity in comparison to the other cues (black bars) in adulthood. Note also broadly similar weightings in “middle infancy” (18-28 weeks) in comparison to older and younger groups.
In summary, therefore, proximity cues appear influential in driving vergence and accommodation in very early infancy but the response to these cues declines with age. The response to disparity as the main cue to both accommodation and vergence, the adult pattern, is in place by 5-9 years of age. Significant developmental changes in the influence of blur were not detectable in this study.
Discussion
This paper describes a unique, complex dataset. While other studies have reported some individual aspects of the data we describe, this is the only study to attempt to use a testing paradigm that applied identical testing conditions from infancy into adulthood to give an overview of developmental change across the two main near visual responses and to the three main stimuli. While of obvious interest to vision scientists, it is equally of relevance to those studying wider aspects of perception or anyone designing studies where visual stimuli or responses are used to study other aspects of infant development.
For the widest audience, our data show that convergence and accommodation can be good even at 6-7 weeks, and broadly adult-like by 8-9 weeks to naturalistic targets: infants were responsive to change in target distance and could use accommodation and convergence to achieve optimal acuity and binocular alignment at whatever distance they fixated. This is somewhat earlier than previous reports(Birch et al., 1985) and vergence, in particular, does not appear to be dependent on emerging stereopsis. Some of the youngest infants showed low gain of accommodation resulting from over-accommodation for distant targets as reported previously (Braddick & Atkinson, 1979; Braddick, Atkinson, Wattam-Bell, Anker, & Norris, 1988; Hainline et al., 1992; Haynes, White, & Held, 1965; Turner et al., 2002), although this was not found consistently in our data. Some of the youngest infants tested responded well to changing target distance, but our data confirm that targets at intermediate distances (around 50 cm) might be optimal when testing infants less than 8 weeks of age. Thereafter, low response gains were consistently the result of under-convergence and accommodation for nearest targets, so any experimenter testing at near with such targets needs to consider whether under-convergence and particularly under-accommodation for a very near task would be an issue.
In very early infancy, impoverished stimuli (e.g. monocular, static or detail-free) were less likely to provide as much data, and mature responses were slower to emerge as the number of cues was reduced. Of these, the dynamic proximity/ looming cues influenced responses maximally in early infancy but then declined in influence. Our youngest age group were of an age when the first reports of directional, cortical, responses to motion cues have been reported (Braddick et al., 2003), and their vergence responses to the Pr target were better than to the other two single-cue targets. It is possible that the looming target is just more interesting to infants, and it does seem to attract their initial attention best. Once that attention had been achieved, we obtained similar amounts of usable data and in each two- or single-cue condition (although those to blur-only were more likely to be erratic), but we found better response gains to proximity cues in the youngest infants. In the older children and adults looming/motion/proximity cues became a weak driver of responses which were little better than baseline. Although other authors have found that infants are sensitive to proximity cues for perceived distance (Banton & Bertenthal, 1997; Yonas & Granrud, 1985; Yonas, Pettersen, & Lockman, 1979), and they still influence perception in adulthood, adults appear to be able to utilise their perceptual value without these cues driving the vergence response (Wismeijer & Erkelens, 2009; Wismeijer et al., 2010; Wismeijer et al., 2008), while our infants did not. Although Yonas et al (2002) found that behavioural responses to “pictorial” cues such as perspective and habitual size, emerged between 5 and 7 months, this did not appear to influence the steady decline of physical visual response the proximity/looming component of our targets. Our dataset gives data on the timescale of the decline of use of the primacy of these cues to drive vergence in childhood, suggesting that this occurs by about 6 months of age. We did not consider non-moving size change cues in our stimuli, so we could not assess the separate effects of dynamic and static cues.
Infants in middle infancy appeared able to respond similarly to the three cues presented. This more balanced weighting of the cues between 8-26 weeks than is found in older children and adults may reflect emerging blur and disparity sensitivity, but also could be developmentally advantageous. IPD, orbit and globe anatomy (and thus eye muscle force vectors (Sevel, 1986), acuity, contrast sensitivity, refractive error (especially hyperopia and therefore accommodation demand), and therefore experience of the three dimensional world in general, all change dramatically during infancy. Being able to use any available cues to depth at a time of such developmental fluidity would provide the best opportunity to respond to, and learn from feedback from these changes in cue quality.
This changing balance of responses to the different cues helps us to understand how the near system develops over time. The stronger use of proximity cues in early infancy might be the result of wide dead zones for blur and disparity detection which would result in proximity cues being the most reliable of the three cues at first. Although blur and disparity can be shown to drive responses before 14 weeks of age (Bharadwaj & Candy, 2009; Riddell et al., 1999; Turner et al., 2002), low acuity and optical blur are present due to immature retinal, cortical and refractive development. Despite the dramatic increases in visual acuity and contrast sensitivity and reduction of the common refractive errors of infancy, somewhat surprisingly, responses to blur could be erratic at all ages (and so some were not even analysed here) and even if they were within our inclusion criteria, blur cues do not drive any better responses in visual maturity than in early infancy, and the desire for optimum acuity does not appear to be a major drive to accommodation in adults. The increasing reliance placed on disparity cues in older children and naïve adults means that if a stimulus (that does not force resolution of threshold detail) is presented to only one eye, not only is vergence prevented, but a participant cannot be assumed to be achieving optimal near vision as they typically under-accommodate (to an even greater extent than do infants).
Before the emergence of stereopsis (Birch, Gwiazda, & Held, 1983) early vergence could only be maintained by gross motor fusion or even two separate foveations (Hainline & Riddell, 1996; Hainline et al., 1992; Riddell, Horwood, Houston, & Turner, 1999) with wider dead zones than would be available when stereopsis matures. In the first weeks of life there is clearly a binocular mechanism in use that is not revealed by VEP and behavioural measures of sensitivity to binocular correlation (Braddick et al., 1980), perhaps supporting the separate foveation theory. This functioning but immature system might also explain the less accurate control of stable alignment before four months of age (Horwood, 2003).
Once stereopsis has emerged, adding to the more global and gross motor fusion driven by images outside Panum’s area, the binocular system is sufficient to resolve both gross motor tasks and achieve precise bifoveal fixation, with detection thresholds and accuracy down to only seconds of arc while blur and “proximity” cues are much less precise. We suggest that a parsimonious system based on increasing statistical regularity and sensitivity provided by maturing systems eventually enables cue use, for motor systems at least, to gradually settle more firmly on the most regular, reliable and accurate signal of target distance; namely binocular disparity. Our data suggests that this process occurs after a period of relative stability in middle infancy between 8-26 weeks of age. We did not find any detectable step-change in vergence accuracy at the age when stereopsis is thought to emerge, which might be expected if stereopsis per se contributed to vergence accuracy, so our data suggest that stereopsis does not significantly add to vergence control. We did not study infants between one and three years of age here, but this seems to be the likely period of transition and (perhaps not co-incidentally), and is the time when many childhood visual problems such as strabismus are first reported.
It is interesting to consider these data in relation to the combination of multiple cues for perceptual judgments that occurs in adults, which results in performance beyond the limits of single cues (Nardini, Bedford, & Mareschal, 2010). From 8-9 weeks mean responses to the most naturalistic condition were not significantly different than adults’ (although with less variance). Our “middle infants” seemed more able to use the full range of single cues we presented, which supports Nardini et al’s contention that a range of sensory conflicts in early life may provide more error signals needed to perform necessary recalibrations which must occur during growth, although our older participants seemed to have pared down their use of some cues in favour of disparity, rather than maximally integrating multiple cues. The differences may be that in visual maturity, disparity is so much more accurate a signal than the other cues we tested that integration these cues would be of little additional advantage; or that we might find evidence of “sensory fusion” in the reduction in speed or precision of responses, rather than averaged motor responses to a particular fixation distance which was assessed. We were also measuring motor responses, rather than threshold perceptual judgements, which can be different (Wismeijer et al., 2008).
Many of our findings are not novel when viewed in isolation. Other authors have reported similar findings in different studies on more closely defined areas. Interest lies in the concurrent data on different aspects of the visual system, changing responses to a standard stimulus set, and in the case of the infants, in the same group of children studied longitudinally. It is known that accommodation may be excessive and relax poorly in the distance (Banks, 1980), but we have shown that infants are converging appropriately at the same time. “All or nothing” accommodation has been noted before (Riddell, Grose-Fifer, Hainline, & Abramov, 1991), and has been interpreted to result from poor attention to static, non-salient distant targets or sub-threshold stimuli. However, we have demonstrated here that convergence is concurrently linear and appropriate (particularly impressive given our pseudo-random order of presentation distance). This suggests that attention is not a limiting factor to responding to the target. As the sensory dead zones narrow dramatically in the first months of life, particularly for blur detection as acuity increases (Teller, 1997), infants could use increasingly precise feedback from both systems to maximise alignment and clarity, resulting in the more stable accommodation and vergence ratios described in older children and adults. It also suggests that the specific coupling between accommodation and convergence so evident in the adult binocular vision clinical literature is not present in infant responses.
Using a standard and composite method also allowed us to track variability in the data. We considered whether the variability in accommodation responses in infancy could be due to measurement or sampling errors. More variable accommodation could result from an instrumental, or measurement error. In view of the extreme youth of the infants and the long testing session, we were unable to make individual accommodation calibrations, as suggested by others (Bharadwaj et al., 2013; Blade & Candy, 2006). However, despite this, our group findings are largely in line with theirs. Calibration errors would only result in gain differences between participants and differences at the limits of the operating range of the Photorefractor, not major differences in linearity of the data with distance or between cues. Responses were generally better in the BlDiPr condition and were reduced and more variable in impoverished conditions, despite an identical data collection method. Adult accommodation responses were much less variable than those of infants, despite similar vergence response variance in infants and adults. This suggests that the differences in accommodative variability between age groups are genuine and cannot simply be a result of measurement errors. While early accommodative variability is likely to be due to wide dead zones in the presence of poor acuity, it is a factor that suggests that fixed ratios between vergence and accommodation only develop with time.
A limitation of the study is that the cues we used were not optimal for any one age group and represented a compromise in order to obtain longitudinal data; some of the stimuli (in terms of spatial frequency of some of the clown target elements for example) may have been beyond the resolution capacity of the youngest infants, and might not have been demanding enough to stretch the adults, but our clown target does represent a naturalistic image with a range of available spatial frequencies. Our blur-minimised, adapted Gabor target, chosen to retain binocularly fusional elements, might have induced some residual accommodation cues and the masked screen edges allowed some residual looming cues. What is more remarkable is that when detail cues were available, accommodation did not increase more.
We were also unable to isolate which of the proximity cues we presented was most influential. Schor et al (1992) have modelled the different elements of the proximity response in adults, but it is possible to speculate that infants will use these elements differently, as some cues (such as texture, shadows, overlay of contours) may need to be learned from experience, while others may be less dependent on an extended learning process, such as dynamic retinal image size change. We did not assess responses to texture gradient, overlap, shadows or perspective and we tried to minimize motion parallax, but even in our somewhat limited proximity cue manipulations, these cues were still more influential in infants than adults.
Our apparatus allowed us to look at the influence of a cue in two complementary ways; when presented in isolation and when removed from the all-cue naturalistic target. Having these two alternative methods of looking at a cue provided some convergent validity within the data. More importantly, two-cue situations are more common in real life than single cue situations and so including these stimuli allow useful clinical predictions and comparisons to be made. For example, disparity cues are absent, defective or suppressed in childhood strabismus, while blur and proximity cues are unaffected, but it is not clear whether atypical response to disparity precedes or is secondary to the strabismus. An atypical developmental trajectory in the emergence of increased disparity weighting might predict increased risk of developing strabismus in later childhood. In severe refractive error, blur cues may be significantly degraded, but disparity and proximity cues remain less affected, but an additional reduced response to disparity in the DiPr condition might also carry increased risk of strabismus.
In conclusion, this paper provides a dataset documenting an overview of how responses in early infancy may serve as a scaffold around which more stable adult patterns can be built. In the light of the early limitations to both the blur and disparity detection systems, size change proximity cues which are available from birth and which would be present despite blur or intermittent misalignment are used as a cue to depth in early infancy. While retaining perceptual value, these cues are less effective as cues to accommodation and convergence after the maturation of disparity detection accurate to a few seconds of arc as demonstrated by data from older children and adults. We were unable to study infants between one and three years of age, but our data suggests that this is the time when the ascendency of disparity occurs.
Supplementary Material
References
- Allard R, Faubert J. Same calculation efficiency but different internal noise for luminance- and contrast-modulated stimuli detection. Journal of Vision. 2006;6(4):322–334. doi: 10.1167/6.4.3. [DOI] [PubMed] [Google Scholar]
- Aslin R. Infant accommodation and convergence. In: Simons K, editor. Early Visual Development, Normal & Abnormal. Oxford University Press; New York: 1993. pp. 30–38. [Google Scholar]
- Banks MS. The development of visual accommodation during early infancy. Child Development. 1980;51(3):646–666. [PubMed] [Google Scholar]
- Banton T, Bertenthal BI. Multiple developmental pathways for motion processing. Optometry and Vision Science. 1997;74(9):751–760. doi: 10.1097/00006324-199709000-00023. [DOI] [PubMed] [Google Scholar]
- Bharadwaj S, Candy T. Cues for the control of ocular accommodation and vergence during postnatal human development. Journal of Vision. 2008;8(16):1–16. doi: 10.1167/8.16.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj S, Sravani N, Little J-A, Narasaiah A, Wong V, Woodburn R, Candy T. Empirical variability in the calibration of slope-based eccentric photorefraction. Journal of the Optical Society of America A. 2013;30(5):923–931. doi: 10.1364/JOSAA.30.000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj SR, Candy TR. Accommodative and vergence responses to conflicting blur and disparity stimuli during development. Journal of Vision. 2009;9(11):1–18. doi: 10.1167/9.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch E, Gwiazda J, Held R. Stereoacity development for crossed and uncrossed disparities in human infants. Vision Research. 1982;22:507–513. doi: 10.1016/0042-6989(82)90108-0. [DOI] [PubMed] [Google Scholar]
- Birch E, Gwiazda J, Held R. The development of vergence does not account for the onset of stereopsis. Perception. 1983;12:331–336. doi: 10.1068/p120331. [DOI] [PubMed] [Google Scholar]
- Birch E, Shimojo S, Held R. Preferential-looking assessment of fusion and stereopsis in infants aged 1-6 months. Investigative Ophthalmology and Visual Science. 1985;26(3):366–370. [PubMed] [Google Scholar]
- Birch E, Stager D. Monocular acuity and stereopsis in infantile esotropia. Investigative Ophthalmol ogy and Visual Science. 1985;26(11):1624–1630. [PubMed] [Google Scholar]
- Blade PJ, Candy TR. Validation of the PowerRefractor for measuring human infant refraction. Optometry and Vision Science. 2006;83(6):346–353. doi: 10.1097/01.opx.0000221402.35099.fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobier W, Howland H, Thompson A, Giunta A, Peck L. Convergence accommodation measured in infants aged 3 to 12 months. Investigative Ophthalmologyand Visual Science. 1995;36(4):S459. [Google Scholar]
- Boothe RG, Dobson V, Teller DY. Postnatal development of vision in human and nonhuman primates. Annual Review of Neuroscience. 1985;8:495–545. doi: 10.1146/annurev.ne.08.030185.002431. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Freeman R, editors. Developmental Neurobiology of Vision. Plenum Press; New York: 1979. Accomodation and acuity in the human infant; pp. 289–300. [Google Scholar]
- Braddick O, Atkinson J, French J, Howland H. A photorefractive study of infant accommodation. Vision Research. 1979;19:1319–1330. doi: 10.1016/0042-6989(79)90204-9. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Julesz B, Kropfl W. Cortical binocularity in infants. Nature. 1980;288:363–365. doi: 10.1038/288363a0. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Wattam-Bell J. Normal and anomalous development of visual motion processing: motion coherence and ‘dorsal-stream vulnerability’. Neuropsychologia. 2003;41(13):1769–1784. doi: 10.1016/s0028-3932(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Wattam-Bell J, Anker S, Norris V. Video-refractive screening of accommodative performance in infants. Investigative Ophthalmology and Visusal Science. 1988;29((suppl):60. [Google Scholar]
- Brookman K. Ocular accommodation in human infants. American Journal of Optometry & Physiological Optics. 1983;60:91–99. doi: 10.1097/00006324-198302000-00001. [DOI] [PubMed] [Google Scholar]
- Buysse S, Casteels I, Dieltiens M, Eggermont E, Missotten L. Ocular findings in prematurely born children at the age of 12. Bulletin de la Societe Belge d’Ophtalmologie. 1994;254:71–78. [PubMed] [Google Scholar]
- Ciuffreda KJ, Kenyon RV. Accommodative Vergence and Accommodation in Normals, Amblyopes and Strabismics. In: Shor C, editor. Vergence Eye Movmements. Basic & Cinical Aspects. Butterworth; Woburn, MA: 1985. pp. 101–173. [Google Scholar]
- Currie DC, Manny RE. The development of accommodation. Vision Research. 1997;37(11):1525–1533. doi: 10.1016/s0042-6989(97)85022-5. [DOI] [PubMed] [Google Scholar]
- Ehrlich D, Braddick O, Atkinson J, Anker S, Weeks F, Hartley T, Rudenski A. Infant Emmetropization: Longitudinal changes in refraction components from nine to twenty months of age. Optometry and Vision Science. 1997;74:822–843. doi: 10.1097/00006324-199710000-00022. [DOI] [PubMed] [Google Scholar]
- Fox R, Aslin RN, Shea SL, Dumais ST. Stereopsis in human infants. Science. 1980;207(4428):323–324. doi: 10.1126/science.7350666. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Torii M, Ukai K, Wolffsohn JS, Gilmartin B. The relationship between CA/C ratio and individual differences in dynamic accommodative responses while viewing stereoscopic images. Journal of Vision. 2009;9(13):21. doi: 10.1167/9.13.21. 21-13. [DOI] [PubMed] [Google Scholar]
- Gallo J, Lennerstrand G. A population-based study of ocular abnormalities in premature children aged 5 to 10 years. American Journal of Ophthalmology. 1991;111(5):539–547. doi: 10.1016/s0002-9394(14)73695-5. [DOI] [PubMed] [Google Scholar]
- Gilmore RO, Hou C, Pettet MW, Norcia AM. Development of cortical responses to optic flow. Visual Neuroscience. 2007;24(6):845–856. doi: 10.1017/S0952523807070769. [DOI] [PubMed] [Google Scholar]
- Green DG, Powers MK, Banks MS. Depth of focus, eye size and visual acuity. Vision Research. 1980;20(10):827–835. doi: 10.1016/0042-6989(80)90063-2. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Thorn F, Bauer J, Held R. Emmetropisation and the progression of manifest refraction in children followed from infancy to puberty. Clinical Vision Sciences. 1993;8:337–344. [Google Scholar]
- Hainline L, Riddell P. Eye alignment and convergence in young infants. In: Vital-Durand F, Atkinson J, Braddick O, editors. Infant Vision. Oxford Science Publications; Oxford: 1996. pp. 221–248. [Google Scholar]
- Hainline L, Riddell P, Grose Fifer J, Abramov I. Development of accommodation and convergence in infancy. Behavioural Brain Research. 1992;49:33–50. doi: 10.1016/s0166-4328(05)80192-5. [DOI] [PubMed] [Google Scholar]
- Haynes H, White B, Held R. Visual accommodation in human infants. Science. 1965;148:528–530. doi: 10.1126/science.148.3669.528. [DOI] [PubMed] [Google Scholar]
- Holmstrom G, Rydberg A, Larsson E. Prevalence and development of strabismus in 10-year-old premature children: a population-based study. Journal of Pediatric Ophthalmol ogy and Strabismus. 2006;43(6):346–352. doi: 10.3928/01913913-20061101-04. [DOI] [PubMed] [Google Scholar]
- Horwood A. Maternal observations of ocular alignment in infants. Journal of Pediatric Ophthalmol ogy and Strabismus. 1993;30:100–105. doi: 10.3928/0191-3913-19930301-09. [DOI] [PubMed] [Google Scholar]
- Horwood A. Neonatal misalignments reflect vergence development but rarely become esotropia. British Journal of Ophthalmology. 2003;87(9):1146–1150. doi: 10.1136/bjo.87.9.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood A, Riddell P. Can misalignments in typical infants be used as a model for infantile esotropia? Investigative Ophthalmology and Visual Science. 2004;45(2):714–720. doi: 10.1167/iovs.03-0454. [DOI] [PubMed] [Google Scholar]
- Horwood A, Riddell P. The use of cues to convergence and accommodation in naïve, uninstructed participants. Vision Research. 2008;48(15):1613–1624. doi: 10.1016/j.visres.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood A, Riddell P. Receding and disparity cues aid relaxation of accommodation. Optometry & Vision Science. 2009;86(11):1276–1286. doi: 10.1097/OPX.0b013e3181bb41de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood A, Riddell P. Hypo-accommodation responses in hypermetropic infants and children. British Journal of Ophthalmology. 2011;95(2):231–237. doi: 10.1136/bjo.2009.177378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung GK, Ciuffreda KJ, Rosenfield M. Proximal contribution to a linear static model of accommodation and vergence. Ophthalmic and Physiological Optics. 1996;16(1):31–41. [PubMed] [Google Scholar]
- Ingram R, Gill L, Goldacre M. Emmetropisation and accommodation in hypermetropic children before they show signs of squint--a preliminary analysis. Bulletin de la Societe Belge d’Ophtalmologie. 1994;253:41–56. [PubMed] [Google Scholar]
- Judge S. How is binocularity maintained during convergence and divergence? Eye. 1996;10((Pt 2):172–176. doi: 10.1038/eye.1996.43. [DOI] [PubMed] [Google Scholar]
- Maxwell JS, Schor CM. Symmetrical horizontal vergence contributes to the asymmetrical pursuit of targets in depth. Vision Research. 2004;44(26):3015–3024. doi: 10.1016/j.visres.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Mayer D, Hansen R, Moore B, Kim S, Fulton A. Cycloplegic refractions in healthy children aged 1 through 48 months. Archives of Ophthalmology. 2001;119(11):1625–1628. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- McClelland JF, Parkes J, Hill N, Jackson AJ, Saunders KJ. Accommodative dysfunction in children with cerebral palsy: a population-based study. Investigative Ophthalmology and Visual Science. 2006;47(5):1824–1830. doi: 10.1167/iovs.05-0825. [DOI] [PubMed] [Google Scholar]
- Mohindra I. A non-cycoplegic refraction technique for infants & young children. Journal of the American Optometric Association. 1977;48:518–523. [PubMed] [Google Scholar]
- Náñez J, Yonas A. Effects of luminance and texture motion on infant defensive reactions to optical collision. Infant Behavior and Development. 1994;17(2):165–174. [Google Scholar]
- Nardini M, Bedford R, Mareschal D. Fusion of visual cues is not mandatory in children. Proceedings of the National Acadamy of Sciences of the United States of America. 2010;107(39):17041–17046. doi: 10.1073/pnas.1001699107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrig B, Julesz B, Kropfl W, Baumgartner G, Anliker M. Development of stereopsis and cortical binocularity in human infants: electrophysiological evidence. Science. 1981;213(4514):1402–1405. doi: 10.1126/science.7268443. [DOI] [PubMed] [Google Scholar]
- Riddell P, Grose-Fifer J, Hainline L, Abramov I. Photorefractive evaluation of infant accommodation and convergence; Paper presented at the Topical mtg of the Optical Society of America, Non-invasive assessment of the visual system; Santa Fe, NM. 1991. [Google Scholar]
- Riddell P, Horwood A, Houston S, Turner J. The response to prism deviations in human infants. Current Biology. 1999;9:1050–1052. doi: 10.1016/s0960-9822(99)80456-4. [DOI] [PubMed] [Google Scholar]
- Riddell PM, Horwood AM, Houston SM, Turner JE. The response to prism deviations in human infants. Current Biology. 1999;9(18):1050–1052. doi: 10.1016/s0960-9822(99)80456-4. [DOI] [PubMed] [Google Scholar]
- Schor CM, Alexander J, Cormack L, Stevenson S. Negative feedback control model of proximal convergence and accommodation. Ophthalmic and Physiological Optics. 1992;12(3):307–318. [PubMed] [Google Scholar]
- Scott W, Kutschke P. Concomitant Strabismus:Esotropias. In: Taylor D, Hoyt C, editors. Pediatric Ophthalmology & Strabismus. Elsevier; Edinburgh: 2005. pp. 883–891. [Google Scholar]
- Sevel D. The origins and insertions of the extraocular muscles: development, histologic features, and clinical significance. Transactions of the American Ophthalmological Society. 1986;84:488–526. [PMC free article] [PubMed] [Google Scholar]
- Sobrado P, Suarez J, Garcia-Sanchez F, Uson E. Refractive errors in children with cerebral palsy, psychomotor retardation, and other non-cerebral palsy neuromotor disabilities. Developmental Medicine and Child Neurology. 1999;41(6):396–403. doi: 10.1017/s0012162299000869. [DOI] [PubMed] [Google Scholar]
- Tarczy-Hornoch K. Accommodative lag and refractive error in infants and toddlers. Journal of the American Association for Pediatric Ophthalmology and Strabismus. 2012;16(2):112–117. doi: 10.1016/j.jaapos.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teller DY. First glances: the vision of infants. The Friedenwald lecture. Investigative Ophthalmology and Visual Science. 1997;38(11):2183–2203. [PubMed] [Google Scholar]
- Teller DY, Palmer J. Infant color vision: motion nulls for red/green vs luminance-modulated stimuli in infants and adults. Vision Research. 1996;36(7):955–974. doi: 10.1016/0042-6989(95)00170-0. [DOI] [PubMed] [Google Scholar]
- Thorn F, Gwiazda J, Cruz A, Bauer J, Held R. Eye alignment, sensory binocularity, and convergence in young infants. Investigative Ophthalmology and Visual Science. 1992;33((suppl):713. [PubMed] [Google Scholar]
- Thorn F, Gwiazda J, Cruz A, Bauer J, Held R. The development of eye alignment, convergence, and sensory binocularity in young infants. Investigative Ophthalmology and Visual Science. 1994;35(2):544–553. [PubMed] [Google Scholar]
- Tsuetaki TK, Schor CM. Clinical method for measuring adaptation of tonic accommodation and vergence accommodation. American Journal of Optometry and Physiological Optics. 1987;64(6):437–449. doi: 10.1097/00006324-198706000-00009. [DOI] [PubMed] [Google Scholar]
- Turner JE, Horwood AM, Houston SM, Riddell PM. Development of the response AC/A ratio over the first year of life. Vision Research. 2002;42(22):2521–2532. doi: 10.1016/s0042-6989(02)00268-7. [DOI] [PubMed] [Google Scholar]
- Tychsen L. Strabismus: The Scientific Basis. In: Taylor D, Hoyt C, editors. Pediatric Ophthalmology & Strabismus. Elsevier; Edinburgh: 2005. pp. 836–848. [Google Scholar]
- Volkmann FC, Dobson MV. Infant responses of ocular fixation to moving visual stimuli. Journal of Experimental Child Psychology. 1976;22(1):86–99. doi: 10.1016/0022-0965(76)90092-8. [DOI] [PubMed] [Google Scholar]
- Wismeijer DA, Erkelens CJ. The effect of changing size on vergence is mediated by changing disparity. Journal of Vision. 2009;9(13):12. doi: 10.1167/9.13.12. 11-10. [DOI] [PubMed] [Google Scholar]
- Wismeijer DA, Erkelens CJ, van Ee R, Wexler M. Depth cue combination in spontaneous eye movements. Journal of Vision. 2010;10(6):25. doi: 10.1167/10.6.25. [DOI] [PubMed] [Google Scholar]
- Wismeijer DA, van Ee R, Erkelens CJ. Depth cues, rather than perceived depth, govern vergence. Experimental Brain Research. 2008;184(1):61–70. doi: 10.1007/s00221-007-1081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhouse J, Pakeman V, Saunders K, Parker M, Fraser W, Lobo S, Sastry P. Visual acuity and accommodation in infants and young children with Down’s syndrome. Journal of Intellectual Disability Research. 1996;40(Pt 1):49–55. doi: 10.1111/j.1365-2788.1996.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Yonas A, Elieff CA, Arterberry ME. Emergence of sensitivity to pictorial depth cues: Charting development in individual infants. Infant Behavior and Development. 2002;25(4):495–514. [Google Scholar]
- Yonas A, Granrud C. The development of sensitivity to kinetic, binocular and pictorial depth information in human infants. In: Ingle D, Jaennerod M, Lee D, editors. Brain mechanisms and spatial vision. Martinus Nijhoff; Dordrecht: 1985. pp. 113–145. [Google Scholar]
- Yonas A, Pettersen L, Lockman J. Young infants’ sensitivity to optical information for collision. Canadian Journal of Psychology. 1979;33:268–276. doi: 10.1037/h0081725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.