Abstract
A remote haploscopic video refractor was used to assess vergence and accommodation responses in a group of 32 emmetropic, orthophoric, symptom free, young adults naïve to vision experiments in a minimally instructed setting. Picture targets were presented at four positions between 2m and 33cm. Blur, disparity and looming cues were presented in combination or separately to asses their contributions to the total near response in a within-subjects design.
Response gain for both vergence and accommodation reduced markedly whenever disparity was excluded, with much smaller effects when blur and proximity were excluded. Despite the clinical homogeneity of the participant group there were also some individual differences.
Keywords: Accommodation, vergence, near cues, naturalistic, photorefraction
Ocular convergence and accommodation occur in response to cues from the visual environment as a target approaches. The main cues are blur and binocular disparity, with a smaller part being played by proximal cues such as looming, motion parallax and overlay of contours. Under typical conditions, all cues to an approaching target are available and provide consistent depth information. In this study, we looked at the contributions of these cues to concurrent convergence and accommodation in a visually normal group of participants. Accommodation and vergence responses to a naturalistic target with full cues to depth were measured, and compared to responses when different cues to depth were removed. The purpose of the study was to determine the range of individual differences in cue use in visually mature individuals with no visuomotor deficits.
Much of the previous research in this area has studied either vergence or accommodation in response to single depth cues, including defocus (blur), disparity or proximal cues. This has provided data for systems models of accommodation, vergence and their interactions (Eadie & Carlin, 1995, Hung, 1992, Schor, 1992). Early studies suggested that blur was the primary drive to accommodation and provided a sufficient cue in isolation (Phillips & Stark, 1977). It was suggested that blur was also the main drive to vergence via the accommodative vergence cross-linkage(Alpern, 1962, Maddox, 1893). More recently, however, disparity cues have been shown to provide the primary drive to vergence (Semmlow & Wetzel, 1979), and there is also evidence to suggest that these provide the main drive to accommodation via the convergence accommodation/convergence (CA/C) crosslink (Crone, 1973, Fincham & Walton, 1957, Judge, 1996, Semmlow & Wetzel, 1979). While retinal disparity and blur have been accepted as driving the accommodation and vergence systems, the role of proximity is less clear. Some studies report variable and idiosyncratic use of proximal cues (Ogle & Martens, 1957), whereas, in other studies, proximal responses have been shown to be linearly related to target distance (Rosenfield, Ciuffreda & Hung, 1991).
In order to assess typical vergence and accommodation responses, it is necessary to assess the role of multiple cues to depth in driving both accommodation and vergence simultaneously. Some researchers have attempted such studies (McLin, Schor & Kruger, 1988a, Okada, Ukai, Wolffsohn, et al., 2006, Rosenfield, et al., 1991, Weiss, Seidemann & Schaeffel, 2004), but this is relatively rare in the literature. In contrast, most previous studies have tended to measure responses to individual cues in isolation (Arnott & O’Callaghan, 1971, Breinin, 1971, Filipovic, 1998, Havertape, Cruz & Miyazaki, 1999, Hung, 1991, Hung, 1997, Hung, Ciuffreda & Rosenfield, 1994, Jiang, 1994, Rosenfield, Ciuffreda & Chen, 1995, Schor, 1983, Schor, 1986, Schor, 1992, Wick, 1985, Wick, & Currie, 1991). While the results of these single cue studies can be related to some clinical conditions, they are likely to have less relevance to uncontrolled, naturalistic responses in typical individuals because they fail to reflect real life situations where it is very rare that only one of the near cues is present or varies in isolation. Multiple cue studies will have more clinical relevance since there are many conditions where, for instance, one cue to appropriate near focus is unavailable, impoverished or conflicting. For example, blur cues can be impoverished due to refractive error or media opacity, but disparity and proximity cues are still available; disparity detection can be disrupted by strabismus but blur and proximal cues are often still intact; and in heterophoria, disparity cues can be in conflict with blur cues. The effect of cue conflict was demonstrated by Okada et al (2006) who found that convergence driven accommodation responses dominated when cue conflict was high, but not in low conflict conditions.
A second problem with many experimental reports is that no attempt is made to control for participants’ higher level perception of the apparent nearness of the target. “Awareness of nearness”, and voluntary factors driven by perceived nearness are known to induce convergence and accommodation (Charman & Tucker, 1977, Mein & Trimble, 1991, Morgan, 1968, Schober, Dehler & Kassel, 1970, Thompson, 1952) and this can be trained as part of conventional orthoptic treatment (Ansons, Trimble, & Davis, 2001, Griffin & Grisham, 2002, Pratt-Johnson & Tillson, 1994). Despite this, experimental participants are frequently staff and students from optometry departments who are likely to be more aware of their accommodation and vergence response than the general population, and many studies require extensive participant training. It is therefore possible that “expert” participants could be invoking undefined higher level conscious control, even when efforts are made to reduce this (Ciuffreda, 1991, Ciuffreda, & Hokoda, 1985, Francis, Jiang, Owens, et al., 2003, Karania & Evans, 2006).
Thirdly, while both early, and some more recent, studies of convergence and accommodation emphasise the variability in the range of normal responses (Fincham & Walton, 1957, Harb, Thorn & Troilo, 2006, Judge, 1996, Ogle & Martens, 1957, Schaeffel, Wilhelm & Zrenner, 1993, Whitefoot & Charman, 1992), it is common in adult studies to tighten experimental control in order to produce more repeatable results. Developmental studies, in contrast, frequently report greater variability in responses (Currie & Manny, 1997, Hainline, Riddell, Grose Fifer, et al., 1992, Tondel, Wang & Candy, 2002, Tondel & Candy, 2007, Turner, Horwood, Houston, et al., 2002), implying that there is a progression from the reported wide variability in developing infants and children to more reliable adult responses. In addition, it is accepted by clinicians that there is a substantial degree of variability in characteristics, symptoms and responses to treatment in all age groups. The differences between developmental, clinical and experimental studies might not result from purely developmental and pathological variation, but could also reflect differences in methodology, particularly in instruction set and experimental control.
In order to bridge the gap between highly controlled, adult, lab-based studies, and developmental and clinical studies there is a clear need for a methodology that can be used to assess the relative contributions of the cues to simultaneous vergence and accommodation across a range of participant groups. We have combined and adapted previously published methods to produce a flexible and non-invasive paradigm to study the response to depth targets when all cues are available, when each is minimised, and when predominantly one single cue is provided in isolation. Here, we report the results from a group of minimally instructed, visually mature, participants. This data provides baseline measures of the relative influences of the main cues to convergence and accommodation and the range of individual differences within this population. From our previous studies (Horwood, Riddell, 2002, Horwood, Turner, Houston, et al., 2001, Turner et al., 2002), we predicted that most participants would show the greatest reduction in convergence and accommodation when the retinal disparity cue was removed, but that there would be a some degree of individual differences in the pattern of response to each cue even in this visually normal population.
Methods
The study was designed according to the tenets of the Declaration of Helsinki, in accordance with institutional ethics regulations and the participants gave fully informed consent.
Participants
We made strenuous efforts to recruit naïve, orthophoric and emmetropic participants. We tested 94 asymptomatic individuals using a battery of tests. Participants who might not have been naïve to manipulation of vergence and accommodation due to previous therapy were excluded. All testing was completed in a single session, with conventional clinical tests being performed between two repeated experimental sessions. All participants had equal visual acuity of at least 0.0 logMAR in each eye tested using a logMAR acuity chart and none were able to overcome more than +0.5D lenses at 6m. All participants had attended an optometrist within the last four years but had not been prescribed spectacles or any other treatment. Heterophoria was measured using alternate prism cover test at 6m and 33cm with subjective confirmation that the phi phenomenon was minimised with the correcting prism. No participant had an exophoria greater than 4Δ for near (mean 0.6Δ± 1.4Δ), any measurable heterophoria at distance, or any esophoria. Prism cover tests were repeated with +3.0D lenses at 33cm and −3.00D lenses at 6m with the participants clearing a 0.1 logMAR letter so that a clinical gradient stimulus AC/A ratio could be assessed. Particular care was taken to allow time for the participants to clear the target before alternate occlusion. AC/A ratios were all less than 3Δ:1D (mean 1.50±1.13Δ/1D). All had at least 60 seconds of arc stereoacuity using the TNO stereotest (mean 50.7±14.1 sec of arc) and all had a near point of accommodation of less than 7cm from the bridge of the nose both binocularly and monocularly (mean 6.15±0.44cm). Fusion was assessed with prisms. At 33cm all participants had a base out blur point of at least 20Δ (mean 37.2±11.5Δ) and break point of at least 35Δ (mean 43.6±10.7Δ), and a base in break point of at least 8Δ (mean 12.4±3.5Δ). At 6m they all had a distance base out prism fusion range of at least 20Δ (22.3±2.4Δ) to break and 18Δ (20.4±2.7Δ) to blur, and a base in range of at least 6Δ to break (mean 7.9±1.5Δ: blur was rarely noticed before break). All could converge binocularly to at least 6cm (mean 5.6±0.6cm). The relatively large standard deviations reflect considerably better responses than our minimum inclusion criteria.
Of the 94 individuals tested, 62 participants were excluded because they had mild refractive errors, asymptomatic heterophorias, mild accommodation or convergence insufficiency, or had received some form of vision therapy in the past. Of the remaining 32 participants who passed the screening, 23 participants were psychology undergraduates aged between 18 and 24 years of age with no history of ocular symptoms, spectacles, or participation in any previous visual experiment. 9 participants were typically developing children aged 8 yrs 8 months to 9 years 10 months who had had no ocular treatment. We wanted to explore two distinct age groups in the young, “visually mature” age range to ascertain whether developmental changes occur between late childhood and adulthood.
The participants were told that the purpose of the experiment was to measure how their eyes responded to pictures at different distances, but were given no further details until the end of the testing session. When asked, no participants were able to accurately describe what had been tested and most erroneously guessed that we had been studying pupil reactions.
Apparatus
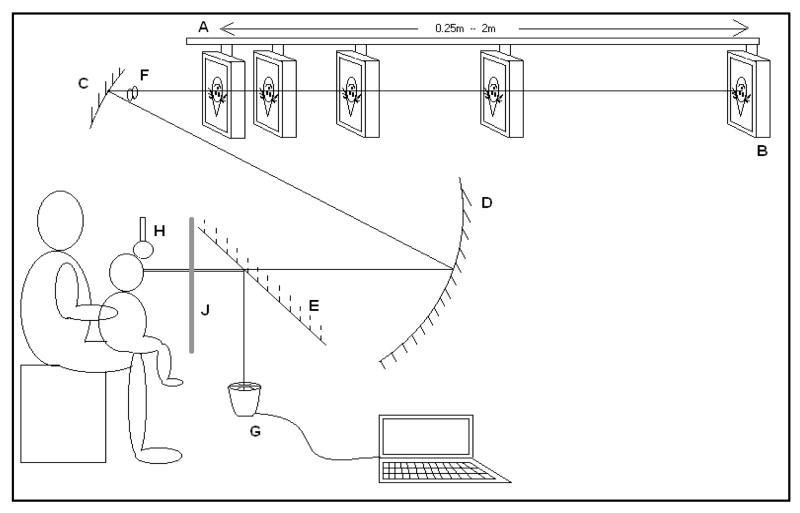
We used an adaptation of the Remote Haploscopic Photorefractor designed by Israel Abramov and Louise Hainline, Infant Study Centre, Brooklyn College of the City University of New York. Our modifications were suggested by experience from our previously published studies (Horwood, et al., 2001, Horwood & Riddell, 2004, Turner et al., 2002) and the availability of new commercially produced equipment (Erdurmus, Yagci, Karadag, et al., 2007, Hunt, Wolffsohn & Gilmartin, 2003, Schimitzek & Lagreze, 2005, Wolffsohn, Hunt & Gilmartin, 2002). The remote haploscopic photorefractor (Figure 1) consists of two optical pathways, one for off-axis infra-red continuous photorefraction and the other for target presentation so that binocular photorefraction can take place independent of target manipulation.
Figure 1.
The Remote Haploscopic Videorefractor. A. Motorised beam. B. Target monitor. C. Upper concave mirror. D. Lower concave mirror. E. Hot mirror. F. Image of participant’s eye where occlusion takes place. G. PlusoptiX SO4 PowerRef II. H. Headrest J. Raisable black cloth screen.
Target Pathway
The equipment is fully enclosed in black painted shuttering except for the aperture through which the target is visible. The room lighting was dimmed so that light levels are low. Dim lighting is necessary to allow the pupils to dilate sufficiently for accurate photorefraction at the closest target distance, but does not result in significant dark adaptation (see later for target details and luminance).
The target was presented on a monitor mounted on a motorised beam that moves between the different fixation distances. The monitor moves in a pseudo random sequence between five different fixation distances (0.33m, 2m, 0.25m, 1m and 0.5m), representing 3, 0.5 , 4, 1, and 2 dioptres (D), or metre angles (MA), demand, so that a near target is always followed and preceded by a far target. Thus, linear responses across target distance demonstrate that participants have detected and responded to both near and distance cues appropriately.
From the monitor, the optical pathway passes through two concave lens mirrors, placed such that the virtual image of the monitor is in front of the participant’s face and a virtual image of the participant’s eyes is in front of the upper mirror (F: Figure 1). The participant sees a single bright image of the target on the screen approaching in the primary position. The participants were not shown the true position of the computer monitor. When asked to touch the 0.33m targets, a separate group of adult participants reached for the image at the correct distance in mid-air (± 1.5 cm) and were frequently surprised when it could not be touched.
The main advantage of such a system is that remote occlusion of one eye is possible. If the image of one eye is occluded at the level of the upper lens mirror (F in Figure 1), it obscures the participant’s view of the target in the same way as if an occluder was placed directly before that eye, but both eyes are still able to be photorefracted simultaneously. After testing, we asked the participants to say if they had noticed anything unusual to determine whether they had been aware of the occlusion. Approximately 30% of the participants were unaware that they had been occluded for part of the experiment. The others had been aware they were only using one eye, but had not been able to work out where or how the occlusion had occurred.
Photorefraction Pathway
We used a commercially available infra-red photorefractor (PlusoptiX S04, Plusoptix GmbH, Nurenberg, Germany). This was designed for child vision screening and incorporates a PowerRefII (R-mode) that makes simultaneous recordings of accommodative state and gaze direction. The PlusoptiX S04 is placed at a testing distance of 1m ± 5cm and uses an infrared source and sensors, and is mounted so that it captures the image of the participant’s eyes via a large 600mm diameter “hot” mirror (Knight Optical UK Ltd: as recommended by the manufacturers of the PlusoptiX SO4: E in Figure 1). This reflects infra-red wavelengths (750-1150nm: reflectance 95%) but allows through visible light at 45° (425-765nm: transmittance 92%). As we were interested in binocular responses, the camera was mounted in the midline between the eyes, but no significant differences have been found between refraction in the midline and along the fixation axis in the range of target demands we used here (Seidemann & Schaeffel, 2003). The centre of the camera was optically aligned with the centre of the target on the monitor. The fixation LEDs on the sensor gun were covered with opaque tape. When no target is presented, the infra red sources could be seen subjectively as very faint red dots, but when any fixation target was on the monitor, these were obscured by the brighter target elements and were invisible to the participant.
Target
We were interested in studying the relative use of the three main cues to vergence and accommodation (blur, proximity and disparity). Two targets were designed: one to stimulate accommodation in a similar fashion to a real-life situation, and the other minimally. Luminance of both targets was 10cd/m2. Although both targets were presented on a black background, the background luminance of the screen was dimly visible against the screen edge. Screen edges were therefore masked with an increasing density filter mask to blur the edge contrast gradient and minimise the screen edges as a stimulus to accommodation.
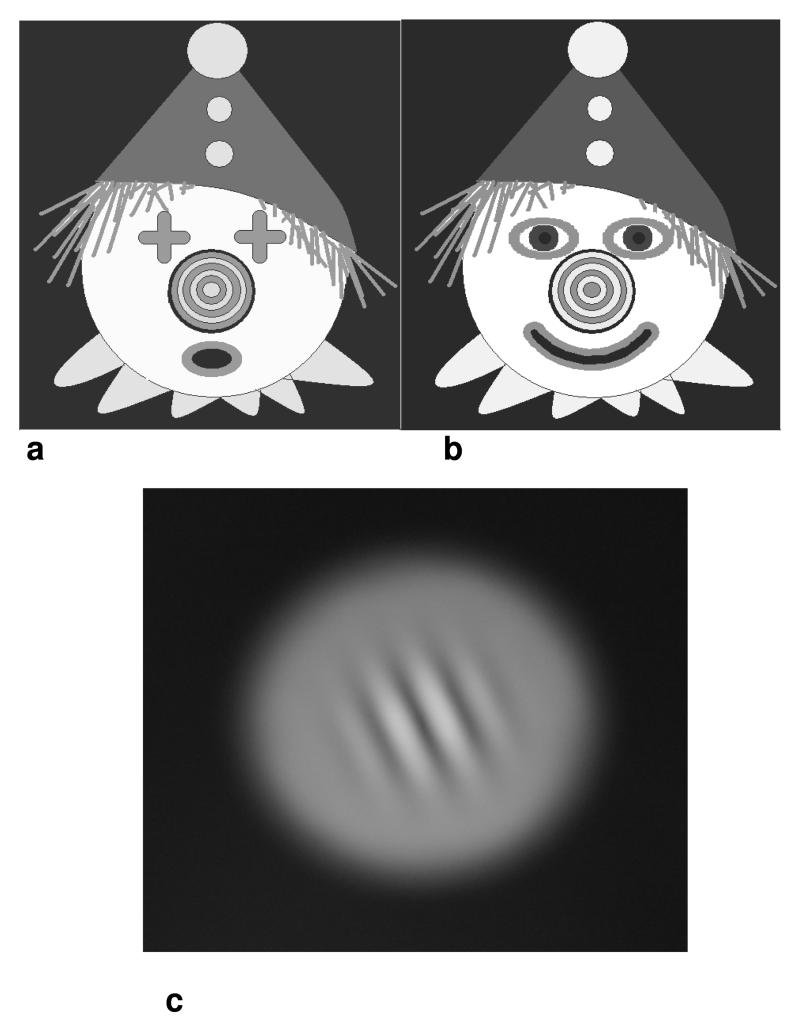
The first target was a brightly coloured picture of a clown containing a range of spatial frequencies, colours and high contrast edges. As the apparatus was designed for use with infants with developing visual acuity and attentional capability, the clown target was designed to contain both high and low spatial frequencies (Figure 2). The central white ‘face’ portion of the clown subtended 3.15° at 2m and 18.26° at 33cm. The rings of the nose were 3mm wide and so subtended approximately 5 min of arc at 2m and 30 min of arc at 33cm, and were edged in a black outline of 1 pixel width (subtending 1 minute of arc at 2m). Thus, high spatial frequencies of near acuity threshold were included in the concentric rings of the nose and lower spatial frequencies in the gross details for the eyes, mouth and hat. This target was chosen in preference to more traditional accommodative targets, for instance a Maltese cross, since it is suitable for use at different distances for participants with both low and high acuity, and will maintain attention in our infant groups. We performed a Fourier transform on frequency content of the clown target which demonstrated that the slope k of the best-fit line to the mean amplitude spectrum plotted on log–log coordinates was approximately -2 (Field, 1987). This is similar to the slope found for artistic paintings (Graham and Field, 2007). A similar slope was produced for the amplitude spectrum from a photograph of a real face against a patterned background. This frequency content contains a range of spatial frequencies that are known to drive accommodation (Ward, 1987). Two versions of the central components of the picture (nose, eyes, mouth) alternated at 1Hz (2a & 2b) to provide an attentional cue for future experiments which will use infant participants.
Figure 2.
a) & b) Clown target alternating at 1Hz containing bright colours, high contrast and range of spatial frequencies. Black outlines to picture elements do not reproduce well in this reduced illustration c) Difference of Gaussian patch. Colour alternated between green and yellow with equal luminance at 1Hz.
We tested the possibility that our clown target was a less demanding accommodative target than an adult near work task such as reading small print. In the pilot phase we compared adult accommodation responses to the clown with those obtained while 39 participants read aloud a 3° patch of 8 point text at 33cm. The participants were not instructed to keep the text clear, but had to use a habitual amount of accommodation to perform the task. A paired t-test showed no significant difference in accommodative response between the text and clown, although accommodative responses were slightly better to the clown target than to the text. (Mean accommodation to clown = 2.76D, mean accommodation to text = 2.57, t(38)=1.322 p=0.194). We therefore feel confident that our near target stimulated similar accommodative responses to those found during short, uninstructed, everyday but demanding, adult close work tasks.
Disparity cues were present when the target was visible to both eyes, and removed by occluding the target before the left eye at the level of the upper mirror.
A second target was used to minimise accommodative cues. We used an image of a difference of Gaussian (DoG) patch against a black background subtending the same visual angle as the clown (Figure 2c). DoG patches have been used by others to investigate open loop accommodation (Kotulak & Schor, 1987, Rosenfield, et al., 1991, Tondel & Candy, 2008, Tsuetaki & Schor, 1987)}. In our study, the DoG patch provided a low spatial frequency, defocused image, while retaining some attentional element which a more diffused image might lack. In order to stimulate the same attentional demand as the alternating clown target as closely as possible, we alternated the colour of the patch between yellow and green (chosen because of their position as near to the centre of the visible spectrum as possible to minimise the duochrome effect).
Proximity cues were made available by using the same size of target at all fixation distances, so that it changed angular subtense as it moved to a new target distance (looming cue). To minimise these proximal cues, the picture was scaled so that it subtended the equivalent angular subtense at all fixation distances as the unscaled clown at 2m (i.e. 3.15° with targets elements of 1 minute of arc). To minimise any residual looming cues from the monitor edges, an opaque black cloth screen (J in Figure 1) was raised to obscure the participant’s view of the monitor during screen movement in the proximity-excluded conditions.
Since we also intend to test infants under the same conditions, when attention may be more limited, we needed to develop a testing sequence that would maximise useful data in less co-operative groups. We therefore divided target presentations into blocks (all cues; one cue removed; and one cue only). We initially presented the all cue condition (“bdp” – blur, disparity and proximity) to obtain a baseline response. We then presented a block of targets with one cue removed in turn (bp(−d); bd(−p); dp(−b)). Order of testing within this block was counterbalanced across trials.
After a rest period, we presented a second block of presentations where the target presented one cue in isolation (b, d or p), by removing the two other cues, again counterbalanced across trial. This block also included a minimal cue (o) condition (scaled, occluded, DoG) to obtain a measure of a “minimal cue baseline” response that might be driven by residual cues which we could not control e.g. auditory cues from the monitor beam motor, residual proximal cues from the masked dim screen edge, residual blur cues from the DoG target as well as higher voluntary influences.
Finally the all cue (bdp) condition was repeated to check for practice or fatigue effects within the session. After a 10 minute rest period, this whole sequence was repeated again with the order of cue(s) removed counterbalanced within blocks.
Data Recording
The examiner started and stopped the PlusoptiX recording, which was continuous for each sequence of the five fixation distances. The participant’s fixation and recording traces were observed during recording. The fixation target was only moved to the next position once the target had stopped and fixation had steadied for a period of at least three seconds and had provided a section of reliable continuous recording. If excessive blinks, off-axis fixations, pupil or lid fluctuations, or light meter adjustments meant that continuous data was lost, the recording period was increased so that a stable section of at least 25 continuous readings (one second) was recorded.
Calibration and Calculations from Raw Data Spreadsheet
Accommodation
We transposed refraction so that we obtained a measure of accommodation in response to target demand (i.e. a –2.0D myopic refraction indicated 2D of accommodation).1 While individual calibration would have been possible with these participants, it will not be possible with the infants and young children we plan to test, and we are particularly keen to compare this adult data with these less co-operative groups, so we used refraction readings provided by the PlusoptiX but adjusted refractive estimates according to group norms derived from a separate calibration group.
Accommodation Calibration
59 separate adult participants with a range of low refractive errors between −0.75 and +1.0 or corrected mild myopes (up to −3.0DS) wearing current contact lenses (mean manifest refraction of group = −0.116D) were refracted using the PlusoptiX while fixing the 2m, 1m, 0.5m and 0.33m clown target. The same participants were then tested using dynamic retinoscopy (monocular estimate method (Eskridge, 1989)), carried out by an experienced retinoscopist (AH) while fixing the same clown target on a similar monitor in similar light levels at the same distances in the same dimly lit laboratory. The tester was unaware of PlusoptiX refractions.
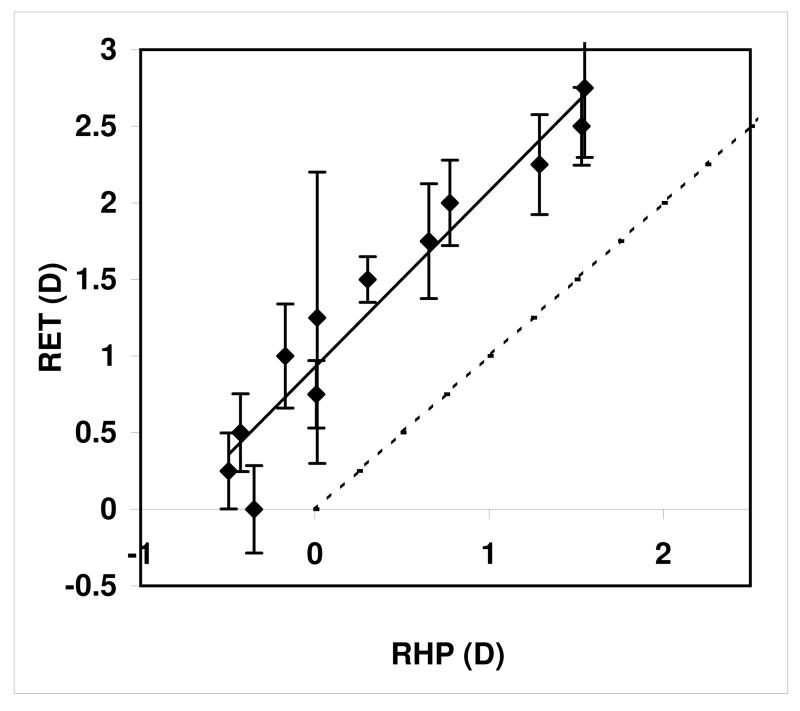
The PlusoptiX estimate of the refraction with the target at 1m was not significantly different from the manifest refraction of the participants (mean −0.127D, paired t-test; t(58)=-0.13, ns) i.e. it made a good estimate of manifest refractive error when tested at the instrument’s recommended testing distance of 1m. We consistently measured a smaller accommodative response to target demand with the RHP in comparison to dynamic retinoscopy (Figure 3), and this increased away from 0 D, as found by Harb et al (2006) using an earlier version of the PowerRefractor.
Figure 3.
RHP vs dynamic retinoscopy of 59 participants fixating targets at 33cm, 50cm,1m and 2m. Error bars indicate 95%CI.
Correlation between the two readings was good (r2=0.695). We used the slope function from Figure 3 to adjust our estimate of true refraction by correcting the RHP measure of accommodation by 1.2385x+0.799: a formula derived from the slope of the fitted line in Figure 3.
Vergence
Although studies have been published validating the refraction data, little has been published to validate gaze measurements using the PlusoptiX. We confirmed that the PlusoptiX calculation of gaze deviation was accurate for our lab by testing a group of 10 adult participants fixating targets at 5° horizontal intervals and confirmed that mean estimate of gaze position was not significantly different from the manufacturer’s value (mean PlusoptiX estimate of gaze change per 1°of target shift normally distributed about mean 1.01° (95%CI 0.97-1.04)).
Vergence Calculations
Angle lambda (the angle between the pupillary axis and the line of sight formed at the centre of the pupil (Millodot, 1997)) varies between individuals, changes throughout growth, and must be taken into account whenever assessing gaze position by corneal reflections. We obtained the best estimate of angle lambda when fixing at infinity by plotting the mean y-intercept of the nasal displacement from the pupil centre averaged across both eyes at all four fixation distances in the all cue (bdp) condition.
We transposed the raw PlusoptiX gaze position data so that version was converted to vergence. As we study participants with widely differing inter-pupillary distances (IPD) and use our data in comparison with accommodation responses, it is desirable to report vergence in terms of metre angles (MA) rather than degrees. We therefore calculated a constant for each participant to transform degrees into metre angles based on individual IPD (from the y-intercept of the PlusoptiX IPD measurements at all test distances using the bdp stimulus).
Vignetting
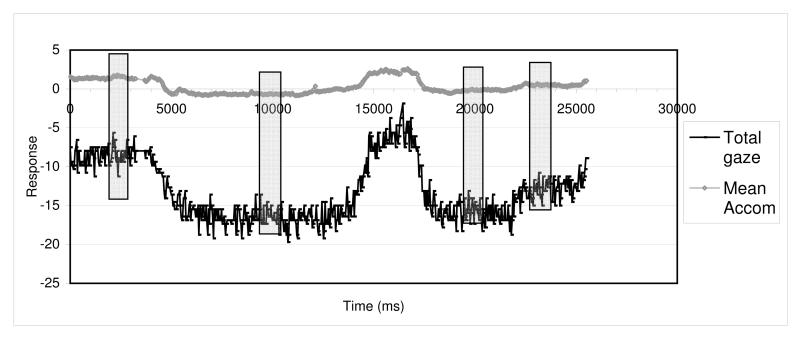
For each testing session, plots of the raw data against time (Figure 4) were produced so that we could identify representative vignettes of stable data to provide an average response. We aimed for vignettes of 25 continuous frames (1.0 sec of stable fixation) at each fixation distance.
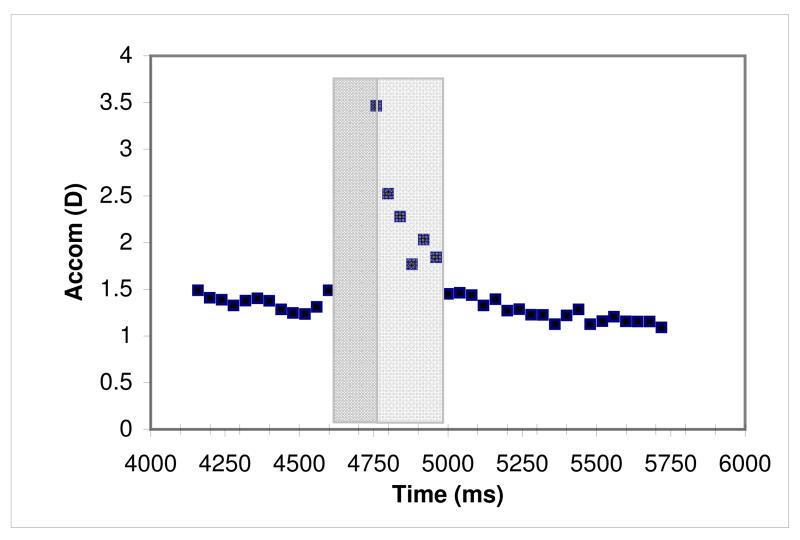
Figure 4.
Example of vignette identification. Plots of partially processed data (before degrees converted to metre angles and angle lambda corrected) used for vignette identification. Responses against time (x-axis) to identify target position and continuous data sections. y-axis scale in dioptres for accommodation and degrees for vergence. Vignettes of 25 continuous data points were selected (shaded) to represent a sample of stable response at each fixation distance
We chose these relatively short vignettes as we will be comparing the adult data with infant and child groups, where prolonged fixation is impossible to guarantee. We were careful to select vignettes that were representative of the response when the participant was attending to the target. Where the participant could watch the target moving (i.e. where proximal cues were present) or where the curtain had obscured the target, there was a transition phase as the eyes responded to the target. Vignettes were only chosen after the response had settled and flattened out for at least 0.5 sec (Tondel & Candy, 2007), but before any tonic changes would be expected to have occurred.
Blinks distort the data and have been removed before analysis by other authors (Day, Strang, Seidel, et al., 2006, Harb et al., 2006). Examination of our data showed that the recovery from the blink spike took up to 5 data points (0.2s) in excess of the portion where data was missing during the blink (Figure 5).
Figure 5.
Sample of actual accommodation data over a blink. Dark shaded area = missing points during blink which fulfil spike identification criterion. Paler shaded area = removed points during blink recovery. Points either side of excluded portion averaged and substituted.
We therefore chose to remove the five data points after the onset of the spike (i.e. if the blink lasted 0.25sec (6 missing data points) plus 5 extra points for recovery = 11 data points (0.44ms) and insert data points that were an average of the two points either side of the blink. Less than 2% of vignettes included a blink and none included more than one blink.
After vingetting, graphs were plotted for monocular and binocular vergence and accommodation (Figure 6). Two scorers independently identified vignettes from 98 separate recording sessions for both vergence and accommodation were correlated using Pearson’s r and Bland Altman analysis(Bland & Altman, 1986, Bland & Altman, 1999). Extremely close correlation was found between individual scorers’ judgement of a representative vignette at the different fixation distances even though vignettes were rarely chosen from exactly the same section of data. For both vergence and accommodation, this analysis showed a high agreement: vergence: r=0.99, mean inter-scorer difference & limits of agreement = 0.037±0.37MA; accommodation r= 0.99, mean inter-scorer difference 0.0095±0.175D.
Figure 6.
Example of a typical response plot after correction for accommodation calibration, angle lambda and IPD. Vergence plotted in metre angles (MA). Uniocular gaze positions summed to show binocular vergence. Uniocular accommodation averaged.
Data Analysis
For both vergence and accommodation analyses, stimulus response graphs for each individual were fitted using linear regression. In some participants, the accommodation data was non-linear between the two furthest testing distances as expected if a proportion of the blur is within depth of focus. Despite this, we chose to fit a linear function since vergence responses were very clearly linear and we wished to analyse both systems in the same manner. In order to compare responses across stimulus conditions, we therefore chose to examine the data in terms of response slope (gain), the y-intercept (reflecting an estimate of focus at infinity), and the strength of the linear relationship between the responses at different target demands (r2).
Data was processed using Microsoft Excel and then statistically examined using repeated measures and between groups analyses. ANOVAs with planned comparisons quote the Greenhouse-Geisser correction where appropriate.
Results
Repeatability
We examined the data for order effects, and repeatability across each target condition. There were no significant differences between first and second measurement sets under any target condition (t-test p>0.4 in all cases). The all cue (bdp) condition was tested four times (at the beginning and end of each of the two testing sessions) and there were no significant differences between any of these repetitions (F((3,114)=0.65, p=0.58). Accommodation was generally more variable than vergence. 95% limits of agreement were +/− 0.17 for vergence slope and +/−0.26 for accommodation slope. In view of this analysis, results from repeated recordings were averaged.
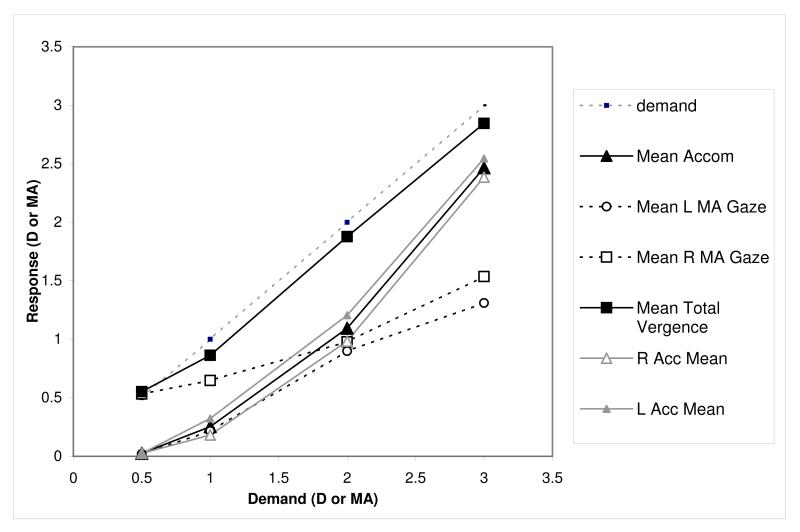
Slopes
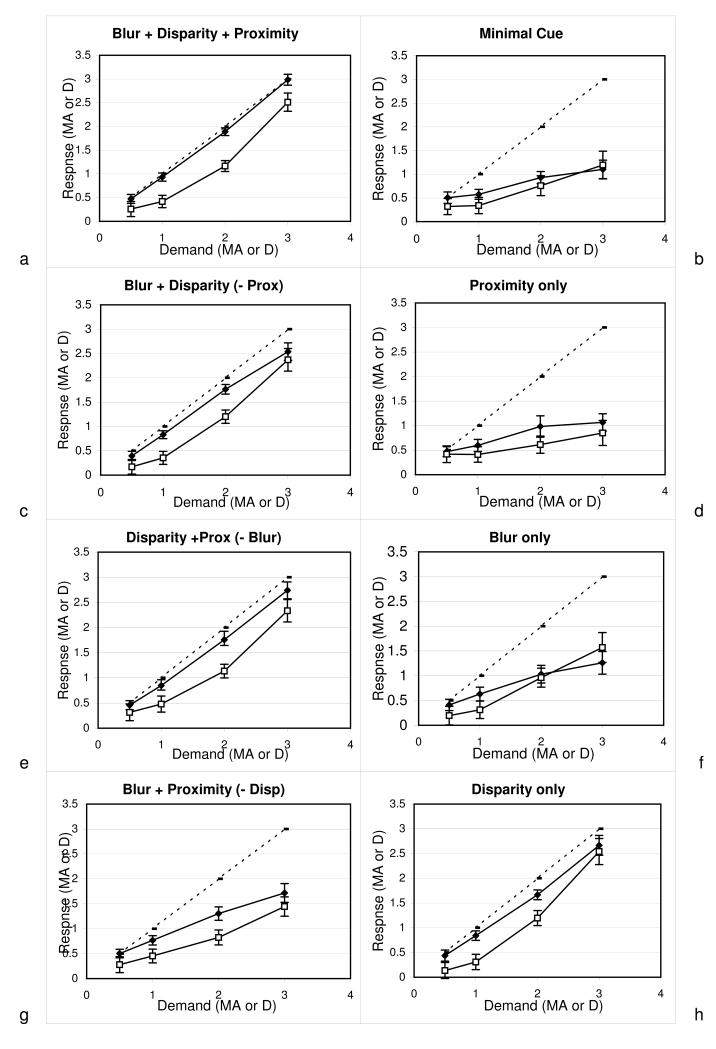
Figure 7 illustrates mean responses at each demand for the eight different cue conditions. Both vergence and accommodation were relatively accurate in all conditions where disparity was present (Fig. 7a, c, e & h), but showed a marked lag when disparity was removed (Fig.7b, d , f, g). When blur was minimised, there was only a small reduction in responses (7e), and responses were poor when blur was the only cue (7f). Manipulating proximity had a weak effect, with only a small reduction in responses when minimised (7c). The proximity-only responses (7d) were marginally worse than the no-cue condition (7b). In the majority of cases, vergence was more accurate than accommodation.
Figure 7.
Response to demand in each stimulus condition. Solid points = vergence, open points = accommodation, dotted line = ideal response to demand. Error bars represent 95%CI.
The slope reflects the proportion of a response that occurs in relation to demand (Table 1). Differences in slope across condition were found. Since the nine children might form a separate group on the basis of developmental stage, a three-way mixed design ANOVA considered cue condition, vergence vs. accommodation response, and child vs. adult responses. The response slopes from the nine children were generally slightly higher than those from the young adults. Mean difference in slope for the children was 0.09 steeper for vergence and 0.18 for accommodation (F(1,30)=7.94, p = 0.008), but no main effect of cue or type of response, and no interactions approached significance. We therefore collapsed data across age for all subsequent analyses.
Table 1.
Response slope (gain) for each target condition. Bold type signifies significant differences between vergence and accommodation on post hoc testing. Italics signify accommodation slope steeper than vergence. Abbreviations for target conditions e.g. bdp = blur, disparity & proximity all present, dp(−b) = disparity and proximity present, blur removed, b = blur only.
| bdp | dp(−b) | bp(−d) | bd(−p) | b | d | p | o | |
|---|---|---|---|---|---|---|---|---|
| Vergence Slope | 1.00 | 0.92 | 0.49 | 0.85 | 0.34 | 0.89 | 0.25 | 0.25 |
| r 2 | 0.97 | 0.95 | 0.89 | 0.93 | 0.75 | 0.92 | 0.67 | 0.68 |
| Accommodation | 0.90 | 0.81 | 0.46 | 0.88 | 0.57 | 0.98 | 0.18 | 0.37 |
| r 2 | 0.91 | 0.90 | 0.78 | 0.90 | 0.78 | 0.89 | 0.60 | 0.65 |
| p (difference between verg & accom slope) | 0.02 | 0.02 | ns | ns | 0.0001 | 0.05 | ns | 0.01 |
A two-way repeated measures ANOVA for slope comparing slope (accommodation or vergence) and cue (the eight target conditions) showed no significant main effects of slope (F(1,31)=0.419, p=0.5), but a highly significant effect of cue (F(1,31) = 109.92 , p<0.0000).
Slopes for both vergence and accommodation were markedly higher (more appropriate) whenever disparity cues were available. There was also a significant interaction between cue condition and accommodative vs. vergence response (F(1,31)=14.85,p<0.0000). Post hoc testing (bold text in Table 1) showed that vergence slope was significantly higher (more appropriate) than accommodation in the all cue (bdp) and blur minimised (dp) conditions and lower in the blur only condition. Vergence slope was also slightly lower than accommodative slope in the disparity only, and minimal cue condition.
A repeated measures ANOVA was used to determine the effects of the three different cues (blur, disparity and proximity) on vergence slopes. This showed highly significant main effects of disparity (F(1,31)= 399.6, p <0.000), blur (F(1,31)=31.2, p <0.000) and proximity (F(1,31)= 12.57 p=0.001), significant two-way interactions between disparity and blur (F(1,31)=22.3, p=0.000), and blur and proximity (F(1,31)= 11.6, p=0.002) but no significant three way interaction.
Planned comparisons (Table 2) looked at the effect of cue removal on the vergence slope. When a single cue was removed from the all cue condition (bdp), the largest reduction in the vergence slope occurred when disparity was the cue removed (Fig 7a compared with 7g). However, removing any other single cue also significantly reduced the vergence slope so that three cues were always better (produced a larger slope) than two cues (Fig 7a compared with 7c, e and g).
Table 2.
Planned comparisons on response slope comparing effects of removing a cue from the stimulus.
| VERGENCE | ACCOMMODATION | |||
|---|---|---|---|---|
| F | p | F | p | |
| Removing DISPARITY | ||||
| BDP vs BP | 246.98 | <0.0001 | 100.19 | <0.0001 |
| DP vs P | 280.310 | <0.0001 | 103.20 | <0.0001 |
| BD vs B | 214.36 | <0.0001 | 51.72 | <0.0001 |
| Removing BLUR | ||||
| BDP vs DP | 8.09 | 0.008 | 9.37 | 0.005 |
| BD vs D | 1.052 | ns | 3.89 | ns |
| BP vs P | 73.17 | 0.0001 | 37.62 | <0.0001 |
| Removing PROXIMITY | ||||
| BDP vs BD | 21.77 | 0.000 | 0.14 | ns |
| BP vs B | 13.78 | 0.001 | 4.18 | 0.049 |
| DP vs D | 0.55 | ns | 9.49 | 0.004 |
When the conditions in which two cues were available are compared to conditions in which a single cue is available, there was no effect on vergence slope when the single cue remaining was disparity (Fig 7c and e compared to 7h). In comparison, when the remaining cue was either proximity or blur, there was a reduction in the vergence slope from the two cue to single cue conditions (Fig 7c, e and g compared with 7 d and f). Thus, the most important cue to vergence across all participants was disparity since loss of the disparity cue resulted in the largest decrease in vergence slope.
A similar analysis of the effects of cue removal on the accommodation slope was carried out (Table 2). There were highly significant main effects of disparity (F(1,31)= 196.2, p <0.000), blur (F(1,31)= 32.3, p <0.000) and proximity (F(1,31)= 11.3, p=0.002), as well as significant two-way interactions between disparity and blur (F(1,31)= 29.8, p=0.00001) and blur and proximity (F(1,31)= 9.6, p =0.004).
When a single cue was removed from the all cue condition (bdp), the largest reduction in slope was seen when disparity was removed (Fig 7a compared with 7g). There was also a significant reduction in the accommodation slope when blur was minimised (Fig 7a compared with 7e), however, there was no change in slope when proximity was removed as a cue to accommodation (Fig 7a compared with 7c).
When the single cue conditions were compared to the conditions in which two cues were available, there was a large reduction in accommodative slope when disparity was removed (Fig 7c and e compared with 7f and d). There was a reduction in accommodative slope when blur was removed leaving proximity as the only cue (Fig 7g compared with 7d). However, there was no reduction in accommodative slope when blur was removed leaving disparity as the only cue (Fig 7c compared with 7h). When proximity was removed as a cue, the same pattern emerged; there was a reduction in accommodative slope when proximity was removed leaving blur as the only cue (Fig 7c compared with 7f) but not when disparity was the only remaining cue (Fig 7e compared with 7h). Thus, disparity is the most important cue to accommodation across all participants.
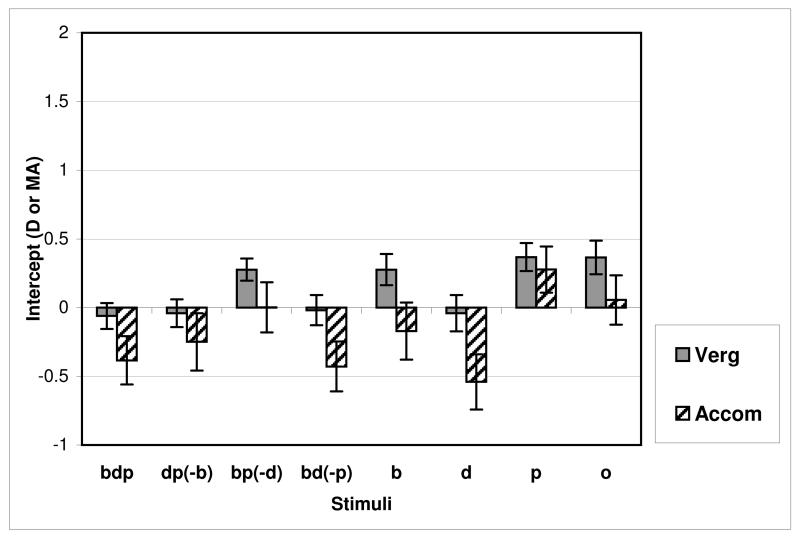
Y-intercepts
The y-intercept represents an estimate of focus and alignment at infinity (zero target demand) and can also estimate manifest refractive error. A two-way ANOVA with cue type (eight stimulus conditions) and response (accommodation or vergence) as factors, showed a significant main effect of cue (F(7,140.4)= 39.35,p<0.000), response (F(1,31)=15.6,p<0.000), and also a significant interaction (F(7,137.06)=6.74, p<0.000). Vergence intercept was close to zero in all conditions where disparity was present, and rose to around 0.25MA when disparity was absent (Figure 8). When disparity was absent, the responses flattened, and the intercepts of both vergence and accommodation increased i.e. this suggested some over convergence and over-accommodation for distance targets. When disparity was present, vergence intercept remained accurate, and accommodation reduced to marginally hyperopic values (as might be expected from this non-spectacle wearing typical population), but when disparity was absent, y-intercepts settled at myopic and slightly converged values (Figure 8).
Figure 8.
Vergence and accommodation intercepts. “bdp” = all cues present, “dp(−b)” = disparity and proximity present, blur removed, “b” = blur only etc.
A repeated measures ANOVA looking at the effect of cue (blur, disparity and proximity) on vergence y-intercept showed a highly significant main effect of disparity (F(1,31)= 88.86, p <0.000), with no significant blur effect (F(1,31)=2.304, ns) and no effect for proximity (F(1,31)= 0.14, ns). There was a significant two-way interaction between disparity and blur (F(1,31)=6.27, p=0.018) but no three way interaction. Removing disparity caused the intercept to increase (Figure 8, bp(−d), b, p and o conditions). Removing blur caused vergence intercept to rise only if disparity was absent.
Similar analysis of accommodation y-intercept showed somewhat different results. Main effects of cue were highly significant for all factors (disparity (F(1,31)= 101.88, p <0.000), blur (F(1,31)= 18.28, p <0.000) and proximity (F(1,31)= 14.45, p=0.001), as well as significant two-way interactions between disparity and blur (F(1,31)=14.45, p=0.001) and blur and proximity (F(1,31)= 4.85, p =0.035). Removing disparity always caused the intercept to rise, but removing blur caused a larger increase in y-intercept if disparity was absent and an even greater increase if proximity was also absent.
Stability and variability of response slopes (r2)
r2 values were calculated for each individual’s response slopes, to provide an estimate of overall linearity and accuracy of response across the different target demands. r2 values were generally high, which, in our pseudo-random order of presentation confirms the overall linearity of the response to demand. Values were always above 0.6 but Table 1 illustrates that responses became progressively less accurate as one, two or three cues were removed, in whichever order this occurred, (significant linear trends for r2 to reduce each time a cue is removed (F(1,31)>37.00, p<0.000 in all cases), but the greatest reduction occurred when disparity was removed, wherever it occurred.
Individual Differences
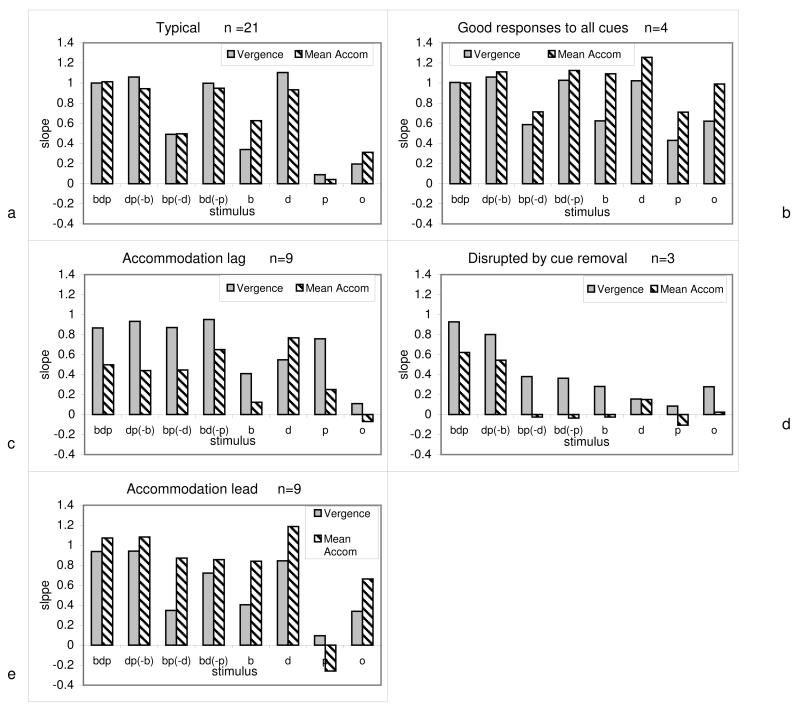
While we noted that there was a pattern of response that was typical amongst our participants, there were also some individual differences in response patterns (Figure 9).
Figure 9.
Examples of different individual response types. Grey bars represent vergence slope, striped bars represent accommodation slope. Letters on y-axis represent cue available (or excluded) e.g. dp(−b) = disparity & proximity present, blur removed. a) typical response in which the vergence and accommodation responses are both reduced when the disparity cue is absent, b) good responses for most stimuli, c) accommodation lag d) disrupted by any cue removal, e) accommodation lead as well as differential response to specific cue removal . Categories are not mutually exclusive.
The most common response was for vergence slope to be accurate whenever disparity was present (21/32 participants; 65%), with only small changes in vergence or accommodation responses when either of the other two stimuli was removed or added (Fig 9a). Accommodation slope was typically similar or slightly lower than vergence, reflecting a lag of accommodation of approximately 0.5D at 3D demand for all cues except blur alone, when accommodation generally exceeded vergence. Responses generally reduced in parallel when a cue was removed, suggesting either a strong cross-linkage or common drive to both systems.
It was very noticeable, however, that there were some less typical responses.. These idiosyncratic patterns were repeatable for an individual over the two testing sessions. When differences in response to cues was considered we found that some participants were little disrupted by the removal of any cue (4/32: 13%: Fig. 9b), while other participants showed a large disruption whenever any cue was removed (3/32: 9%: Fig.9d). When differences in accommodative responses were examined, we found that some participants showed a lead of accommodation (9/32: 28%: Fig 9e), while others showed a considerable lag, with accommodation slopes more than 0.2 less than vergence (9/32: 28%: Fig 9c).
Finally, when we compared vergence and accommodation we found that some individuals (n = 15 e.g. Fig 9d) showed broadly similar disruption across vergence and accommodation, whatever the cue removed, while others showed differential degradation depending on the type of stimulus disruption (n = 17: e.g.Fig.9c& e). This highlights the individual nature of the use of cues to vergence and accommodation in such a homogeneous, visually normal population.
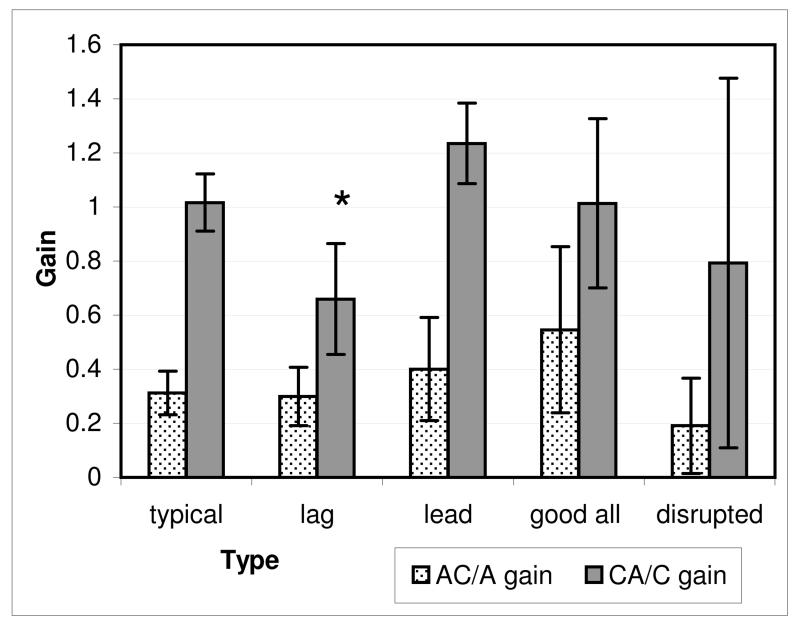
We considered whether conventional indicators of association between vergence and accommodation such as AC/A or CA/C ratio might characterise these patterns. The vergence slope in the blur-only condition represents a response AC/A relationship and the accommodation slope in the disparity-only condition represents CA/C relationship (Figure 10).
Figure 10.
AC/A and CA/C relationship between groups in Figure 9 (±SE). Accommodative vergence represented by gain of vergence response in the “blur only” condition. Vergence accommodation represented by gain of accommodation response in “disparity only” condition. Accommodative vergence gain not significantly different between groups. Vergence accommodation significantly lower in the “lag” group (asterisked). .
When the accommodation lag and lead groups were compared with the typical pattern it can be seen that CA/C in the accommodative lag group is significantly less than that of the typical group (t=3.33, p=0.002) and the lead group (t=4.01, p=0.001). This suggests that not only were accommodation responses in these individuals poorer overall (a criterion for inclusion in the lag group), but accommodation also provided less drive to vergence. There was no correlation between the clinical stimulus AC/A ratios measured and our laboratory measure.
Discussion
There is a substantial literature on vergence, accommodation and their interactions (see e.g. Schor & Cuiffreda (1985) for a now classic review volume), but relatively little research investigates the interfaces between experimental, clinical and developmental studies, mainly as a result of methodological difficulties. The results presented here provide a further step in this process by providing an overview of the contributions of the three main cues to accommodation and vergence in a naïve population carefully screened to exclude any visuomotor deficits.
Response slopes
The younger participants produced overall steeper response slopes for both vergence and accommodation, but this difference was consistent across cue condition and did not differ significantly between vergence and accommodation. Accommodation slopes appeared slightly steeper in the children than those of vergence, but in this relatively small sample, the interaction did not reach significance. It was striking that the overall pattern of responses to the different cue conditions was indistinguishable from that of the adults.
Our within-subjects design strengthens our main findings. The mean responses in each target condition confirmed the primacy of disparity as the main drive to both vergence and accommodation. Removing disparity as a cue caused a large reduction in the slopes of both responses. Both vergence and accommodation were accurate in all conditions where disparity was present. Indeed, disparity was able to drive accommodation as much as vergence when it was the only cue available. This result supports the views of Fincham & Walton (1957), Stark (1983), and Judge (1996), who hypothesised a strong role for disparity cues in driving accommodation and vergence, and refutes the older views of Maddox (1893) who hypothesised that blur would drive both systems. While Maddox’s model is not well supported by our, and others’, mean data, it is often cited by clinical texts (Ansons et al., 2001, Griffin & Grisham, 2002, Pratt-Johnson & Tillson, 1994, von Noorden, 1985) and, for example, seems to be influential in the case of accommodative strabismus. One possible explanation for this would be that cue use in clinical populations, such as strabismics and amblyopes, is different to that in typical adults, as suggested by Kenyon et al (1980, 1981). If these clinical populations do use the cues to vergence and accommodation differently, it would be of interest to investigate whether differences in cue use and response are causal or secondary to the onset of abnormalities.
Blur had a much smaller, but still significant, effect than disparity on response slopes. As an isolated cue it significantly increased slope from baseline (Fig 7f compared with Fig 7b). When added to proximity, blur also resulted in significant increase in slope (Fig 7d compared to Fig 7g). When both disparity and proximity remained present, minimising the blur cues had a small detrimental effect on accommodation but, interestingly, resulted in an even larger mean decrease in vergence (Fig.7a compared to 7e).
We considered whether this small effect of blur in comparison to disparity could be due to a weakness in our target; either resulting from an inability of the DoG target to eliminate accommodative cues sufficiently, or from insufficient detail in the clown target, but we feel that this is not the reason for our findings. The poor slopes to the DoG target in the two disparity-free conditions (“o” and “p”) reflect mean responses, and although some individuals are more affected by cue removal than others, in 34% of cases both vergence and accommodation slope was less than 0.15, suggesting that the DoG target is truly a weak cue. We are also confident that the clown target provides a good accommodation stimulus, as shown by the comparable responses to this target and when reading small text in the pilot studies. Although the DoG target was of low contrast and spatial frequency to minimise accommodative stimulus, its spatial frequency and grating contrast gradient does change at the different fixation distances in the unscaled condition (lower spatial frequency for near) and may contribute to the residual accommodation response in the same way that Okada et al (2006) found that when vergence cues were in conflict with accommodation cues, more accommodation occurred when the target was blurred. However, the poor responses to the blur only condition (scaled, occluded clown target) where high frequencies and contrast were available, in comparison to the minimal cue condition, suggest that these changes in spatial frequency are a weak cue especially in conditions when cues are not in conflict.
Proximity alone appears to have no influence on accommodation and vergence slope over and above the baseline level of the minimal cue condition, despite the looming cues of the target being intuitively a powerful stimulus on casual observation. Indeed, mean accommodation responses in the minimal cue condition (Fig.7b) were significantly better than in the proximity-only (Fig.7d) condition. However, proximity does have a small main effect, reducing accommodation and vergence slopes when removed from the all-cue condition (Fig 7a compared with 7c). Our data agree with Weiss et al (2004) who also found that looming is a weak cue to vergence and accommodation, while earlier studies had suggested that looming has a greater ability to drive these responses (Kruger & Pola, 1985, McLin, Schor & Kruger, 1988b, North, Henson & Smith, 1993). It is possible that evidence for proximity as a strong drive to vergence and accommodation is only found in open loop conditions (Hung, Ciuffreda & Rosenfield, 1996). Since the vergence and accommodation loops were closed in many of our conditions, we would not expect to see a large effect of proximity. It is also possible that in this visually normal group, where disparity is so dominant, proximity is of little importance.
While the screen edges were masked, the remaining dim outline of the screen edges might still have provided minimal looming cues even in the scaled conditions and is a possible source of the residual responses found in this condition. However, in the scaled condition, not only is the image obscured by the cloth screen during target movement, but the screen edge will increase in angular subtense while the scaled target does not. This might be expected to provide conflicting cues to accommodation and vergence and therefore to result in lower slopes in the minimal cue condition when compared to proximity only, where looming is retained; but this was not found to be the case in this study. We were unable to quantify what drives the minimal “o” responses. It is likely to be a combination of residual blur cues given by the DoG grating, the screen edges that we were unable to fully mask and “top down” voluntary influences driven by familiarity with the task learned from the initial “bdp” stimulus condition. Although the low gains in the minimal cue condition show we have not fully eliminated all near cues, this does not detract from a main finding in this study, which is that providing blur cues (clown target), and proximity cues (looming) cause so little improvement in vergence and accommodative responses in comparison to the relatively flat “o” (minimal cue) condition.
In summary, in mature, non-clinical, populations, the relative contributions of blur and proximity are weak in comparison to disparity when driving vergence and accommodation to naturalistic targets. The relative weights of each cue might be investigated further using cue conflict paradigms (Okada et al 2006).
y- intercepts
As long as disparity was present, y-intercepts, representing focus at infinity, were very close to zero MA for vergence and slightly hyperopic for accommodation. In order to compare vergence and accommodation we chose to use a linear fit. It is possible that by fitting a non-linear function to the accommodation data, the absolute values of these y-intercepts could be increased slightly. However the overall pattern of responses between cue conditions would be unchanged. As with response slopes, disparity had a greater effect than blur on moving the intercepts in a negative direction i.e. at infinity vergence estimate was closer to zero (perfect alignment) and refraction slightly hyperopic, as might be expected in such a typical group. Accommodation y-intercept was more sensitive to changes in blur or proximity than was vergence. When disparity was absent, y-intercepts rose to slightly positive levels, representing focus closer than infinity and closer to the levels of dark or open-field focus (Baker, Brown & Garner, 1983, Leibowitz & Owens, 1975, Owens, 1979), as might be expected in a reduced stimulus situation. These positive levels could also include elements of instrumental proximal responses (Rosenfield & Ciuffreda, 1991) that are overridden by stronger disparity cues when present. These orthophoric participants showed a vergence y-intercept of approximately 0.3MA (2Δ esodeviation) in the minimal cue and proximity-only conditions.
Individual Variability
We made strenuous efforts to ensure that our participants would be considered visually normal even by stringent clinical measures. While it was possible to identify a “typical” response pattern (Fig 9a) there were also a small number of participants who showed different individual responses. Specifically, four participants showed little degradation of response whatever cue was removed and so contributed to the residual slopes in the “o” and “p” conditions. These participants were not as dependent on disparity as our typical participants since they were able to use blur and proximity, and possibly voluntary, cues to drive accommodation and vergence even when disparity was not present. Three other participants’ responses were very disrupted whichever cue was removed. These participants behave similarly to infants who have also been shown to decrease their accommodative and vergence responses when individual cues are removed (Currie & Manny, 1997, Turner et al., 2002). There were no appreciable age differences between these small groups of participants.
Approximately half the participants’ accommodation and vergence responses changed concurrently in relation to different stimulus conditions, while the others showed differential responses, with vergence being more affected by one cue and accommodation by another. It was not possible to identify any clinical correlates to differentiate these two groups or to identify a particular cue with a pattern of change in vergence but not accommodation, or vice versa.
Previous studies from our laboratory (Horwood et al., 2001, Horwood & Riddell, 2004, Turner et al., 2002), as well as the individual variability that classic strabismus and paediatric ophthalmology texts emphasise, suggest that there may be many “styles” of interpretation of near cues, and also that flexibility in the use of near cues enables comfortable near vision under a range of commonly occurring circumstances (such as monocular viewing or uncorrected refractive error). Published models suggest how this might occur (Schor 1992, Hung et al 1996) but little attention is paid to whether this is task- or “individual style”- related and it is obscured in studies which consider mean data only. Our data suggest that, when different cue conditions are tested within participant, the majority of visually normal, individuals show the same pattern of response.
We were unable to find many clinical correlates with the laboratory data apart from the lower CA/C ratio in the accommodative lag group, which may provide some support for the model suggested by Schor(1999) but which we feel is not incompatible with our contention that some individuals are less responsive to blur. This has been suggested in the case of myopes (Radhakrishnan, Allen & Charman, 2007), but has not been suggested to contribute to typical individual variability in normal responses. This lack of clinical correlation is not necessarily surprising in this symptom free, normal group with no atypical participants, but it is possible that by studying clinical groups such as strabismus and refractive error we may find more marked and significant differences that characterise clinical diagnosis. Blur is known to be more important for individuals with poor disparity detection due to suppression (Kenyon, Ciuffreda & Stark, 1980); proximity may play a larger, or disproportionate, role in some clinical conditions such as intermittent exotropia as suggested by Kushner (1988, 1999). There also may be a typical developmental progression in infants.
This study provides a baseline with which atypical groups and developmental progression can be compared. The strength of this naturalistic study is that comparison of the relative contributions of the different near cues across cues can be made because the target presentation method, instruction set (minimal) and measurement method can be held constant. Conventional clinical tests and experimental methods allow the effects of individual cues to be investigated, but do not usually look at them all under the same conditions. Here we have identified the full range of typical visually mature naïve responses and limits of normality before researching typical infant development and clinical groups. We hypothesise that being able to use all or any of the near cues reduces risk of clinical problems, while over- or under-reliance on one cue may lead to greater clinical difficulties. This type of research has the potential for use in predicting risk of later abnormalities or refining screening programmes.
Acknowledgement
We would like to thank Professor Philip Smith for statistical advice. This research was supported by a Department of Health Research Capacity Development Fellowship award PDA 01/05/031 to AMH.
Footnotes
We were unable to fix pupil size in this paradigm and so we accept that we cannot control for apparent accommodation leads and lags due to spherical aberration which vary with pupil size Buehren, T., & Collins, M.J. (2006). Accommodation stimulus-response function and retinal image quality. Vision Res, 46, 1633-1645.. However, over the range of target distances tested here, the mean leads and lags that might be expected to result from spherical aberration are less than +0.5D which is close to the measurement tolerance of the paradigm.
References
- Alpern M. Accommodation:evaluation of the theories of presbyopia. In: Davson H, editor. The Eye. Vol. 3. Academic Press; London: 1962. pp. 191–229. [Google Scholar]
- Ansons A, Trimble R, Davis H, Mein J. Diagnosis and management of ocular motility disorders. Blackwell Science; Oxford: 2001. [Google Scholar]
- Arnott E, O’Callaghan K. Further investigations of the AC/A ratio. Brit Orthoptic J. 1971;28:11–22. [Google Scholar]
- Baker R, Brown B, Garner L. Time course and variability of dark focus. Invest Ophthalmol Vis Sci. 1983;24:1528–1531. [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;8476:307–310. [PubMed] [Google Scholar]
- Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- Breinin G. Accommodative strabismus and the AC/A ratio. Am J Ophthalmol. 1971;71:303. doi: 10.1016/0002-9394(71)90403-x. [DOI] [PubMed] [Google Scholar]
- Buehren T, Collins MJ. Accommodation stimulus-response function and retinal image quality. Vision Res. 2006;4:1633–1645. doi: 10.1016/j.visres.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Charman W, Tucker J. Dependence of accommodation response on the spatial frequency spectrum of the observed object. Vision Research. 1977;17:129–139. doi: 10.1016/0042-6989(77)90211-5. [DOI] [PubMed] [Google Scholar]
- Ciuffreda K. The Glenn A. Fry Invited Lecture. Accommodation to gratings and more naturalistic stimuli. Optometry & Vis.Sci. 1991;68:243–260. doi: 10.1097/00006324-199104000-00001. [DOI] [PubMed] [Google Scholar]
- Ciuffreda KJ, Hokoda SC. Effect of instruction and higher level control on the accommodative response spatial frequency profile. Ophthalmic Physiol Opt. 1985;5:221–223. [PubMed] [Google Scholar]
- Crone R. Diplopia. American Elsevier; New York: 1973. The control of eye movements; p. 89. [Google Scholar]
- Currie D, Manny R. The development of accommodation. Vision Res. 1997;37:1525–1533. doi: 10.1016/s0042-6989(97)85022-5. [DOI] [PubMed] [Google Scholar]
- Day M, Strang NC, Seidel D, Gray LS, Mallen EA. Refractive group differences in accommodation microfluctuations with changing accommodation stimulus. Ophthalmic Physiol Opt. 2006;26:88–96. doi: 10.1111/j.1475-1313.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- Eadie AS, Carlin PJ. Evolution of control system models of ocular accommodation, vergence and their interaction. Med Biol Eng Comput. 1995;33:517–524. doi: 10.1007/BF02522508. [DOI] [PubMed] [Google Scholar]
- Erdurmus M, Yagci R, Karadag R, Durmus M. A comparison of photorefraction and retinoscopy in children. J AAPOS. 2007;11:606–611. doi: 10.1016/j.jaapos.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Eskridge JB. Clinical objective assessment of the accommodative response. J Am Optom Assoc. 1989;60:272–275. [PubMed] [Google Scholar]
- Field D. Relations between the statistics of natural images and the response properties of cortical cells. J Opt Soc Am. 1987;4:2379–94. doi: 10.1364/josaa.4.002379. [DOI] [PubMed] [Google Scholar]
- Filipovic T. The accommodative convergence/accommodation (AC/A) and near convergence/distance(NC/D) ratios in esotropia. J Pediatric Ophthalmol & Strab. 1998;35:91–95. doi: 10.3928/0191-3913-19980301-07. [DOI] [PubMed] [Google Scholar]
- Fincham E, Walton J. The reciprocal actions of accommodation and vergence. J Physiol. 1957;137:488–508. doi: 10.1113/jphysiol.1957.sp005829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis E, Jiang B, Owens D, Tyrrell R. Accommodation and vergence require effort-to-see. Optometry & Vision Science. 2003;80:467–473. doi: 10.1097/00006324-200306000-00014. [DOI] [PubMed] [Google Scholar]
- Graham D, Field D. Statistical regularities of art images and natural scenes: spectra, sparseness and nonlinearities. Spatial Vision. 2007;21:149–164. doi: 10.1163/156856807782753877. [DOI] [PubMed] [Google Scholar]
- Griffin J, Grisham J. Binocular Anomalies: Diagnosis and Vision Therapy. Butterworth-Heinemann; Boston: 2002. [Google Scholar]
- Hainline L, Riddell P, Grose Fifer J, Abramov I. Development of accommodation and convergence in infancy. Behavioural Brain Research. 1992;49:33–50. doi: 10.1016/s0166-4328(05)80192-5. [DOI] [PubMed] [Google Scholar]
- Harb E, Thorn F, Troilo D. Characteristics of accommodative behavior during sustained reading in emmetropes and myopes. Vision Res. 2006;46:2581–2592. doi: 10.1016/j.visres.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havertape S, Cruz O, Miyazaki E. Comparison of methods for determining the AC/A ratio in accommodative esotropia. J Pediatric Ophthalmol & Strab. 1999;36:178–183. doi: 10.3928/0191-3913-19990701-05. [DOI] [PubMed] [Google Scholar]
- Horwood A, Riddell P. The role of near cues in neonatal misalignments. Invest.Ophth. Vis.Sci. 2002 e-abstract 4707. [Google Scholar]
- Horwood A, Turner J, Houston S, Riddell P. Variations in accommodation and convergence responses in a minimally controlled photorefractive setting. Optometry & Vis Sci. 2001;78:791–804. doi: 10.1097/00006324-200111000-00009. [DOI] [PubMed] [Google Scholar]
- Horwood AM, Riddell PM. Can misalignments in typical infants be used as a model for infantile esotropia? Invest Ophthalmol Vis Sci. 2004;45:714–720. doi: 10.1167/iovs.03-0454. [DOI] [PubMed] [Google Scholar]
- Hung G. Linear model of accommodation and vergence can account for discrepancies between AC/A measures using the fixation disparity and phoria methods. Ophthalmic Physiol Opt. 1991;11:275–278. [PubMed] [Google Scholar]
- Hung G. Quantitative analysis of the accommodative convergence to accommodation ratio: Linear and static models. IEEE Transactions on Biomedical engineering. 1997;44:306–316. doi: 10.1109/10.563300. [DOI] [PubMed] [Google Scholar]
- Hung G, Ciuffreda K, Rosenfield M. Static model of proximal accommodation and vergence. Invest Ophth Vis Sci. 1994;35:1280. [Google Scholar]
- Hung G, Ciuffreda K, Rosenfield M. Proximal contributions to a linear static model of accommodation and vergence. Ophthalmic Physiol Opt. 1996;16:31–41. [PubMed] [Google Scholar]
- Hung GK. Adaptation model of accommodation and vergence. Ophthalmic Physiol Opt. 1992;12:319–326. [PubMed] [Google Scholar]
- Hunt OA, Wolffsohn JS, Gilmartin B. Evaluation of the measurement of refractive error by the PowerRefractor: a remote, continuous and binocular measurement system of oculomotor function. Br J Ophthalmol. 2003;87:1504–1508. doi: 10.1136/bjo.87.12.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B-C. A method for correction of CA/A ratio based on linear model of accommodation and vergence. Optom Vision Sci. 1994;71:192–197. doi: 10.1097/00006324-199403000-00008. [DOI] [PubMed] [Google Scholar]
- Judge S. How is binocularity maintained during convergence and divergence? Eye. 1996;10:172–176. doi: 10.1038/eye.1996.43. [DOI] [PubMed] [Google Scholar]
- Karania R, Evans BJ. The Mallett Fixation Disparity Test: influence of test instructions and relationship with symptoms. Ophthalmic Physiol Opt. 2006;26:507–522. doi: 10.1111/j.1475-1313.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- Kenyon R, Ciuffreda K, Stark L. Dynamic vergence eye movements in strabismus and amblyopia: symmetric vergence. Invest Ophthalmol Vis Sci. 1980;19:60–74. [PubMed] [Google Scholar]
- Kenyon R, Ciuffreda K, Stark L. Dynamic vergence eye movements in strabismus and amblyopia: asymmetric vergence. Brit. J. Ophthalmol. 1980;65:167–176. doi: 10.1136/bjo.65.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotulak J, Schor C. The effects of optical vergence, contrast, and luminance on the accommodative response to spatially bandpass filtered targets [published erratum appears in Vision Res 1988;27(10):361] Vision Res. 1987;27:1797–1806. doi: 10.1016/0042-6989(87)90108-8. [DOI] [PubMed] [Google Scholar]
- Kruger PB, Pola J. Changing target size is a stimulus for accommodation. J Opt Soc Am A. 1985;2:1832–1835. doi: 10.1364/josaa.2.001832. [DOI] [PubMed] [Google Scholar]
- Kushner B. Exotropic deviations: a functional classification and approach to treatment. Am Orthoptic J. 1988;38:81–93. [Google Scholar]
- Kushner B. Diagnosis and treatment of exotropia with a high Accommodation convergence- accommodation ratio. Arch Ophth. 1999;117:221–224. doi: 10.1001/archopht.117.2.221. [DOI] [PubMed] [Google Scholar]
- Leibowitz HW, Owens DA. Anomalous myopias and the intermediate dark focus of accommodation. Science. 1975;189:646–648. doi: 10.1126/science.1162349. [DOI] [PubMed] [Google Scholar]
- Maddox E. Investigations on the relationship between convergence and accommodation of the eyes. J Anat. 1893;20:475–505. 565–484. [PMC free article] [PubMed] [Google Scholar]
- McLin L, Schor C, Kruger P. Changing size (looming) as a stimulus to accommodation and vergence. Vision Res. 1988a;28:883–898. doi: 10.1016/0042-6989(88)90098-3. [DOI] [PubMed] [Google Scholar]
- McLin LN, Jr., Schor CM, Kruger PB. Changing size (looming) as a stimulus to accommodation and vergence. Vision Res. 1988b;28:883–898. doi: 10.1016/0042-6989(88)90098-3. [DOI] [PubMed] [Google Scholar]
- Mein J, Trimble R. Diagnosis and Management of Ocular Motility Disorders. Blackwell Scientific Publications; Oxford: 1991. [Google Scholar]
- Millodot M. Dictionary of Optometry & Visual Science. Butterworth-Heinemann; Oxford: 1997. p. 16. [Google Scholar]
- Morgan M. Accommodation and vergence. Am J Optom Arch Am Acad Optom. 1968;45:417–454. doi: 10.1097/00006324-196807000-00002. [DOI] [PubMed] [Google Scholar]
- North R, Henson D, Smith T. Influence of proximal, accommodative and disparity stimuli upon the vergence system. Ophthalmic Physiol Opt. 1993;13:239–243. doi: 10.1111/j.1475-1313.1993.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Ogle K, Martens T. On the accommodative convergence and the proximal convergence. Arch Ophth. 1957;128:702–715. doi: 10.1001/archopht.1957.00930050714011. [DOI] [PubMed] [Google Scholar]
- Okada Y, Ukai K, Wolffsohn JS, Gilmartin B, Iijima A, Bando T. Target spatial frequency determines the response to conflicting defocus- and convergence-driven accommodative stimuli. Vision Res. 2006;46:475–484. doi: 10.1016/j.visres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Owens DA. The Mandelbaum effect: evidence for an accommodative bias toward intermediate viewing distances. J Opt Soc Am. 1979;69:646–652. doi: 10.1364/josa.69.000646. [DOI] [PubMed] [Google Scholar]
- Phillips S, Stark L. Blur: a sufficient accommodative stimulus. Doc Ophthalmol. 1977;43:65–89. doi: 10.1007/BF01569293. [DOI] [PubMed] [Google Scholar]
- Pratt-Johnson J, Tillson G. Management of Strabismus and Amblyopia. Thieme; New York: 1994. [Google Scholar]
- Radhakrishnan H, Allen PM, Charman WN. Dynamics of Accommodative Facility in Myopes. Invest. Ophthalmol. Vis. Sci. 2007;48:4375–4382. doi: 10.1167/iovs.07-0269. [DOI] [PubMed] [Google Scholar]
- Rosenfield M, Ciuffreda K, Chen H. Effect of age on the interaction between the AC/A and CA/C ratios. Ophthalmic Physiol Opt. 1995;15:451–455. [PubMed] [Google Scholar]
- Rosenfield M, Ciuffreda K, Hung G. The linearity of proximally induced accommodation and vergence. Invest Ophth Vis Sci. 1991;32:2985–2991. [PubMed] [Google Scholar]
- Rosenfield M, Ciuffreda KJ. Effect of surround propinquity on the open-loop accommodative response. Invest Ophthalmol Vis Sci. 1991;32:142–147. [PubMed] [Google Scholar]
- Schaeffel F, Wilhelm H, Zrenner E. Inter-individual variability in the dynamics of natural accommodation in humans: relation to age and refractive errors. J Physiol. 1993;461:301–320. doi: 10.1113/jphysiol.1993.sp019515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimitzek T, Lagreze WA. Accuracy of a new photo-refractometer in young and adult patients. Graefes Arch Clin Exp Ophthalmol. 2005;243:637–645. doi: 10.1007/s00417-004-1056-y. [DOI] [PubMed] [Google Scholar]
- Schober H, Dehler H, Kassel R. Accommodation during observations with optical instruments. J Opt Soc Am. 1970;60:103–107. [Google Scholar]
- Schor C. Analysis of tonic and accommodative vergence disorders of binocular vision. Am J Optom Physiol Opt. 1983;60:1–14. doi: 10.1097/00006324-198301000-00001. [DOI] [PubMed] [Google Scholar]
- Schor C. The Glenn A.Fry Award Lecture: Adaptive regulation of accommodative vergence and vergence accommodation. Am J Optom & Physiological Optics. 1986;63:587–609. [PubMed] [Google Scholar]
- Schor C. A dynamic model of cross-coupling between accommodation and convergence - simulations of step and frequency responses. Optom Vision Sci. 1992;69:258–269. doi: 10.1097/00006324-199204000-00002. [DOI] [PubMed] [Google Scholar]
- Schor C. The influence of interactions between accommodation and convergence on the lag of accommodation. Ophthalmic Physiol Opt. 1999;19:134–150. doi: 10.1046/j.1475-1313.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- Schor CM, Cuiffreda K. Vergence Eye Movmements. Basic & Cinical Aspects. Butterworth; Woburn,MA: 1985. [Google Scholar]
- Seidemann A, Schaeffel F. An evaluation of the lag of accommodation using photorefraction. Vision Res. 2003;43:419–430. doi: 10.1016/s0042-6989(02)00571-0. [DOI] [PubMed] [Google Scholar]
- Semmlow J, Wetzel P. Dynamic contributions of the components of binocular vergence. J Opt Soc Am. 1979;69:639–645. doi: 10.1364/josa.69.000639. [DOI] [PubMed] [Google Scholar]
- Stark L. Normal and abnormal vergence. In: Schor C, Cuiffreda K, editors. Vergence Eye Movements. Butterworth; Woburn,MA: 1983. pp. 3–14. [Google Scholar]
- Thompson D. Measurements with cover test versus troposcope. Am Orthopt J. 1952;2:47–52. [PubMed] [Google Scholar]
- Tondel G, Candy T. Accomodation and vergence latencies in human infants. Vision Research. 2008 doi: 10.1016/j.visres.2007.11.016. in press, doi:10.1016/j.visres.2007.1011.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondel G, Wang J, Candy T. Infants’ Ability To Track Ramp Accommodative Stimuli. Investigative Ophthalmology & Vision Science. 2002;43(Suppl.):2865. (Abstract) [Google Scholar]
- Tondel GM, Candy TR. Human infants’ accommodation responses to dynamic stimuli. Invest Ophthalmol Vis Sci. 2007;48:949–956. doi: 10.1167/iovs.06-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuetaki TK, Schor CM. Clinical method for measuring adaptation of tonic accommodation and vergence accommodation. Am J Optom Physiol Opt. 1987;64:437–449. doi: 10.1097/00006324-198706000-00009. [DOI] [PubMed] [Google Scholar]
- Turner J, Horwood A, Houston S, Riddell P. Development of the response AC/A ratio over the first year of life. Vision Research. 2002;42:2521–2532. doi: 10.1016/s0042-6989(02)00268-7. [DOI] [PubMed] [Google Scholar]
- von Noorden G. Amblyopia, a multi-disciplinary approach. Invest Ophthalmol Vis Sci. 1985;26:1704–1716. [PubMed] [Google Scholar]
- Ward P. The effect of spatial frequency on steady-state accommodation. Ophthalmic Physiol Opt. 1987;7:211–7. [PubMed] [Google Scholar]
- Weiss M, Seidemann A, Schaeffel F. Stimulating human accommodation without changes in focus. Ophthalmic Physiol Opt. 2004;24:207–217. doi: 10.1111/j.1475-1313.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- Whitefoot H, Charman WN. Dynamic retinoscopy and accommodation. Ophthalmic Physiol Opt. 1992;12:8–17. [PubMed] [Google Scholar]
- Wick B. Clinical factors in proximal vergence. Am J Optom Physiol Opt. 1985;62:1–18. doi: 10.1097/00006324-198501000-00001. [DOI] [PubMed] [Google Scholar]
- Wick B, Currie D. Dynamic demonstration of proximal vergence and proximal accommodation. Optom Vision Sci. 1991;68:163–167. doi: 10.1097/00006324-199103000-00001. [DOI] [PubMed] [Google Scholar]
- Wolffsohn JS, Hunt OA, Gilmartin B. Continuous measurement of accommodation in human factor applications. Ophthalmic Physiol Opt. 2002;22:380–384. doi: 10.1046/j.1475-1313.2002.00050.x. [DOI] [PubMed] [Google Scholar]