Abstract
We report the results of a subgroup analysis of the Benefits of Universal Glove and Gown (BUGG) trial. In 20 ICUs, the reduction in acquisition of methicillin-resistant Staphylococcus aureus observed in the BUGG trial was observed in units also using chlorhexidine bathing and in those that previously performed active surveillance.
Keywords: MRSA, VRE, contact precautions, chlorhexidine bathing, infection control, active surveillance
Introduction
Healthcare-associated infections (HAIs) cause significant patient morbidity and mortality.(1) Methicillin-resistant Staphylococcus aureus (MRSA) is the most common antibiotic-resistant bacteria causing HAIs and is more difficult to treat than methicillin-susceptible S. aureus.(2) Trials of MRSA prevention have generally not evaluated more than one intervention. Trials evaluating active surveillance culturing have not found MRSA reduction.(3, 4) Trials of chlorhexidine (CHG) patient bathing found variable impact on overall infections, skin contaminants, MRSA and vancomycin –resistant Enterococcus (VRE).(5, 6) The BUGG trial, a single trial of universal gloving and gowning found a reduction in MRSA transmission but not VRE transmission.(7)
To determine the interaction between universal gloving and gowning with CHG bathing and other infection control interventions, we conducted a subgroup analysis of the BUGG trial.
Methods
The study was a subgroup analysis of the BUGG cluster randomized ICU trial. From September 2011 to December 2011, ICUs collected baseline data on the primary outcome of MRSA acquisition. For randomization and analysis, ICUs were pair-matched on baseline rates of MRSA and VRE acquisition. Within each pair, one ICU was randomized to the intervention and the other to the control group.(7) The intervention period was from January to October 2012.
We recruited medical, surgical or combined medical-surgical ICUs for adult patients from academic and community hospitals in the United States through the Society for Healthcare Epidemiology of America (SHEA) Research Network excluding those performing active surveillance culturing for MRSA or VRE during the study. Information on infection control practices from infection control staff was obtained during site visits at each ICU.(7)
The intervention occurred at the cluster level of ICU. During the study period, all healthcare workers (e.g., nurses, physicians, respiratory therapists) in the 10 ICUs assigned to the intervention group were required to wear gloves and gowns when entering any patient room. The 10 ICUs in the control group followed usual standard of care, which consists of healthcare workers following CDC contact precautions guidelines (gloves and gowns) for patients known to have infection or colonization with antibiotic-resistant bacteria. ICUs were stratified according to whether or not they performed chlorhexidine patient bathing, were classified as academic hospitals based on affiliation with a medical school and whether they had performed active surveillance for MRSA prior to entering the study.
All patients had ICU admission and ICU discharge surveillance cultures collected for MRSA (nasal swab). For each patient, MRSA acquisition was defined as having a surveillance culture on ICU admission that was negative for MRSA, with a subsequent surveillance culture on ICU discharge for the same admission that was positive for MRSA. Poisson mixed effects models were used to perform all subgroup analyses. The models included fixed effects for time period (baseline period versus study period), random group assignment (control versus intervention), subgroup category, two-way interaction terms between these variables and a three-way interaction term. A random intercept was included to account for heterogeneity among ICUs. A separate model was fit for each subgroup variable. We tested the coefficient of the three-way interaction term to determine whether the effect of the intervention differed by subgroup category. All tests were two-sided with the threshold for statistical significance being p < .05. The University of Maryland School of Medicine Institutional Review Board (IRB) served as the central IRB. All participating ICUs received approval from their local IRBs.
Results
Twenty ICUs participated in the study and none withdrew. There were 26,180 ICU admissions and 92,241 swabs for detection of MRSA. During the study period, compliance with obtaining nasal cultures was 95.73% at admission and 84.44% at discharge. Compliance with wearing gloves in the intervention ICUs was 86.18% (2787/3234), and compliance with wearing gowns was 85.14% (2750/3230). In the control group, 10.52% of patients were on contact precautions.
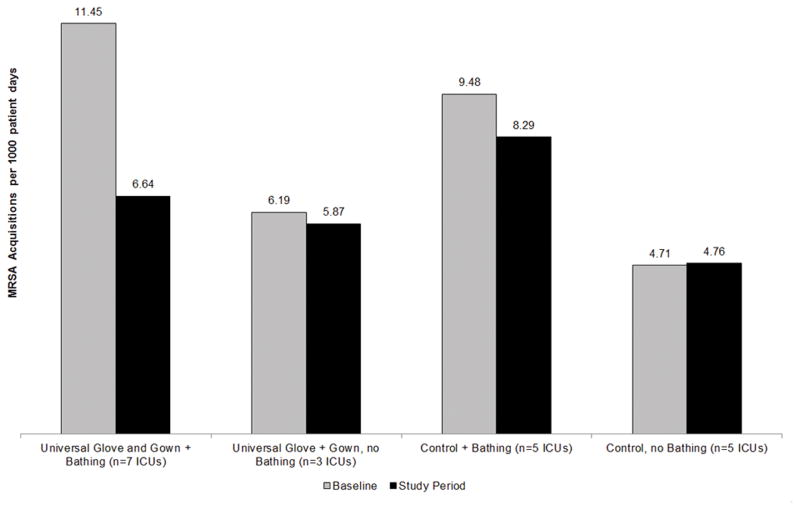
Figure 1 and Table 1 demonstrate the results of the intervention stratified by infection control practices including CHG bathing, academic versus non-academic setting and presence of MRSA surveillance prior to study initiation. No units began new infection prevention practices during the study period. Five units in the control arm practiced CHG bathing (3 used wipes, 2 CHG soap) whereas seven units in the intervention arm practiced CHG bathing (4 used wipes, 3 CHG soap). We found no statistically significant differences in units that practiced CHG bathing versus units that did not practice CHG bathing on the effect of universal gloving and gowning to reduce acquisition of MRSA. However, among units that practiced CHG bathing, the reduction in MRSA was greater in intervention ICUs than in control ICUs (rate difference −3.62, 95% CI −8.96 to 1.73, p=0.18). Among units that did not practice CHG bathing, the change in MRSA acquisition was similar in intervention ICUs and control ICUs (rate difference −0.37, 95% CI −4.37 to 3.62, p=0.85).
Figure 1.
Frequency of MRSA acquisition in ICU patients during baseline and study periods representing the effect of universal glove and gown on patients in intensive care units that did or did not use chlorhexidine bathing.
Table 1.
Baseline and study period rates of MRSA acquisition per 1000 patient days in those sites randomized to the intervention of universal glove and gown use compared to control sites as subanalyzed by other infection control methods used at sites during the baseline and study periods.
| Site characteristics/infection control measures | N | Baseline MRSA rate (95% CI) | Study period MRSA rate (95% CI) | Change (95% CI) | Difference (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Chlorhexidine bathing | 12 | −3.62 (−8.96, 1.73)* | 0.18 | |||
|
| ||||||
| Intervention sites | 7 | 11.45 (5.97, 21.95) | 6.64 (3.54, 12.44) | −4.81 (−9.01, −0.62) | ||
| Control sites | 5 | 9.48 (4.67, 19.27) | 8.29 (4.36, 15.77) | −1.2 (−5.02, 2.63) | ||
| No chlorhexidine bathing | 8 | −0.37 (−4.37, 3.62)* | 0.85 | |||
| Intervention sites | 3 | 6.19 (2.76, 13.86) | 5.87 (2.95, 11.7) | −0.32 (−3.72, 3.08) | ||
| Control sites | 5 | 4.71 (3.1, 7.16) | 4.76 (2.63, 8.63) | 0.05 (−1.95. 2.06) | ||
| Difference in effect (CHG bathing – no CHG bathing) | −3.24 (−9.5, 3.01) | 0.31** | ||||
|
| ||||||
| Academic Medical Center | 15 | −3.86 (−8.77, 1.05)* | 0.12 | |||
|
| ||||||
| Intervention sites | 8 | 10.29 (3.83, 27.65) | 6.36 (2.4, 16.87) | −3.93 (−8.49, 0.63) | ||
| Control sites | 7 | 5.92 (2.15, 16.28) | 5.86 (2.19, 15.66) | −0.06 (−2.00, 1.87) | ||
| Non-Academic Medical Center | 5 | 2.12 (−4.99, 9.24)* | 0.56 | |||
| Intervention sites | 2 | 6.37 (1.8, 22.55) | 6.38 (2.15, 18.93) | 0.01 (−5.24, 5.26) | ||
| Control sites | 3 | 9.45 (4.89, 18.29) | 7.34 (2.89, 18.67) | −2.11 (−7.11, 2.89) | ||
| Difference in effect (Academic – Non-Academic) | −5.98 (−14.07, 2.1) | 0.15** | ||||
|
| ||||||
| MRSA active surveillance prior to trial | 9 | −8.16 (−16.42, 0.1)* | 0.05 | |||
|
| ||||||
| Intervention sites | 3 | 14.27 (6.35, 32.06) | 7.26 (3.36, 15.69) | −7.01 (−14.48, 0.47) | ||
| Control sites | 6 | 5.27 (2.40, 11.59) | 6.42 (3.12, 13.23) | 1.15 (−1.24, 3.54) | ||
| No MRSA active surveillance prior to trial | 11 | 0.64 (−3.62, 4.89)* | 0.77 | |||
| Intervention sites | 7 | 8.06 (3.81, 17.04) | 5.98 (2.92, 12.24) | −2.08 (−4.95, 0.78) | ||
| Control sites | 4 | 8.71 (5.45, 13.91) | 5.99 (3.16, 11.35) | −2.72 (−5.56, 0.12) | ||
| Difference in effect (MRSA AS vs. no MRSA AS) | −8.8 (−17, −0.59) | 0.04** | ||||
Negative # reflects greater reduction in the intervention group than in the control group from the baseline period to the study
p for interaction
Among units that performed MRSA active surveillance prior to the start of the study, the reduction in MRSA acquisition was greater in intervention ICUs than in control ICUs (rate difference −8.16, 95% CI 16.42 to 0.01, p=0.05). In contrast, among units that did not practice active MRSA surveillance prior to the start of the study, the change in MRSA acquisition was similar in intervention ICUs and control ICUs (rate difference 0.64, 95% CI 3.62 to 4.89, p=0.77). This resulted in a significant difference in effects of −8.80 (95% CI 17 to −0.59, p for interaction=0.04). We did not find a significant interaction when comparing the intervention effect between academic and non-academic ICUs (p=0.15).
Discussion
In a subgroup analysis of the BUGG trial, we found that the effect of universal gloving and gowning on MRSA acquisition was not modified by whether a unit was already using CHG bathing or was an academic medical center. Those units that were using CHG bathing during the study period had larger, although not statistically significant, decreases in MRSA acquisition than did units not using CHG bathing. Facilities that had been performing active surveillance culturing immediately prior to the BUGG study had a significantly greater reduction in MRSA acquisition with universal gloving and gowning than did their counterparts.
Although there was limited power for sub-analysis of a 20 ICU cluster study, the estimated effect of universal gloving and gowning was greater in units using CHG bathing. This may suggest that there are additional benefits to performing both chlorhexidine bathing and universal gloving and gowning in the reduction of MRSA acquisition.(6, 7) The reason for the increased benefit from universal gloving and gowning as a change from active surveillance culturing for MRSA is unclear. Units employing MRSA active surveillance may have had higher rates of MRSA or, as some have argued, ineffective active surveillance for MRSA may distract staff from general horizontal infection prevention practices.(8) These results suggest that the benefits of CHG bathing and universal gloving and gowning may be additive. The decision to implement a method of infection prevention should depend on frequency of HAIs and MDROs at an individual hospital or ICU. Units with low rates of both HAIs and MDROs using baseline prevention methods may choose not to implement any additional interventions, while units with high rates of HAIs and MDROs may wish to implement one or both of CHG bathing and universal gloving and gowning. This study was limited by statistical power and a non-random adoption of infection prevention practices.
In conclusion, in a sub-analysis of the BUGG study, we found that there are additive benefits to performing both chlorhexidine bathing and universal gloving and gowning to reduce MRSA in some ICUs.
Acknowledgments
Financial Support. This study was supported by contract # HHSA290200600015 Task Order No. 5. from the Agency for Healthcare Research and Quality (AHRQ), by grant # 1K08HS18111-01 (Dr. Morgan) from AHRQ and by grant #s 5K25AG034216-03 (Dr. Shardell) and 5K24AI079040-05 (Dr. Harris) from the National Institutes of Health (NIH).
Footnotes
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, management, analysis and interpretation of the data; or in the preparation of the manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ or NIH.
Potential conflicts of interest. ADH has been a consultant for Premier, Cubist and Sanogiene and is an editor for UpToDate, Online. DJM has served as a consultant for Welch Allyn and Sanogiene. DHK serves as a consultant for Pfizer and Astellas; has received honoraria or speaking fees from Pfizer, Astellas and Glaxo Smith-Kline; and has received research support from Pfizer and Akers Bioscience. DKW has received research support from Cubist and Biomerieux, payment for a lecture from 3M Healthcare and serves as a consultant for Centene Corp. and Sagentia. No other authors have financial disclosures.
References
- 1.Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible staphylococcus aureus bacteremia: A meta-analysis. Clin Infect Dis. 2003;36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 3.Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364:1407–1418. doi: 10.1056/NEJMoa1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derde LP, Cooper BS, Goossens H, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: An interrupted time series study and cluster randomised trial. Lancet Infect Dis. 2014;14:31–39. doi: 10.1016/S1473-3099(13)70295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: A multicentre, cluster-randomised, crossover trial. Lancet. 2013;381:1099–1106. doi: 10.1016/S0140-6736(12)61687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368:2255–2265. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: A randomized trial. JAMA. 2013;310:1571–1580. doi: 10.1001/jama.2013.277815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edmond MB, Wenzel RP. Targeted decolonization to prevent ICU infections. N Engl J Med. 2013;369:1471. doi: 10.1056/NEJMc1309704. [DOI] [PubMed] [Google Scholar]