Abstract
Pre- and postnatal calorie restriction is associated with postnatal growth restriction, reduced circulating leptin concentrations and perturbed energy balance. Hypothalamic regulation of energy balance demonstrates enhanced orexigenic (NPY, AgRP) and diminished anorexigenic (POMC, CART) neuropeptide expression (PN21) setting the stage for subsequent development of obesity in female Sprague-Dawley rats. Leptin replenishment during the early postnatal period (PN2-PN8) led to reversing the hypothalamic orexigenic:anorexigenic neuropeptide ratio at PN21 by only reducing the orexigenic (NPY, AgRP) without affecting the anorexigenic (POMC, CART) neuropeptide expression. This hypothalamic effect was mediated via enhanced leptin receptor (ObRb) signaling that involved increased pSTAT3/STAT3 but reduced PTP1B. This was further confirmed by an increase in body weight at PN21 in response to intracerebroventricular administration of antisense ObRb oligonucleotides (PN2-PN8). The change in the hypothalamic neuropeptide balance in response to leptin administration was associated with increased oxygen consumption, carbon dioxide production and physical activity which resulted in increased milk intake (PN14) with no change in body weight. This is in contrast to the reduction in milk intake with no effect on energy expenditure and physical activity observed in controls. We conclude that pre- and postnatal calorie restriction perturbs hypothalamic neuropeptide regulation of energy balance setting the stage for hyperphagia and reduced energy expenditure, hallmarks of obesity. Leptin in turn reverses this phenotype by increasing hypothalamic ObRb signaling (sensitivity) and affecting only the orexigenic arm of the neuropeptide balance.
Keywords: Fetal programming, leptin, energy expenditure, hypothalamus, STAT3, NPY, AgRP, POMC, CART
Introduction
Obesity has become an expanding epidemic within the past decade (James 2008). Obesity among children and adolescents has continued to increase in prevalence. Data from NHANES (National Health and Nutrition Examination Surveys) of a period between 1976–1980, to that between 1999–2006 shows increasing prevalence of severe obesity in all age groups: 0.4% to 2.2% among 2–5 year olds, 1.8% to 4.7% among 6–11 year olds, and 1.1% to 6.3% among 12–19 year olds (Claire Wang et al. 2011). Obesity is associated with cardiovascular risk factors such as diabetes mellitus, dyslipidemia, and hypertension resulting in the metabolic syndrome (Good et al. 2008; Messerli 1982; Poirier et al. 2006). Obesity is also a known risk factor for development of certain cancers (Key et al. 2010; La Vecchia et al. 1984; Lapidus et al. 1988) and cognitive impairments.
While in-utero obesity presents with a large for gestational age phenotype encountered in gestational diabetes or maternal obesity, the other end of the spectrum consisting of gestational and neonatal malnutrition also causes adiposity in later life. In a study of more than 400 babies, small-for-gestational age infants developed obesity at twice the rate of appropriate-for-gestational age babies (12% vs 6%) (Meas et al. 2008). Thus, perinatal nutrition significantly impacts childhood and adult phenotypes. Early nutritional restriction during the critical period of postnatal life has lasting effects into the adult with trans-generational inheritance (Hales and Barker 2001). Pre- and early postnatal caloric restriction with subsequent rapid catch-up growth predetermines adult-onset obesity and related diseases (Barker 2007; Roseboom et al. 2001). Since obesity is pre-programmed in postnatal life, targeted therapies must be directed towards this critical window prior to expression of the adult phenotype.
Disruption of energy balance is a hallmark of obesity and regulation of energy balance is under hypothalamic control (Faulconbridge and Hayes 2011; Harrold 2004; Hill et al. 2012; Vickers et al. 2005). Our previous rodent studies have demonstrated that late gestation maternal (pre-) and postnatal caloric restriction in the offspring perturbs circulating leptin concentrations which in turn affect the hypothalamic balance between the orexigenic and anorexigenic neuropeptides (Shin et al. 2012). This imbalance sets the stage for hyperphagia and diminution of energy expenditure, forerunners of obesity, particularly if high caloric diet is consumed ad lib (Garg et al. 2012; Shin et al. 2012). Moreover, other groups have shown that providing leptin to the postnatal rat ameliorates the adult phenotype of hyperphagia and obesity (Vickers et al. 2005). This is similar to the dramatic effect of leptin administration in children born with leptin deficiency (Bluher et al. 2009; Farooqi et al. 1999; Licinio et al. 2004). However leptin therapy in obese adults failed to achieve loss of body weight related to leptin resistance (Hukshorn et al. 2000; Hukshorn et al. 2002). While postnatal leptin administration has been successful in reversing the obese phenotype in rodents (Vickers et al. 2005), the mechanism of action in the hypothalamus by which the phenotype is altered has not been thoroughly investigated.
We therefore hypothesized that postnatal leptin administration in the pre- and postnatal calorie restricted rat offspring will restore the perturbed orexigenic:anorexigenic neuropeptide ratio (expression and action), and thereby the energy balance. To test this hypothesis, we employed our pre- and postnatal calorie restricted rodent model and provided daily leptin therapy in the early postnatal period. We observed a reduction in the enhanced orexigenic peptide expression (neuropeptide Y [NPY] and agouti-related peptide [AgRP]) with no change in the diminished anorexigenic (pro-opiomelanocorticotropin [POMC] and cocaine amphetamine-related transcript [CART]) neuropeptides. These changes were associated with enhanced energy expenditure and physical activity with a recovery of energy intake in an attempt to match energy expenditure. Thus, postnatal leptin administration reversed the perturbed hypothalamic neuropeptide imbalance characteristic of pre- and postnatal caloric restriction most likely by affecting the orexigenic but not the anorexigenic neuropeptides via increased leptin receptor sensitivity.
Materials and Methods
Animals
Gestationally timed Sprague-Dawley rats (Charles River Laboratories, Hollister, CA) were housed in individual cages and exposed to 12-h light/dark cycles at 21–23°C. As approved by the University of California, Los Angeles Animal Research Committee, NIH guidelines for the Care and Use of Animals were followed. The pregnant dams were allowed at least one day of acclimatization before experimental manipulation. Animals were fed standard rat chow (composition: carbohydrate 63.9%, fat 4% and protein 14.5%) and were allowed ad lib access to water.
Calorie Restriction Model
Pregnant dams were separated into two groups and allowed ad lib access to standard chow and water or were given 50% of daily calorie intake (11 grams/day) as well as ad lib access to water from gestational day (E)11 to E21. At birth, only female pups were culled six per litter to ensure no inter-litter nutritional variability. The pups born to ad lib feeding control mothers were reared by control mothers, and pups born to calorie-restricted mothers were reared by calorie-restricted mothers. During lactation, calorie-restricted mothers were continued on 50% daily calorie intake (20 grams/day) from postnatal day (PN) 1 to 21 of the suckling offspring (Fig. 1A).
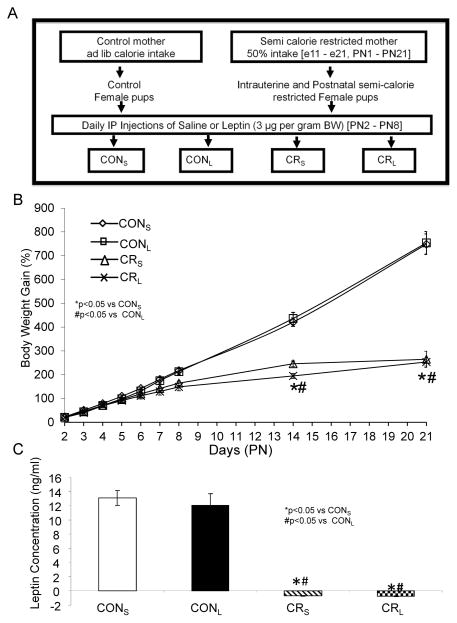
Figure 1. Experimental design (A), growth curves (B) and circulating plasma leptin concentrations (C).
A. Scheme demonstrating the experimental design: Four experimental groups were created: CONS, ad lib access to food during pre- and post-natal stages, saline injected; CONL, ad lib access to food during pre- and post-natal stages, leptin-injected; CRS, 50% calorie-restriction during pre- and post-natal stages, saline-injected; CRL, 50% calorie-restriction during pre- and post-natal stages. B. Percent weight gain in pups from the four experimental groups: CONS, CONL, CRS and CRL ranging from PN2 to PN21 are shown. n = 12–21 per group. From PN2 through PN21, calorie restricted groups weighed less than control at all ages, but no intergroup differences were seen with leptin intervention. C. Plasma leptin concentrations: Both CRS and CRL expressed negative values. N=8 in each group, *or #p<0.05 vs CONS or CONL respectively.
Leptin administration
Within the control and calorie-restricted groups, different litters were assigned to receive daily intraperitoneal (IP) injections with the use of an insulin syringe (28G1/2- Becton Dickinson, Franklin, NJ) from PN2 to PN8 of either a saline vehicle 50 μl.day−1 or freshly reconstituted leptin (Dr. A. F. Parlow, Harbor-UCLA, Torrance, CA) at 3 μg.g body weight−1.day−1.50 μl saline−1. Four experimental groups were created (Fig. 1A): control-saline (CONS), control-leptin (CONL), calorie restriction-saline (CRS), and calorie restriction-leptin (CRL).
Energy Metabolic Characteristics
a. Milk intake
Milk intake by the pups in all experimental groups was assessed at PN7 and PN14. Pups were separated from mothers and kept for 5 hours at 27±2°C on a temperature-controlled heating pad. The pups were then returned to their mothers for one hour of suckling (Fukushima et al. 2006; Shin et al. 2012). The difference in body weights before and after suckling reflected milk intake (g.g body weight−1). b. Indirect caloric measurement
Energy expenditure was measured by an indirect open circuit calorimeter (Oxymax, Columbus Instruments, Columbus, OH) at PN14, as previously described (Shin et al. 2012). Calorimeter was calibrated and weighed rat pups were placed individually in sealed chambers. The expired air was analyzed every 6 minutes using an electrochemical O2 analyzer and a CO2 sensor (Oxymax). O2 consumption (VO2) and CO2 produced (VCO2) were measured by indirect calorimeter and expressed as milliliters of O2 or CO2 × hour−1 × body weight in kg. The respiratory exchange ratio (RER) was calculated as VCO2/VO2. Energy expenditure (kcal/h/kg) was calculated using a rearrangement of the Weir equation as supplied by Columbus Instruments: (3.815 + 1.232 × RER) × VO2. A mean of 12–14 determined values per rat were averaged for the final value. Photocells (Opto-M3 Activity Monitor) with 16 IR beams intersecting the chamber in the X and Z-axes processed and tabulated activity counts associated with ambulation. The activity data was obtained concurrently in the time period when calorimetric measurements were performed and averaged. The method scores counts as ambulation when the animal traverses the cage, breaking a series of IR beams in Y-axes. All beam interruptions are scored as Total Activity in X and Z axes which provides all ambulation and counts associated with stereotypy activity (grooming, scratching, etc). Z-Axis represents rearing.
Anthropometric and Metabolic Characteristics
Pups were weighed daily from PN2 to PN9 and again at PN14 and PN21. At PN21, animals were anesthetized using inhalational isoflurane, and blood was collected. The plasma was separated by centrifugation at 4°C, aliquoted, and stored at −70°C until further analysis. Plasma leptin concentrations were quantified by enzyme linked immuno-absorbent assays (ELISA) using rat leptin antibodies (Millipore, Billerica, MA).
Western blot analysis of hypothalamic signaling molecules
At PN21, hypothalami were separated microscopically from whole brain using established anatomical landmarks (Paxinos and Watson 1998) and then homogenized in cell lysis buffer (Cell Signaling Technology, Danvers, MA). Protein content was measured by the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). Homogenates (30 μg of protein) were subjected to sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and the separated proteins were transferred to nitrocellulose membrane filters (Bio-Rad, Hercules, CA). The blotted membranes were sequentially incubated in 3% bovine serum albumin or 5% nonfat dry milk and the primary antibody consisting of the rabbit anti-STAT3, anti-SOCS3 (1:1000 each, Cell Signaling Technology, Danvers, MA), anti-PTP1B IgG (1:1000, BD Bioscience, San Jose, CA) and mouse anti-vinculin (1:40,000, internal loading control; Sigma, St. Louis, MO) on the same membrane. The proteins were visualized in Typhoon 9410 Phosphorimager (GE Healthcare Biosciences, Piscataway, NJ) by blotting with the enhanced chemiluminescence (ECL) plus detection kit (GE Healthcare BioSciences Corp., Piscataway, NJ) following horseradish peroxidase-labeled anti-rabbit IgG for anti-STAT3 or anti-mouse IgG for anti-PTP1B and anti-vinculin (GE Healthcare Biosciences Corp., Piscataway, NJ). Each protein was quantified by using ImageQuant 5.2 software (GE Healthcare Biosciences, Piscataway, NJ) and normalized to vinculin which served as the internal control.
Total mRNA extraction
Total RNA was extracted from hypothalami at PN21 using the QIAGEN miRNeasy Mini kit (QIAGEN, CA). The yield, integrity and RNA purity were determined spectrophotometrically (NanoDrop, Wilmington, DE) and by formaldehyde-agarose gel electrophoresis.
Microarray analyses
One μg of total RNA was used to generate microarray probes by standard Affymetrix protocol (Enzo Diagnostics; Farmingdale, NY) which were hybridized to the Affymetrix Rat 1.0 gene arrays (Affymetrix; Santa Clara, CA). Two independent replicated experiments were carried out for all the treatments. The data was normalized using the Robust Multichip Average algorithm with the Affymetrix software Expression Console. The normalized data files and corresponding text files were uploaded into the dCHIP program (Li and Wong 2001) for pair-wise comparisons. Probesets were filtered to exclude low expressed genes. Data was deposited and is available in the Gene Expression Omnibus (Accession No.: GSE55384) at: http:www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=adszmmcgxhqjvmx&acc=GSE55384.
mRNA quantification by quantitative real-time polymerase chain reaction
First strand cDNA was synthesized from 1 μg of DNase-treated total RNA using Superscript II reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA), as previously described (Shin et al. 2012). Quantitative real time PCR was performed as previously described (Shin et al. 2012; Thamotharan et al. 2005). Primers and Taqman probes for detection of specific neuropeptide genes were designed using Primer Express Software (Applied Biosystems, Foster, CA). Target genes including gene bank accession numbers were previously described (Shin et al. 2012). In addition, for CART (Genebank accession no.: U10071) with the 65 bp PCR product size, primers and probe are as follows: forward, 5′ cccgagccctggacatcta 3′; reverse, 5′ gccttggcagctccttctc 3′; probe, 5′ ctgccgtggatgatgcgtccc 3′. These designed forward and reverse primers generate corresponding DNA fragments after amplification. Taqman probes were synthesized and labeled with fluorescent dye, 6-carboxyfluorescein (FAM) on the 5′-end and N, N, N′, N′-tetramethyl-6-carboxyrhodamine (TAMRA) on the 3′- end (Applied Biosystems, Foster CA). Taqman PCR was carried out using a StepOnePlus real time PCR system (Applied Biosystems, Foster, CA). Real time PCR quantification was then performed using Taqman glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or eukaryotic 18S rRNA (Applied Biosystems, Foster, CA) as internal controls. PCR amplifications were performed in triplicates. The amplification cycles consisted of 12 min at 95°C (hot start), followed by 40 cycles at 95°C for 30 sec (denaturation), 56°C for ObRb; 58°C for NPY and CART; and 60°C for AgRP and POMC over 30 sec (annealing), and 72°C for 30 sec (extension), using reagents from Applied Biosystems (Foster, CA). Relative quantification of PCR products were based on value differences between the target and GAPDH or 18S rRNA control using the comparative CT method, as previously described (Shin et al. 2012).
Brain sample preparation
Brains at PN21 were fixed by perfusion with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 after anesthetization by intraperitoneal injection of sodium pentobarbital (50 mg/kg), as previously described (Shin et al. 2012; Varma et al. 2004). Whole brains were embedded in 2% agar for immunohistochemical analysis (Fung et al. 2010) or embedded in paraplast wax after dehydration through graded alcohols for in-situ hybridization (Shin et al. 2012).
Immunohistochemistry
Coronal brain sections (30 μm thickness) obtained by a vibratome (Leica VT 1000S; Leica Microsystems, Deerfield, IL) were subjected to immunofluorescent staining using the rabbit anti-pSTAT3 antibody (1:500 dilution, Cell Signaling Technology, Danvers, MA) as the primary antibody with the fluorescent tag being rhodamine (red) (Jackson Immunoresearch, West Grove, PA). 4′, 6-diamino-2-phenylindole dihydrochloride (DAPI) was used for nuclear DNA staining (blue). Slides with sections were examined under a Nikon E-600 fluorescence microscope (Nikon, Melville, NY, USA) equipped with a cooled charged-coupled device camera as previously described (Shin et al. 2004).
In situ Hybridization
a. cRNA probes
Rat CART cDNA (+58 to +390, Gene bank accession No. U10071) was generated by reverse transcription (RT)-PCR, using the first-strand cDNA synthesized from 1 μg of DNase-pretreated rat hypothalamic RNA, as previously described (Shin et al. 2012). Primers used for CART were 5′-cccgggtctagactacctttgctgggtgccggtgcc-3′ (forward) and 5′-gggcccgcatgctcacaagcacttcaagaggaaaga-3′ (reverse). PCR products were sub-cloned in pGEM-3Zf(+) vector with Xba I and Sph I sites, as previously described (Shin et al. 2012). Antisense RNA probes were prepared by in-vitro transcription from the fragment of cDNA digested with Bam HI using Sp6 RNA polymerase, in the presence of 350 mM digoxigenin (DIG)-linked UTP in a 20 μl reaction mixture (Roche Applied Science, Penzberg, Germany), as previously described (Shin et al. 2012; Shin et al. 1998). A sense probe was similarly generated with T7 RNA polymerase from the Bam HI-digested cDNA fragments and served as the negative control.
b. In situ hybridization
Serial sections (10 μm thickness) obtained using a microtome (Leica RM2235; Leica Microsystems, Deerfield, IL), were mounted on poly-L-lysine-coated slides and stored at room temperature until use. In-situ hybridization was performed as previously described (Shin et al. 2012; Shin et al. 1998). Briefly, after de-waxing and rehydration, tissue sections were digested with 5 μg/ml proteinase K at room temperature for 20 min, then fixed in 0.4% paraformaldehyde at 4°C for 10 min and incubated overnight at 53°C in a hybridization solution containing DIG-labeled CART riboprobes, 50% formamide, 10 mM Tris-HCl, pH 7.5, 300 mM NaCl, 1 mM EDTA, 0.25% SDS, 1× Denhardt’s solution, 200 μg/ml yeast tRNA, and 10% dextran sulfate. After hybridization, tissue sections were washed in 2× SSC, 50% formamide at 55°C for 30 min, incubated with 1 μg/ml RNase A solution at 37°C for 30 min, and washed once in 2 × SSC and twice in 0.2 × SSC at 50°C for 20 min each. After hybridization, sections with DIG-labeled CART riboprobes were incubated with polyclonal sheep anti-DIG Fab antibody conjugated with alkaline phosphatase (Roche Applied Science). Detection of the label was accomplished with NBT (nitroblue tetrazolium chloride) and BCIP (5-bromo-4-chloro-3-indolylphosphate; Roche Applied Science). Color development was carried out at room temperature for 2 hours, and the sections were washed in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.5). Slides were mounted with a mixture containing 24% polyvinyl alcohol, 12% glycerol, and 59 mM Tris-HCl, pH 7.5 (Shin et al. 1998). Controls consisted of the corresponding sense RNA probes employed in serial sections and were noted to abolish the final immunoreaction.
DNA Methylation of Hypothalamic CART Gene Promotor
To examine DNA methylation of the promoter region of the hypothalamic CART gene, genomic DNA was extracted, and subjected to bisulfite modification as previously described (Shin et al. 2012). DNA methylation was quantified by pyrosequencing. Pyrosequencing for allele quantification (PSQ H96A; Biotage AB, Kungsgatan, Sweden) is a real-time sequencing-based DNA analysis software that quantifies multiple and individual consecutive CpG sites (Shin et al. 2012). Briefly, 1,000 ng of sample DNA was bisulfite treated using the Zymo DNA Methylation kit (Zymo Research, Orange, CA). Bisulfite-treated DNA was eluted in a 10-μl volume, and 1 μl of it was used for each PCR. PCR was performed using 10 × PCR buffer, 3.0 mM MgCl2, 200 μM of each dNTP, 0.2 μM each of forward and reverse primers, 1.25 U of Hot Start DNA polymerase (Qiagen), and ~10 ng bisulfite converted DNA per 50 μl reaction. PCR cycling conditions were 94°C for 15 min; 45 cycles of 94°C for 30 sec, 53°C for 30 sec, 72°C for 30 sec; 72°C for 5 min; and the amplification products were maintained at 4°C. PCR was performed with one of the PCR primers biotinylated to convert the PCR product to single-stranded DNA templates. The PCR products (each 10 μl) were sequenced by the Pyrosequencing PSQ96 HS system (Biotage AB, Uppsala, Sweden), following the manufacturer’s instructions. The methylation status of each locus was analyzed individually as T/C SNP in QCpG software (Biotage AB, Uppsala, Sweden).
Intracerebroventricular injection of antisense ObRb oligonucleotides
To test the functional role of leptin receptor (ObRb) by antisense oligonucleotides, antisense (5′-tttctgacacgtcatctt-3′) targeting ObRb or scrambled (5′-gagcacgtttcaagacaa-3′) nucleotides were intracerebroventricularly injected in a volume of 2.5 μl containing 1 μg oligonucleotides respectively daily from PN2-PN8 using landmarks as previously described (Chauhan 2002; Chauhan and Siegel 2007; Kumar et al. 2000; Shin et al. 2012). These landmarks consisted of coordinates set at 0.4 mm lateral to the midline, 0.4 mm posterior to the bregma and 2.5 mm ventral to the skull surface (Varma et al. 2004).
Data analysis
All data is expressed as Mean±SEM. All four experimental groups were compared, following establishment of normality, by analysis of variance and F values were determined. Once significance was established, inter-group differences were validated by the Fisher’s paired least significance difference test. Final p values were considered significant at < 0.05.
Results
Anthropometric and Metabolic Characteristics
Body weight gain from PN2 through PN21 in the four experimental groups is seen in Fig 1B. Weight gain was reduced in CRS versus CONS, however administration of leptin did not alter the weight gain pattern in either group (CONL and CRL) versus the respective saline groups (CONS and CRS) (Fig 1B). Circulating leptin concentrations also decreased in CRS versus CONS at PN21, however earlier administration of leptin (PN2 to PN8) in CONL and CRL did not affect the circulating leptin concentrations at PN21 when compared to their respective saline groups (CONS and CRS) (Fig 1C).
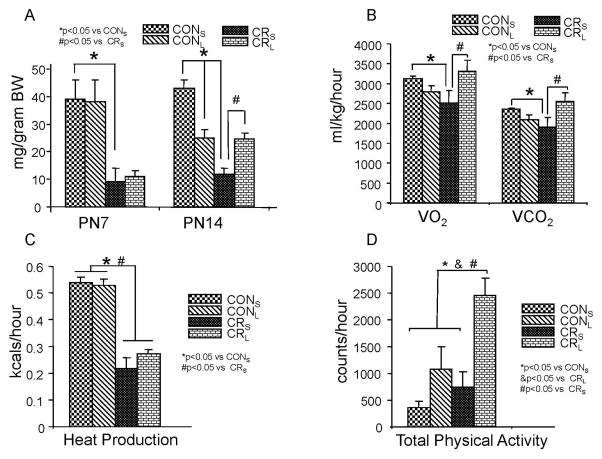
To examine metabolic changes in response to leptin treatment, energy intake, O2 consumption, CO2 production, heat production and total activity were measured (Fig. 2). Energy intake measurements demonstrated that the control group consumed more calories at both PN7 and PN14 versus the age-matched calorie restricted group (Fig 2A). Leptin intervention demonstrated no difference at PN7 in CONL versus CONS and CRL versus CRS, although both CR groups were significantly lower than their respective control groups (Fig 2A). This finding is expected given the imposed calorie restriction in lactating mothers at PN7 per study design. However at PN14, CONL consumed lesser calories than CONS. In contrast, CRL consumed 75% more calories than CRS despite ongoing maternal calorie restriction. CRS in turn consumed much lower amounts of calories compared to both CONS and CONL groups (Fig 2A). This implies that the control group exhibits leptin sensitivity even after cessation of leptin administration, while the calorie-restricted group appears to lack leptin sensitivity with respect to energy intake. An opposing divergence in leptin sensitivity between CON and CR is also seen with energy expenditure. Oxygen consumption (ml/kg/hr), CO2 production (ml/kg/hr), and heat production (kcals/hr) decreased in CRS versus the respective CONS (Fig 2B & C). With leptin administration, no change in VO2, VCO2 and heat production of CONL versus CONS is seen (Fig 2B & C), supporting a lack of leptin sensitivity in this regard. Leptin treatment led to an increase in VO2 and VCO2 with no change in heat production in CRL versus CRS (Fig 2B & C), demonstrating leptin sensitivity. In addition, total physical activity increased in CRS versus CONS. Leptin led to ~80% enhanced total physical activity in CRL versus CRS but also versus the two control groups CONS and CONL (Fig 2D). This increase in physical activity achieved by leptin administration in CRL may be responsible for the augmented energy intake seen (Fig 2A).
Figure 2. Energy intake (A), O2 consumption (VO2) and CO2 production (VCO2) (B), heat production (C) and total physical activity (D) are shown in four experimental groups.
A. Energy intakes are shown in grams/gram body weight per one hour at P7 and P14 (n=12 each per age and group). At PN7, milk intake is decreased in calorie-restriction (CRS) vs control (CONS), and no intergroup differences are seen with leptin intervention (CONL and CRL versus CRS). At PN14, milk intake is still decreased in calorie-restriction (CRS) vs control (CONS). At PN14, with leptin intervention, CONL consumes less than CONS but CRL consumes more than CRS. B. VO2 and VCO2 as ml/kg/hour in four experimental groups at PN14 are shown (n=9 per group). Calorie restriction (CRS) demonstrated decreased VO2 and VCO2 when compared to control (CONS). Leptin treatment had no effect in control (CONL vs CONS) but with calorie-restriction, CRL had increased VO2 and VCO2 versus CRS. C. Heat production as kcals/hr in four experimental groups at PN14 (n=9 per group). Heat production is lower in calorie-restriction (CRS) compared to control (CONS). No difference is seen with leptin intervention in control (CONL versus CONS) and caloric-restriction (CRS versus CONS) groups. D. Total physical activity as an average of ambulatory activity level counts measured as counts of movements in X and Z axes in the metabolic chamber of four experimental groups at PN14 (n=9 per group). No difference in total physical activity was seen in control (CONS) versus calorie-restriction (CRS), until leptin intervention was imposed when CRL was more active than both control groups, CONS and CONL, and its saline counterpart, CRS.
Hypothalamic neuropeptides and leptin signaling
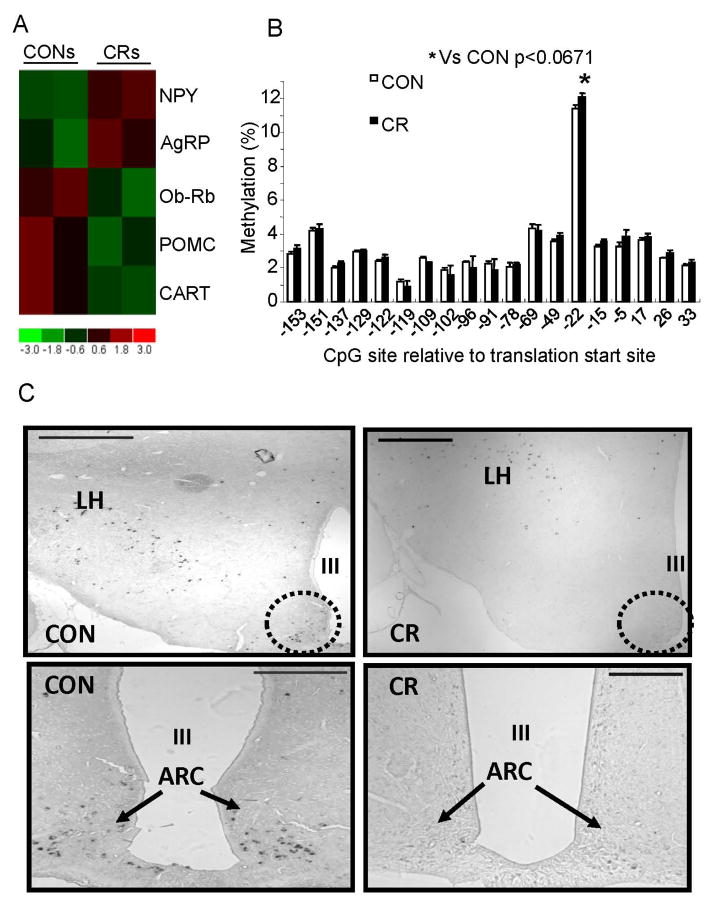
Microarray expression profiles revealed perturbations in select orexigenic (NPY and AgRP) and anorexigenic (POMC and CART) hypothalamic neuropeptides and ObRb (long form of the leptin receptor) (Fig 3A). Hypothalamic NPY and AgRP increased and ObRb, POMC and CART decreased in CRs when compared to CONs (Fig 3A). Since DNA methylation of all these genes except CART was previously examined (Shin et al. 2012), DNA methylation of the CART gene promoter was assessed by bisulfite modification and quantified by pyrosequencing (Fig 3B). One CpG site was differentially methylated, with methylation trending to an increase in CR versus CON (both not injected), although not statistically significant (p<0.067) (Fig 3B). In keeping with this trend, in-situ hybridization revealed a dramatic reduction with disappearance of CART mRNA within the arcuate nucleus in CR versus CON, while no such change was seen in the lateral hypothalamus (De Jonghe et al.) (Fig 3C).
Figure 3. Hypothalamic gene expression profiles, DNA methylation of hypothalamic CART promoter region and CART gene expression in CON and CR groups.
A. Microarray analysis: Orexigenic (NPY and AgRP) and anorexigenic (POMC and CART) neuropeptides including leptin receptor gene, ObRb, expression by microarray analyses is shown. Heat map displays CONs (left two columns) and CRs (right two columns) labeled at the top. At the bottom is shown the heatmap index, with red indicating up-regulation as compared to control (CONs); green down-regulation as compared to control (CONs). NPY and AgRP were increased while POMC and CART were decreased in CRs compared to CONs. B. DNA methylation of the CART gene: Genomic DNA from the hypothalamic CART promoter region was analyzed for differential methylation by bisulfite conversion and pyrosequencing.*p< 0.067 compared to CON. C. In situ hybridization histochemistry, demonstrates CART gene expression (arrows point to the black staining) in the arcuate nucleus (dotted circle) of CON and CR groups. LH = lateral ventricle, III = third ventricle. Scale bars = 200 μm
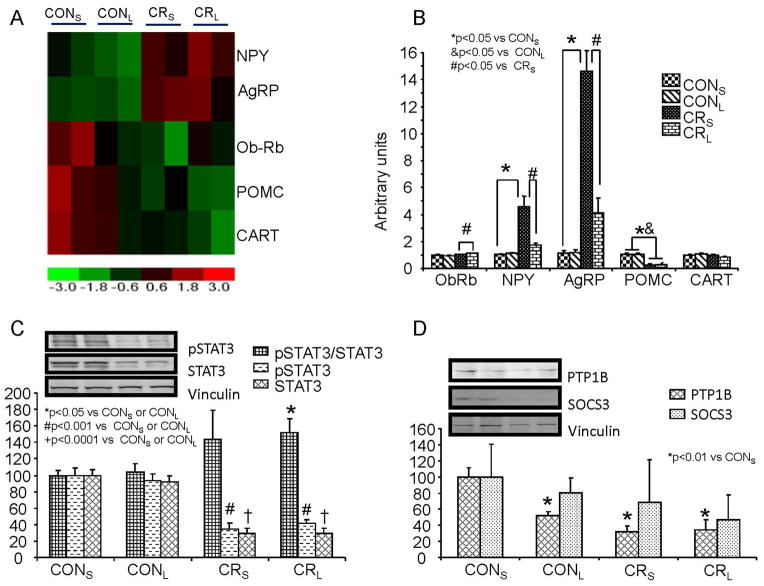
Next microarray expression profiles in leptin versus saline injected CON and CR groups revealed changes in hypothalamic neuropeptides (Fig 4A) that were validated by reverse-transcription and qPCR quantification. NPY and AgRP expression was increased, while ObRb, POMC and CART expression were decreased in CRS versus both CONS and CONL. Following leptin administration NPY and AgRP expression was decreased in CRL versus CRS approaching the two control groups, namely CONS and CONL (Fig 4B). In contrast, POMC and CART expression did not change in CRL versus CRS (Fig 4B). Hypothalamic ObRb mRNA was unchanged in CRS versus CONS (Fig 4B). However, leptin treatment led to a small but statistically significant increase in ObRb in CRL vs CRS, while not changing CONL and CONS. We then examined leptin receptor signaling by interrogating changes in post-receptor downstream molecules, such as p(phosphorylated)STAT3, PTP1B and SOCS3. Immunoblotting with antibodies against signaling proteins STAT3, pSTAT3, SOCS3 and PTP1B revealed perturbations (Fig 4 C & D). pSTAT3 or STAT3 decreased in CRS versus CONS, however no difference was seen between CRS and CRL. pSTAT3:STAT3 ratio on the other hand increased in CRL versus either CONS or CONL (Fig 4C). Although no difference in PTP1B concentrations between CRS and CRL was evident, PTP1B decreased in CONL and both CRS and CRL versus CONS (Fig 4D). In contrast, while a trend towards a decrease in CRL was seen no statistically significant changes in SOCS3 were observed between the four groups (Fig 4D).
Figure 4. Hypothalamic gene expression profiles, validation of mRNA expression of hypothalamic neuropeptides and ObRb related signaling peptides.
A. Expression profile of hypothalamic orexigenic (NPY and AgRP) and anorexigenic (POMC and CART) neuropeptides in four experimental groups is shown (n=2 per group). The heat map displays CONS, CONL, CRS, and CRL labeled at the top of the columns. Rows demonstrate hypothalamic NPY, AgRP, ObRb, POMC and CART expression. At the bottom is the heatmap index, with red indicating up-regulation as compared to the control (CONS); green down-regulation as compared to control (CONS). NPY and AgRP are upregulated in CRS compared to CONS; ObRb, POMC and CART in CRS are downregulated compared to control (CONS). With leptin treatment in calorie-restricted pups (CRL), the degree of upregulation of NPY and AgRP appears blunted (lower) versus that seen in CRS (CRL<CRS). In contrast, ObRb reduction in CRL is much less than that seen in CRS (CRL>CRS), while the reduction in POMC and CART expression appears greater in CRL versus CRS (CRL<CRS). B. mRNA quantification by reverse transcription and real time PCR of hypothalamic ObRb and orexigenic and anorexigenic neuropeptides in four experimental groups at PN21 (n=12 per group). ObRb, no difference between CONS and CRS is observed. When leptin is administered, no effect is seen in CONL versus CONS, but a slight increase occurs in CRL vs CRS. NPY and AgRP, calorie-restriction (CRS) increased NPY and AgRP expression versus CONS, but leptin intervention (CRL) blunted this response (CRS) of NPY and AgRP, thereby approaching control (CONS) values. POMC, calorie restriction (CRS) had an opposite effect, decreasing expression versus control (CONS). No further change is seen with leptin (CRL versus CRS). No difference in hypothalamic CART expression was seen with either calorie-restriction (CRS) or leptin intervention (CRL). C & D. Western blot analysis demonstrates hypothalamic STAT3, phosphorylated (p)STAT3 (C), PTP1B & SOCS3 proteins (D) in four experimental groups at PN21 (n=6 per group). Calorie restriction (CRS) reduced STAT3 and pSTAT3, however no difference in the two groups was noted with leptin treatment (CONL versus CONS and CRL versus CRS). However, when the ratio of pSTAT3 to total STAT3 was examined, CRS, not significant, but CRL expressed an increase versus CONS and CONL. PTP1B, demonstrated a reduction in CONL, CRS and CRL versus CONS, however no difference between CRL and CRS was observed. SOCS3, while demonstrating a trend towards a decline in CONL, CRS and CRL versus CONS, was not statistically significant. The insets demonstrate representative Western blots with vinculin serving as an internal loading control. Separate blots are indicated by black boxes.
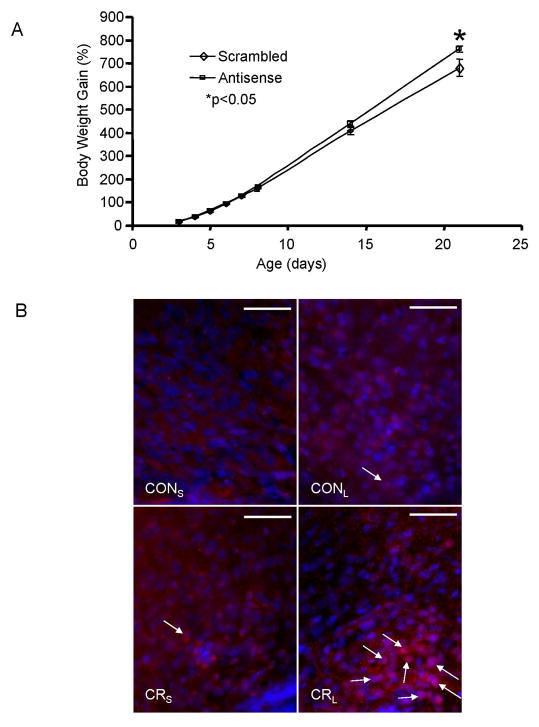
Antisense oligonucleotides targeting ObRb and scrambled oligonucleotides were injected intracerebroventricularly (Fig 5A). Body weight gain increased by PN21 in the group that received the antisense oligonucleotide when compared to the scrambled oligonucleotide group (Fig 5A), supporting a role for ObRb in regulating weight gain during the postnatal period. In addition, the acute post-receptor signaling response to leptin administration in both CON and CR groups was examined in brain sections that were subjected to pSTAT3 immunohistochemistry 45 min following a single dose of leptin administration in CON and CR (Fig 5B). CRL demonstrated strong pSTAT3 signals compared to the other three groups, CONS, CONL or CRS (Fig 5B).
Figure 5. Leptin signaling - ObRb and pSTAT3.
A. Growth curves (percent body weight gain) of pups that received intracerebroventricular injections of antisense or scrambled ObRb oligonucleotides. B. Immunofluorescence staining using the rabbit anti-pSTAT3 antibody in PN21 hypothalami. Pups from each experimental group (CON and CR) were subjected to only a single intraperitoneal leptin (CONL and CRL) or saline (CONS and CRS) administration. To examine the acute leptin effect, after 45 min, pups were anesthetized and perfused with 4% paraformaldehyde fixative solution. Arrows indicate pSTAT3 positive staining (red) in peri- or nuclear regions of cells within the arcuate nucleus. DAPI (blue) stained the nuclei. Scale bars = 50 μm
Discussion
We have demonstrated that early postnatal parenteral leptin administration replenishes low circulating leptin concentrations during a critical window of hypothalamic development in the offspring exposed to pre- and postnatal calorie restriction. The impact of exogenous leptin intervention lasts beyond the therapeutic period in both the CON and CR offspring. While the effect of leptin in CON appears to be limited to milk intake alone, the effect on the energy balance and hypothalamic regulatory mechanisms of the CR offspring appear corrective of the existing derangements. These findings provide the mechanistic basis for previous observations where early postnatal leptin administration reversed the adult phenotype of hyperphagia, inactivity and resultant obesity (Vickers et al. 2005). We and others have demonstrated that pre- and postnatal caloric restriction sets the stage for hypothalamic neuropeptides to counteract this adverse nutritional state by mediating hyperphagia and physical inactivity. When ad lib nutrition and more importantly high caloric diet is made available post-weaning, the stage is set for initial “catch-up” growth and subsequent mal-distribution of adiposity with onset of chronic diseases and complications.
In utero, the main source of leptin is the placenta. Ex-utero during the suckling phase, the major source of leptin is mother’s milk and to some extent subcutaneous fat depots if they exist (Rayner et al. 1997). Pre- and postnatal calorie restriction with reduced availability of mother’s milk and low subcutaneous fat stores results in reduced circulating leptin concentrations. In addition, the normal postnatal leptin surge that occurs at PN10 in mice is advanced to PN7 with a reduction in the amplitude of this surge as previously observed (Delahaye et al. 2008; Smith and Waddell 2003; Yura et al. 2005).
Leptin is a key trophic factor that triggers the development of hypothalamic arcuate nuclear feeding circuits, by stimulating growth of projections from the arcuate nucleus to secondary neurons located deeper within the hypothalamus such as the dorsal median nucleus (DMN), paraventricular nucleus (PVN), and lateral hypothalamus (Bouret et al. 2004a; Bouret et al. 2004b). Hypothalamic regulation of energy balance is sensitive to circulating leptin as evidenced by decreased food/milk intake and increased energy expenditure in response to exogenous leptin. Pre- and postnatal calorie restriction in response to decreased calorie (milk) intake was associated with decreased body weight and circulating leptin concentrations. This led to decreased energy expenditure (oxygen consumption), CO2 production, and heat production, perhaps in an energy conservation mode. In association with this phenotype, pre- and postnatal calorie restriction perturbs the balance between hypothalamic orexigenic:anorexigenic neuropeptide expression. Thus low circulating leptin concentrations were associated with an increase in orexigenic neuropeptide expression (NPY, AgRP) and a decrease in anorexigenic neuropeptide expression (POMC, CART). This hypothalamic expression pattern is facilitative of increased food intake and decreased energy expenditure, pre-runner characteristics of obesity. Thus despite a reduction in postnatal body weight, the hypothalamic regulation of energy balance is pre-programmed towards developing obesity in the presence of adequate or increased caloric availability. To avoid the path towards developing obesity, we undertook replenishment of leptin in the offspring exposed to pre- and postnatal calorie restriction. Since sex-specificity of leptin-sensitive neurotrophic effect resided in female postnatal murine hypothalamus (Mela et al. 2012; Shin et al. 2012), we focused on the female offspring in this study. Besides our previous investigations related to postnatal exposures involving leptin, neuropeptide Y or glucocorticoids that perturb the rat hypothalamic equipoise, uniformly affected the adult phenotype in females alone (He et al. 2004; Varma et al. 2004; Varma et al. 2003). Even in the human, there are gender-based differences in the regulation and action of circulating leptin. In obese individuals, circulating leptin concentrations are higher in women than men (Kennedy et al. 1997; Wabitsch et al. 1997). Additionally female calorie-restricted rat offspring has the propensity of presenting as obese mothers during pregnancy and displaying an aberrant intra-uterine metabolic environment to the next generation.
We demonstrated that body weight during the suckling phase was not affected by leptin administration in both CON and CR groups, although the CR offspring exposed to exogenous leptin trended in gaining less weight versus the saline-treated counterpart. This is consistent with a previous report involving control pups (Yura et al. 2005). In this prior study, postnatal leptin administration to CON pups however subsequently led to a higher body weight in the adult offspring when reared on a high fat diet. (Yura et al. 2005).
Previously leptin was also observed to impose the anorexic effect only post-weaning and not during the immediate postnatal period (Ahima and Hileman 2000). In our present study, during leptin treatment (PN7), no effect on milk intake was evident, however after cessation of the leptin treatment (PN14), a milk intake-reducing effect of leptin is evident during the postnatal stage in CON but increased milk intake is seen in the CR group. This increased milk intake in CR parallels the increased energy expenditure and physical activity seen.
Increased energy expenditure and physical activity in CR reflects the leptin-induced decrease in hypothalamic orexigenic neuropeptide expression (NPY, AgRP - known to reduce energy expenditure and physical activity) without any effect on anorexigenic neuropeptides (POMC, CART – known to enhance energy expenditure and physical activity). This resistance of POMC and CART expression from increasing in response to leptin may be a protective mechanism against further reducing energy intake. This may explain the increased milk intake in PN14 CRL versus CRS. More recently, oral administration of leptin throughout the postnatal period after only a modest 20% restriction of calories during conception (E1-E12 days) led to recovery of the hypothalamic structure and neuropeptides at PN25 (Konieczna et al. 2013). In our present study, this effect of exogenous leptin over a shorter postnatal period (PN2-PN8) following late gestational calorie restriction (E11 to E21) on hypothalamic NPY and AgRP is mediated by enhanced post-ObRb (Cottrell et al. 2010) signaling seen as increased pSTAT3 and reduced PTP1B and SOCS3 (both inhibitors of leptin signaling). However the dramatic reduction in hypothalamic pSTAT3 seen in CRS versus CONS in the absence of exogenous leptin may be responsible for the link between low circulating leptin concentrations-reduced ObRb signaling and decreased hypothalamic POMC and CART with increased NPY and AgRP. Hypothalamic NPY and AgRP are known to demonstrate functional selectivity to carbohydrates during the post-weaning period (Leibowitz et al. 2005).
To prove that ObRb signaling is responsible for the observed changes in the hypothalamic neuropeptides and the resultant body weight phenotype, we observed an increase in body weight at PN21 in response to early postnatal intracerebroventricular administration of antisense oligonucleotides targeting ObRb, similar toa previous study in adult rats (Shih et al. 2003). Other groups administered ObRb antagonists and observed dysregulation of ObRb mediated neurotropic effect in the hypothalamus (Mela et al. 2012) and in other organs (Attig et al. 2011). However, upon replenishment of the low circulating leptin concentrations in the CR group with exogenous leptin, correction of only the increased orexigenic neuropeptides but not the decreased anorexigenic neuropeptides was noted in our present study. The reason for this selective leptin effect in the postnatal hypothalamus is unknown, but perhaps protective against further reduction in milk intake and extinction.
We speculate that this may also relate to leptin induced epigenetic modifications that were not investigated in the present study. Maternal undernutrition imposed during conception led to histone modifications and hypomethylation of the hypothalamic POMC promoter although POMC mRNA expression was unchanged (Stevens et al. 2010; Stevens et al. 2011). However, our previous studies of gestational calorie restriction did not alter NPY and POMC promoter DNA methylation (Shin et al. 2012), although a trend towards hypermethylation of a single CpG site in the CART promoter which was associated with a reduction in arcuate nuclear CART expression was only evident by in-situ hybridization but not by reverse transcription-real time PCR. Thus, epigenetic modifications in response to pre- and postnatal calorie restriction and leptin treatment may become evident along with specific hypothalamic nuclear expression of POMC when unraveled in future studies.
In summary, our studies demonstrate that leptin replenishment in intrauterine and postnatal calorie restricted states reverses the deleterious imbalance in hypothalamic neuropeptides responsible for altering energy intake and expenditure. This important intervention during a critical period of hypothalamic plasticity has the potential of reversing hyperphagia and inactivity seen prior to the development of obesity. Our present study is a proof-of-principle that supports the value of developing targeted therapies in reversing preprogrammed mechanisms responsible for the subsequent development of obesity. Our results also pave the way towards developing leptin-like molecules that will prove safe to administer during the early postnatal period of development in interrupting the trajectory towards obesity.
Acknowledgments
We thank Dr. A. F. Parlow of the Harbor-UCLA Research and Education Institute (Torrance, CA) and the NIDDK’s National Hormone & Peptide Program for the recombinant mouse leptin; Alexander H. Tyan and Regina Lee for help maintaining the animal model, Amit Ganguly and Shanthie Thamotharan for tissue collection from rats. This work was supported by grants from NIH, HD-25024 (to S.U.D.) and HD-41230 (to S.U.D). L. C. Gibson was supported by NIH T32 HD07549 (to S.U.D.) and Marshall Klaus Perinatal Research Award (2010).
References
- Ahima RS, Hileman SM. Postnatal regulation of hypothalamic neuropeptide expression by leptin: implications for energy balance and body weight regulation. Regul Pept. 2000;92(1–3):1–7. doi: 10.1016/s0167-0115(00)00142-7. [DOI] [PubMed] [Google Scholar]
- Attig L, Larcher T, Gertler A, Abdennebi-Najar L, Djiane J. Postnatal leptin is necessary for maturation of numerous organs in newborn rats. Organogenesis. 2011;7(2):88–94. doi: 10.4161/org.7.2.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Bluher S, Shah S, Mantzoros CS. Leptin deficiency: clinical implications and opportunities for therapeutic interventions. J Investig Med. 2009;57(7):784–788. doi: 10.2310/JIM.0b013e3181b9163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci. 2004a;24(11):2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004b;304(5667):108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- Chauhan NB. Trafficking of intracerebroventricularly injected antisense oligonucleotides in the mouse brain. Antisense Nucleic Acid Drug Dev. 2002;12(5):353–357. doi: 10.1089/108729002761381320. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, Siegel GJ. Antisense inhibition at the beta-secretase-site of beta-amyloid precursor protein reduces cerebral amyloid and acetyl cholinesterase activity in Tg2576. Neuroscience. 2007;146(1):143–151. doi: 10.1016/j.neuroscience.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claire Wang Y, Gortmaker SL, Taveras EM. Trends and racial/ethnic disparities in severe obesity among US children and adolescents, 1976–2006. Int J Pediatr Obes. 2011;6(1):12–20. doi: 10.3109/17477161003587774. [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Mercer JG, Ozanne SE. Postnatal development of hypothalamic leptin receptors. Vitam Horm. 2010;82:201–217. doi: 10.1016/S0083-6729(10)82011-4. [DOI] [PubMed] [Google Scholar]
- De Jonghe BC, Hayes MR, Banno R, Skibicka KP, Zimmer DJ, Bowen KA, Leichner TM, Alhadeff AL, Kanoski SE, Cyr NE, Nillni EA, Grill HJ, Bence KK. Deficiency of PTP1B in POMC neurons leads to alterations in energy balance and homeostatic response to cold exposure. Am J Physiol Endocrinol Metab. 2011;300(6):E1002–1011. doi: 10.1152/ajpendo.00639.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahaye F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, Laborie C, Lesage J, Vieau D. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149(2):470–475. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341(12):879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- Faulconbridge LF, Hayes MR. Regulation of energy balance and body weight by the brain: a distributed system prone to disruption. Psychiatr Clin North Am. 2011;34(4):733–745. doi: 10.1016/j.psc.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima N, Yokouchi K, Kawagishi K, Moriizumi T. Effect of maternal deprivation on milk intake in normal and bilaterally facial nerve-injured developing rats. Neurosci Res. 2006;54(2):154–157. doi: 10.1016/j.neures.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Fung C, Evans E, Shin D, Shin BC, Zhao Y, Sankar R, Chaudhuri G, Devaskar SU. Hypoxic-ischemic brain injury exacerbates neuronal apoptosis and precipitates spontaneous seizures in glucose transporter isoform 3 heterozygous null mice. J Neurosci Res. 2010;88(15):3386–3398. doi: 10.1002/jnr.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M, Thamotharan M, Dai Y, Thamotharan S, Shin BC, Stout D, Devaskar SU. Early postnatal caloric restriction protects adult male intrauterine growth-restricted offspring from obesity. Diabetes. 2012;61(6):1391–1398. doi: 10.2337/db11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good D, Morse SA, Ventura HO, Reisin E. Obesity, hypertension, and the heart. J Cardiometab Syndr. 2008;3(3):168–172. doi: 10.1111/j.1559-4572.2008.00011.x. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Harrold JA. Hypothalamic control of energy balance. Curr Drug Targets. 2004;5(3):207–219. doi: 10.2174/1389450043490460. [DOI] [PubMed] [Google Scholar]
- He J, Varma A, Weissfeld LA, Devaskar SU. Postnatal glucocorticoid exposure alters the adult phenotype. Am J Physiol Regul Integr Comp Physiol. 2004;287(1):R198–208. doi: 10.1152/ajpregu.00349.2003. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation. 2012;126(1):126–132. doi: 10.1161/CIRCULATIONAHA.111.087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan JN, Li J, Han Y, Chen K, Wu N, Zhao AZ. Adipocyte-selective reduction of the leptin receptors induced by antisense RNA leads to increased adiposity, dyslipidemia, and insulin resistance. J Biol Chem. 2003;278(46):45638–45650. doi: 10.1074/jbc.M304165200. [DOI] [PubMed] [Google Scholar]
- Hukshorn CJ, Saris WH, Westerterp-Plantenga MS, Farid AR, Smith FJ, Campfield LA. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab. 2000;85(11):4003–4009. doi: 10.1210/jcem.85.11.6955. [DOI] [PubMed] [Google Scholar]
- Hukshorn CJ, van Dielen FM, Buurman WA, Westerterp-Plantenga MS, Campfield LA, Saris WH. The effect of pegylated recombinant human leptin (PEG-OB) on weight loss and inflammatory status in obese subjects. Int J Obes Relat Metab Disord. 2002;26(4):504–509. doi: 10.1038/sj.ijo.0801952. [DOI] [PubMed] [Google Scholar]
- James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263(4):336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, Pan Q, Garvey WT. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997;82(4):1293–1300. doi: 10.1210/jcem.82.4.3859. [DOI] [PubMed] [Google Scholar]
- Key TJ, Spencer EA, Reeves GK. Symposium 1: Overnutrition: consequences and solutions. Obesity and cancer risk. Proc Nutr Soc. 2010;69(1):86–90. doi: 10.1017/S0029665109991698. [DOI] [PubMed] [Google Scholar]
- Konieczna J, Garcia AP, Sanchez J, Palou M, Palou A, Pico C. Oral leptin treatment in suckling rats ameliorates detrimental effects in hypothalamic structure and function caused by maternal caloric restriction during gestation. PLoS One. 2013;8(11):e81906. doi: 10.1371/journal.pone.0081906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar VB, Farr SA, Flood JF, Kamlesh V, Franko M, Banks WA, Morley JE. Site-directed antisense oligonucleotide decreases the expression of amyloid precursor protein and reverses deficits in learning and memory in aged SAMP8 mice. Peptides. 2000;21(12):1769–1775. doi: 10.1016/s0196-9781(00)00339-9. [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Franceschi S, Decarli A, Gallus G, Tognoni G. Risk factors for endometrial cancer at different ages. J Natl Cancer Inst. 1984;73(3):667–671. [PubMed] [Google Scholar]
- Lapidus L, Helgesson O, Merck C, Bjorntorp P. Adipose tissue distribution and female carcinomas. A 12-year follow-up of participants in the population study of women in Gothenburg, Sweden. Int J Obes. 1988;12(4):361–368. [PubMed] [Google Scholar]
- Leibowitz SF, Sepiashvili K, Akabayashi A, Karatayev O, Davydova Z, Alexander JT, Wang J, Chang GQ. Function of neuropeptide Y and agouti-related protein at weaning: relation to corticosterone, dietary carbohydrate and body weight. Brain Res. 2005;1036(1–2):180–191. doi: 10.1016/j.brainres.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A. 2001;98(1):31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O’Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, Krauss RM, Veldhuis JD, Wagner AJ, DePaoli AM, McCann SM, Wong ML. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci U S A. 2004;101(13):4531–4536. doi: 10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meas T, Deghmoun S, Armoogum P, Alberti C, Levy-Marchal C. Consequences of being born small for gestational age on body composition: an 8-year follow-up study. J Clin Endocrinol Metab. 2008;93(10):3804–3809. doi: 10.1210/jc.2008-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela V, Diaz F, Gertler A, Solomon G, Argente J, Viveros MP, Chowen JA. Neonatal treatment with a pegylated leptin antagonist has a sexually dimorphic effect on hypothalamic trophic factors and neuropeptide levels. J Neuroendocrinol. 2012;24(5):756–765. doi: 10.1111/j.1365-2826.2012.02279.x. [DOI] [PubMed] [Google Scholar]
- Messerli FH. Cardiovascular effects of obesity and hypertension. Lancet. 1982;1(8282):1165–1168. doi: 10.1016/s0140-6736(82)92234-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- Rayner DV, Dalgliesh GD, Duncan JS, Hardie LJ, Hoggard N, Trayhurn P. Postnatal development of the ob gene system: elevated leptin levels in suckling fa/fa rats. Am J Physiol. 1997;273(1 Pt 2):R446–450. doi: 10.1152/ajpregu.1997.273.1.R446. [DOI] [PubMed] [Google Scholar]
- Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185(1–2):93–98. doi: 10.1016/s0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- Shih CD, Au LC, Chan JY. Differential role of leptin receptors at the hypothalamic paraventricular nucleus in tonic regulation of food intake and cardiovascular functions. J Biomed Sci. 2003;10(4):367–378. doi: 10.1159/000071156. [DOI] [PubMed] [Google Scholar]
- Shin BC, Dai Y, Thamotharan M, Gibson LC, Devaskar SU. Pre- and postnatal calorie restriction perturbs early hypothalamic neuropeptide and energy balance. J Neurosci Res. 2012;90(6):1169–1182. doi: 10.1002/jnr.23013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin BC, McKnight RA, Devaskar SU. Glucose transporter GLUT8 translocation in neurons is not insulin responsive. J Neurosci Res. 2004;75(6):835–844. doi: 10.1002/jnr.20054. [DOI] [PubMed] [Google Scholar]
- Shin BC, Suzuki M, Inukai K, Anai M, Asano T, Takata K. Multiple isoforms of the regulatory subunit for phosphatidylinositol 3-kinase (PI3-kinase) are expressed in neurons in the rat brain. Biochem Biophys Res Commun. 1998;246(2):313–319. doi: 10.1006/bbrc.1998.8606. [DOI] [PubMed] [Google Scholar]
- Smith JT, Waddell BJ. Developmental changes in plasma leptin and hypothalamic leptin receptor expression in the rat: peripubertal changes and the emergence of sex differences. J Endocrinol. 2003;176(3):313–319. doi: 10.1677/joe.0.1760313. [DOI] [PubMed] [Google Scholar]
- Stevens A, Begum G, Cook A, Connor K, Rumball C, Oliver M, Challis J, Bloomfield F, White A. Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology. 2010;151(8):3652–3664. doi: 10.1210/en.2010-0094. [DOI] [PubMed] [Google Scholar]
- Stevens A, Begum G, White A. Epigenetic changes in the hypothalamic pro-opiomelanocortin gene: a mechanism linking maternal undernutrition to obesity in the offspring? Eur J Pharmacol. 2011;660(1):194–201. doi: 10.1016/j.ejphar.2010.10.111. [DOI] [PubMed] [Google Scholar]
- Thamotharan M, Shin BC, Suddirikku DT, Thamotharan S, Garg M, Devaskar SU. GLUT4 expression and subcellular localization in the intrauterine growth-restricted adult rat female offspring. Am J Physiol Endocrinol Metab. 2005;288(5):E935–947. doi: 10.1152/ajpendo.00342.2004. [DOI] [PubMed] [Google Scholar]
- Varma A, He J, Shin BC, Weissfeld LA, Devaskar SU. Postnatal intracerebroventricular exposure to leptin causes an altered adult female phenotype. Am J Physiol Endocrinol Metab. 2004;287(6):E1132–1141. doi: 10.1152/ajpendo.00228.2004. [DOI] [PubMed] [Google Scholar]
- Varma A, He J, Weissfeld L, Devaskar SU. Postnatal intracerebroventricular exposure to neuropeptide Y causes weight loss in female adult rats. Am J Physiol Regul Integr Comp Physiol. 2003;284(6):R1560–1566. doi: 10.1152/ajpregu.00557.2001. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146(10):4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- Wabitsch M, Blum WF, Muche R, Braun M, Hube F, Rascher W, Heinze E, Teller W, Hauner H. Contribution of androgens to the gender difference in leptin production in obese children and adolescents. J Clin Invest. 1997;100(4):808–813. doi: 10.1172/JCI119595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, Fujii S. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005;1(6):371–378. doi: 10.1016/j.cmet.2005.05.005. [DOI] [PubMed] [Google Scholar]