Abstract
There has been considerable interest in the role of the lymphoma microenvironment. Despite the use of highly active antiretroviral therapy (HAART), AIDS-related diffuse large-B-cell lymphoma remains common and HIV-relatedHIV-associated classical Hodgkin's lymphoma is increasing in incidence. Less is known about the impact HIV and HAART have on the lymphoma microenvironment. AIDS-related diffuse large B-cell lymphoma is highly angiogenic, demonstrates increased lymphoblastic histology, proliferation, increased activated cytotoxic T cells, reduced CD4+ and FOXP3+ T cells, but no differences in tumor-associated macrophages. Early initiation of HAART improves immunosurveillance, but cases without viral antigens appear able to avoid immunologic reaction. Increased T cell infiltrates seen with HAART treatment in HIV-related classical Hodgkin's lymphoma may contribute to malignant cell growth.
Keywords: angiogenesis, HIV-related diffuse large B-cell lymphoma, Hodgkin's lymphoma, immunosurveillance, impact of highly active antiretroviral therapy, p17, Tat, tumor microenvironment
The burden of AIDS-related lymphoma (ARL) remains substantial even as we enter the fourth decade of the HIV/AIDS epidemic. The introduction of highly active antiretroviral therapy (HAART) has dramatically improved the outcomes of patients living with HIV. As the survival of people living with HIV increases, malignancy has become a leading cause of death in this population [1]. Between 25 and 40% of people living with HIV will develop cancer, with approximately 10% of these attributed to ARL. Infection-related cancer is likely to become an increasingly important complication of long-term HIV infection [2,3].
The introduction of HAART has resulted in a dramatic decrease in opportunistic infections, Kaposi sarcoma and primary CNS lymphoma. However, the situation surrounding AIDS-related diffuse large B-cell lymphoma (AR-DLBCL), Burkitt's lymphoma (BL) and HIV-associated Hodgkin's lymphoma (HIV-HL) has proven to be more complex. The decline in ARLs has been less pronounced than might be predicted with a simple relationship between HIV-associated immunosuppression and lymphoma [4]. There has been a steady but gradual decline in cases of AR-DLBCL and BL incidence, declining only slightly, has increased as a proportion of HIV-associated non-Hodgkin's lymphoma [5]. In contrast, the rate of HIV-HL is increasing despite the introduction of HAART. It remains unclear why HL has emerged to this extent in the HAART era, and as the survival of HIV-infected individuals increases, HL is likely to become an increasingly important complication of HIV infection [6].
There is increasing recognition of the importance of the local environment of tumor cells in cancer biology and disease progression. Within this tumoral microenvironment, immune-related cells, cells of the stromal microenvironment in addition to angiogenic vascular cells, engage in complex cross-talk with tumor cells, thereby providing a critically important milieu for tumor growth, invasion and metastasis. The roles these components play and the communication they engage in within the microenvironment have not been well elucidated and fresh insights are needed to identify new targets for drug discovery and development. In sporadic DLBCL, gene expression profiling (GEP) has amply established the existence of molecular subtypes with distinct genetic signatures and clinical outcomes [7]. These GEP studies also highlighted the importance of the tumor microenvironment; a study examined two distinct signatures derived from the nonmalignant cells within DLBCL and determined that these could independently predict outcome [8]. The first of these signatures, termed stromal-1, was prognostically favorable in patients treated with both cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and rituximab plus CHOP (R-CHOP) and included genes that are coequally expressed in many normal mesenchymal tissues and mostly associated with the extracellular matrix. Infiltration by myeloid lineage cells was a characteristic attribute of this signature. In contrast, DLBCL tumors with the prognostically unfavorable stromal-2 signature included genes involved in endothelial cell (EC) biology and adipocyte function and correlated with increased blood vessel density.
Much less work has been done in the setting of HIV-associated lymphomas and there is good reason to expect significant differences. Studies examining immunohistochemical prognostic markers validated in sporadic DLBCL found that they were not predictive in AR-DLBCL suggesting fundamental differences in pathophysiology [9]. These changes are likely to be related to and reflect in the mechanisms by which the tumor cells interact with their environment. The main cellular targets of HIV are the CD4+ T cells, dendritic cells, and cells of macrophage lineage; cells which play important roles in the microenvironments of both Hodgkin's and non-Hodgkin's lymphomas [10]. HIV does not infect the neoplastic B-cells; instead, the increased lymphomagenesis seen in HIV is hinged on its ability to manipulate the host immune system and microenvironment. Given the impact of HIV on key microenvironment components and the mechanisms involved in the neoplastic complications of AIDS, it seems logical to compare tumor microenvironments in the context of HIV with HIV-negative cases and also compare the pre and post HAART eras. A greater understanding of this area should yield further insight into HIV-associated lymphoma pathophysiology, perhaps highlight interactions fundamental to lymphoma subtypes as a whole, and indicate new therapeutic strategies.
This review will focus initially on the microenvironment of AR-DLBCL, before considering HIV-HL. Burkitt's lymphoma will not be covered but deserves mention as a lymphoma of increasing importance in the context of HIV and HAART where up to now the tumor microenvironment remains essentially unexplored.
The microenvironment in HIV-associated DLBCL
HIV-1 increases the risk for systemic DLBCL, the most common form of ARL, by 60–200-fold [11]. Notable differences between AR-DLBCL and DLBCL occurring in the general population include a higher frequency of extranodal disease and prominent association with Epstein–Barr virus (EBV) and HHV-8 gamma herpesviruses. Very few studies have focused on the microenvironment in AR-DLBCL.
Using paraffin-embedded tissue we compared the microenvironment in AR-DLBCL with sporadic cases and also compared the pre and post HAART eras, correlating this with clinical data [12]. AR-DLBCL showed inferior progression-free and overall survival. The outlook improved after HAART, however, HIV-positive status remained a negative prognostic factor for overall but not progression-free survival. Clinical factors associated with increased survival after AR-DLBCL included early stage disease, good performance status, low age adjusted international prognostic index (aaIPI), lack of B symptoms, HIV index 0–1, no prior AIDS defining illness, treatment with HAART and rituximab-containing chemotherapy. Of interest, the CD4 count <100 cells/mm3 at presentation was not statistically significant. Results of multivariate analysis revealed only two factors retained their prognostic significance, low aaIPI 0–1 and treatment with rituximab containing regimen.
Our comparison revealed a number of interesting findings (Table 1). This review will focus on two of them. Firstly, the AR-DLBCL microenvironment was characterized by much higher angiogenic activity than sporadic cases. This correlated with immunoblastic and plasmablastic morphology as well as with EBV positivity. Secondly, when compared with sporadic cases there were reduced T-helper (CD4+) and T-regulatory (FOXP3+) cells, but markedly higher numbers of CD8+ T cells. Notably, cases expressing viral antigen (LMP1 [EBV-related] and/or p24 [HIV-related]) contained much higher numbers of CD8+ T cells implicating potential differences in immunosurveillance in virally driven tumors.
Table 1.
Common themes: features of AR-DLBCL and HIV-HL microenvironments.
| AR-DLBCL | HIV-associated HL | ||
|---|---|---|---|
| Immunoblastic histology | ↑ | Mixed cellularity | ↑ |
| Proliferation index | ↑ | ||
| Angiogenesis | ↑ | ||
| Expression of viral antigens | ↑ | Expression of viral antigens | ↑ |
| CD4+ T cells | ↓ | CD4+ T cells | ↓ |
| CD8+ T cells | ↑ | CD8+ T cells | ↑ |
| FOXP3+ T cells | ↓ | FOXP3+ T cells | ↓ |
| Tumor-associated macrophages | → | Tumor-associated macrophages | → |
| M2 macrophages | ↑ | M2 macrophages | ↑ |
Angiogenesis
The finding of higher angiogenic activity in AR-DLBCL and correlation with immunoblastic and plasmablastic morphology as well as EBV suggests a link between angiogenesis and the presence of HIV, EBV or both. This finding is consistent with those of Nyagol et al. who examined the role of VEGF and HIV-1 Tat protein in angiogenesis in AR-DLBCL and Burkitt's lymphoma [13]. VEGF expression has been associated with angiogenesis across a range of tumors [14]. It is of particular interest in the context of HIV due to the Tat protein's ability to bind and activate downstream signaling at the VEGF receptor VEGFR-2 [15]. Furthermore, Tat protein can be secreted by HIV-infected cells so this mechanism does not require direct infection of the tumor cell [16]. The presence of Tat within the AR-DLBCL microenvironment was been previously documented and was also seen in our own study [12,17]. Nyagol et al. demonstrated the ability of Tat to down-regulate VEGF promoter activity in human 293 and Burkitt-derived Raji cell lines in vitro. In addition, the authors documented decreased VEGF expression in the context of increased micro-vessel density in HIV-1 positive tumors, implying a possible negative feedback loop and suggesting Tat may be influencing the microenvironment. They did not observe a correlation between EBV status and microvessel formation. Supporting this hypothesis studies in non-HIV related DLBCL have noted increased VEGF expression in DLBCL correlating with increased microvessel density and non-Germinal B-Cell morphology [18]. Further work in this area would be useful to demonstrate the relevance of this mechanism in AR-DLBCL, including documenting the expression of VEGFR-2 in this context. The intimate role HIV Tat appears to play within AR-DLBCL angiogenesis has parallels with the close relationship between HIV and the angioproliferative tumor Kaposi's sarcoma [19].
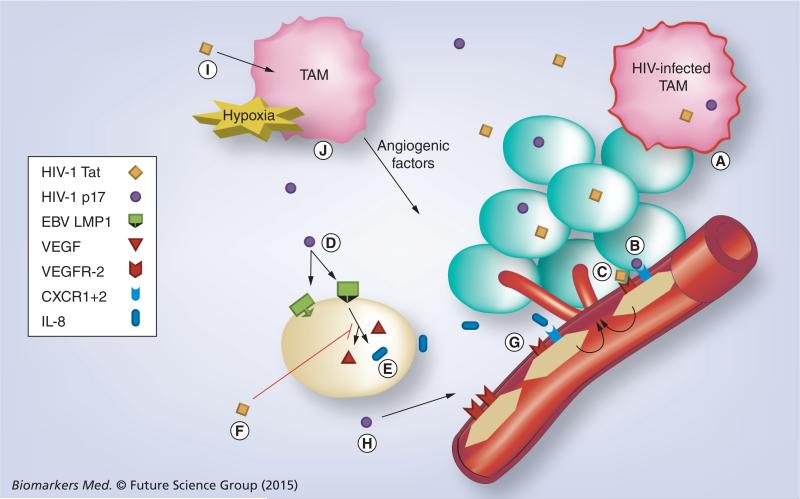
HIV-1 has also been implicated in angiogenesis via other secreted factors [20]. Data suggest that endothelium dysfunction relies on the action of HIV-1 proteins rather than on a direct effect of the virus itself and the HIV-1 matrix protein p17 is known to deregulate the biological activity of different immune cells. p17 mimics IL-8 chemokine activity by binding to the IL-8 receptor CXCR1 and CXCR2 and promotes the formation of capillary-like structures on human ECs. Further supporting a role for p17 in HIV-1-induced aberrant angiogenesis is the detection of p17 as a single protein in blood vessels and EC nuclei of HIV-1 infected patients [20,21]. Angiogenesis is a complex process. This evidence points to possible mechanisms and highlights some of the roles HIV may be playing in manipulating the tumor environment (Figure 1).
Figure 1. Proposed mechanisms of angiogenesis in AIDS-related diffuse large B-cell lymphoma and the role of HIV.
(A) HIV-infected tumor-associated macrophages (TAM) secrete HIV-1 viral factors Tat and p17. (B) HIV-1 p17 binds IL-8 receptors CXCR1 and CXCR2 stimulating angiogenesis†. (C) HIV-1 Tat binds VEGF receptor VEGFR2 stimulating angiogenesis independent of homeostatic controls on VEGF production. (D) HIV-1 p17 upregulates EBV LMP1 expression on EBV-infected tumor cells†. (E) LMP-1 increases VEGF and IL-8 production promoting angiogenesis. (F) HIV-1 Tat suppresses VEGF production, but not IL-8. (G) Tumor-produced IL-8 binds CXCR1+2 promoting angiogenesis†. (H) HIV-1 p17 upregulates VEGFR2 expression on epithelial cells†. (I) HIV-1 Tat induces secretion of IFN-γ in macrophages and activated CD8+ T lymphocytes†. (J) Uncertain additional role of Tumor-associated macrophages and mediation of angiogenesis†.
†Requires further investigation in context of AR-DLBCL.
EBV has also been shown to play a role in angiogenesis and our data suggest that neovascularization is tumor-driven and linked to immunoblastic features and the EBV status of tumor. Accumulating data from experimental studies provide helpful groundwork for the biologic link between EBV infection and the process of new-vessel development. The EBV-associated latent membrane protein LMP1 is known for its ability to cause transformation, proliferation and immortalization of B-cells. Signaling by LMP1 can alternatively upregulate HIF1 through Siah proteins resulting in VEGF production and in this way promote angiogenesis [22]. Increased VEGF was not a feature of the data provided by Nyagol et al.; however, LMP1 is heavily implicated in angiogenesis, promoting secretion of factors including IL-8 and IL-6 addition to VEGF in Nasopharyngeal carcinoma and lymphoblastoid cell lines [23–25]. The role of this mechanism in AR-DLBCL is supported by IL-8 being identified as part of the cytokine signature of AR-DLBCL [26]. In addition, HIV secreted p17 has been shown to upregulate LMP1 expression in cell lines [27]. The role it plays in the context of HIV needs further exploration. Finally, in cells undergoing lytic infection, a lytic viral gene has also been identified as angiogenesis activator [23].
We considered the role of macrophages, as there is considerable evidence to indicate that initiation of angiogenesis, the so-called angiogenic switch, is mediated by factors released by the tumor and often by macrophages attracted to it. No correlation was seen between blood-vessel density in AR-DLBCL and CD68+ macrophages. Likewise, no association was noted between microvessels and CD163, lymphocytic infiltration, Ki67, c-Myc status or CD4 count.
T cells, immunosurveillance & more on macrophages
There are changes in the morphology of DLBCL in AR-DLBCL. Before HAART, immunoblastic DLBCL was seen in virtually all cases, while in the HAART era, among HIV-infected patients, 22% had centroblastic, 48.8% immunoblastic, 9.7% plasmablastic lymphoma, and in the remaining 19.5% histology was unclassified. Among patients without HIV, centroblastic DLBCL accounted for 69.7% and immunoblastic for 11.7%, whereas unclassified cytology was noted in 18.6%.
From our current perspective on immunosurveillance, infiltrating lymphocytes play a key role in the host defense against tumor. Specifically, activated cytotoxic and helper T cells are responsible for immune responses within the tumor microenvironment. In the context of AR-DLBCL we expect distinctive changes in the microenvironment given the abnormalities in cell-mediated immunity caused by HIV. Enumeration of immune cells within AR-DLBCL biopsies compared with DLBCL without HIV showed proportionally fewer CD3+ T lymphocytes and markedly reduced CD4+ and FOXP3+ T-cells. As a result of CD4+ depletion, CD8+ T cells forms the predominant type of the T-cell infiltrate. Tumor-infiltrating cytotoxic T lymphocytes (CTLs), identified by staining for TIA1, perforin and granzyme B (GrB) demonstrated that TIA1+ CTL were more frequent among HIV-negative patients whereas granzyme expression was significantly higher in AR-DLBCL. Staining for CD56+, a characteristic glycoprotein of NK and NKT cells, showed a paucity of positive cells in both groups, ruling out other cytolytic-cell types. Thus, AR-DLBCL is characterized by a diminution in the proportion of helper and regulatory T cells and a disproportional enrichment in CTL, especially activated GrB+ CTL.
We were interested to see how this relationship changed with the introduction of HAART. Historically, the median survival pre HAART was less than 12 months, whereas after HAART it increased to 15–34 months [28]. This improvement is generally attributed to the restoration of cell-mediated immunity. HAART-era patients had higher CD4 counts and fewer AIDS-defining conditions than those presenting pre-HAART. Within the microenvironment, higher numbers of CD4+ T cells were present in HAART-era specimens (p < 0.001; although still reduced when compared with sporadic DLBCL), whereas expression of other lymphocytic markers was not significantly different. Surprisingly, only a weak correlation was observed between blood CD4+ T-cell counts and those seen in tumor biopsies. Two factors may account this weak correlation. First, the cancer–host immunological interaction is complex. CD4 count is not an unbiased measure of immunodeficiency and development of AIDS-defining illness is more accurately predicted by decay of tissue lymphocytes and involution of lymphoid than blood CD4 kinetics [29]. Second, blood lymphocytes represent only 2% of the total lymphocyte pool and their composition differs from that within the organs during progressive HIV disease [30].
To further characterize the role and nature of the infiltrating T cells, we stained for markers of exhaustion. PD1 and its ligand PD-L1 mediate a key inhibitory pathway on activated T cells characterized by diminished cytotoxicity, decreased cytokine production and increased apoptosis. T-cell exhaustion is present in chronic viral infections, where it has a negative impact on viral clearance, and in various cancers including B cell malignancies, where it is involved in tumor-mediated immune evasion [31,32]. CD274 (PD-L1) expression was seen at comparable levels on the tumor cells in HIV-positive and uninfected cases. CD279 (PD1) expression, however is seen on a higher proportion of AR-DLBCL than in sporadic cases.
Profiling the viral expression within AR-DLBCL tissue was pursued through staining for EBER and LMP1 for EBV, LANA1 for HHV-8 and p24 for HIV-1 infection. Coinfection with EBV or HHV-8 affected 60% of patients whereas approximately 20% were negative for EBV, HHV-8 and HIV-1 p24. These findings were consistent with those of Morton et al. who detected EBV in 63% of AR-DLBCL and found it correlated strongly with activated B-cell (ABC) type [33]. These results may have implications for pathogenesis because coinfection with γ-herpesviruses is a major cofactor for the development of ARLs. Biopsies positive for LMP1 and/or p24 contained a markedly higher number of CTL than cases negative for either viral antigen. Characteristically, the number of GrB+ CTL was lower in cases without LMP1 and p24 expression. CTL infiltration of EBER-positive, LMP1-negative and p24-negative specimens did not differ from the pattern seen in EBER-negative, p24-negative specimens. Therefore, cytotoxic cell infiltration of AR-DLBCL appears dependent at least in part on the presence of LMP1 or p24 viral antigens. The picture that emerges illustrates viral antigen-expressing DLBCL as more likely to raise a cytotoxic response than viral antigen-negative or nonviral cases. Since a major function of granzymes is the maintenance of immunosurveillance, our data support the idea that surveillance of infection-linked cancer normally depends on reducing viral burden through killing virus-infected cells [34]. Our findings might be also be taken as an argument that HIV patients, despite their immunosuppression, still have residual capability for immunological defense against viral cancer mounted by CTL.
Studying tumor macrophages revealed no differences in their numbers between HIV-infected and uninfected patients. In addition, we did not find between-group difference in the two AIDS periods and we did not observe a correlation between macrophages and survival. However, this should be interpreted with caution because the propagating HIV-1 within macrophages in combination with the chronic inflammation, systemic activation and opportunistic infections could cause dynamic variations in macrophages. Our data did show evidence of a low level of infected macrophages with p24 and Tat positivity. Macrophages are a major target of HIV and a source of virus production [10]. Overall, infected macrophages were found in 39% of samples, typically from HAART-naive patients. This figure is consistent with other studies [35]. HIV replication strongly represses CD163 expression, orientating macrophages towards a pro-inflammatory phenotype in HIV infection [36]. However, there was coordinate expression of CD68 and CD163 in the AR-DLBCL microenvironment, suggesting that the tumor-associated macrophages are alternatively activated (M2). Macrophages have been shown to play important roles in tumor progression as viral reservoirs and have even been implicated in ARL tumorigenesis with transplanted tumor-associated macrophages from ARL patients causing lymphoma in SCID mice (while T cells did not have this effect) [35]. Furthermore, in other settings, such as in Kaposi's sarcoma, HIV-1 Tat stimulates macrophage and activated CD8+ T Lymphocyte production of IFN-γ, which has angiogenic properties, a role that has not been explored in AR-DLBCL [19]. Hence, even though no significant difference in absolute numbers was noted their role in AR-DLBCL warrants closer examination.
Future directions in the AR-DLBCL microenvironment
Taken together, the study of the microenvironment in AR-DLBCL provides interesting insights into the pathophysiology of AR-DLBCL, providing a rationale for the consideration of novel therapeutics targeting the tumor microenvironment and angiogenesis. For example, future efforts could focus on characterizing the function of the tumor-infiltrating CD8+ T cells, especially with the advent of EBV-directed cytotoxic T-cell therapies targeting LMP antigens as a potential therapeutic for EBV-associated lymphomas [37]. In addition, it would be compelling to assess gene expression signatures from the host microenvironment cells in AR-DLBCL, as has been done in sporadic cases.
Regulating angiogenesis involves a complicated interplay of cells and cytokines, and studies that better elucidate the mechanics of this are needed; inhibiting angiogenesis by monoclonal antibodies to VEGF, or by using small-molecule inhibitors, may be interesting future strategies to investigate, particularly in AR-DLBCL. Further elucidating the pathophysiology of angiogenesis in AR-DLBCL could lead to improvements in our knowledge of the factors that control the angiogenic switch and enable these factors to be modified. Additional studies will be needed to define the relative roles of the malignant cell, EBV, HIV-1 and possibly their synergism. What has practical implications is that increased angiogenesis represents an attractive target that could be exploited therapeutically. The monoclonal antibody to VEGF, bevacizumab, is currently being tested in clinical trials involving sporadic DLBCL and HIV-associated Kaposi sarcoma [8,38]. It will be illuminating to see the impact of this. Furthermore, HIV-protease inhibitors can directly affect angiogenesis due to inhibition of matrix metalloprotease activity [39]. HAART containing a protease inhibitor may theoretically benefit the patient twofold by controlling HIV replication and tat and by limiting angiogenesis.
The increased numbers of activated CTLs observed raises questions around immunosurveillance. In viral-antigen-positive AR-DLBCL, judging from the insufficiency of CTLs to kill infected cells, pathways that impede cytotoxicity should take place. A recent study in mice pointed to the strategic role of CD4+ T cells in eliminating LMP1-positive cells and in keeping LMP1-driven lymphomas in check [40]. In fact, CD4+ T cells had superior in vivo cytolytic activity against LMP1-transformed cells. The killing activity developed by CD4+ T cells is likely mediated by class II-dependent degranulation of granzymes [41]. Extrapolating our findings on the composition of microenvironment and taking into account that both CD4+ and CD8+ T cells are necessary for cytotoxicity, the lack of LMP1-reactive CD4+ T cells could be the Achilles’ heel of the tumor-killing response against LMP1-positive AR-DLBCL. On the basis of this hypothesis, substantial increments in CD4+ T cells, through early initiation of HAART, should improve immunosurveillance and reduce the incidence of this type of ARL. As such, AR-DLBCL with viral antigens should be more likely to have an adjunctive antitumor benefit after improvement of immunosurveillance. AR-DLBCL without viral antigens, like DLBCL without infective basis, is characterized by a low percentage of granzyme-positive CTL and thus may be best viewed as able to hide from immunosurveillance. After surveying additional immunosuppressive mechanisms, we noted PDL1 expression in 28% of AR-DLBCL. The significance of PD1-PDL1 in the immunotherapy of AR-DLBCL remains to be defined.
Finally, there is a lack of simple immune biomarkers that can be applicable in the clinical setting to assist with patient stratification. Automated analysis is the preferred method for immunohistochemistry studies and for demonstrating the role played by immune cells such as CD4+ and FOXP3+ T cells in predicting response to chemoimmunotherapy in DLBCL and cHL [42]. Large automated assessments of the microenvironment would yield results of great interest in both AR-DLBCL and HIV-HL.
Hodgkin's lymphoma
In contrast to AR-DLBCL, the rate of HIV-associated Hodgkin's lymphoma (HL) is increasing following the introduction of HAART. In a prospective cohort of 11,112 HIV-infected patients, the standardized incidence ratio for HL was 4.5 in the pre-HAART era (1983–1996), 11.1 during the early HAART era (1996–2001) and 32.0 between 2002 and 2007 [43]. Evidence from observational studies indicates an association between HAART and HL. Although the growth of an ageing population may have contributed, the risk for HIV-HL remains markedly above that for age-matched general population, suggesting involvement of additional factors in the context of HIV. The high incidence after the availability of HAART has been called an epidemiological paradox, because by reconstituting the immune system, HAART was expected to reduce the occurrence of HIV-HL [44]. The current hypothesis is that incidence declines in severely immunosuppressed HIV-infected patients with low CD4+ cells because the malignant Reed-Sternberg cells are not able to recruit CD4+ lymphocytes required for their survival. [44–46] An alternative hypothesis suggests that potentially with advanced immunosuppression, HIV-HL might remain occult until the immune system is sufficiently reconstituted to respond to the Reed–Sternberg cells [47]. These hypotheses are not completely satisfactory and require testing but suggest that the answer for the rising tide of HIV-HL in the HAART era may be hidden in the tumor microenvironment. In the absence of an understanding of what constitutes a permissive microenvironment in the HAART era, it is unlikely that we can tackle the rising incidence of HIV-HL.
Classical HL (cHL) is unique among the lymphomas in that the bulk of the infiltrate comprises not the malignant Reed-Sternberg cells but inflammatory cells, including T cells, B cells, macrophages, neutrophils, eosinophils, fibroblasts and plasma cells in various proportions. The neoplastic Reed–Sternberg cells typically constitute less than 1% of the cells, regardless of the HIV status of the tumor [48,49]. Multiple lines of evidence support a causal relation between the composition of microenvironment and chemotactic factors secreted by Reed–Sternberg cells. Reed–Sternberg cells produce many cytokines and chemokines, resulting in an influx of activated CD4+ cells, macrophages and other cells. Moreover, Reed–Sternberg cells respond to the inflammatory cells in their vicinity. Thus, recruited inflammatory cells may provide essential feedback signals that stimulate proliferation and inhibit apoptosis of Reed–Sternberg cells. [45]
Much interest has focused on the microenvironment of cHL in the general population over the past decade. The results from these studies have become of particular interest as they suggest that targeting the microenvironment might be a useful strategy in the treatment of cHL [50,51]. The introduction of biological agents including those that target the tumor cells (e.g., brentuximab vedotin, HDACi, bortezomib) as well as agents that can disrupt the interplay of Reed–Sternberg cells with the non-neoplastic cells in the microenvironment (e.g., lenalidomide, rituximab, antiangiogenic drugs, PD1 and PD-L1 inhibitors, agonist anti-CD137 antiantibodies) might represent promising new therapeutic paradigms. In addition, researchers from several centers, including our own, have shown that the cells of the microenvironment such as CD68+ macrophages, CD20+ B lymphocytes, FOXP3+ T-regulatory cells and activated (GrB+) cytotoxic T cells may represent clinically useful biomarkers for accurately predicting the outcome of treatment in cHL, independently of clinical prognostic-factor systems [52–54].
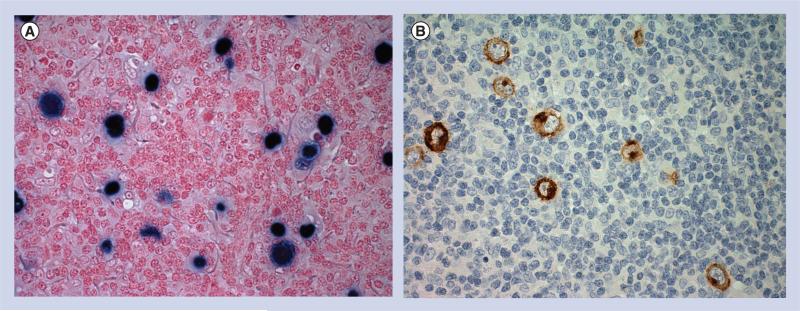
There are significant clinico-pathological differences between HIV-positive and HIV-negative HL, which may suggest important differences in underlying biological and pathophysiological mechanisms [43,44]. A major difference is that the Reed-Sternberg cells in HIV-HL are almost always EBV positive (>95%), whereas only one third of HIV-negative HL specimens express EBV (Figure 2). Surprisingly, the area of tumor microenvironment research is very limited in HIV-HL in the HAART era. The details of its composition and basic characteristics, biologic mechanisms and prognostic microenvironmental biomarkers are remaining gaps in our knowledge. One small study has demonstrated that HIV-HL cases have reduced numbers of CD4+ T cells, CD56+ and CD57+ cells, CD123+ cells as well as B cells, but an increased numbers of FOXP3+ CD8+ cells. No significant differences were seen in the number of CD8− T cells, FOXP3+ T cells and macrophages [55]. There are also a significantly increased number of CD163+ macrophages in HIV-HL. Cases exhibiting a sarcomatoid pattern of the reactive infiltrate exhibited significantly greater numbers of macrophages, associating the sarcomatoid pattern with the presence of spindle-shaped macrophages. Whereas, rosetting of CD4+ T cells around Reed–Sternberg cells was frequently observed in cHL in immunocompetent patients, rosetting in a subset of HIV-HL cases appeared to involve cytoplasmic protrusions of spindle-shaped macrophages [56].
Figure 2. HIV-associated Hodgkin's lymphoma.
(A) EBER nuclear blue stain (EBV in situ hybridization). (B) LMP1 stain (immunoperoxidase).
EBV: Epstein–Barr virus.
Many questions remain unanswered, including a clear picture of the mechanisms by which the unique tumor microenvironment is formed in this disease, the role of viral factors, what makes EBV so key to HIV-HL pathogenesis and what this can tell us about HIV-HL and cHL as a whole. It remains unclear what is the role of new treatments and in particular, the effect of new anti-retroviral agents such as maraviroc. We may conclude, therefore, that further in-depth analysis of the tumor microenvironment is urgently needed in HIV-HL.
Future perspective.
With the introduction of HAART, the outcome of HIV-associated lymphomas is improving, but the incidence has not decreased as expected and the incidence of HIV-associated cHL is increasing. Cancer is now the leading cause of death in people living with HIV.
The tumor microenvironment on AR-DLBCL and HIV-associated cHL demonstrated significant differences from sporadic cases, even with the introduction of HAART and increased circulating CD4 cells. Strategies to increase immune responses such as using anti-PDL1 antibodies need investigation and may improve outcome.
The increased angiogenesis observed represents a novel therapeutic target.
Executive summary.
Introduction
Incidence of AIDS-related lymphoma has not fallen as expected with highly active antiretroviral therapy; AIDS-related diffuse large B-cell lymphoma (AR-DLBCL) and Burkitt's lymphoma have declined only gradually and HIV-associated Hodgkin's lymphoma (HIV-HL) is becoming more common.
Study of the microenvironment led to insights into sporadic DLBCL and classical Hodgkin's lymphoma but few studies address the context of HIV.
HIV does not directly infect tumor cells but influences the microenvironment.
Studying how HIV and ARLs interact should give insight into ARL and lymphoma as a whole.
The microenvironment in HIV-associated DLBCL
The AR-DLBCL microenvironment differs significantly from the microenvironment of sporadic DLBCL.
Major differences include increased angiogenesis and changes in the numbers, ratios and character of tumor-associated T lymphocytes.
Angiogenesis
AR-DLBCL exhibits higher angiogenic activity than sporadic DLBCL.
Secreted HIV-1 factors including Tat and p17 may significantly influence angiogenesis by mimicking VEGF and IL-8 angiogenic pathways.
EBV LMP1 and macrophages may also play important roles.
T cells, immunosurveillance & more on macrophages
The AR-DLBCL microenvironment contains reduced numbers of CD4+ and FOXP3+ lymphocytes and is enriched with CD8+ lymphocytes of an activated type.
Activated CD8+ lymphocytes correlate with viral antigen expression raising important questions for immunosurveillance.
Macrophages are likely to play a significant, but currently poorly defined role.
Future directions in the AR-DLBCL microenvironment
Further characterization of angiogenic pathways would provide important information.
The role of activated CD8+ lymphocytes is another important line of investigation.
Insights into both areas might yield exciting results due to the advent of new therapeutic options.
Hodgkin's lymphoma
The rising incidence of HIV-HL needs further explanation.
Significant advances have been made in understanding of the Classical Hodgkin's lymphoma microenvironment.
Early studies into HIV-HL microenvironment show reduced CD4+ T cells but increased CD163+ macrophages and possibly increased CD8+FOXP3+ T cells but many questions remain in the context of HIV.
Acknowledgments
Supported by NIH grant no. P01 CA81538 from the National Cancer Institute to JG Gribben.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317–324. doi: 10.1002/cncr.20354. [DOI] [PubMed] [Google Scholar]
- 2.Tirelli U, Bernardi D. Impact of haart on the clinical management of AIDS-related cancers. Eur. J. Cancer. 2001;37(10):1320–1324. doi: 10.1016/s0959-8049(01)00106-x. [DOI] [PubMed] [Google Scholar]
- 3.Grulich AE, Van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 4.Rabkin CS. Aids and cancer in the era of highly active antiretroviral therapy (HAART). Eur. J. Cancer. 2001;37(10):1316–1319. doi: 10.1016/s0959-8049(01)00104-6. [DOI] [PubMed] [Google Scholar]
- 5.Gopal S, Patel MR, Yanik EL, et al. Temporal trends in presentation and survival for HIV-associated lymphoma in the antiretroviral therapy era. J. Natl Cancer Inst. 2013;105(16):1221–1229. doi: 10.1093/jnci/djt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the united states. J. Natl Cancer Inst. 2011;103(9):753–762. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 8.Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N. Engl. J. Med. 2008;359(22):2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chadburn A, Chiu A, Lee JY, et al. Immunophenotypic analysis of AIDS-related diffuse large B-cell lymphoma and clinical implications in patients from AIDS malignancies consortium clinical trials 010 and 034. J. Clin. Oncol. 2009;27(30):5039–5048. doi: 10.1200/JCO.2008.20.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stebbing J, Gazzard B, Douek DC. Where does HIV live? N. Engl. J. Med. 2004;350(18):1872–1880. doi: 10.1056/NEJMra032395. [DOI] [PubMed] [Google Scholar]
- 11.Dunleavy K, Wilson WH. How I treat HIV-associated lymphoma. Blood. 2012;119(14):3245–3255. doi: 10.1182/blood-2011-08-373738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liapis K, Clear A, Owen A, et al. The microenvironment of AIDS-related diffuse large B-cell lymphoma provides insight into the pathophysiology and indicates possible therapeutic strategies. Blood. 2013;122(3):424–433. doi: 10.1182/blood-2013-03-488171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyagol J, De Falco G, Lazzi S, et al. HIV-1 tat mimetic of vegf correlates with increased microvessels density in AIDS-related diffuse large B-cell and burkitt lymphomas. J. Hematop. 2008;1(1):3–10. doi: 10.1007/s12308-008-0002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002;20(21):4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 15.Scheidegger P, Weiglhofer W, Suarez S, et al. Signalling properties of an HIV-encoded angiogenic peptide mimicking vascular endothelial growth factor activity. Biochem. J. 2001;353(Pt 3):569–578. doi: 10.1042/0264-6021:3530569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bornkamm GW. Epstein–Barr virus and the pathogenesis of Burkitt's lymphoma: more questions than answers. Int. J. Cancer. 2009;124(8):1745–1755. doi: 10.1002/ijc.24223. [DOI] [PubMed] [Google Scholar]
- 17.Lazzi S, Bellan C, De Falco G, et al. Expression of rb2/p130 tumor-suppressor gene in AIDS-related non-Hodgkin's lymphomas: implications for disease pathogenesis. Hum. Pathol. 2002;33(7):723–731. doi: 10.1053/hupa.2002.125372. [DOI] [PubMed] [Google Scholar]
- 18.Gratzinger D, Zhao S, Marinelli RJ, et al. Microvessel density and expression of vascular endothelial growth factor and its receptors in diffuse large B-cell lymphoma subtypes. Am. J. Pathol. 2007;170(4):1362–1369. doi: 10.2353/ajpath.2007.060901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Da Silva SR, De Oliveira DE. HIV, EBV and kshv: Viral cooperation in the pathogenesis of human malignancies. Cancer Lett. 2011;305(2):175–185. doi: 10.1016/j.canlet.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Caccuri F, Giagulli C, Bugatti A, et al. HIV-1 matrix protein p17 promotes angiogenesis via chemokine receptors cxcr1 and cxcr2. Proc. Natl Acad. Sci. USA. 2012;109(36):14580–14585. doi: 10.1073/pnas.1206605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caccuri F, Rueckert C, Giagulli C, et al. HIV-1 matrix protein p17 promotes lymphangiogenesis and activates the endothelin-1/endothelin B receptor axis. Arterioscler. Thromb. Vasc. Biol. 2014;34(4):846–856. doi: 10.1161/ATVBAHA.113.302478. [DOI] [PubMed] [Google Scholar]
- 22.Kondo S, Seo SY, Yoshizaki T, et al. Ebv latent membrane protein 1 up-regulates hypoxia-inducible factor 1alpha through siah1-mediated down-regulation of prolyl hydroxylases 1 and 3 in nasopharyngeal epithelial cells. Cancer Res. 2006;66(20):9870–9877. doi: 10.1158/0008-5472.CAN-06-1679. [DOI] [PubMed] [Google Scholar]
- 23.Hong GK, Kumar P, Wang L, et al. Epstein–Barr virus lytic infection is required for efficient production of the angiogenesis factor vascular endothelial growth factor in lymphoblastoid cell lines. J. Virol. 2005;79(22):13984–13992. doi: 10.1128/JVI.79.22.13984-13992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paydas S. Strong cross-talk between angiogenesis and EBV: Do we need different treatment approaches in lymphoma cases with EBV and/or high angiogenic capacity. Med. Oncol. 2012;29(3):2159–2165. doi: 10.1007/s12032-011-0065-z. [DOI] [PubMed] [Google Scholar]
- 25.Ren Q, Sato H, Murono S, Furukawa M, Yoshizaki T. Epstein–Barr virus (EBV) latent membrane protein 1 induces interleukin-8 through the nuclear factor-kappa b signaling pathway in EBV-infected nasopharyngeal carcinoma cell line. Laryngoscope. 2004;114(5):855–859. doi: 10.1097/00005537-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Miyauchi K, Urano E, Yoshiyama H, Komano J. Cytokine signatures of transformed B cells with distinct Epstein–Barr virus latencies as a potential diagnostic tool for B cell lymphoma. Cancer Sci. 2011;102(6):1236–1241. doi: 10.1111/j.1349-7006.2011.01924.x. [DOI] [PubMed] [Google Scholar]
- 27.Martorelli D, Muraro E, Mastorci K, et al. A natural HIV p17 protein variant up-regulates the lmp-1 EBV oncoprotein and promotes the growth of EBV-infected B lymphocytes: Implications for EBV-driven lymphomagenesis in the HIV setting. Int. J. Cancer. 2015 doi: 10.1002/ijc.29494. doi:10.1002/ijc.29494 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 28.Bower M, Gazzard B, Mandalia S, et al. A prognostic index for systemic AIDS-related non-Hodgkin lymphoma treated in the era of highly active antiretroviral therapy. Ann. Intern. Med. 2005;143(4):265–273. doi: 10.7326/0003-4819-143-4-200508160-00007. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg YJ, Zack PM, Leon EC, et al. Immunological and virological changes associated with decline in CD4/CD8 ratios in lymphoid organs of siv-infected macaques. AIDS Res. Hum. Retroviruses. 1994;10(7):863–872. doi: 10.1089/aid.1994.10.863. [DOI] [PubMed] [Google Scholar]
- 30.Sopper S, Nierwetberg D, Halbach A, et al. Impact of simian immunodeficiency virus (SIV) infection on lymphocyte numbers and T-cell turnover in different organs of rhesus monkeys. Blood. 2003;101(4):1213–1219. doi: 10.1182/blood-2002-06-1644. [DOI] [PubMed] [Google Scholar]
- 31.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 32.Riches JC, Davies JK, Mcclanahan F, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121(9):1612–1621. doi: 10.1182/blood-2012-09-457531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morton LM, Kim CJ, Weiss LM, et al. Molecular characteristics of diffuse large B-cell lymphoma in human immunodeficiency virus-infected and -uninfected patients in the pre-highly active antiretroviral therapy and pre-rituximab era. Leuk. Lymphoma. 2014;55(3):551–557. doi: 10.3109/10428194.2013.813499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Podack ER. Execution and suicide: cytotoxic lymphocytes enforce draconian laws through separate molecular pathways. Curr. Opin. Immunol. 1995;7(1):11–16. doi: 10.1016/0952-7915(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 35.Huysentruyt LC, Mcgrath MS. The role of macrophages in the development and progression of AIDS-related non-Hodgkin lymphoma. J. Leukoc. Biol. 2010;87(4):627–632. doi: 10.1189/jlb.0809564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porcheray F, Samah B, Leone C, Dereuddre-Bosquet N, Gras G. Macrophage activation and human immunodeficiency virus infection: HIV replication directs macrophages towards a pro-inflammatory phenotype while previous activation modulates macrophage susceptibility to infection and viral production. Virology. 2006;349(1):112–120. doi: 10.1016/j.virol.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 37.Bollard CM, Gottschalk S, Torrano V, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein–Barr virus latent membrane proteins. J. Clin. Oncol. 2014;32(8):798–808. doi: 10.1200/JCO.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uldrick TS, Wyvill KM, Kumar P, et al. Phase II study of bevacizumab in patients with HIV-associated Kaposi's sarcoma receiving antiretroviral therapy. J. Clin. Oncol. 2012;30(13):1476–1483. doi: 10.1200/JCO.2011.39.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sgadari C, Monini P, Barillari G, Ensoli B. Use of HIV protease inhibitors to block Kaposi's sarcoma and tumor growth. Lancet Oncol. 2003;4(9):537–547. doi: 10.1016/s1470-2045(03)01192-6. [DOI] [PubMed] [Google Scholar]
- 40.Zhang B, Kracker S, Yasuda T, et al. Immune surveillance and therapy of lymphomas driven by Epstein–Barr virus protein lmp1 in a mouse model. Cell. 2012;148(4):739–751. doi: 10.1016/j.cell.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quezada SA, Simpson TR, Peggs KS, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 2010;207(3):637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coutinho R, Clear AJ, Mazzola E, et al. Revisiting the immune microenvironment of diffuse large B-cell lymphoma using a tissue microarray and immunohistochemistry: robust semi-automated analysis reveals CD3 and Foxp3 as potential predictors of response to R-CHOP. Haematologica. 2014;100(3):363–369. doi: 10.3324/haematol.2014.110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobson CA, Abramson JS. HIV-associated Hodgkin's lymphoma: prognosis and therapy in the era of cart. Adv. Hematol. 2012;2012:507257. doi: 10.1155/2012/507257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spina M, Carbone A, Gloghini A, Serraino D, Berretta M, Tirelli U. Hodgkin's disease in patients with HIV infection. Adv. Hematol. 2011 doi: 10.1155/2011/402682. pii: 402682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108(12):3786–3791. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gloghini A, Carbone A. Why would the incidence of HIV-associated Hodgkin lymphoma increase in the setting of improved immunity? Int. J. Cancer. 2007;120(12):2753–2754. doi: 10.1002/ijc.22650. [DOI] [PubMed] [Google Scholar]
- 47.Kis LL, Takahara M, Nagy N, Klein G, Klein E. Cytokine mediated induction of the major Epstein–Barr virus (EBV)-encoded transforming protein, lmp-1. Immunol. Lett. 2006;104(1–2):83–88. doi: 10.1016/j.imlet.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Thompson LD, Fisher SI, Chu WS, Nelson A, Abbondanzo SL. HIV-associated Hodgkin lymphoma: a clinicopathologic and immunophenotypic study of 45 cases. Am. J. Clin. Pathol. 2004;121(5):727–738. doi: 10.1309/PNVQ-0PQG-XHVY-6L7G. [DOI] [PubMed] [Google Scholar]
- 49.Greaves P, Gribben JG. Lymphoid neoplasia. Laser-capturing the essence of Hodgkin lymphoma. Blood. 2012;120(23):4451–4452. doi: 10.1182/blood-2012-09-455873. [DOI] [PubMed] [Google Scholar]
- 50.Greaves P, Clear A, Owen A, et al. Defining characteristics of classical Hodgkin lymphoma microenvironment T-helper cells. Blood. 2013;122(16):2856–2863. doi: 10.1182/blood-2013-06-508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greaves P, Clear A, Coutinho R, et al. Expression of foxp3, CD68, and CD20 at diagnosis in the microenvironment of classical Hodgkin lymphoma is predictive of outcome. J. Clin. Oncol. 2013;31(2):256–262. doi: 10.1200/JCO.2011.39.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greaves P, Clear A, Coutinho R, et al. Expression of foxp3, CD68, and CD20 at diagnosis in the microenvironment of classical Hodgkin lymphoma is predictive of outcome. J. Clin. Oncol. 2013;31(2):256–262. doi: 10.1200/JCO.2011.39.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steidl C, Farinha P, Gascoyne RD. Macrophages predict treatment outcome in Hodgkin's lymphoma. Haematologica. 2011;96(2):186–189. doi: 10.3324/haematol.2010.033316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral foxp3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin's lymphoma. Haematologica. 2008;93(2):193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]
- 55.Koulis A, Trivedi P, Ibrahim H, Bower M, Naresh KN. The role of the microenvironment in human immunodeficiency virus-associated classical Hodgkin lymphoma. Histopathology. 2014;65(6):749–756. doi: 10.1111/his.12458. [DOI] [PubMed] [Google Scholar]
- 56.Hartmann S, Jakobus C, Rengstl B, et al. Spindle-shaped CD163+ rosetting macrophages replace CD4+ T-cells in HIV-related classical Hodgkin lymphoma. Mod. Pathol. 2013;26(5):648–657. doi: 10.1038/modpathol.2012.217. [DOI] [PubMed] [Google Scholar]