Abstract
Glycans mediate many critical, long-term biological processes such as stem cell differentiation. However, few methods are available for the sustained remodeling of cells with specific glycan structures. We report a new strategy that enables the long-lived presentation of defined glycosaminoglycans on cell surfaces using HaloTag proteins (HTPs) as anchors. By controlling the sulfation patterns of heparan sulfate (HS) on pluripotent embryonic stem cell (ESC) membranes, we demonstrate that specific glycans cause ESCs to undergo accelerated exit from self-renewal and differentiation into neuronal cell types. Thus, the stable display of glycans on HTP scaffolds provides a powerful, versatile means to direct key signaling events and biological outcomes such as stem cell fate.
Keywords: embryonic stem cells, heparan sulfate, cell-surface engineering, stem cell differentiation, cell signaling
The ability to control embryonic stem cell (ESC) differentiation holds great promise as a renewable source of replacement cells and tissues to treat diseases such as heart disease, diabetes, and neurodegeneration.[1] However, realizing the full potential of stem cells will require new strategies for directing differentiation, as well as a better understanding of the molecular mechanisms that guide the development of specific cell lineages and fates.
Heparan sulfate (HS) glycosaminoglycans (GAGs) have recently been identified as important regulators of stem cell differentiation.[2] HS GAGs are a ubiquitous class of extracellular polysaccharides comprised of uronic acid and glucosamine disaccharide units. The sugar backbone is further modified by various sulfotransferase enzymes, potentially giving rise to hundreds of sulfation patterns. This rich structural diversity enables HS GAGs to interact selectively with proteins, including those involved in stem cell differentiation such as fibroblast growth factors (FGFs), bone morphogenic proteins (BMPs), and wingless-type MMTV integration site family members (Wnts).[2c, 3] Notably, specific sulfation patterns of HS have been implicated in the progression of ESCs from self-renewal to a differentiated state. For example, undersulfated HS is found on pluripotent cells,[2d] whereas highly sulfated HS is associated with differentiated cells and has been proposed to promote interactions between soluble FGF and BMP factors and their receptors.[2c] However, the precise sulfated epitopes and mechanisms involved in the generation of specific cell lineages remain unclear. We postulated that the presentation of particular HS GAG structures on ESC surfaces might enable the selective activation of signaling pathways and thereby induce desirable cell fates. Such an approach would also provide novel insights into the structure-function relationships of HS GAGs and their roles in stem cell biology.
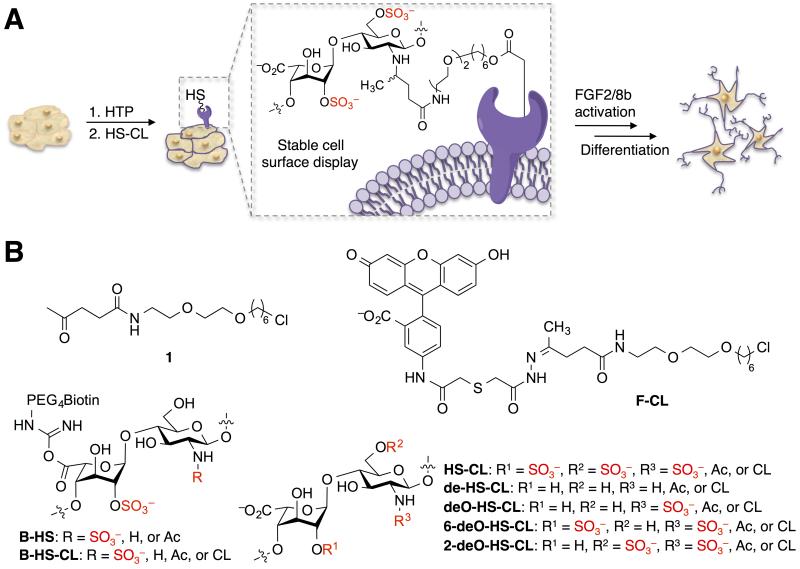
Elegant studies have recently shown that the short-term display of synthetic HS glycopolymers can promote stem cell specification to form intermediate, neural rosettes.[4] However, directing the generation of fully differentiated, mature cell types will likely require the development of new methods to enable the long-term, stable presentation of defined HS GAGs. Two powerful approaches for cell-surface glycan engineering have recently been developed that employ lipid-functionalized polysaccharides[5] and synthetic glycopolymers.[6] While both strategies can elicit short-term cellular responses, the lipid tail anchor limits the membrane lifetime of exogenous glycans to several hours. Herein, we developed a method to tailor cell surfaces with specific HS derivatives using membrane-bound HaloTag proteins (HTPs) as anchors (Figure 1). Molecules covalently attached to HTPs displayed prolonged cell-surface lifetimes of greater than one week, circumventing the temporal limitation of lipid anchors. Moreover, mouse ESCs remodeled with heparin/highly sulfated HS underwent accelerated exit from self-renewal and commitment to a neural lineage through early activation of extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) signaling pathways. These results highlight the potential to elucidate the functional roles of HS GAGs and direct cell differentiation by remodeling the glycocalyx of stem cells.
Figure 1.
A) Strategy for presenting HS GAGs on cell membranes using HTP anchors to direct stem cell differentiation. B) Molecules used in this study.
HTP is a modified alkane dehalogenase that forms a covalent adduct with chloroalkane substrates.[7] The HTP technology has been adapted for diverse applications, ranging from cancer diagnostics to chemical proteomics.[8] However, most reported applications have used HTP technology to append molecules that serve as detection or capture agents. We chose to exploit the HTP platform to modulate biological processes in living cells.
We first investigated the membrane lifetime of molecules conjugated to HTPs. Boc-protected 1-(2-(2-aminoethoxy)ethoxy)-6-chlorohexane[8c] was deprotected with trifluoroacetic acid and combined with N-hydroxysuccinimidyl levulinate to obtain chloroalkane linker (CL) 1 (Figure 1B and Scheme S1 in the Supporting Information). Condensation of 1 with a fluorescein-hydrazide derivative gave CL-conjugated fluorescein (F-CL; Figure 1B and Scheme S1). To test the approach, we stably expressed hemagglutinin (HA)-tagged HTP in Chinese hamster ovary (CHO) cell membranes by fusing it to the platelet-derived growth factor receptor (PDGFR) transmembrane domain. Cells were then incubated with F-CL for 1 h at 37 °C, and individual wells were fixed and imaged every 12 h for 8 days. Remarkably, we observed a strong fluorescence signal that persisted for at least 8 days after only a single F-CL treatment (Figure 2A). In contrast, no fluorescence signal was observed when cells were treated with the fluorescein-hydrazide derivative alone or with cells lacking HTP (Figure S1). These results indicate that the display of HTP-conjugates is specific and long-lived despite membrane turnover, highlighting the potential of this approach to exert long-lasting effects on cellular function.
Figure 2.
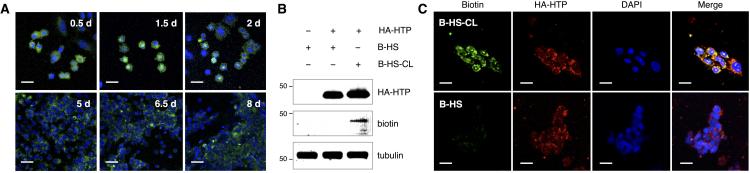
Extended cell-surface display by HTP anchoring. A) CHO cells stably expressing HTP were functionalized with a single treatment of F-CL (shown in green) and imaged over 8 days. Cell nuclei were co-stained at each time point with DAPI (shown in blue). B) Western blot detection and C) fluorescence imaging of hemagglutinin (HA)-tagged HTP and biotinylated HS. Stably transfected CHO cells were labeled with biotinylated HS with or without the chloroalkane linker (B-HS-CL or B-HS, respectively). Tubulin was used as a control for equal protein loading in B. Scale bars represent 20 μm.
We next examined whether the technology could be used to present highly sulfated HS GAGs on cell surfaces. Although the glucosamine sugars in heparin/HS are mostly N-acetylated or N-sulfated, the free amine exists in low abundance (1-3%), which provides a convenient functional handle for attaching the chloroalkane linker in a single step. We biotinylated HS (B-HS)[9] and conjugated it to 1 via reductive amination chemistry (B-HS-CL; Figure 1B and Scheme S2). CHO cells stably expressing HTP were incubated with B-HS or B-HS-CL at 37 °C for 6 h. Cells were lysed and subjected to blotting analysis using an anti-HA antibody and streptavidin-IRDye800. Importantly, biotinylated HS was detected only from HTP-expressing cells treated with B-HS-CL, but not B-HS, which lacks the chloroalkane moiety (Figure 2B). Immunocytochemical analysis of cells further confirmed the presence of biotinylated HS on HTP-expressing cells incubated with B-HS-CL, but not B-HS (Figure 2C). Together, these studies demonstrate the selective display of HS GAGs on cell surfaces using HTP anchors.
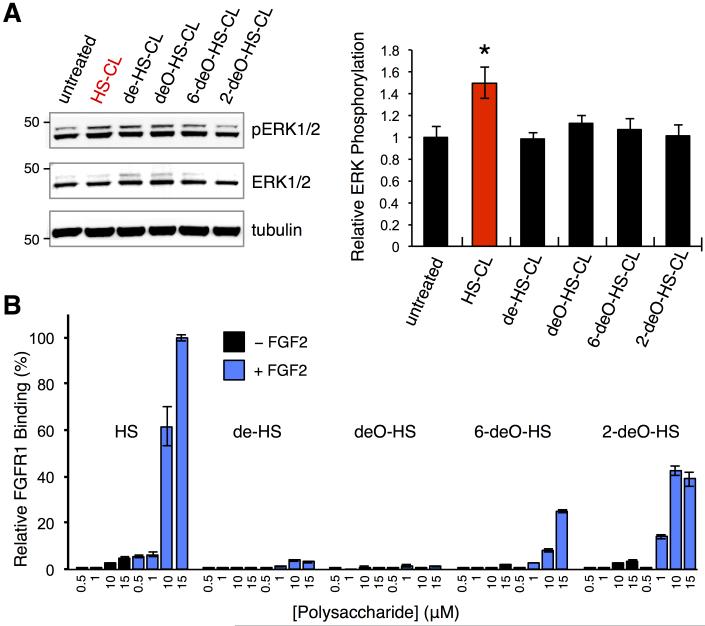
With a method for the long-lived presentation of GAGs in hand, we examined the ability of our non-natural presentation of HS GAGs to stimulate stem cell signaling pathways. HS GAGs regulate FGF-FGF receptor (FGFR) signaling events involved in stem cell differentiation by assisting in the assembly of active growth factor-receptor complexes.[10] Activation of FGF2-FGFR1, in turn, initiates several intracellular signaling pathways, including the ERK/MAPK cascade.[11] A distinct period of increased ERK1/2 phosphorylation through FGF signaling triggers ESCs to exit self-renewal and transition to a neural cell fate.[12] To display particular sulfated HS structures on ESCs and stimulate the FGF2/FGFR1 pathway, we conjugated 1 to various heparin/HS polysaccharides. The commercially available polysaccharides (12-13 kDa) were derived from a single natural source and chemically desulfated to produce heparin/HS with defined sulfation motifs. Specifically, CL-functionalized heparin/highly sulfated HS (HS-CL), fully desulfated heparin/HS (de-HS-CL), de-O-sulfated heparin/HS (deO-HS-CL), de-6-O-sulfated heparin/HS (6-deO-HS-CL), and de-2-O-sulfated heparin/HS (2-deO-HS-CL) were readily generated in one step under standard reductive amination conditions (Figure 1B and Scheme S2). A homogeneous population of pluripotent mouse ESCs were obtained commercially (ATCC) and transiently transfected with the HTP construct. The ESCs were treated with heparinase II to remove endogenous HS GAGs and incubated overnight in serum-depleted medium containing the various HS-CL derivatives. Cells were then stimulated with FGF2 and assayed for ERK1/2 activation by immunoblotting with antibodies against phosphorylated (pThr202/pTyr204) and total ERK1/2. To our delight, ESCs engineered to display heparin/highly sulfated HS exhibited a 1.5-fold increase in ERK1/2 phosphorylation compared to untreated cells (Figure 3A). HS-CL had no effect on ESCs lacking HTP, confirming that ERK1/2 activation required the HTP anchors (Figure S2). Moreover, ERK1/2 activation was dependent on the sulfation pattern, as cells engineered with other selectively desulfated heparin/HS variants showed no significant increase in phospho-ERK1/2 levels (Figure 3A).
Figure 3.
Cell-surface presentation of HS GAGs on ESCs induces FGF2-mediated ERK1/2 activation. A) Representative immunoblots (left) and quantification (right) of ERK1/2 phosphorylation levels in ESCs remodeled with the indicated HS GAGs and stimulated with FGF2. Phospho-ERK levels were normalized with respect to total ERK levels for each condition and compared to untreated ESCs. Tubulin was used as a control for equal protein loading. Data represent the mean ± S.E.M. (*P < 0.05) from three experiments. B) FGFR1-Fc binding to glycan microarrays in the presence (blue bars) or absence (black bars) of FGF2. Data represent the mean ± S.E.M. from ten replicate microarray spots.
Structural and biochemical studies have led to conflicting reports on the pivotal determinants of HS-FGF2-FGFR1 complexation and ERK1/2 activation.[10b,13] For example, crystallographic studies suggested that 6-O-sulfation is critical for both ligand and receptor binding,[10b] while certain biochemical studies indicated that downstream ERK1/2 activation elicited by the complex is not significantly attenuated by loss of the 6-O-sulfate group.[13b] Interestingly, the same crystallographic studies found that the other sulfate groups are also important for interactions with FGF2, suggesting that all three of the sulfate groups may be required for formation of the ternary HS-FGF2-FGFR1 complex.[10b] To clarify the role of sulfation, we used GAG microarrays[14] to probe the ternary interaction more closely. Microarrays printed with various concentrations of the HS derivatives were incubated with an FGFR1-Fc fusion protein in the presence or absence of FGF2. Binding of FGFR1-Fc was visualized using an anti-Fc antibody conjugated to AlexaFluor 647. We found that FGFR1 bound preferentially to heparin/highly sulfated HS only in the presence of FGF2, suggesting the formation of a ternary complex (Figure 3B). Consistent with our ERK1/2 activation studies (Figure 3A), binding of FGFR1 was significantly attenuated by either 6-O- or 2-O-desulfation of HS. Taken together, our studies suggest that both 2-O- and 6-O-sulfation of HS are critical for FGF2-FGFR1-mediated ERK1/2 activation in ESCs. Thus, an HTP-based approach can be used to hijack endogenous signaling pathways and deconvolute the sulfation requirements of complex, GAG-mediated processes.
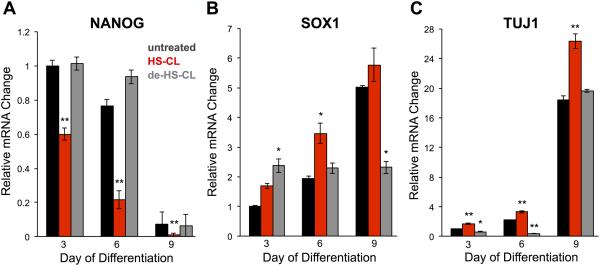
Finally, we investigated whether the HTP-dependent presentation of specific heparin/HS epitopes could promote stem cell differentiation into mature cell types. Dopaminergic neurons from fetal sources have shown long-term cell survival and preliminary clinical benefits when implanted into patients with Parkinson’s disease.[15] As such, the development and refinement of in vitro systems to generate neuronal cell populations could have widespread biomedical implications for the treatment of neurological diseases. The differentiation of ESCs into dopaminergic neurons is promoted by FGF8b.[16] Interestingly, our microarray studies indicated that FGF8b and its receptor FGFR3c are capable of forming ternary complexes with heparin/highly sulfated HS, while complex assembly was attenuated with 6-de-O-sulfated or 2-de-O-sulfated heparin/HS and was not observed with fully de-O-sulfated heparin/HS (Figure S3). Pluripotent mouse ESCs transiently expressing HTP were treated with heparinase II and then grown in neural induction medium supplemented with FGF2 and HS-CL. For comparison, ESCs were treated identically, but in the absence of HS-CL (untreated) or replacing HS-CL with de-HS-CL. After two days, the FGF2-containing medium was substituted with neural induction medium containing FGF8b. We monitored the differentiation process at specific time points by profiling the gene expression levels of specific markers using real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR, Table S1). The transcription factors NANOG and SOX1 are well-established markers for pluripotency and self-rewewal exit/neural lineage commitment, respectively, whereas TUJ1 is widely used as a specific marker for mature, fully differentiated neurons.[17] In parallel, the presence of NANOG, SOX1, and TUJ1 in cells were imaged by immunocytochemistry (Figure S4). We found that NANOG levels declined most rapidly in ESCs treated with HS-CL, consistent with accelerated loss of pluripotency (Figure 4A). In contrast, the levels remained high until day 9 in untreated cells and cells treated with de-HS-CL. The decrease in NANOG levels at day 9 for untreated and de-HS-CL-treated cells is most likely due to de novo HS biosynthesis following the single heparinase treatment. Consistent with accelerated loss of pluripotency, the decline in NANOG levels in HS-CL-treated cells was accompanied by a corresponding increase in the neuroectoderm-specific marker SOX1 (Figure 4B). At day 6, SOX1 levels were significantly higher in HS-CL-treated cells compared to untreated or de-HS-CL-treated cells, suggesting that HS-CL-treated cells had undergone accelerated exit from self-renewal and commitment to an intermediate, neuroectoderm state. Importantly, cells remodeled with HS-CL also showed significantly higher levels of TUJ1 compared to untreated or de-HS-CL-treated cells at all time points (Figure 4C), indicative of faster progression to a mature, differentiated neuronal phenotype. As expected, the cells also developed more elaborate neurite processes compared to those from untreated cells and de-HS-CL treated cells (Figure S5). Interestingly, cells remodeled with de-HS-CL showed significantly lower amounts of SOX1 at day 9 and TUJ1 at days 3 and 6 compared to untreated cells, consistent with studies suggesting that undersulfated HS may help to maintain pluripotency and restrict differentiation.[2d] Together, our results illustrate the power of using long-term, cell-surface display of HS GAGs to activate specific signaling events and drive the differentiation of stem cells into mature neuronal populations.
Figure 4.
Remodeling the glycocalyx of ESCs with highly sulfated HS induces accelerated self-renewal exit, neural lineage commitment, and differentiation into mature, neuronal cells. qRT-PCR quantification of mRNA levels for A) pluripotent marker NANOG, B) neuroectoderm marker SOX1, and C) neuronal marker TUJ1. Data points were normalized to the housekeeping genes GAPDH and SDHA for cross comparison and to the untreated condition at day 3 for data presentation. Values represent the mean ± S.E.M. (*P < 0.05, **P < 0.01 when compared to the untreated control at each time point) from two independent experiments.
Here, we developed a new method for the long-lived presentation of specific HS molecules on cell surfaces. This HTP-anchoring platform significantly increases the lifetime of glycans displayed on cells and allows for the modulation of biological processes on time scales inaccessible by other methods. The synthetic ease of the approach also offers a major advantage over current, synthetically intensive techniques by affording a one-step strategy to functionalize molecules for cell-surface display. Furthermore, the versatile and bioorthogonal HaloTag anchor provides a general approach to stably append a diverse range of bioactive molecules. We note that the current method does not fully recapitulate native proteoglycans, in which the GAG chains are attached via their non-reducing end to specific core proteins. Future studies could seek to mimic proteoglycans more faithfully by exploring the fusion of HTP to distinct proteoglycan domains. Despite the differences, the GAG-HTP conjugates show a remarkable capacity to activate signaling pathways in stem cells. We found that ESCs engineered with heparin/highly sulfated HS undergo accelerated exit from self-renewal and commitment to neural lineages through activation of FGF/ERK-mediated signaling pathways, whereas cells displaying undersulfated HS experience delayed responses to differentiation cues and restricted differentiation potential. Our findings underscore the potential for remodeling the glycocalyx of ESCs to provide insights into the factors and mechanisms that drive stem cell differentiation toward neuronal and perhaps other cell fates. In the future, this platform could be extended to an in vitro differentiation system to obtain homogeneous neuronal populations as cell replacement therapies. Gene delivery methods to express HTP on specific cell populations could allow for the sustained, precise control of signaling in many other important physiological settings. Thus, our method of long-term glycan remodeling may provide a general means to regulate carbohydrate-mediated processes in vivo on time scales currently unattainable by any other method.
Supplementary Material
Footnotes
This research was supported by National Institutes of Health grant R01-GM093627 (L.H.W.) and National Science Foundation Graduate Research Fellowship DEG-1144469 (M.E.G.). We thank Greg Miller for microarray assistance. We also thank Fred Tan, Elizabeth Jensen and the lab of Peter Dervan for qRT-PCR assistance and helpful discussions.
References
- [1].a) Hansson EM, Lindsay ME, Chien KR. Cell Stem Cell. 2009;5:364–377. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]; b) D'Amour KA, Bang AG, Eliazer S, Kelly OG. Nature. 2006 [Google Scholar]; c) Kim J-H, Auerbach JM, Rodríguez-Gómez JA, Velasco I, Gavin D, Lumelsky N, Lee S-H, Nguyen J, Sánchez-Pernaute R, Bankiewicz K, McKay R. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- [2].a) Smith RAA, Meade K, Pickford CE, Holley RJ, Merry CLR. Biochem. Soc. Trans. 2011;39:383–387. doi: 10.1042/BST0390383. [DOI] [PubMed] [Google Scholar]; b) Kraushaar DC, Yamaguchi Y, Wang L. J. Biol. Chem. 2010;285:5907–5916. doi: 10.1074/jbc.M109.066837. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kraushaar DC, Rai S, Condac E, Nairn A, Zhang S, Yamaguchi Y, Moremen K, Dalton S, Wang L. J. Biol. Chem. 2012;287:22691–22700. doi: 10.1074/jbc.M112.368241. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Forsberg M, Holmborn K, Kundu S, Dagalv A, Kjellen L, Forsberg-Nilsson K. J. Biol. Chem. 2012;287:10853–10862. doi: 10.1074/jbc.M111.337030. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Pickford CE, Holley RJ, Rushton G, Stavridis MP, Ward CM, Merry CLR. Stem Cells. 2011;29:629–640. doi: 10.1002/stem.610. [DOI] [PubMed] [Google Scholar]
- [3].Lin X. Development. 2004;131:6009–6021. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- [4].Huang ML, Smith RAA, Trieger GW, Godula K. J. Am. Chem. Soc. 2014;136:10565–10568. doi: 10.1021/ja505012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pulsipher A, Griffin ME, Stone SE, Brown JM, Hsieh-Wilson LC. J. Am. Chem. Soc. 2014;136:6794–6797. doi: 10.1021/ja5005174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rabuka D, Forstner MB, Groves JT, Bertozzi CR. J. Am. Chem. Soc. 2008;130:5947–5953. doi: 10.1021/ja710644g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, Simpson D, Mendez J, Zimmerman K, Otto P, Vidugiris G, Zhu J, Darzins A, Klaubert DH, Bulleit RF, Wood KV. ACS Chem. Biol. 2008;3:373–382. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- [8].a) Jia J, Wang W, Meng W, Ding M, Ma S, Wang X. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095444. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Brigham JL, Perera BGK, Maly DJ. ACS Chem. Biol. 2013;8:691–699. doi: 10.1021/cb300623a. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) So M.-k., Yao H, Rao J. Biochem. Biophys. Res. Commun. 2008;374:419–423. doi: 10.1016/j.bbrc.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Glabe CG, Harty PK, Rosen SD. Anal. Biochem. 1983;130:287–294. doi: 10.1016/0003-2697(83)90590-0. [DOI] [PubMed] [Google Scholar]
- [10].a) Spivak-Kroizman T, Lemmon MA, Dikic I, Ladbury JE, Pinchasi D, Huang J, Jaye M, Crumley G, Schlessinger J, Lax I. Cell. 1994;79:1015–1024. doi: 10.1016/0092-8674(94)90032-9. [DOI] [PubMed] [Google Scholar]; b) Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, Yayon A, Linhardt RJ, Mohammadi M. Mol. Cell. 2000;6:743–750. doi: 10.1016/s1097-2765(00)00073-3. [DOI] [PubMed] [Google Scholar]
- [11].Lanner F, Rossant J. Development. 2010;137:3351–3360. doi: 10.1242/dev.050146. [DOI] [PubMed] [Google Scholar]
- [12].a) Stavridis MP, Lunn JS, Collins BJ, Storey KG. Development. 2007;134:2889–2894. doi: 10.1242/dev.02858. [DOI] [PubMed] [Google Scholar]; b) Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith A. Development. 2007;134:2895–2902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- [13].a) Kreuger J, Salmivirta M, Sturiale L, Gimenez-Gallego G, Lindahl U. J. Biol. Chem. 2001;276:30744–30752. doi: 10.1074/jbc.M102628200. [DOI] [PubMed] [Google Scholar]; b) Sugaya N, Habuchi H, Nagai N, Ashikari-Hada S, Kimata K. J. Biol. Chem. 2008;283:10366–10376. doi: 10.1074/jbc.M705948200. [DOI] [PubMed] [Google Scholar]
- [14].Rogers CJ, Clark PM, Tully SE, Abrol R, Garcia KC, Goddard WA, III, Hsieh-Wilson LC. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9747–9752. doi: 10.1073/pnas.1102962108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].a) Mendez I, Viñuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, Isacson O. Nat. Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Freed CR, Greene PE, Breeze RE, Tsai W-Y, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. N. Engl. J. Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- [16].Yan YP, Yang DL, Zarnowska ED, Du ZW, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Proc. Natl. Acad. Sci. U. S. A. 2011;108:7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.