Abstract
Background
Few studies have assessed metabolic and body composition alterations in young perinatally HIV-infected African children on antiretroviral therapy (ART). We compared metabolic profiles and regional fat of children on a combination of ritonavir-boosted lopinavir (lopinavir/ritonavir), lamivudine, and stavudine to those switched to nevirapine, lamivudine, and stavudine.
Methods
This study evaluated metabolic and body composition outcomes in 156 HIV-infected children completing a randomized trial that assessed the continued use of lopinavir/ritonavir-based or switch to NVP-based ART in Johannesburg, South Africa (2005–2010). Fasting total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides were measured. Total and regional body fat (BF) were estimated by anthropometry and bioelectrical impedance analysis (BIA). A clinical assessment for lipodystrophy was conducted. Modified intent-to-treat analyses were conducted.
Results
156 children (mean age 5.1±0.8 years, mean duration of treatment 4.2±0.7 years, mean time since randomization 3.4±0.7 years) were enrolled. 85 were randomized to the lopinavir/ritonavir- and 71 to the nevirapine-group. The lopinavir/ritonavir-group had lower mean HDL (1.3±0.4 vs. 1.5±0.4 mmol/L, p<0.001) and higher mean TC (4.4±1.0 vs. 4.1±0.8 mmol/L, p=0.097), LDL (2.6±0.9 vs. 2.3±0.7mmol/L, p=0.018), and triglycerides (1.1±0.4 vs. 0.8±0.3 mmol/L, p<0.001). The lopinavir/ritonavir-group had more total BF by mean skinfold sum (43±11.1 vs. 39±10.1 mm, p=0.031) and BF% by BIA (17.0±7.0 vs. 14.1±8.0%, p=0.022). Thirteen (8.4%) met criteria for lipodystrophy.
Conclusions
Unfavorable alterations in lipid profile and triglycerides, and differences in fat are detectable in young HIV-infected South African children receiving lopinavir/ritonavir-based vs. those switched to nevirapine-based regimens. Interventions to mitigate these alterations are warranted to reduce long-term cardiovascular disease risk.
Keywords: HIV, Antiretroviral Therapies, Body Composition, Lipids
INTRODUCTION
Unfavorable alterations in lipid and glucose metabolism, well established risk factors for cardiovascular disease (CVD), are reported among children and adolescents with perinatally acquired HIV-infection receiving antiretroviral therapy (ART).1–8 Significant proportions of ART-treated children have elevated total cholesterol (TC), low density lipoprotein (LDL), triglycerides and abnormal glucose and insulin homeostasis.1–8 The etiology of the metabolic derangements is multi-factorial, and specific ART drugs, particularly protease inhibitors (PIs), are an important cause of dyslipidemia and impaired glucose homeostasis.1–3,7–10 Exposure to nucleoside (NRTI) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) have also been associated with dyslipidemia.7
Abnormal body fat distribution, or lipodystrophy, also occurs in HIV-infected children both alone and in combination with metabolic abnormalities and may also contribute to increased CVD risk.2,11–16 A number of drugs, including thymidine analogue NRTIs and PIs, are associated with lipodystrophy.17 With greatly improved survival due to effective therapies, developing treatment strategies for children that optimize long-term viral suppression and limit long-term adverse effects is an important goal.
Few studies have evaluated lipid profiles and insulin-glucose metabolism or body fat distribution in young children receiving ART in sub-Saharan Africa, where over 90% of HIV-infected children reside.18 The objectives of this study are to examine the lipid profiles, insulin sensitivity, markers of inflammation, and regional fat distribution of HIV-infected children in South Africa who were initiated on a PI-based regimen prior to age 2 years and randomly assigned to continue on a PI-based regimen or switch to an NNRTI-based regimen. These parameters are also compared in children with and without lipodystrophy.
PATIENTS AND METHODS
Study Design
This cross-sectional study evaluated HIV-infected children at the final study visit of a randomized clinical trial (NEVEREST 2) that assessed continued ritonavir-boosted lopinavir (lopinavir/ritonavir)-based therapy vs. switching to nevirapine-based therapy, conducted from 2005 to 2010.19 In NEVEREST 2, children who maintained a viral load (VL) <400 copies/mL for ≥3 months while on their primary lopinavir/ritonavir-containing regimen were randomized to one of two groups. Children randomized to the lopinavir/ritonavir-group continued to receive lopinavir/ritonavir (230 mg/m2), lamivudine (4 mg/kg), and stavudine (1 mg/kg) whereas the nevirapine-group children switched to nevirapine (120 mg/m2 once per day for the first 2 weeks and thereafter 200 mg/m2 every 12 hours), lamivudine, and stavudine, the two regimens recommended for pediatric use in South Africa.20 The study was conducted at the Empilweni Clinical Research Unit at Rahima Moosa Mother and Child Hospital in Johannesburg, South Africa and was approved by the Institutional Review Boards of Columbia University and the University of the Witwatersrand. Each child’s guardian provided signed informed consent.
Measurements
Weight (kg) was measured using a digital scale and height (cm) was measured using a stadiometer. Body mass index (BMI) was calculated as weight (kg) divided by squared height (m2). Weight-for-age (WAZ), height-for-age (HAZ), and BMI-for-age z-scores (BAZ) were determined using WHO standards.21 Blood pressure was measured using an electronic sphygmomanometer with a pediatric cuff (Angelus, Model number SN-AJ710150) and compared with age and sex reference values.22 A clinical assessment for lipodystrophy was completed by two trained physicians (RS, LM) and children were classified as having lipodystrophy (LD+), possible lipodystrophy (possible LD), and no signs of lipodystrophy (LD−) if ≥2, 1, or 0 of the following features of lipoatrophy (sunken cheeks, temporal wasting, skinny limbs, wasting of buttocks) or lipohypertrophy (increased abdominal girth, dorsal cervical enlargement, breast enlargement) were present.12
Following an overnight fast (≥8 hours), TC, LDL, high-density lipoprotein (HDL), triglycerides, C-reactive-protein (CRP), and insulin were measured using the Roche COBAS®INTEGRA 400 system and venous blood glucose with a handheld glucometer. VL by quantitative HIV-1 RNA PCR (Cobas Ampliprep Taqman V2, Roche), absolute CD4 count, and CD4 percentages (Beckman Coulter Flow Analyzer) were also determined. TC categories were set as <4.4 mmol/L (<170 mg/dL) for acceptable, 4.4–5.1 mmol/L (170–199 mg/dL) for borderline, and ≥5.2 mmol/L (≥200 mg/dL) for elevated.23 A cut off value of 0.9 mmol/L (<35 mg/dL) was used for abnormal HDL concentrations.24 LDL categories were set as <2.9 mmol/L (<110 mg/dL) for acceptable, 2.9–3.3 mmol/L (110–129 mg/dL) for borderline, and ≥3.4 mmol/L (≥130 mg/dL) for elevated. Triglycerides >1.69 mmol/L (>150 mg/dL) and CRP >3 mg/L were considered elevated.23–25 Impaired fasting glucose was defined as ≥6.11 mmol/L (≥110 mg/dL).26 Homeostasis model assessment of insulin resistance (HOMA-IR) >3.16 was used to determine insulin resistance.27 All impaired fasting glucose measurements were repeated for confirmation.
Anthropometrics, including circumferences and skinfold thicknesses, were measured by two trained physicians (RS, LM) unblinded to treatment using the average of triplicate standardized measurements. Circumferences were measured to the nearest 0.1 cm using a flexible tape measure with a spring tension attachment at the mid-upper arm (MUAC), mid- upper thigh (MTC), mid-waist (MWC), and maximum hip (MHC). Skinfolds, including bicep skinfold (BSF), tricep skinfold (TSF), subscapular skinfold (SSF), suprailiac skinfold (SISF), umbilical skinfold (USF), and mid-thigh skinfold (MTSF), were measured with a Harpendon caliper (Baty International, England). Cumulative skinfold sum (SFS) was calculated by adding BSF, TSF, SSF, SISF, USF, and MTSF.
Extremity fat area (cm2), including upper arm fat area (AFA), percentage of fat in upper arm (%AF), upper leg fat area (LFA), and percentage of fat in upper leg (%LF) were calculated.28 Regional (arm, trunk, leg) fat as proportions of total body fat were calculated as follows: arm [(BSF+TSF)/SFS], trunk [(SSF+SISF+USF)/SFS], and leg [MTSF/SFS]. The following trunk-extremity skinfold ratios were calculated as indicators of central subcutaneous fat as compared to extremity fat: trunk-arm [(SSF+SISF)/(BSF+TSF+SSF+SISF)] and trunk-leg [(SSF+SISF)/(MTSF+SSF+SISF)].29 Total body resistance was measured by single-frequency bioimpedance analysis (BIA) (Quantum II, RJL Systems, Clinton Township, MI) and used in a prediction equation to estimate percent body fat (%BF).30
Statistical Analysis
Modified intent-to-treat analyses of the treatment groups (lopinavir/ritonavir vs. nevirapine) were conducted using the Wilcoxon rank-sum test for non-normally distributed and t-test for normally-distributed continuous variables. Chi-squared or Fisher’s exact tests were used for categorical variables. These tests were also used for comparisons across lipodystrophy categories. Multiple linear regression was performed to assess differences in arm, trunk, and leg fat across lipodystrophy groups, adjusting for total fat, sex, and age. All statistical tests were 2-sided and a p-value <0.05 was considered statistically significant. Analyses were performed using SAS version 9.1.3 (SAS Institute Inc, Cary, North Carolina).
RESULTS
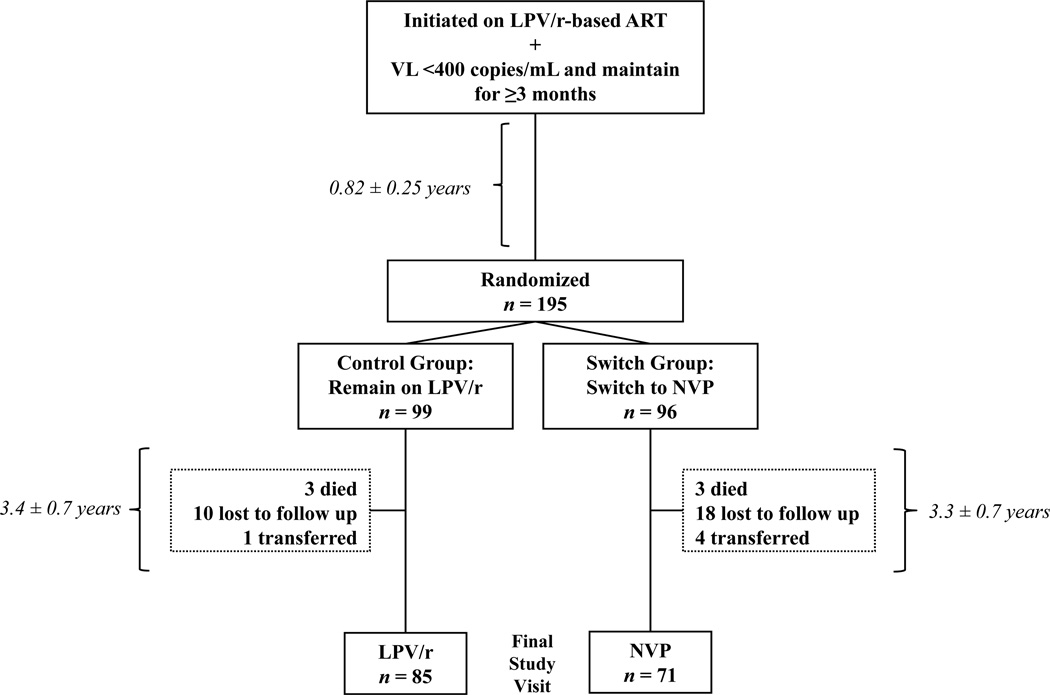
Of the 195 children randomized in the NEVEREST 2 clinical trial, 156 completed the final study visit and were included in this analysis (Figure 1). Six(3.1%) children died, 28(14.4%) were lost to follow up, and 5(2.6%) transferred out. At the time of this assessment, subjects were between 3.3 and 7.3 years old, 52% were male, and 85 were previously randomized to the lopinavir/ritonavir-group and 71 to the nevirapine-group (Table 1). There were no differences in total time on ART or time since randomization between the groups. WAZ, HAZ, or BAZ were similar between groups at randomization and at the time of this assessment. At the final study visit, 5(5.9%) in the lopinavir/ritonavir-group and 7(9.9%) in the nevirapine- group had moderate or severe malnutrition i.e. WAZ <–2.21 Sixteen(18.8%) lopinavir/ritonavir-group and 13(18.3%) nevirapine-group children had a HAZ <–2. No differences in proportion of children with abnormal systolic (38.8 vs. 26.8%, p=0.112) or diastolic (42.3 vs. 35.2%, p=0.363) blood pressure were detected. Most children (88%) had a VL<50 copies/mL. There were no virologic differences between the groups. Compared to the lopinavir/ritonavir-group, the nevirapine-group had a higher median CD4 cell count. There were no differences in lipodystrophy classification between lopinavir/ritonavir- and nevirapine-groups.
Figure 1.
Flow diagram of study participants in NEVEREST 2 (2005–2010) in Johannesburg, South Africa detailing the number of subjects excluded from the analysis, the reason for their exclusion, and distribution of included subjects by randomization group. Time intervals are provided in mean ± standard deviation.
Table 1.
Characteristics at the time of assessment of 156 perinatally HIV-infected South African children previously suppressed on ritonavir-boosted lopinavir (LPV/r)-based treatment and randomized to either maintain the LPV/r-based regimen or switch to a nevirapine (NVP)-based regimen
| Randomization group | |||||
|---|---|---|---|---|---|
| characteristic | Combined (n=156) |
Lpv/r (n=85) |
NVP (n=71) |
P-valuea | |
| Males | n(%) | 81 (51.9) | 41 (48.2) | 40 (56.3) | 0.313 |
| Age, years | Mean±SD | 5.1±0.8 | 5.2±0.8 | 5.0±0.8 | 0.095 |
| Total Time on ART, years | Mean±SD | 4.2±0.7 | 4.2±0.7 | 4.1±0.7 | 0.338 |
| Time Since Randomization, years | Mean±SD | 3.4±0.7 | 3.4±0.7 | 3.3±0.7 | 0.469 |
| Weight, kg | Mean±SD | 17.1±2.9 | 17.2±3.0 | 16.8±2.8 | 0.379 |
| Weight-for-Age z-score | Mean±SD | −0.67±1.0 | −0.67±0.9 | −0.66±1.0 | 0.912 |
| Height, cm | Mean±SD | 105.3±7.2 | 105.7±7.0 | 104.9±7.4 | 0.537 |
| Height-for-age z-score | Mean±SD | −0.98±1.1 | −1.02±1.0 | −0.93±1.2 | 0.575 |
| BMI, kg/m2 | Mean±SD | 15.3±1.5 | 15.4±1.6 | 15.2±1.4 | 0.529 |
| BMI-for-age z-score | Mean±SD | −0.1±1.0 | −0.09±1.0 | −0.11±1.1 | 0.884 |
| Blood Pressure, mmHg Systolic Diastolic |
Mean±SD |
98.5 ± 13.9 62.6 ± 11.6 |
99.6 ± 13.7 63.2 ± 12.3 |
97.0 ± 14.1 61.8 ± 10.6 |
0.294 0.464 |
| Viral Load, copies/mL <50 50–999 ≥1000 |
n (%) |
137 (87.8) 12 (7.7) 7 (4.5) |
72 (84.7) 8 (9.4) 5 (5.9) |
65 (91.6) 4 (5.6) 2 (2.8) |
0.450 |
| CD4 percent, % | Median (IQR) |
36.7 (32.7,41.3) |
35.9 31.2,40.6 |
37.2 33.0,41.5 |
0.185 |
| CD4 count, cells/mm3 | Median (IQR) |
1451 (1048, 1749) |
1356 (962, 1620) |
1480 (1174,1773) |
0.047 |
| LD Classification LD Possible LD No LD |
n (%) |
13 (8.3) 18(11.5) 125 (80.1) |
6 (7.1) 8 (9.4) 71 (83.5) |
7 (9.9) 10 (14.1) 54 (76.1) |
0.505 |
Abbreviations: SD, standard deviation; IQR, inter-quartile range
LPV/r- and NVP-groups were compared using the Wilcoxon rank sum test and t-test for continuous variables and chi-squared or Fisher's exact test for categorical variables
Several metabolic measurements differed between the treatment groups (Table 2). The mean TC was greater in the lopinavir/ritonavir-group, as was the proportion with elevated TC. Significantly lower mean HDL concentrations and higher mean LDL concentrations were observed in the lopinavir/ritonavir-group. Additionally, a significantly higher ratio of TC:HDL was observed in the lopinavir/ritonavir-group. The mean triglyceride concentration and proportion with abnormal triglycerides were also greater in the lopinavir/ritonavir-group. Mean CRP and proportion with elevated CRP were lower for the lopinavir/ritonavir-group. There were no differences in recent (i.e. past 1 month) or acute illnesses between the groups.
Table 2.
Metabolic and other laboratory assessments of 156 perinatally HIV-infected South African children suppressed on ritonavir-boosted lopinavir (LPV/r)-based treatment and randomized to either maintain the LPV/r-based regimen or switch to a nevirapine (NVP)-based regimen
| Randomization Group | |||||
|---|---|---|---|---|---|
| Measure | combined (n=156) |
LPV/r (n=85) |
NVP (n=71) |
p-valuea | |
| Total Cholesterol, mmol/L(mg/dL) | Mean±SD | 4.3±0.9 (166±36) | 4.4±1.0 (171±39) | 4.1±0.8 (161±31) | 0.097 |
| Acceptable: <4.4 (<170) | n (%) | 97 (62.2) | 45 (52.9) | 52 (73.2) | 0.030 |
| Borderline: 4.4–5.1 (170–199) | 37 (23.7) | 24 (28.2) | 13 (18.3) | ||
| Elevated: ≥5. (≥200) | 22 (14.1) | 16 (18.8) | 6 (8.5) | ||
| HDL, mmol/L(mg/dL) | Mean±SD | 1.4±0.4 (54±15) | 1.3±0.4 (51±14) | 1.5±0.4 (59±16) | <0.001 |
| Abnormal: <0.9 (<35) | n (%) | 9 (5.8) | 7 (8.2) | 2 (2.8) | 0.183 |
| Normal:≥0.9 ≥ 35) | 147 (94.2) | 78 (91.8) | 69 (97.2) | ||
| LDL, mmol/L(mg/dL) | Mean±SD | 2.4±0.8 (94±32) | 2.6±0.9 (100±34) | 2.3±0.7 (88±27) | 0.018 |
| Acceptable: <2.9 (<110) | n (%) | 112 (71.8) | 56 (65.9) | 56 (78.9) | 0.156 |
| Borderline: 2.9–3.3 (110–129) | 26 (16.7) | 16 (18.8) | 10 (14.1) | ||
| Elevated: ≥ 3.4 ≥ 130) | 18 (11.5) | 13 (15.3) | 5 (7.0) | ||
| Total Cholesterol : HDL Ratio | Mean±SD | 3.3±1.0 | 3.6±1.1 | 2.9±0.9 | <0.001 |
| Triglycerides, mmol/L(mg/dL) | Mean±SD | 0.9±0.4 (84±36) | 1.1±0.4 (94±39) | 0.8±0.3 (72±29) | <0.001 |
| Normal: ≤1.69 (≤150) | n (%) | 143 (91.7) | 74 (87.1) | 69 (97.2) | 0.038 |
| Abnormal: >1.69 (>150) | 13 (8.3) | 11 (12.9) | 2 (2.8) | ||
| C-Reactive Protein, mg/L | Mean±SD | 6.3±15.3 | 3.5±6.1 | 9.6±21.4 | 0.023 |
| Normal: ≤ 3 | n (%) | 115 (73.7) | 69 (81.2) | 46 (64.8) | 0.021 |
| Elevated: >3 | 41 (26.3) | 16 (18.8) | 25 (35.2) | ||
| Glucose, mmol/L(mg/dL) | Mean±SD | 5.3±0.5 (96±8.2) | 5.4±0.4 (97±7.4) | 5.3±0.5 (96±9.1) | 0.566 |
| Normal: <6.11 (<110) | n (%) | 154 (98.7) | 84 (98.8) | 70 (98.6) | 0.898 |
| Impaired: ≥6.11 (≥110) | 2 (1.3) | 1 (1.2) | 1 (1.4) | ||
| HOMA-IR | Mean±SD | 1.05±0.7 | 1.04±0.6 | 1.08±0.8 | 0.716 |
| Normal: ≤ 3.16 | n (%) | 152 (98.1) | 85 (100.0) | 67 (95.7) | 0.090 |
| Insulin Resistance: >3.16 | 3 (1.9) | 0 (0.0) | 3 (4.3) | ||
Abbreviations: SD, standard deviation; IQR, inter-quartile range
LPV/r- and NVP-groups were compared using the Wilcoxon rank sum test and t-test for continuous variables and chi-squared or Fisher’s exact test for categorical variables
In addition, multiple abnormalities were more common in the lopinavir/ritonavir group. More children in the lopinavir/ritonavir-group had elevated TC and LDL (13.2 vs. 5.5%, p=0.054) and elevated TC and triglycerides (5.9 vs. 0.0%, p=0.038) compared to children in the nevirapine-group. Mean glucose and HOMA-IR did not differ between the groups. A repeat impaired fasting glucose was detected in 1(1.2%) child in the lopinavir/ritonavir-group and 1(1.4%) child in the nevirapine-group. None in the lopinavir/ritonavir-group had insulin resistance compared to 3(4.3%) in the nevirapine-group (p=0.09).
Complete body composition measurements were available for 139 children (Table 3). Children in the lopinavir/ritonavir-group had a greater amount of body fat than children in the nevirapine-group, as measured by mean SFS. A similar pattern in total BIA-determined %BF was observed. Leg fat area and percentage of fat in upper leg were both significantly greater in the lopinavir/ritonavir-group. In addition, while the arm fat proportion of total fat was not different between the groups, the trunk fat proportion of total fat was lower for the lopinavir/ritonavir-group and the leg fat proportion of total fat was significantly greater for the lopinavir/ritonavir-group. Although there was no difference in trunk-arm skinfold ratios between the groups, the lopinavir/ritonavir-group had a lower trunk-leg skinfold ratio compared to the nevirapine-group.
Table 3.
Body composition measures of 156 perinatally HIV-infected South African children suppressed on ritonavir-boosted lopinavir (LPV/r)-based treatment and randomized to either maintain the LPV/r-based regimen or switch to a nevirapine (NVP)-based regimen (Mean±SD)
| Randomization Group | |||||
|---|---|---|---|---|---|
| Measure | combined (n=156) |
LPV/r (n=85) |
NVP (n=71) |
P-value a | |
| MUAC – Mid Upper Arm Circumference, cm | 15.5±2.1 | 15.8±2.2 | 15.2±1.9 | 0.073 | |
| MTC – Mid Thigh Circumference, cm | 29.7±3.4 | 30.1±3.8 | 29.3±2.9 | 0.143 | |
| MWC – Mid Waist Circumference, cm | 51.1±3.7 | 51.4±4.0 | 50.8±3.5 | 0.325 | |
| MHC – Maximum Hip Circumference, cm | 53.9±6.0 | 54.2±7.3 | 53.6±4.0 | 0.556 | |
| MWC – MHC Ratio | 1.02±1.0 | 1.08±1.3 | 0.95±0.1 | 0.373 | |
| BSF – Bicep Skinfold, mm | 5.29±1.7 | 5.67±1.8 | 4.84±1.5 | 0.002 | |
| TSF – Tricep Skinfold, mm | 7.06±1.7 | 7.24±1.8 | 6.86±1.7 | 0.180 | |
| SSF – Subscapular Skinfold, mm | 6.24±1.7 | 6.47±1.7 | 5.97±1.6 | 0.069 | |
| SISF – Suprailiac Skinfold, mm | 6.40±2.6 | 6.58±2.8 | 6.18±2.2 | 0.329 | |
| USF – Umbilical Skinfold, mm | 6.71±4.5 | 7.01±5.4 | 6.31±2.9 | 0.320 | |
| MTSF – Mid Thigh Skinfold, mm | 9.80±3.2 | 10.4±3.3 | 9.1±3.0 | 0.018 | |
| SFS – Skinfold Sum, mm | 41.3±10.8 | 43.0±11.1 | 39.0±10.1 | 0.031 | |
| %BF = %Body Fat by BIA | 15.7±8.0 | 17.0±7.0 | 14.1±8.0 | 0.022 | |
| Extremity Fat Area | |||||
| Arm | AFA - Arm Fat Area, cm2 | 5.5±1.9 | 5.8±2.1 | 5.2±1.7 | 0.078 |
| %AF - % of Fat in Arm | 29.0±8.3 | 29.1±8.3 | 28.9±8.3 | 0.837 | |
| Leg | LFA - Leg Fat Area, cm2 | 14.8±5.8 | 15.7±6.0 | 13.6±5.3 | 0.023 |
| %LF - %of Fat in Leg | 20.7±6.4 | 21.8±6.7 | 19.4±5.7 | 0.023 | |
| Regional Fat Proportions of Total Body Fat | |||||
| Arm | [(BSF+TSF) / SFS] | 0.30±0.04 | 0.30±0.05 | 0.30±0.04 | 0.734 |
| Trunk | [(SSF +SISF+USF) / SFS] | 0.46±0.06 | 0.45±0.06 | 0.47±0.05 | 0.114 |
| Leg | [MTSF / SFS] | 0.24±0.04 | 0.24±0.04 | 0.23±0.03 | 0.046 |
| Trunk-Extremity Skinfold Ratios | |||||
| Trunk-Arm | [(SSF+SISF) / (BSF+TSF+SSF+SISF)] | 0.50±0.06 | 0.50±0.06 | 0.51±0.05 | 0.256 |
| Trunk-Leg | [(SSF + SISF) / (MTSF + SSF+SISF)] | 0.56±0.06 | 0.55±0.07 | 0.57±0.05 | 0.034 |
Abbreviations: SD, standard deviation
LPV/r- and NVP-groups were compared using t-tests
Thirteen(8.3%) children were classified as LD+ and 18(11.5%) as possible LD. Of the 13 LD+ children, all had signs of lipoatrophy and 6 also had signs of lipohypertrophy. There were no differences in age, sex, age at ART initiation, total time on ART, randomization group, WAZ, HAZ, BAZ, or proportion with VL <50 copies/mL between the three lipodystrophy groups.
No differences in TC, HDL, LDL, TC:HDL ratio, CRP, glucose, or HOMA-IR were detected across lipodystrophy groups (data not shown). However, mean triglycerides and proportion with abnormal triglycerides was higher for LD+ than LD− children as well as for possible LD than LD− children.
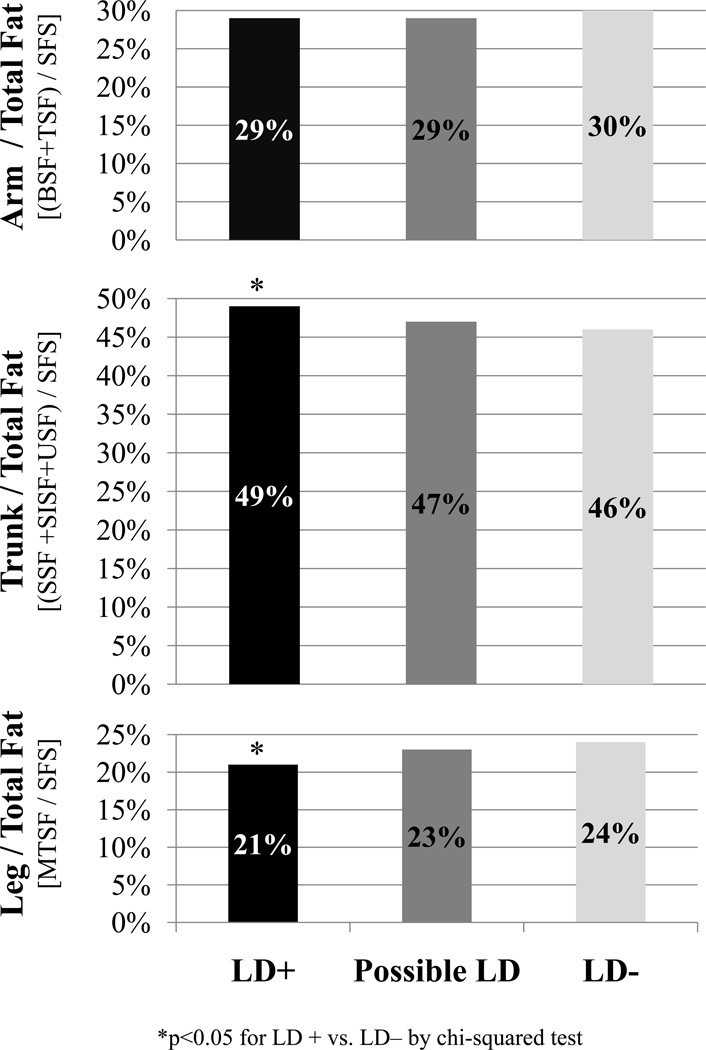
Overall, LD+ children had significantly less body fat compared to LD− children as measured by SFS. A similar pattern was observed using BIA-based estimates of total BF%, although this did not achieve statistical significance. As presented in Figure 2, no differences were seen in arm fat; however, LD+ children had a greater trunk fat proportion of total fat and less leg fat as a proportion of total fat compared to LD− children. A similar pattern of differences in arm (p=0.04), trunk (p=0.01), and leg (p=0.05) fat between LD+ and LD− children was detected after adjusting for total fat, sex, and age by means of multiple linear regression. LD+ children also had significantly greater trunk-arm and trunk-leg skinfold ratios compared to LD− children.
Figure 2.
Regional fat (as % of total) by skinfolds of children with lipodystrophy (LD+), possible LD, or no LD (LD−), as determined by clinician assessment. Children were classified as LD+, possible LD, and LD− if >2, 1, or 0 of features of lipoatrophy (i.e. sunken cheeks, temporal wasting, skinny limbs, wasting of buttocks) or lipohypertrophy (i.e increased abdominal girth, dorsal cervical enlargement, or breast enlargement) were present.
DISCUSSION
In this study of young perinatally HIV-infected South African children initiated and suppressed on PI-based ART and then randomized to either remain on the initial PI-based regimen or switch to a NNRTI-based regimen, the group remaining on the lopinavir/ritonavir-based regimen had unfavorable concentrations of lipoproteins and triglycerides compared to those switching to the nevirapine-based regimen. These results confirm and extend our prior, shorter term observations and other published studies. 1,3,4,31,32 With extended survival and the requirement of lifelong ART, these metabolic alterations may pose long-term risk with respect to CVD, as CVD often results from exposure to risk factors that have their origins in childhood.33 Serum lipid concentrations, insulin resistance, hypertension, and obesity early in life contribute to the development of atherosclerotic lesions in children and adolescents.34,35 In addition, studies among adults contracting HIV indicate that HIV infection per se may be associated with greater CVD possibly due to chronic immune activation.36 Also, increased intima-media thickness, an indicator of atherosclerosis, has been reported in studies of HIV-infected children and adolescents receiving ART.37 The constellation of risk factors seen during childhood and adolescence among those with perinatally-acquired HIV suggests that developing strategies for CVD risk reduction during childhood are warranted.
The elevated CRP reported in this study has also been previously reported.37 While CRP might have been expected to cluster with alterations in lipids and triglycerides or regional fat changes depot, our finding of elevated CRP in the NNRTI-group compared to the PI-group is not without precedent nor is the long-term significance known.38,39
In this cohort of children receiving stavudine-backbone ART, continuation on PI-based regimen was associated with greater overall subcutaneous fat, and less central (trunk) fat in relation to leg fat in comparison to children switched to an NNRTI-based regimen. This difference in subcutaneous regional fat was detectable even though the groups had a similar overall WAZ, BMI and proportion of children with lipodystrophy. Since children in our study were randomly assigned to continue on a PI-based regimen or switch to an NNRTI-based regimen, our findings provide less confounded evidence that PI-based regimens are associated with greater amounts of subcutaneous fat compared to NNRTI than available from prior non- randomized studies. 2,13,40,41 It is not possible to determine which of the patterns of changes in adipose tissue we observed is a departure from normal, since our study did not include a healthy comparison group and appropriate skinfold standards are not available for young South African children. In the present study no relationship was detected between adipose and metabolic measures. Whether the clustering of these features will evolve with additional time and exposure to ARV remains to be determined.
Lipodystrophy is cosmetically stigmatizing and can adversely affect adherence to ART.42 Finding a substantial proportion of young children with lipodystrophy has implications for future adherence, especially during adolescence when awareness of physical appearance is heightened. Most studies indicate the prevalence of lipodystrophy increases with age, duration of therapy, and puberty. 5,15 In addition, stavudine, possibly due to inhibition of mitochondrial DNA polymerase has been implicated in lipoatrophy, and for this and other reasons, WHO advises phasing out or discontinuing stavudine.43,44 Of interest, anthropometry appears to confirm the distinctive regional fat pattern of lipodystrophy observed by clinician assessment. Standardized clinical and anthropometric assessments for early detection of changes in regional fat are a valuable addition to clinical studies.
There are a number of limitations in this study. The metabolic and body composition measurements did not assess visceral adiposity, were obtained at the final visit of this trial, and were not available prior to randomization. For those switched back to nevirapine, observation time was relatively short. In addition, family history of lipid disorders, diet and exercise may be relevant to our findings but were not assessed. In addition, although significant proportions of subjects had abnormal blood pressure, interpretation is difficult as the norms applied were from US children. Furthermore, the measurement of glucose-insulin homeostasis used is relatively insensitive compared to more invasive tests.8 Finally, a single CRP measurement may not be reflective of long-term inflammatory status.
Lopinavir/ritonavir is highly effective in suppressing HIV and an essential part of treatment for children in resource-limited settings. WHO includes lopinavir/ritonavir as part of first-line treatment for HIV-infected children with prior exposure to NNRTI used for prevention of mother-to-child transmission and for those who experience treatment failure with NNRTIs.43 The long-term impact of the body fat and lipid alterations bears further study, as do efforts to devise safe and effective approaches for prevention and management of these abnormalities. It is also essential to expand access to newer antiviral agents with potentially fewer metabolic effects to children in resource-limited settings.43,45
What is Already Known on this Topic
Changes in lipid, glucose, and triglyceride metabolism, and regional fat are well described among older children and adolescents with perinatally acquired HIV-infection receiving antiretroviral therapy in high income countries.
There are limited studies of younger HIV-infected children in sub-Saharan Africa, where >90% of HIV-infected children live and where there is increasing availability of ART.
What This Study Adds
Hypercholesterolemia and hypertriglyceridemia were detectable in HIV-infected children who were initiated on antiretroviral therapy before the age of 2 years.
These metabolic markers were significantly higher among patients on lopinavir/ritonavir-based than nevirapine-based ART.
Lipodystrophy is visible in 8.4% of children and was confirmed by simple anthropometry.
Acknowledgments
Funding
This study was supported in part by the National Institute of Child Health and Human Development [grant numbers HD 47177, HD 61255], and Secure the Future Foundation [grant number RES 219].
References
- 1.Carter RJ, Wiener J, Abrams EJ, et al. Dyslipidemia among perinatally HIV-infected children enrolled in the PACTS-HOPE cohort: 1999–2004: a longitudinal analysis. J Acquir Immune Defic Syndr. 2006;41(4):453. doi: 10.1097/01.qai.0000218344.88304.db. [DOI] [PubMed] [Google Scholar]
- 2.Aldrovandi GM, Lindsey JC, Jacobson DL, et al. Morphologic and metabolic abnormalities in vertically HIV-infected children and youth. AIDS. 2009;23(6):661. doi: 10.1097/QAD.0b013e3283269dfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strehlau R, Coovadia A, Abrams EJ, et al. Lipid Profiles in Young HIV-infected Children Initiating and Changing Antiretroviral Therapy. J Acquir Immune Defic Syndr. doi: 10.1097/QAI.0b013e318243760b. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sztam KA, Jiang H, Jurgrau A, Deckelbaum RJ, Foca MD. Early Increases in Concentrations of Total, LDL, and HDL Cholesterol in HIV-infected Children Following New Exposure to Antiretroviral Therapy. J Pediatr Gastroenterol Nutr. 2011;52(4):495. doi: 10.1097/MPG.0b013e3181f5e9d4. [DOI] [PubMed] [Google Scholar]
- 5.Jaquet D, Lévine M, Ortega-Rodriguez E, et al. Clinical and metabolic presentation of the lipodystrophic syndrome in HIV-infected children. AIDS. 2000;14(14):2123. doi: 10.1097/00002030-200009290-00008. [DOI] [PubMed] [Google Scholar]
- 6.Dimock D, Thomas V, Cushing A, et al. Longitudinal assessment of metabolic abnormalities in adolescents and young adults with HIV-infection acquired perinatally or in early childhood. Metabolism. 2010 doi: 10.1016/j.metabol.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chantry CJ, Hughes MD, Alvero C, et al. Lipid and glucose alterations in HIV-infected children beginning or changing antiretroviral therapy. Pediatrics. 2008 doi: 10.1542/peds.2007-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bitnun A, Sochett E, Dick PT, et al. Insulin Sensitivity and {beta}-Cell Function in Protease Inhibitor-Treated and-Naive Human Immunodeficiency Virus-Infected Children. J Clin Endocrinol Metab. 2005;90(1):168. doi: 10.1210/jc.2004-0125. [DOI] [PubMed] [Google Scholar]
- 9.Noor MA, Seneviratne T, Aweeka FT, et al. Indinavir acutely inhibits insulin-stimulated glucose disposal in humans: a randomized, placebo-controlled study. AIDS. 2002;16(5):F1. doi: 10.1097/00002030-200203290-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Romano R, Rudich A, Török D, et al. Agent and cell-type specificity in the induction of insulin resistance by HIV protease inhibitors. AIDS. 2003;17(1):23. doi: 10.1097/00002030-200301030-00005. [DOI] [PubMed] [Google Scholar]
- 11.Innes S, Levin L, Cotton M. Lipodystrophy syndrome in HIV-infected children on HAART. South Afr J HIV Med. 2009;10(4):76. doi: 10.4102/sajhivmed.v10i4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Paediatric Lipodystrophy Group. Antiretroviral therapy, fat redistribution and hyperlipidaemia in HIV-infected children in Europe. AIDS. 2004;18(10):1443–1451. doi: 10.1097/01.aids.0000131334.38172.01. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson DL, Patel K, Siberry GK, et al. Body fat distribution in perinatally HIVinfected and HIV-exposed but uninfected children in the era of highly active antiretroviral therapy: outcomes from the Pediatric HIV/AIDS Cohort Study. Am J Clin Nutr. 2011 Dec;94(6):1485–1495. doi: 10.3945/ajcn.111.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniels SR, Morrison JA, Sprecher DL, Khoury P, Kimball TR. Association of body fat distribution and cardiovascular risk factors in children and adolescents. Circulation. 1999 Feb 2;99(4):541–545. doi: 10.1161/01.cir.99.4.541. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez Torres AM, Munoz Muniz R, Madero R, Borque C, Garcia-Miguel MJ, De Jose Gomez MI. Prevalence of fat redistribution and metabolic disorders in human immunodeficiency virus-infected children. Eur J Pediatr. 2005 May;164(5):271–276. doi: 10.1007/s00431-004-1610-y. [DOI] [PubMed] [Google Scholar]
- 16.Alam N, Cortina-Borja M, Goetghebuer T, Marczynska M, Vigano A, Thorne C. Body fat abnormality in HIV-infected children and adolescents living in Europe: prevalence and risk factors: Fat abnormality in children. J Acquir Immune Defic Syndr. 2012 Mar 1;59(3):314–324. doi: 10.1097/QAI.0b013e31824330cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallewa JE, Wilkins E, Vilar J, et al. HIV-associated lipodystrophy: a review of underlying mechanisms and therapeutic options. J Antimicrob Chemother. 2008 Oct;62(4):648–660. doi: 10.1093/jac/dkn251. [DOI] [PubMed] [Google Scholar]
- 18.WHO. Towards Universal Access: Scaling up priority HIV/AIDS interventions in the health sector. 2010
- 19.Coovadia A, Abrams EJ, Stehlau R, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial. JAMA. 2010 Sep 8;304(10):1082–1090. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.South African National Department of Health. [Accessed August 2011];South African Guidelines, Factsheet Section 10, Antiretroviral. 2007 http://www.doh.gov.za/docs/factsheets/guidelines/hiv/part5.pdf.
- 21.WHO. [Accessed May 2011];Child Growth Standards and WHO Anthro and Macros. 2007 http://www.who.int/childgrowth/en/
- 22.Kaelber DC, Pickett F. Simple table to identify children and adolescents needing further evaluation of blood pressure. Pediatrics. 2009;123(6):e972. doi: 10.1542/peds.2008-2680. [DOI] [PubMed] [Google Scholar]
- 23.Daniels S, Greer F. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122(1):198. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 24.Kavey REW, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation. 2003;107(11):1562. doi: 10.1161/01.cir.0000061521.15730.6e. [DOI] [PubMed] [Google Scholar]
- 25.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003 Jan 28;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association. Type 2 diabetes in children and adolescents. Diabetes Care. 2000;23:381–389. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- 27.Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115(4):e500. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 28.Rolland-Cachera M, Brambilla P, Manzoni P, et al. Body composition assessed on the basis of arm circumference and triceps skinfold thickness: a new index validated in children by magnetic resonance imaging. Am J Clin Nutr. 1997;65(6):1709. doi: 10.1093/ajcn/65.6.1709. [DOI] [PubMed] [Google Scholar]
- 29.Van Lenthe FJ, Kemper HCG, Van Mechelen W, Twisk JWR. Development and tracking of central patterns of subcutaneous fat in adolescence and adulthood: the Amsterdam Growth and Health Study. Int J Epidemiol. 1996;25(6):1162. doi: 10.1093/ije/25.6.1162. [DOI] [PubMed] [Google Scholar]
- 30.Horlick M, Arpadi SM, Bethel J, et al. Bioelectrical impedance analysis models for prediction of total body water and fat-free mass in healthy and HIV-infected children and adolescents. Am J Clin Nutr. 2002;76(5):991. doi: 10.1093/ajcn/76.5.991. [DOI] [PubMed] [Google Scholar]
- 31.Aurpibul L, Puthanakit T, Lee B, Mangklabruks A, Sirisanthana T, Sirisanthana V. Lipodystrophy and metabolic changes in HIV-infected children on non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Antivir Ther. 2007;12(8):1247–1254. [PubMed] [Google Scholar]
- 32.Purnell JQ, Zambon A, Knopp RH, et al. Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. AIDS. 2000 Jan 7;14(1):51–57. doi: 10.1097/00002030-200001070-00006. [DOI] [PubMed] [Google Scholar]
- 33.Nicklas T, Von Duvillard S, Berenson G. Tracking of serum lipids and lipoproteins from childhood to dyslipidemia in adults: the Bogalusa Heart Study. Int J Sports Med. 2002;23(s 1):39–43. doi: 10.1055/s-2002-28460. [DOI] [PubMed] [Google Scholar]
- 34.Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med. 1998;338(23):1650. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood. JAMA. 2003;290(17):2271. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 36.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011 Feb 18;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross AC, Storer N, Ann OR. Longitudinal Changes in Carotid Intima-Media Thickness and Cardiovascular Risk Factors in Human Immunodeficiency Virus-Infected Children and Young Adults Compared With Healthy Controls. Pediatr Infect Dis J. 2010;29(7):634. doi: 10.1097/inf.0b013e3181d770c4. [DOI] [PubMed] [Google Scholar]
- 38.Cook DG, Mendall MA, Whincup PH, et al. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000 Mar;149(1):139–150. doi: 10.1016/s0021-9150(99)00312-3. [DOI] [PubMed] [Google Scholar]
- 39.Guimaraes MM, Greco DB, Figueiredo SM, Foscolo RB, Oliveira AR, Jr, Machado LJ. High-sensitivity C-reactive protein levels in HIV-infected patients treated or not with antiretroviral drugs and their correlation with factors related to cardiovascular risk and HIV infection. Atherosclerosis. 2008 Dec;201(2):434–439. doi: 10.1016/j.atherosclerosis.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Arpadi SM, Bethel J, Horlick M, et al. Longitudinal changes in regional fat content in HIV-infected children and adolescents. AIDS (London, England) 2009;23(12):1501. doi: 10.1097/QAD.0b013e32832b7e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dzwonek AB, Lawson MS, Cole TJ, Novelli V. Body fat changes and lipodystrophy in HIV-infected children: impact of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2006 Sep;43(1):121–123. doi: 10.1097/01.qai.0000230523.94588.85. [DOI] [PubMed] [Google Scholar]
- 42.Ammassari A, Antinori A, Cozzi-Lepri A, et al. Relationship between HAART adherence and adipose tissue alterations. J Acquir Immune Defic Syndr. 2002;31:S140. doi: 10.1097/00126334-200212153-00011. [DOI] [PubMed] [Google Scholar]
- 43.WHO. Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access. Recommendations for a Public Health Approach: 2010 revision. World Health Organization; 2010. [PubMed] [Google Scholar]
- 44.Squires KE. An introduction to nucleoside and nucleotide analogues. Antivir Ther. 2001;6(Suppl 3):1–14. [PubMed] [Google Scholar]
- 45.Mobius U, Lubach-Ruitman M, Castro-Frenzel B, et al. Switching to atazanavir improves metabolic disorders in antiretroviral-experienced patients with severe hyperlipidemia. J Acquir Immune Defic Syndr. 2005 Jun 1;39(2):174–180. [PubMed] [Google Scholar]