Abstract
Purpose
To evaluate the accuracy of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code for small for gestational age (SGA) recorded in administrative healthcare records using birthweight and gestational age information recorded in electronic medical records.
Methods
We used billing and medical records from females aged 13-55 years who delivered at a tertiary care center in the U.S. between 2004 and 2011. Information on birthweight, gestational age at birth, and ICD-9-CM code for SGA, 656.5x, was abstracted from the database. Each infant's birthweight percentile for gestational age was calculated based on published U.S. references; infants below the 10th percentile were classified as SGA. The performance characteristics of SGA ICD-9-CM diagnosis code against SGA classification based on birthweight and gestational age were calculated, for all deliveries and by strata of demographic and delivery characteristics.
Results
We identified 51 292 singleton live birth deliveries. The prevalence of SGA infants calculated from birthweight and gestational age at birth was higher (13%) than the prevalence based on ICD-9-CM code (2%). Sensitivity of the SGA ICD-9-CM code was 14.2%; specificity was 99.7%; positive predictive value was 86.8% and negative predictive value was 88.4%. Stratification by demographic and delivery characteristics yielded similar results.
Conclusions
Identification of SGA infants using ICD-9-CM code, 656.5x, from administrative healthcare records has low sensitivity but high specificity; the accuracy did not differ across demographic and delivery characteristics. Thus, although this source of information would underestimate the prevalence of SGA, it could produce valid relative risk estimates.
Keywords: healthcare records, sensitivity, specificity, gestational age, small for gestational age, ICD-9-CM codes
Introduction
Being born small for gestational age (SGA)1 is associated with substantial neonatal morbidity and mortality, higher healthcare costs among newborns, as well as a range of chronic diseases later in life.2-5 Established risk factors for SGA include nulliparity, maternal cigarette smoking and cocaine use, chronic hypertension and pre-eclampsia.6-11 Epidemiologic studies have also indicated that certain medications during pregnancy, including antihypertensives,12, 13 opioids,14, 15 anticonvulsants,16, 17 and antiretrovirals,18, 19 may be associated with significant increases in this adverse pregnancy outcome; however, for many of these and other medications, the potential effects on fetal growth is controversial and further research is needed.
In order to identify infants who are born SGA, information on both the weight and the gestational age at birth are required. These critical details however are often not available in administrative healthcare databases that are increasingly being used to study the safety of medications during pregnancy, 20 such as the Medicaid Analytic eXtract and the Healthcare Cost and Utilization Project Nationwide Inpatient Sample;21, 22 the databases have information on healthcare utilization claims, including pharmacy dispensing and inpatient and outpatient diagnosis and procedure codes. Therefore, in pharmacoepidemiology studies that utilize administrative healthcare data, a better understanding of the performance characteristics of the diagnosis code used to classify infants as SGA is crucial.
We evaluated the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code for SGA, 656.5x, recorded in the maternal delivery admission records compared to the definition of SGA using recorded birthweight and gestational age at birth in a large sample of delivery admissions at a tertiary care center.
Methods
Data source
We used an existing medical record database of women who delivered at Brigham and Women's Hospital (BWH), a tertiary care center located in Boston, U.S. The database was constructed by linking electronic medical data recorded by the labor nurses at the time of delivery (including birthweight, gestational age, and parity) with billing data for the delivery admission (including demographic information and ICD-9-CM diagnosis and procedure codes). The project was approved by Brigham and Women's Hospital and Harvard School of Public Health Institutional Review Boards.
Study cohort
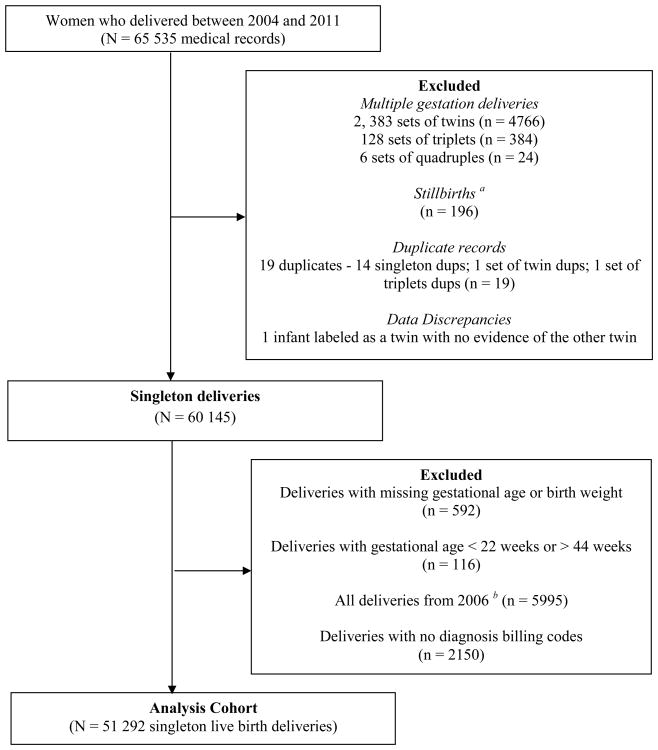
The study source population consisted of females aged 13-55 years who delivered from January, 2004 through December, 2011. From the source population we excluded multiple gestation deliveries, since these are highly correlated with SGA, and also stillbirths, as SGA is not well defined for fetal deaths. We also excluded records with inconsistent data entries (for example, duplicate delivery records), and those with missing information on either the infant's birthweight or gestational age at birth [Figure 1]. All singleton live birth deliveries for a woman that occurred during the study period and were captured in the database were included in our final study population. A cohort that included multiples was considered in secondary analysis.
Figure 1. Study source population.
a Stillbirths were identified from the following ICD-9-CM diagnosis codes: 656.4x, v27.1, v27.3, v27.4, v27.6, and v27.7
b All deliveries from 2006 were deleted since > 80% of deliveries from that year had no diagnosis billing codes
Classification of small for gestational age infants based on ICD-9-CM codes
Information on SGA was obtained directly from the database based on ICD-9-CM code 656.5x. Per standard hospital procedure, trained medical coders enter the code (for billing purposes) based on delivery admission and progress notes recorded by the labor and delivery nurses in the maternal electronic hospital discharge records. BWH follows the American College of Obstetricians and Gynecologists (ACOG) standards,23 and the gestational age information on maternal records is generally based on the estimated date of confinement (EDC) reported by the patient and multiple other sources that contain this information, including outpatient records from respective obstetrics and gynecology offices. There are neonatal codes that indicate SGA that can be applied to the infant record; however we did not have access to infant records in the database used for our study.
Classification of small for gestational age infants based on recorded birthweight and gestational age at birth
We obtained information on birthweight and gestational age at birth from the linked medical records and used it to determine the percentile for birthweight for each infant based on their gestational age. For our primary analysis SGA infants were defined from published U.S. references as those with a birthweight below the 10th percentile for their gestational age.24 In secondary analyses, two other methods were used to define SGA infants. First, we used varying thresholds for SGA as birthweight below the 2nd, 5th, 7th, 12th, and 15th percentile for gestational age based on some suggested cutoffs in the literature.25 Second, to assess the accuracy of gestational age in our data source (which is rounded off to the nearest integer in the database and may therefore not be the most precise representation of gestational age), we used information on the delivery date and the EDC to generate a second alternative estimate for gestational age (and used the 10th birthweight percentile cutoff from U.S. references to identify SGA infants).
Study covariates
Information on maternal age, race (self-reported at time of admission), parity, calendar year of delivery, and delivery characteristics (induced vs. not induced labor, cesarean vs. vaginal delivery)) was obtained from the database. The presence of maternal chronic and pregnancy-related conditions (pre-existing diabetes mellitus, pre-existing hypertension, gestational diabetes mellitus, preeclampsia, and previous cesarean delivery) was identified from the recordings of the appropriate ICD-9-CM codes in the database.
Statistical analysis
We calculated the performance characteristics (sensitivity, specificity, PPV, and NPV) and 95% confidence intervals of the ICD-9-CM diagnosis code for SGA against SGA classification based on information on birthweight and gestational age; first for all singleton deliveries and then stratified by demographic and delivery characteristics. In secondary analyses we first assessed how well the ICD-9-CM code performed across different threshold definitions for SGA. Second, we assessed whether the performance characteristics for all deliveries were sensitive to the estimate for gestational age (gestational age as recorded in the database vs. gestational age we calculated using the delivery date and the EDC). Third, we constructed a prediction model to identify factors that predict the correct assignment of SGA status by first using univariable logistic regression models to identify characteristics associated with correct SGA assignment; variables with a 2-sided p-value <0.10 were included in the multivariable prediction model, and those with a p-value >0.05 in the adjusted model were removed one at a time, starting with the variable that had the largest p-value. Finally, we also assessed how effect estimates when investigating factors associated with SGA may be impacted by using only the ICD-9-CM code for SGA (e.g., in the absence of information on birthweight and gestational age); to illustrative this we estimated the prevalence and unadjusted relative risk for SGA by strata of risk factors for SGA (race, Black vs. White; age, ≤ 35 years vs. > 35 years; gravida, primigravida vs. multigravida; pre-existing hypertension; pre-eclampsia; and gestational diabetes mellitus). All analyses were repeated in a cohort that included multiple gestation deliveries. Analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC).
Results
From the medical record database of women who delivered between 2004 and 2011, we identified 65 535 maternal records from which we excluded 5174 multiple gestation deliveries, 196 stillbirths, 592 deliveries with missing gestational age or birthweight, and 136 deliveries from further data cleaning. Furthermore, deliveries with missing diagnosis billing codes were excluded (all 5995 deliveries from 2006 since more than 80% had this information missing, and an additional 2150 deliveries from the other study years). Our final study population consisted of 51 292 singleton live birth deliveries that occurred from 2004 - 2005 and 2007 – 2011 [Figure 1].
The characteristics of the final cohort of 51 292 deliveries are shown in Table 1. Overall, the proportion of women who delivered in each calendar year was similar across the study years. The average age at delivery was 31 years, with age ranging from 13 to 55 years; 54% of women were White.
Table 1. Characteristics of singleton deliveries from 2004 – 2005 and 2007 - 2011 (N = 51 292).
| Characteristics | Number of women/deliveries (%) |
|---|---|
| Demographics | |
| Calendar year of delivery | |
| 2004 | 8 067 (16) |
| 2005 | 7 632 (15) |
| 2007 | 7 588 (15) |
| 2008 | 7 411 (14) |
| 2009 | 7 395 (14) |
| 2010 | 7 124 (14) |
| 2011 | 6 075 (12) |
| Maternal age at delivery, mean (range) | 31 (13 – 55) |
| Maternal race | |
| White | 27 744 (54) |
| Black | 7 679 (15) |
| Hispanic | 7 580 (15) |
| Asian | 4 431 (9) |
| Other | 1 637 (3) |
| Unknown | 2 027 (4) |
| Primigravida | 18 051 (35) |
| Chronic/pregnancy-induced conditions | |
| Pre-existing diabetes mellitus a | 303 (0.6) |
| Pre-existing hypertension b | 1 029 (2) |
| Previous cesarean section c | 7 332 (14) |
| Gestational diabetes mellitus d | 2 322 (5) |
| Preeclampsia/gestational hypertension e | 4 453 (9) |
| Delivery outcomes | |
| Birth weight | |
| <1500 | 757 (2) |
| 1500 – 2499 | 2 661 (5) |
| 2500 – 4000 | 43 202 (84) |
| >4000 | 4 672 (9) |
| Gestational age at delivery f | |
| <32 weeks | 651 (1) |
| 32 – 36 weeks | 1 859 (4) |
| =>37 weeks | 48 782 (95) |
| Cesarean delivery | 15 434 (30) |
| Induced labor | 15 800 (31) |
Pre-existing diabetes mellitus (ICD-9-CM codes: 250.x, 648.0x)
Pre-existing hypertension (ICD-9-CM codes: 401.x-405.x, 642.0x-642.2x, 642.7x)
Previous cesarean section (ICD-9-CM codes: : 654.2x)
Gestational diabetes mellitus (ICD-9-CM codes: 648.8x)
Preeclampsia (ICD-9-CM codes: : 642.3x-642.9x)
Gestational age at delivery based on completed weeks.
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification
Note: All deliveries from 2006 were excluded from the final study population since >80% had no diagnosis billing codes.
There were 6780 (13%) deliveries of SGA infants (<10th birthweight percentile for gestational age) based on recorded birthweight and gestational age at birth as reported in the medical record database, compared to 1113 (2%) SGA infants based on the ICD-9-CM code [Table 2]. The ICD-9-CM code for SGA had a sensitivity of 14%, specificity of 100%, PPV of 87% and NPV of 88% when compared to using recorded birthweight and gestational age at birth [Table 2]. Stratified analysis of the performance characteristics overall yielded similar results, with specificities greater than 99%, PPVs above 84% and NPVs above 75% for all strata. The sensitivities remained low overall, but notable variations were seen in strata for induced labor (28%) vs. non-induced labor (7%), cesarean (19%) vs. vaginal (12%) delivery, and preterm (23%) vs. term (13%) delivery.
Table 2. Performance characteristics of the SGA ICD-9-CM code 656.5x in a medical record database compared to SGA based on recorded birthweight and gestational age: singleton deliveries (N = 51 292).
| SGA: ICD-9-CM codes Total (%) |
SGA: BW & GA Total (%) |
Sensitivity (%) (95% CI) |
Specificity (%) (95% CI) |
PPV (%) (95% CI) |
NPV (%) (95% CI) |
|
|---|---|---|---|---|---|---|
| All deliveries | 1131 (2) | 6780 (13) | 14.2 (13.4, 15.0) | 99.7 (99.6, 99.7) | 86.8 (84.8, 88.8) | 88.4 (88.1, 88.7) |
| Preterm deliveries (<37 weeks) | 187 (7) | 758 (30) | 22.7 (19.7, 25.6) | 99.2 (98.8, 99.6) | 92.6 (88.9, 96.4) | 74.9 (73.1, 76.6) |
| Term Deliveries (=> 37 weeks) | 926 (2) | 6022 (12) | 13.2 (12.3, 14.0) | 99.7 (99.6, 99.7) | 85.6 (83.4, 87.9) | 89.1 (88.8, 89.3) |
| Induced labor | 762 (5) | 2345 (15) | 28.0 (26.2,29.8) | 99.2 (99.1, 99.4) | 86.2 (83.7, 88.6) | 88.7 (88.3, 89.3) |
| Non-induced labor | 350 (1) | 4428 (13) | 6.9 (6.2, 7.7) | 99.9 (99.8, 99.9) | 88.2 (84.9, 91.6) | 88.3 (87.9, 88.6) |
| Cesarean delivery | 463 (3) | 2174 (14) | 19.1 (17.4, 20.8) | 99.7 (99.6, 99.8) | 90.2 (87.6, 92.9) | 88.2 (87.7, 88.7) |
| Vaginal delivery | 650 (2) | 4585 (13) | 12.0 (11.0, 12.9) | 99.7 (99.6, 99.7) | 84.4 (81.6, 87.1) | 88.5 (88.2, 88.8) |
| Primigravida | 505 (3) | 2900 (16) | 15.1 (13.8, 16.4) | 99.6 (99.4, 99.7) | 86.5 (83.6, 89.5) | 85.9 (85.4, 86.4) |
| Multigravida | 608 (2) | 3879 (12) | 13.6 (12.5, 14.6) | 99.7 (99.7, 99.8) | 87.0 (84.4, 89.7) | 89.7 (89.4, 90.1) |
| Maternal age ≤ 35 years | 880 (2) | 5540 (13) | 13.6 (12.7, 14.5) | 99.6 (99.5, 99.7) | 85.8 (83.5, 88.1) | 88.2 (87.9, 88.5) |
| Maternal age > 35 years | 233 (2) | 1240 (12) | 17.1 (15.0, 19.2) | 99.7 (99.6, 99.8) | 90.1 (87.3, 94.7) | 89.4 (88.7, 90.0) |
| Race (White) | 491 (2) | 2770 (10) | 15.4 (14.0, 16.7) | 99.7 (99.7, 99.8) | 86.8 (83.9, 89.8) | 91.4 (91.1, 91.7) |
| Race (Black) | 233 (3) | 1419 (18) | 14.5 (12.7, 16.3) | 99.6 (99.4, 99.7) | 87.8 (83.7, 92.0) | 83.7 (82.9, 84.6) |
| Race (Hispanic) | 168 (2) | 1169 (15) | 12.7 (10.8, 14.6) | 99.7 (99.6, 99.8) | 88.9 (84.2, 93.6) | 86.3 (85.5, 87.0) |
| Race (Other a/Unknown) | 218 (3) | 1394 (17) | 13.3 (11.3, 15.4) | 99.5 (99.3, 99.7) | 85.1 (79.7, 90.5) | 84.6 (83.7, 85.5) |
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; SGA = small-for-gestational age; BW = birthweight; GA = gestational age; PPV = positive predictive value; NPV = negative predictive value; CI = confidence interval
Other race includes race = “Asian” and race = “Other” as shown in Table 1.
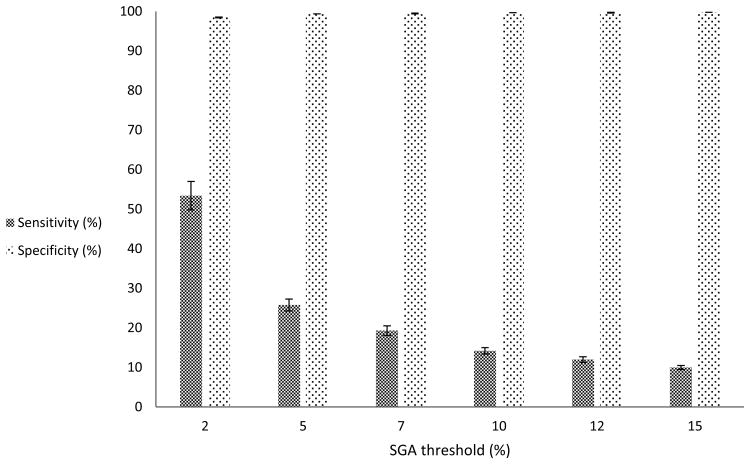
In secondary analyses, our first results from using different thresholds for SGA continued to show high and stable specificity for the ICD-9-CM code 656.5x (>99%) [Figure 2]. However, we observed a rapid decline in the sensitivity of the code from the 2nd percentile threshold for defining SGA to the 15th percentile threshold (53% vs. 10%). Our second results from assessing whether the performance characteristics for all deliveries were sensitive to the estimate for gestational age (gestational age as recorded in the database vs. gestational age we calculated using the delivery date and the EDC) showed a decrease in the prevalence of SGA to 8%; the ICD-9-CM code for SGA had a slightly higher sensitivity of 21%, specificity of 99%, PPV of 76% and NPV of 94%. In our third results, infants who were more likely to have correct assignment of SGA status were those whose mothers were White compared to other races (adjusted odds ratio [aOR] = 1.8, 95% confidence interval [CI]: 1.7, 1.9), or had pre-existing diabetes mellitus (aOR= 1.7, 95% CI: 1.1, 2.5); infants less likely to have correct assignment of SGA were those whose mothers had pre-existing hypertension (aOR= 0.6, 95% CI: 0.5, 0.7) or pre-eclampsia (aOR= 0.5, 95% CI: 0.4, 0.5) compared to infants whose mothers did not have these conditions [Table 3]. Our fourth results from assessing how effect estimates when investigating factors associated with SGA may be impacted by using only the ICD-9-CM code for SGA (e.g., in the absence of information on birthweight and gestational age) showed varied SGA prevalence between each strata of risk factors for SGA; however, the unadjusted relative risk estimates were similar between each strata. Finally, in the re-analyses that included multiple gestations deliveries, the overall results were similar to those of singletons only [Table 4].
Figure 2. Varying thresholds for defining SGAa for all deliveries (N = 51 292).
a SGA based on recorded birthweight and gestational age
Table 3. Estimates of prevalence and unadjusted risk ratios for SGA comparing SGA based on ICD-9-CM code 656.5x vs. birthweight and gestational age: illustrative examples by strata of SGA risk factors.
| SGA based on: | ||||
|---|---|---|---|---|
| ICD-9-CM code 656.5x | birthweight and gestational age | |||
|
| ||||
| Prevalence (%) |
Risk Ratio (95% CI) |
Prevalence (%) |
Risk Ratio (95% CI) |
|
| Gravida | ||||
| Primigravida | 2.8 | 16.1 | ||
| Multigravida | 1.8 | 1.5 (1.4, 1.7) | 11.7 | 1.4 (1.3, 1.4) |
| Maternal age | ||||
| ≤ 35 years | 2.1 | 13.4 | ||
| > 35 years | 2.4 | 0.9 (0.8, 1.0) | 12.5 | 1.1 (1.0, 1.1) |
| Race | ||||
| White | 1.8 | 10.0 | ||
| Black | 3.0 | 1.7 (1.5, 2.0) | 18.5 | 1.8 (1.7, 2.0) |
| Pre-existing diabetes mellitus | ||||
| Yes | 3.1 | 14.7 | ||
| No | 2.9 | 1.1 (0.6, 1.9) | 16.1 | 0.9 (0.7, 1.2) |
| Pre-existing hypertension | ||||
| Yes | 7.9 | 26.9 | ||
| No | 2.8 | 2.8 (2.3, 3.5) | 15.9 | 1.7 (1.5, 1.9) |
| Gestational diabetes mellitus | ||||
| Yes | 3.5 | 18.9 | ||
| No | 2.9 | 1.2 (1.0, 1.5) | 16.0 | 1.2 (1.1, 1.3) |
| Pre-eclampsia | ||||
| Yes | 6.1 | 31.3 | ||
| No | 2.6 | 2.4 (2.1, 2.7) | 14.5 | 2.2 (2.1, 2.3) |
SGA = small-for-gestational age; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; CI = confidence interval
Table 4. Performance characteristics of the SGA ICD-9-CM code 656.5x in a medical record database compared to SGA based on recorded birthweight and gestational age: singleton and multiple gestation deliveries (N = 55 589).
| SGA: ICD-9-CM codes Total (%) |
SGA: BW & GA Total (%) |
Sensitivity (%) (95% CI) |
Specificity (%) (95% CI) |
PPV (%) (95% CI) |
NPV (%) (95% CI) |
|
|---|---|---|---|---|---|---|
| All deliveries | 1615 (3) | 8972 (16) | 15.0 (14.3, 15.8) | 99.4 (99.4, 99.5) | 83.5 (81.7, 85.3) | 85.9 (85.6, 86.2) |
| Preterm deliveries (<37 weeks) | 485 (11) | 1622 (36) | 24.4 (22.3, 26.4) | 96.9 (96.3, 97.5) | 81.4 (78.0, 84.9) | 69.6 (68.2, 71.0) |
| Term Deliveries (=> 37 weeks) | 1130 (2) | 7350 (14) | 13.0 (12.2, 13.7) | 99.6 (99.5, 99.7) | 84.3 (82.2, 86.5) | 87.2 (86.9, 87.5) |
| Induced labor | 873 (5) | 2766 (17) | 27.1 (25.5, 28.8) | 99.1 (99.0, 99.3) | 85.9 (83.6, 88.2) | 87.1 (86.6, 87.6) |
| Non-induced labor | 741 (2) | 6187 (16) | 9.7 (8.9, 10.4) | 99.6 (99.5, 99.6) | 80.6 (77.7, 83.4) | 85.4 (85.0, 85.7) |
| Cesarean delivery | 886 (5) | 3886 (21) | 18.8 (17.6, 20.1) | 98.9 (98.8, 99.1) | 82.6 (80.1, 85.1) | 82.4 (81.9, 82.9) |
| Vaginal delivery | 727 (2) | 5063 (14) | 12.1 (11.2, 13.0) | 99.6 (99.6, 99.7) | 84.5 (81.8, 87.1) | 87.6 (87.3, 87.9) |
| Primigravida | 715 (4) | 3796 (19) | 15.7 (14.6, 16.9) | 99.3 (99.1, 99.4) | 83.5 (80.8, 86.2) | 83.2 .82.6, 83.7) |
| Multigravida | 900 (3) | 5175 (14) | 14.5 (13.6, 15.5) | 99.5 (99.4, 99.6) | 83.4 (81.0, 85.9) | 87.4 (87.0, 87.7) |
| Maternal age ≤ 30 years | 1222 (3) | 6984 (16) | 14.6 (13.7, 15.4) | 99.5 (99.4, 99.5) | 83.1 (81.0, 85.2) | 86.1 (85.8, 86.5) |
| Maternal age > 30 years | 393 (3) | 1988 (17) | 16.7 (15.1, 18.3) | 99.4 (99.2, 99.5) | 84.5 (80.9, 88.1) | 84.9 (84.3, 85.6) |
| Race (White) | 812 (3) | 4224 (14) | 15.7 (14.6, 16.8) | 99.4 (99.3, 99.5) | 81.7 (79.0, 84.3) | 88.1 (87.7, 88.4) |
| Race (Black) | 305 (4) | 1636 (20) | 16.1 (14.4, 17.9) | 99.4 (99.2, 99.6) | 86.6 (82.7, 90.4) | 82.4 (81.6, 83.3) |
| Race (Hispanic) | 210 (3) | 1354 (17) | 13.7 (11.8, 15.5) | 99.6 (99.5, 99.8) | 88.1 (83.7, 92.5) | 84.8 (84.0, 85.6) |
| Race (Other a/Unknown) | 281 (3) | 1719 (20) | 13.5 (11.9, 15.1) | 99.3 (99.1, 99.5) | 82.6 (78.1, 87.0) | 82.4 (81.6, 83.2) |
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; SGA = small-for-gestational age; BW = birthweight; GA = gestational age; PPV = positive predictive value; NPV = negative predictive value; CI = confidence interval
Other race includes race = “Asian” and race = “Other” as shown in Table 1.
Discussion
The ICD-9-CM code for poor fetal growth has not been previously validated, which is a necessary first step before it can be used in pharmacoepidemiology studies based on administrative healthcare data. Our results show that the code, 656.5x, recorded in the maternal delivery admission record, has a PPV of 87% for SGA, and a sensitivity of 14%.
The high PPV of the ICD-9-CM code for SGA is consistent with the validation studies of the codes for many other conditions. If an ICD-9-CM code for a condition is recorded, the diagnosis is likely to have been made by a clinician and recorded in the patients' chart.26 Likewise, a relatively low sensitivity for ICD-9-CM codes has been reported for many diseases and complications in validation studies.27-29 While the low sensitivity of a code precludes its use in defining the prevalence or risk difference for an outcome associated with a particular exposure, the code still has utility in defining relative risks as we showed in our example assessing the risk of SGA among several strata of SGA risk factors. If a condition can be identified using an ICD-9-CM code with high specificity, as is the case with the SGA code, and misclassification is non-differential between exposed and unexposed, then estimates of the relative risk will be nearly unbiased. Thus, the SGA code examined here can be reasonably applied in many pharmacoepidemiology studies that are based on administrative data alone, such as ones examining the relative risk of SGA in association with a particular drug exposure. However, if misclassification is differential with respect to exposure, then relative risk estimates will be biased. This could arise if a certain drug was suspected to be associated with SGA which could result in more active surveillance for the condition and robust documentation of its presence, and potentially lead to differential misclassification.
A PPV of approximately 90% was present in each of the subgroups analyzed. Sensitivity of the code was somewhat higher in preterm compared to term deliveries, cesarean compared to vaginal deliveries, and induced deliveries compared to non-induced deliveries. This may reflect clinician documentation of intrauterine growth retardation (IUGR) and subsequent SGA in circumstances where management decisions (such as performing an indicated preterm delivery, induction, or cesarean delivery) are based on the presence of these conditions. The sensitivity was also increased when more extreme thresholds for defining SGA were imposed. For example, when the threshold for SGA was set at the second percentile, the sensitivity was in excess of 50%. This again is consistent with recording of the code in clinical circumstances where management is likely to be altered.
Our study is subject to certain limitations inherent in its design. First, it was conducted at a single center and thus reflects the coding practices and population associated with this center. Indeed, the populations treated at the center may not be representative of the general delivering population in the U.S., with a higher fraction of women with high-risk conditions (because it is a tertiary care center with a level 3 NICU) and larger fraction of women of advanced maternal age. That said, the PPV for SGA did not change substantially in the various subgroups that we analyzed, suggesting no heterogeneity in PPV according to patient characteristics or approaches to obstetric management. An additional limitation of the study is one inherent in the ICD-9-CM code itself. The definition for the code states that it represents “poor fetal growth” or “small for dates”. There is no strict percentile for infant growth that is included in the definition. Therefore, while we conducted our primary analysis using the traditional threshold for defining SGA as birthweight below the 10th percentile, we explored the performance characteristics of the code across a range of cutoffs for defining SGA, based on some of the suggested cutoffs from previous studies to identify clinically important poor fetal growth.25 Also, because these codes are recorded in the maternal record, they are probably most commonly applied in situations where the clinician suspects intrauterine growth restriction. We validated the code against SGA defined by percentile of infant weight at birth. In some circumstances, IUGR and SGA may not be equivalent.30 For example, IUGR may be diagnosed when an infant's growth trajectory changes (even if the infant does not meet criteria for SGA) and SGA may be diagnosed in the absence of IUGR if the parents are of small constitution. That said, in most clinical circumstances these conditions will overlap. Finally, we observed a substantial difference in the prevalence of SGA using gestational age as recorded in the administrative dataset compared to gestational age calculated from recorded EDC and date of birth. This difference may be due to the fact that gestational age and EDC estimates are informed by a variety of data, including LMP, obstetrical ultrasound data and obstetrical examination. EDCs based on LMP are frequently adjusted after ultrasound data is available; therefore, because ultrasound estimates of EDC are based on fetal measurements, we would expect the adjustment of EDC on the basis of fetal growth to lower the prevalence of SGA.
We showed that the ICD-9-CM administrative code for SGA has high PPV (87%) but low sensitivity (14%). The accuracy was non-differential for a range of risk factors evaluated. Our study suggests this code can be used in a valid fashion in some circumstances (e.g., assuming non-differential misclassification between exposed and unexposed) to quantify the relative risk of SGA associated with prenatal exposures such as maternal use of medications. Future work should be directed towards confirming these findings in samples from other hospitals.
Key Points.
ICD-9-CM diagnosis code indicating small for gestational age (SGA) has high positive predictive value, but limited sensitivity.
When information on birthweight and gestational age is not available, such as in administrative health care databases, the ICD-9-CM code may be used in an unbiased fashion to estimate the relative risk of SGA associated with particular exposures (e.g. medication exposures during pregnancy).
The low sensitivity of the SGA code prevents its use in quantifying prevalence estimates of SGA associated with a particular exposure.
Acknowledgments
Research Sponsors: Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (Award Number K08HD075831)
Footnotes
Prior postings and presentations: NONE
Contributor Information
Kelesitse Phiri, Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA.
Sonia Hernandez-Diaz, Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA.
Lawrence C. Tsen, Department of Anesthesia, Perioperative and Pain Medicine, Brigham and Women's Hospital, Boston, MA, USA.
Karen M. Puopolo, Department of Newborn Medicine, Brigham and Women's Hospital, Boston, MA, USA.
John D. Seeger, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Harvard Medical School, Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA.
Brian T. Bateman, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Harvard Medical School, Boston, MA, USA
References
- 1.Carberry AE, Gordon A, Bond DM, Hyett J, Raynes-Greenow CH, Jeffery HE. Customised versus population-based growth charts as a screening tool for detecting small for gestational age infants in low-risk pregnant women. Cochrane Database Syst Rev. 2011;(12):CD008549. doi: 10.1002/14651858.CD008549.pub2. doi:CD008549. [DOI] [PubMed] [Google Scholar]
- 2.Saenger P, Czernichow P, Hughes I, Reiter EO. Small for gestational age: short stature and beyond. Endocr Rev. 2007;28:219–251. doi: 10.1210/er.2006-0039. [DOI] [PubMed] [Google Scholar]
- 3.La Merrill M, Stein CR, Landrigan P, Engel SM, Savitz DA. Prepregnancy body mass index, smoking during pregnancy, and infant birth weight. Ann Epidemiol. 2011;21:413–420. doi: 10.1016/j.annepidem.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luyckx VA, Bertram JF, Brenner BM, et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet. 2013;382:273–283. doi: 10.1016/S0140-6736(13)60311-6. [DOI] [PubMed] [Google Scholar]
- 5.Lim G, Tracey J, Boom N, et al. CIHI survey: Hospital costs for preterm and small-for-gestational age babies in Canada. Healthc Q. 2009;12:20–24. doi: 10.12927/hcq.2013.21121. [DOI] [PubMed] [Google Scholar]
- 6.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson JM, Clark PM, Robinson E, et al. Risk factors for small-for-gestational-age babies: The Auckland Birthweight Collaborative Study. J Paediatr Child Health. 2001;37:369–375. doi: 10.1046/j.1440-1754.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- 8.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(Suppl 2):S125–40. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 9.Bada HS, Das A, Bauer CR, et al. Low birth weight and preterm births: etiologic fraction attributable to prenatal drug exposure. J Perinatol. 2005;25:631–637. doi: 10.1038/sj.jp.7211378. [DOI] [PubMed] [Google Scholar]
- 10.Chiaffarino F, Parazzini F, Chatenoud L, et al. Alcohol drinking and risk of small for gestational age birth. Eur J Clin Nutr. 2006;60:1062–1066. doi: 10.1038/sj.ejcn.1602419. [DOI] [PubMed] [Google Scholar]
- 11.McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynaecol. 2009;23:779–793. doi: 10.1016/j.bpobgyn.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Meidahl Petersen K, Jimenez-Solem E, Andersen JT, et al. beta-Blocker treatment during pregnancy and adverse pregnancy outcomes: a nationwide population-based cohort study. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001185. Print 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magee LA, Duley L. Oral beta-blockers for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2003;(3):CD002863. doi: 10.1002/14651858.CD002863. [DOI] [PubMed] [Google Scholar]
- 14.Finnegan LP, Hagan T, Kaltenbach KA. Scientific foundation of clinical practice: opiate use in pregnant women. Bull N Y Acad Med. 1991;67:223–239. [PMC free article] [PubMed] [Google Scholar]
- 15.Nezvalova-Henriksen K, Spigset O, Nordeng H. Effects of codeine on pregnancy outcome: results from a large population-based cohort study. Eur J Clin Pharmacol. 2011;67:1253–1261. doi: 10.1007/s00228-011-1069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ornoy A, Zvi N, Arnon J, Wajnberg R, Shechtman S, Diav-Citrin O. The outcome of pregnancy following topiramate treatment: a study on 52 pregnancies. Reprod Toxicol. 2008;25:388–389. doi: 10.1016/j.reprotox.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Hernández-Díaz S, Mittendorf R, Smith CR, Hauser WA, Yerby M, Holmes LB. Association Between Topiramate and Zonisamide Use During Pregnancy and Low Birth Weight. 2014;123:1–9. doi: 10.1097/AOG.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 18.Townsend CL, Cortina-Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and premature delivery in diagnosed HIV-infected women in the United Kingdom and Ireland. AIDS. 2007;21:1019–1026. doi: 10.1097/QAD.0b013e328133884b. [DOI] [PubMed] [Google Scholar]
- 19.Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012;206:1695–1705. doi: 10.1093/infdis/jis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmsten K, Huybrechts KF, Mogun H, et al. Harnessing the Medicaid Analytic eXtract (MAX) to Evaluate Medications in Pregnancy: Design Considerations. PLoS One. 2013;8:e67405. doi: 10.1371/journal.pone.0067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Medicare and Medicaid Services. Research, Statistics, Data and Systems.Medicaid Analytic eXtract (MAX) General Information. http://www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/MedicaidDataSourcesGenInfo/MAXGeneralInformation.html.
- 22.Agency for Healthcare Research and Quality; Rockville, MD: Jul, 2014. HCUP Databases. Healthcare Cost and Utilization Project (HCUP) www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed] [Google Scholar]
- 23.Committee opinion no 611: Method for estimating due date. Obstet Gynecol. 2014;124:863–866. doi: 10.1097/01.AOG.0000454932.15177.be. [DOI] [PubMed] [Google Scholar]
- 24.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Simonet F, Luo ZC. Optimal birth weight percentile cut-offs in defining small- or large-for-gestational-age. Acta Paediatr. 2010;99:550–555. doi: 10.1111/j.1651-2227.2009.01674.x. [DOI] [PubMed] [Google Scholar]
- 26.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58:323–337. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Goff SL, Pekow PS, Markenson G, Knee A, Chasan-Taber L, Lindenauer PK. Validity of using ICD-9-CM codes to identify selected categories of obstetric complications, procedures and co-morbidities. Paediatr Perinat Epidemiol. 2012;26:421–429. doi: 10.1111/j.1365-3016.2012.01303.x. [DOI] [PubMed] [Google Scholar]
- 28.Romano PS, Yasmeen S, Schembri ME, Keyzer JM, Gilbert WM. Coding of perineal lacerations and other complications of obstetric care in hospital discharge data. Obstet Gynecol. 2005;106:717–725. doi: 10.1097/01.AOG.0000179552.36108.6d. [DOI] [PubMed] [Google Scholar]
- 29.Yasmeen S, Romano PS, Schembri ME, Keyzer JM, Gilbert WM. Accuracy of obstetric diagnoses and procedures in hospital discharge data. Am J Obstet Gynecol. 2006;194:992–1001. doi: 10.1016/j.ajog.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 30.Lee PA, Chernausek SD, Hokken-Koelega AC, Czernichow P. International Small for Gestational Age Advisory Board. International Small for Gestational Age Advisory Board consensus development conference statement: management of short children born small for gestational age, April 24-October 1, 2001. Pediatrics. 2003;111:1253–1261. doi: 10.1542/peds.111.6.1253. [DOI] [PubMed] [Google Scholar]