Abstract
Purpose
Progestins have been used in the treatment of recurrent endometrial adenocarcinoma for almost 50 years. Some endometrial carcinomas respond to hormonal therapy, but the mechanism of action remains incompletely known. We wished to determine the efficacy of progestins to induce a histologic response in endometrioid carcinomas and explore its effects on histologic and immunohistochemical measures of growth and cell death.
Methods
The Gynecologic Oncology Group initiated a study of 75 women with endometrioid endometrial adenocarcinoma, 59 of whom received the progestin, medroxyprogesterone acetate (MPA) for 21-24 days immediately prior to hysterectomy and had available slides. Initial biopsies and hysterectomies were H&E stained and immunostained for estrogen receptor (ER) and progesterone receptor (PR), progesterone receptor Beta (PRB), Bcl-2, Ki-67, and cleaved caspase-3 (Casp3). A histologic response was defined subjectively, following which specific histologic measurements and semi-quantitative scores of immunohistologic variables of initial biopsies were compared to post-treatment slides.
Results
Only one complete histologic response was seen, but 37 tumors (63%) had a partial histologic response. Specific histologic changes included the following: a decrease in the nuclear grade, the number of mitotic figures, nucleoli, and mean gland cellularity, and acquisition of more abundant eosinophilic cytoplasm, squamous metaplasia, and secretion. The tumors that displayed a subjectively defined histologic response following treatment differed initially from those that did not only with respect to initial nuclear grade and the mitotic index. Statistically significant differences in the specific histologic features in carcinomas of responders versus non-responders following treatment were found only with respect to acquisition of pale eosinophilic cytoplasm and luminal secretion.
More than 90% of tumors were initially ER positive and 76% were PR positive. The initial presence of ER or PR was not related to subjective histologic response. PR and PRB were significantly down-regulated following progestin therapy, as were Ki-67 and Bcl-2. However, ER ad Casp3 did not change significantly. Tumors that displayed a histologic response had significantly lower pre-treatment levels of Ki-67. Mean Ki-67 and Bcl-2 decreases following MPA were greater in histologic responders than non-responders, but not decreases in ER, PR, PRB and Casp3.
The histologic response in the tumors and their stroma differed quantitatively and qualitatively from that of the adjacent benign endometrium, where decidual change accompanied luminal secretion and secretory exhaustion of glands.
Conclusion
Three weeks of MPA therapy induces partial histologic responses in most endometrioid adenocarcinomas. Previously suggested features of histologic response do not capture the entire spectrum of changes seen. Down regulation of ER, PR, PRB, Ki-67 and Bcl-2 occurs without a significant change in Casp3. These alterations suggest that progestins act by differentiation of neoplastic cells with diminished proliferation rather than tumor cell death. Since stromal decidualization was confined to areas surrounding benign glands, a paracrine effect may be involved in complete response to progestins.
Keywords: therapy endometrium, carcinoma, cancer, progestin, histology, response
INTRODUCTION
Progestins are sometimes used to treat endometrial hyperplasia, occasionally are the primary therapy for non-surgical candidates with endometrial carcinoma, and often are employed in the treatment of advanced or metastatic endometrial adenocarcinoma. Unfortunately, the response rate for metastatic disease has generally been only about 25%, and the responses are usually not durable, averaging less than one year [1, 2]. In contrast, numerous investigators during the past 50 years have found initial response rates to progestins of 62% to 79% in women with atypical hyperplasia or Grade 1 endometrioid adenocarcinoma, of whom about 25% to 89% later had recurrence of tumor [3-10]. However, neither the specific mechanism nor the general pathways by which progestins act on hyperplastic or neoplastic endometrial cells have been elucidated. It is believed that progestin action requires the presence of specific high affinity receptors in the target cells, that there is translocation of receptor and ligand to the nucleus, and that this complex binds to DNA where it alters downstream transcription of RNA for undefined proteins. But it is unknown what proportions of tumor cells must express PR, whether a paracrine effect (e.g. signaling from endometrial stromal cells) is important in mediating progestin action, whether progestins decrease cells proliferation, and whether progestins are tumoricidal or act to cause temporary or permanent terminal differentiation of endometrial cells.
During the past thirty years, most studies have demonstrated a relationship between the presence and quantity of steroid receptors and the degree of histologic differentiation, the nuclear and architectural grade, FIGO stage, and survival [11-13]. The availability of monoclonal antibodies to steroid receptors has made it possible to determine the distribution of receptors within the endometrium carcinomas. Surprisingly, these conceptual and technical advances have not yet yielded an improved hormonal therapy [1, 2]. This may in part reflect the paucity of data concerning the mechanisms of action by which steroid hormones affect cell proliferation, differentiation, and cell death in patients with endometrial cancer.
Given our lack of knowledge regarding the mechanism of action, we wished to initiate a study that would permit us to learn more about the relatively short-term histologic responses of endometrial carcinomas to progestins and some of the mechanisms involved in the response. Specifically, the goals of this study were as follows: 1) to determine the efficacy of a progestin commonly used to treat endometrial hyperplasia or carcinoma, medroxyprogesterone acetate (MPA), to induce a subjectively defined histologic response in endometrioid adenocarcinomas of the uterine corpus, 2) to determine whether the probability of response is related to the initial presence of progesterone receptor (PR); 3) to evaluate whether MPA down-regulates ER and PR in endometrioid adenocarcinomas; 4) to examine the effects of MPA on selected histologic and immunohistochemical measures of growth and apoptosis, including gland cellularity, nuclear grade, nucleoli, metaplasia, secretion, Ki-67, Bcl-2 and cleaved caspase 3 (Casp3); and by doing so, 5) to explore the mechanism by which MPA acts.
MATERIALS AND METHODS
In 2004, the Gynecologic Oncology Group (GOG) activated a group-wide protocol to investigate the relationship between short-term MPA exposure and morphologic and immunohistochemical changes in endometrioid endometrial adenocarcinoma (all grades and stages). To be eligible, patients must have had an endometrioid endometrial adenocarcinoma diagnosed by curettage or biopsy within eight weeks of enrollment, with the diagnosis confirmed by the study pathologist (RJZ), and the availability of 16 unstained charged slides of about 5 micrometers thickness of the tumor from the diagnostic sample. Each woman enrolled on the study was given MPA, 400 mg, intramuscularly, between 21 and 24 days before the date of the planned hysterectomy. Sixteen unstained slides were then obtained from a section of the endometrial adenocarcinoma within the hysterectomy specimen. Informed consent was obtained from each patient entered on the protocol, and the protocol received IRB approval at each participating institution.
The unstained slides were submitted from each patient for central laboratory histologic and immunohistochemical staining for each tumor. In addition to hematoxylin and eosin stains, immunohistochemical stains were performed with antibodies directed against Ki-67, Bcl-2, cleaved caspase 3 (Casp3), estrogen receptor (ER), progesterone receptor (PR), and the B isoform of progesterone receptor (PRB).
Definition of histologic response
To determine the presence of a histologic response, the slide from the initial sample was compared to the slide from the matching hysterectomy specimen. A complete histologic response was defined as the absence of identifiable adenocarcinoma in the hysterectomy specimen section. A partial histologic response was subjectively defined in advance of the study based on criteria slightly modified from Wheeler et al. as the presence of a complex proliferation of glands that retain the architectural characteristics of adenocarcinoma, but with features of secretion, decreased nuclear stratification, or the presence of eosinophilic, squamous or mucinous metaplasia, when this was absent in the initial sample [14]. Either a complete or partial histologic response was considered a histologic response in the analysis of data.
Scoring of histologic changes
All of the histologic changes were separately scored in the original endometrial sample and the section of tumor from the hysterectomy by two pathologists (RJZ and WT). The International Federation of Gynecologists and Obstetricians (FIGO) histologic grade was determined for each tumor based on the initial endometrial sample. The nuclear grade was assessed as 1, 2, or 3, based on the system previously published[15]. Nucleoli were scored as absent or present. The percentages of tumor that displayed cytoplasmic eosinophilia and squamous or mucinous metaplasia were estimated, with less than 10% considered negative and 10% or more as positive. Gland cellularity was assessed by counting the number of cells in one-quarter of a high powered field (HPF) (average of three fields). The mitotic index was calculated as the number of mitoses per HPF (average of three fields). Secretion was reported as absent, subnuclear, apical or luminal. In addition to tumor associated characteristics, the presence of atrophy, hyperplasia, secretory change in benign glands, and decidual change in the stroma was recorded in the post-treatment sections of uterus.
Immunohistochemistry
All immunohistochemical analyses were performed on 3-4μm thick sections from formalin-fixed, paraffin-embedded tissue. Immunohistochemistry for Ki-67 (M7240, Dako, Carpenteria, CA), Bcl-2 (M08876, Dako, Carpenteria, CA) and cleaved caspase-3 (#9661, Cell Signaling Technology, Danvers, MA) was performed by first using heat-induced epitope retrieval in 1 mM EDTA buffer pH 8.0 for 20 minutes with 20 minutes of cooling at RT. This was followed by a 10 minute 3% peroxidase block. Incubation with the primary antibodies (Ki-67 1:200/30’, Bcl-2 1:150/30’, cleaved caspase-3 1:100/60’) was then performed at room temperature. This was followed by incubation with Envision+(labeled polymer) goat anti-mouse or rabbit/HRP (Dako Cytomation, Carpinteria, CA) for 30 minutes at room temperature. Staining was completed with development of DAB+ substrate (Dako Cytomation, Carpinteria, CA) for 10 minutes followed by counterstaining for 6 minutes in Mayer’s modified hematoxylin (Dako Cytomation, Carpinteria, CA). FFPE samples of follicular lymphoma (Bcl-2) and normal tonsil (Ki-67 and cleaved caspase-3) were used as positive tissue controls.
The ER and PR antibodies were obtained from Zymed and Dako, while the antibody to PRB isoform was from Cell Signaling, Danvers, MA.
Scoring of immunohistochemistry
The unstained sides for immunohistochemistry were divided and sent to two different labs, with one set going to the specialized GOG laboratory for hormone receptors, directed by one of the authors (KL), where the protocols were optimized by EGF, and the slides were evaluated and scored by MI. The product score (0-3.0) was determined for ER, PR, and PRB, based upon the intensity of the intranuclear stain (0, 1+, 2+, 3+) multiplied by the proportion of nuclei staining (0-1.) A score of 0.2 was considered positive. The remaining slides for immunohistochemical stains for Ki-67, Bcl-2, and Casp3 were performed and scored in the laboratory of RJZ and WT according to the following systems: for Ki-67, the number of positive staining nuclei per high powered field (HPF) was counted (average of three fields); for Casp3, the number of positive staining nuclei per high powered field (HPF) was counted (average of six fields); for Bcl-2, cases were scored (0-2.0) using the product of the intensity of cytoplasmic staining (0, 1+ and 2+), and the proportion of cells staining (0-1.0) based on the distribution of staining.
Statistical analyses
Changes from baseline were tested using McNemar’s test for binary variables and paired t-test for continuous data. Differences between responders and non-responders with respect to pre-treatment values were tested using chi-square test for binary data and two-sample t-test for continuous data. Differences between responders and non-responders with respect to changes from pre- to post-treatment were tested using chi-square test based on paired differences for binary data and analysis of covariance (with baseline value in the model) for continuous data. Analysis of variance was used to compare continuous parameters across grades. Results were considered statistically significant if p<0.05 (two-sided).
RESULTS
Between 2004 and 2008, 75 women were enrolled on this protocol, of whom 16 were not evaluable due to inadequate pathology materials, wrong cell type, or absence of treatment, leaving a study population of 59. The baseline characteristics are depicted in Table 1. The mean age of the women was 61 years, of whom 54 self-identified as white. Fifty-three percent of the tumors were grade 1, 29% were grade 2, and 15% were grade 3.
Table 1.
Comparison of Baseline Characteristics
| All Patients (N=59) | |
|---|---|
|
| |
| Age (y), mean (SD) | 61 (9.0) |
| min - max | 37 - 87 |
|
| |
| Race, n (%) | |
| Unknown | 1 (1.7%) |
| Asian | 1 (1.7%) |
| Black | 1 (1.7%) |
| American Indian | 2 (3.4%) |
| White | 54 (91.5%) |
|
| |
| Tumor Grade, n (%) | |
| 1 | 31 (52.5%) |
| 2 | 17 (28.8%) |
| 3 | 9 (15.3%) |
| Not graded | 2 (3.4%) |
SD: standard deviation
Histologic response
After 21 to 24 days of MPA administration, a complete histologic response was seen in 1 specimen (2%), while a partial histologic response was subjectively identified in 37 specimens (63%) (Figs.1 and 2), and no response was seen in 21 specimens (36%) (Fig. 3). A histologically classified complete or partial response was not related to the initial histologic grade (Table 2).
Fig 1.


(A) FIGO grade 1 endometrioid adenocarcinoma (#684), pre-treatment (all photomicrographs have been taken at the same magnification). (B) Post-treatment (#684), partial histologic response includes loss of nuclear stratification, occasional luminal secretion and acquisition of abundant eosinophilic cytoplasm.
Fig 2.
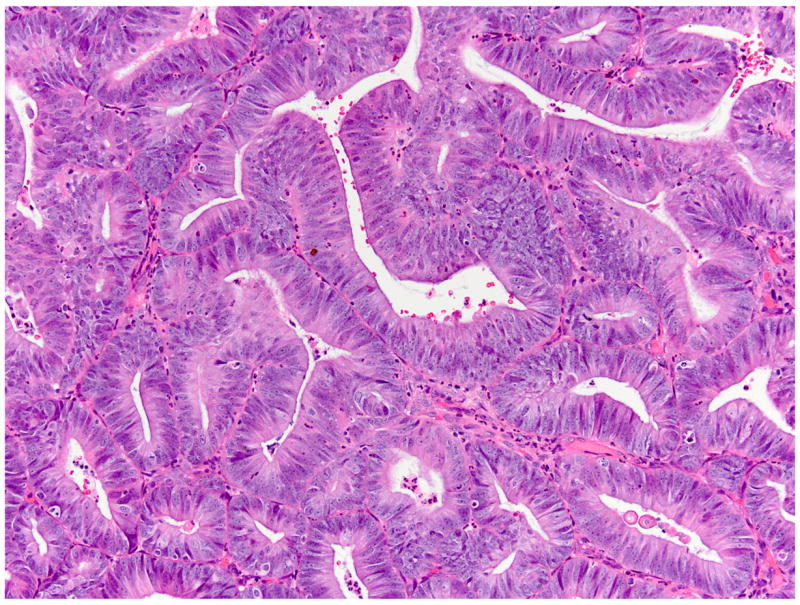

(A) FIGO grade 1 endometrioid adenocarcinoma (#960), pre-treatment. (B) Post-treatment (#960), partial histologic response includes both acquisition of abundant eosinophilic cytoplasm and squamous differentiation.
Fig 3.


(A) FIGO grade 1 endometrioid adenocarcinoma (#733), pre-treatment. (B) Post-treatment (#733), no histologic response is identified, with persistence of nuclear stratification and no change in cell cytoplasm.
Table 2.
Histologic Response by Histologic Grade, ER, PR, and PRB
| Complete or Partial Histologic Response | p-value† | |||
|---|---|---|---|---|
| N | n | % | ||
| Grade - Pretreatment (H&E) | ||||
| 1 | 38 | 27 | 71.1% | 0.352 |
| 2 | 13 | 7 | 53.8% | |
| 3 | 8 | 4 | 50.0% | |
| Baseline ER Positive Based on Aggregate Score (>0.2 vs. <=0.2) | ||||
| <=0.2 | 3 | 2 | 66.7% | 0.962 |
| >0.2 | 50 | 34 | 68.0% | |
| Baseline PR Positive Based on Aggregate Score (>0.2 vs. <=0.2) | ||||
| <=0.2 | 8 | 5 | 62.5% | 0.721 |
| >0.2 | 45 | 31 | 68.9% | |
| Baseline PRB Positive Based on Aggregate Score (>0.2 vs. <=0.2) | ||||
| <=0.2 | 15 | 8 | 53.3% | 0.114 |
| >0.2 | 37 | 28 | 75.7% | |
Note: Percentages are row percentages.
p-value from chi-square test.
H&E: hematoxylin and eosin
Histologic changes
Specific quantifiable, statistically significant histologic changes that occurred in the carcinomas following administration of progestin included the following: 1) a decrease in mean gland cellularity (from 204 cells per gland to 151 cells per gland); 2) a decrease in the frequency of nucleoli; 3) a decrease in the number of mitotic figures (from 2.68 to 0.82 per HPF); 4) a decrease in high nuclear grade; 5) acquisition of more abundant eosinophilic cytoplasm; 6) increased squamous metaplasia; and 7) the presence of intraluminal secretion (Figs.1B and 2B)(Table 3). The tumors that displayed a partial or complete histologic response following treatment differed initially from those that did not respond only with respect to initial nuclear grade and mitotic index (Table 4). Statistically significant differences in the specific histologic features in carcinomas of responders versus non-responders following treatment were found only with respect to acquisition of pale eosinophilic cytoplasm, and luminal secretion (Table 4).
Table 3.
Histologic Characteristics (N=59)
| Variable | Pre-treatment | Post-treatment | Change | p-value† |
|---|---|---|---|---|
| Presence (%) of: | ||||
| Nucleoli (H&E) | 27.1% | 15.3% | -11.9% | 0.020 |
| Nuclear grade (2 or 3) (H&E) | 50.8% | 35.6% | -15.3% | 0.003 |
| Mucinous Metaplasia (H&E) >10% | 3.4% | 5.1% | 1.7% | 0.655 |
| Squamous Metaplasia (H&E) >10% | 6.8% | 23.7% | 16.9% | 0.008 |
| Apical Secretion (H&E) | 1.7% | 3.4% | 1.7% | 0.564 |
| Luminal Secretion (H&E) | 11.9% | 50.8% | 39.0% | <0.001 |
| Subnuclear Secretion (H&E) | 6.8% | 1.7% | -5.1% | 0.083 |
| Pale eosinophilic (H&E) +2 or +3 | 3.4% | 71.2% | 67.8% | <0.001 |
| Non-neoplastic Endometrium - Atrophy (H&E) | 1.7% | 3.4% | 1.7% | 0.564 |
| Non-neoplastic Endometrium - Decidua (H&E) | 0.0% | 23.7% | 23.7% | <0.001 |
| Non-neoplastic Endometrium - Hyper (H&E) | 32.2% | 6.8% | -25.4% | <0.001 |
| Mean (SE) of: | ||||
| Mitotic Index (per HPF) | n=40 | n=38 | n=37 | |
| 2.68 (0.38) | 0.82 (0.17) | -1.59 (0.26) | <0.001 | |
| Gland Cellularity (Cells/Gland in 1/4 HPF) | n=39 | n=37 | n=36 | |
| 203.5 (10.3) | 150.7 (6.5) | -49.4 (10.2) | <0.001 |
p-value for change from pre- to post-treatment, based on McNemar’s test for presence (%) variables, and from paired t-test for means.
SE: standard error, HPF: high power field, H&E: hematoxylin and eosin
Table 4.
Histologic Characteristics in Responders (N=38) and Non-responders (N=21)
| Pre-treatment | Post-treatment | Change | Within-group p-value† | p-values for Comparison of Responders and Non-responders with Respect to
|
|||
|---|---|---|---|---|---|---|---|
| Baseline‡ | Change§ | ||||||
| Presence (%) of: | |||||||
|
| |||||||
| Nucleoli (H&E) | Responder | 23.7% | 7.9% | -15.8% | 0.014 | 0.425 | 0.096 |
| Non-responder | 33.3% | 28.6% | -4.8% | 0.564 | |||
|
| |||||||
| Nuclear grade (2 or 3) (H&E) | Responder | 39.5% | 21.1% | -18.4% | 0.008 | 0.019 | 0.096 |
| Non-responder | 71.4% | 61.9% | -9.5% | 0.157 | |||
|
| |||||||
| Mucinous Metaplasia (H&E) >10% | Responder | 5.3% | 5.3% | 0.0% | 1.000 | 0.285 | 0.655 |
| Non-responder | 0.0% | 4.8% | 4.8% | 0.317 | |||
|
| |||||||
| Squamous Metaplasia (H&E) >10% | Responder | 7.9% | 18.4% | 10.5% | 0.157 | 0.647 | 0.593 |
| Non-responder | 4.8% | 33.3% | 28.6% | 0.014 | |||
|
| |||||||
| Apica Secretion (H&E) | Responder | 0.0% | 5.3% | 5.3% | 0.157 | 0.175 | 0.083 |
| Non-responder | 4.8% | 0.0% | -4.8% | 0.317 | |||
|
| |||||||
| Luminal Secretion (H&E) | Responder | 15.8% | 71.1% | 55.3% | <0.001 | 0.210 | <0.001 |
| Non-responder | 4.8% | 14.3% | 9.5% | 0.157 | |||
|
| |||||||
| Subnuclear Secretion (H&E) | Responder | 7.9% | 2.6% | -5.3% | 0.157 | 0.647 | 0.564 |
| Non-responder | 4.8% | 0.0% | -4.8% | 0.317 | |||
|
| |||||||
| Pale eosinophilic (H&E) +2 or +3 | Responder | 5.3% | 100% | 94.7% | <0.001 | 0.285 | <0.001 |
| Non-responder | 0.0% | 19.0% | 19.0% | 0.046 | |||
|
| |||||||
| Non-neoplastic Endometrium - Atrophy (H&E) | Responder | 2.6% | 2.6% | 0.0% | 1.000 | 0.453 | 0.564 |
| Non-responder | 0.0% | 4.8% | 4.8% | 0.317 | |||
|
| |||||||
| Non-neoplastic Endometrium - Decidua (H&E) | Responder | 0.0% | 34.2% | 34.2% | <0.001 | 1.000 | 0.001 |
| Non-responder | 0.0% | 4.8% | 4.8% | 0.317 | |||
|
| |||||||
| Non-neoplastic Endometrium - Hyper (H&E) | Responder | 36.8% | 5.3% | -31.6% | <0.001 | 0.305 | 0.029 |
| Non-responder | 23.8% | 9.5% | -14.3% | 0.180 | |||
|
| |||||||
| Mean (SE) of: | |||||||
|
| |||||||
| Mitotic Index (per HPF) | Responder | n=25 | n=24 | n=24 | |||
| 1.83 (0.30) | 0.53 (0.14) | -1.35 (0.31) | <0.001 | 0.015 | 0.191 | ||
| Non-responder | n=15 | n=14 | n=13 | ||||
| 4.09 (0.79) | 1.31 (0.36) | -2.03 (0.48) | 0.001 | ||||
|
| |||||||
| Gland Cellularity (Cells/Gland in 1/4 HPF) | Responder | n=24 | n=23 | n=23 | |||
| 192.2 (12.7) | 142.4 (8.5) | -50.2 (14.1) | 0.002 | 0.170 | 0.133 | ||
| Non-responder | n=15 | n=14 | n=13 | ||||
| 221.6 (17.1) | 164.4 (9.4) | -48.0 (14.1) | 0.005 | ||||
p-value for change from pre- to post-treatment within responders and nonresponders separately, based on McNemar’s test for presence (%) and on paired t-test for means.
p-value for difference between responders and non-responders with respect to pre-treatment value based on chi-square test for presence (%) on two-sample t-test for means.
p-value for difference between responders and non-responders with respect to change from pre- to post-treatment based on chi-square test using paired differences for presence (%) and based on ANCOVA with baseline value in model for means.
SE: standard error, HPF: high power field, H&E: hematoxylin and eosin
In 21 cases, non-neoplastic endometrium, as well as carcinoma, was present in the hysterectomy specimen. Glandular secretion was present in 7 of these cases and decidual change in 14 cases, while atrophy or hyperplasia was identified in 2 and 3 cases, respectively. The decidual change was entirely confined to the stroma surrounding benign appearing glands in 12 of the 14 cases (Fig. 4). It is of interest that decidual change was more likely to occur in the benign portions of endometrium adjacent to carcinomas that showed a histologic response (Table 4).
Fig 4.


(A) FIGO grade 2 endometrioid adenocarcinoma (#957), post-treatment, with cytoplasmic vacuolization and luminal secretion. The stroma is composed of small cells with scant cytoplasm. (B) Benign appearing endometrium adjacent to the tumor (#957), post treatment. There is extensive pre-decidual change of the stroma.
Immunohistochemical measurements
The relationships between initial histologic grade and pre-treatment ER, PR, PRB, Ki-67, Casp3, and Bcl-2 are presented in Table 5. Lower histologic grade was statistically significantly associated with higher pre-treatment PR, PRB, and lower pre-treatment Ki-67 and Casp3.
Table 5.
Initial Histologic Grade versus Pre-treatment IHC Characteristics
| Grade - Pre-treatment (H&E)
|
p-value† | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||||
|
| ||||||||||
| n | Mean | SE | n | Mean | SE | n | Mean | SE | ||
| ER, Pre-Treatment | 33 | 2.3 | 0.13 | 12 | 2.0 | 0.28 | 8 | 1.5 | 0.27 | 0.064 |
| PR, Pre-Treatment | 33 | 2.1 | 0.16 | 12 | 1.6 | 0.30 | 8 | 0.9 | 0.36 | 0.009 |
| PRB, Pre-Treatment | 33 | 1.7 | 0.19 | 12 | 1.0 | 0.28 | 7 | 0.3 | 0.14 | 0.003 |
| Ki-67, Pre-Treatment | 33 | 181.1 | 19.65 | 12 | 223.6 | 24.81 | 8 | 301.1 | 42.10 | 0.023 |
| Cleaved Caspase-3, Pre-Treatment | 35 | 9.5 | 1.23 | 11 | 8.3 | 1.70 | 8 | 17.4 | 4.89 | 0.040 |
| Bcl-2, Pre-Treatment | 35 | 0.8 | 0.14 | 12 | 0.3 | 0.16 | 8 | 0.3 | 0.12 | 0.078 |
p-value from analysis of variance (ANOVA) comparing characteristic across grades.
Fifty of the 53 (94%) pre-treatment specimens upon which immunohistochemistry was performed were considered ER positive (value greater than 0.2), while 45 (76%) were PR positive (Table 2). The presence or absence of a histologic response was not related to initial ER, PR or PRB positivity (either as a binary division or when divided by tertiles of ER and PR scores [Table 2 and additional data, not shown]). A statistically significant decrease was identified in the mean ER, PR, PRB, Ki-67, and Bcl-2 as well as a non-significant decrease in Casp3 following 21 days of progestin treatment (Table 6).
Table 6.
Immunohistologic Characteristics for ER, PRE, PRB, Ki-67, Bcl-2, and Cleaved Caspase prior to and after MPA
| Pre-treatment | Post-treatment | Change | p-values for Comparison of Responders and Non-responders with Respect to | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | p-value† | Pre-treatment‡ | Change§ | ||
| ER | Total (n=53) | 2.07 | 0.11 | 1.30 | 0.12 | -0.77 | 0.14 | <.001 | ||
| Responder (n=36) | 1.99 | 0.14 | 1.31 | 0.15 | -0.68 | 0.17 | <.001 | 0.305 | 0.651 | |
| Non-responder (n=17) | 2.25 | 0.21 | 1.29 | 0.21 | -0.96 | 0.22 | <.001 | |||
| PR | Total (n=53) | 1.78 | 0.14 | 0.66 | 0.11 | -1.12 | 0.15 | <.001 | ||
| Responder (n=36) | 1.87 | 0.17 | 0.79 | 0.14 | -1.08 | 0.16 | <.001 | 0.368 | 0.141 | |
| Non-responder (n=17) | 1.60 | 0.26 | 0.38 | 0.19 | -1.22 | 0.34 | 0.002 | |||
| PRB | Total (n=52) | 1.36 | 0.15 | 0.43 | 0.11 | -0.94 | 0.14 | <.001 | ||
| Responder (n=36) | 1.52 | 0.18 | 0.52 | 0.15 | -1.00 | 0.16 | <.001 | 0.118 | 0.565 | |
| Non-responder (n=16) | 1.00 | 0.29 | 0.21 | 0.10 | -0.80 | 0.25 | 0.006 | |||
| Ki-67 | Total (n=53) | 208.83 | 15.76 | 136.13 | 16.35 | -72.70 | 15.54 | <.001 | ||
| Responder (n=34) | 179.95 | 18.74 | 96.43 | 15.59 | -83.52 | 16.43 | <.001 | 0.013 | 0.014 | |
| Non-responder (n=19) | 260.51 | 24.86 | 207.16 | 30.34 | -53.35 | 32.05 | 0.113 | |||
| Bcl-2 | Total (n=55) | 0.60 | 0.10 | 0.24 | 0.06 | -0.36 | 0.09 | <.001 | ||
| Responder (n=36) | 0.66 | 0.12 | 0.18 | 0.05 | -0.48 | 0.10 | <.001 | 0.405 | 0.035 | |
| Non-responder (n=19) | 0.48 | 0.17 | 0.35 | 0.14 | -0.13 | 0.15 | 0.412 | |||
| Cleaved Caspase | Total (n=54) | 10.40 | 1.17 | 8.12 | 1.10 | -2.28 | 1.20 | 0.063 | ||
| Responder (n=36) | 9.21 | 1.28 | 6.48 | 1.14 | -2.73 | 1.47 | 0.071 | 0.151 | 0.098 | |
| Non-responder (n=18) | 12.79 | 2.35 | 11.40 | 2.24 | -1.39 | 2.14 | 0.526 | |||
The mean pre-treatment values of ER, PR, PRB, Bcl-2 and Casp3 in the tumors that displayed a histologic response were not statistically different from those that did not. However, the initial index of Ki-67 reactivity was higher in non-responders than responders (261 vs. 180, p=0.013). Surprisingly, when changes in post-treatment values of non-responding tumors were compared to responding tumors, only the decrease in Bcl-2 and Ki-67 reached statistical significance (Table 6).
Both the pre-treatment and post-treatment mitotic indices were statistically significantly correlated with Ki-67 (p=0.002 and p<0.001, respectively), but there was no significant correlation between Bcl-2 and Casp3 (p>0.1) (data not shown).
DISCUSSION
For more than 50 years, women with endometrial hyperplasia or carcinoma have been treated by progestins with variable success [3-10], but the mechanism of action remains incompletely known [16]. In this study we attempted both to assess subjectively the presence and frequency of a histologic response and to examine selected quantifiable histologic and immunohistologic characteristics to progestin treatment at one time point of 21 days. We found features of a complete or partial histologic response in more than 64% of tumors. In addition, we were surprised by the presence of relatively subtle, but quantifiable, histologic changes in almost all of the carcinomas, including both those that were classified as having a histologic response and those that were not.
Complete histologic resolution of carcinoma was very uncommon (2%); while partial histologic responses were quite frequent (63%). Descriptively, the incomplete histologic response could most readily be recognized as a process having the overall architectural features of a carcinoma with cells having either more abundant eosinophilic cytoplasm or squamous or mucinous metaplasia, accompanied by slightly less nuclear atypia, nuclear stratification or mitotic activity. Subnuclear or apical cytoplasmic vacuoles were not present, and luminal secretion occurred rarely. The observed changes did not closely correspond to those present in the adjacent benign endometrium, where secretory exhaustion and luminal secretion was frequent and often accompanied by a decidual reaction in the stroma. Neither the typical histologic changes associated with secretory phase endometrium nor the features of secretory carcinoma were identified within the neoplasms. Without specific knowledge of progestin therapy, it is unlikely that a pathologist would have suggested it upon examination of most of these carcinomas.
Prior investigators have found initial response rates of 62% to 79% in women with atypical hyperplasia or Grade 1 endometrioid adenocarcinoma, of whom about 25% to 89% later had recurrence of tumor [3-10]. Their study populations often were largely composed of young women with mean ages of about 30 years who wished to preserve fertility, significantly different than the mean age of 61 years in this study. While the definition of a complete pathologic response is relatively straightforward, requiring the absence of identifiable residual neoplastic cells, the histologic characteristics of a partial response remain poorly defined and have varied markedly among the prior studies. This reflects at least two variables, as follows: histologic changes may vary depending on the type and duration of the progestin therapy; and some architectural or cytologic features that appear histologically abnormal may, nevertheless, be associated with terminal differentiation. For example, following progestin therapy, cytologically bland cells may still form highly complex, cribriform glands that are architecturally malignant but might be biologically benign. Consequently, both prior observations and their interpretations have varied. In 1959, Kistner first described atypical secretory change with pseudodecidual reaction in the stroma in atypical hyperplasias and carcinoma in situ following 3-10 weeks of progestin therapy [17], while shortly thereafter, Varga and Henriksen described squamous differentiation as a common consequence of progestin therapy [18]. Kamoi et al. examined the sequence of changes in carcinomas at five time-points from 4 -16 weeks following progestin therapy [19]. At four weeks, they observed cytoplasmic swelling accompanied by eosinophilic granular change in the absence of subnuclear vacuolization, squamous morules, and occasional fragmentation of glands, as well as occasional decidual change. The mitotic rate was dramatically less than prior to treatment. Saegusa and Okayasu noted either reversion to normal secretory phase histology or squamous morule formation in responders but minimal change in non-responders during the first 10 weeks of MPA treatment [20, 21]. They also found a decrease in the mitotic index but not apoptotic index. Wheeler et al. identified histologic alterations following 12-24 weeks of progestin treatment that included a decreased gland to stroma ratio, decreased gland cellularity, decreased mitotic activity, loss of cytologic atypia, and a variety of metaplasias [14]. We adopted the criteria of Wheeler et al. with minor modification for the definition of partial histologic response in this study [14].
In contrast to the subtle subjectively observed histologic response, the quantifiable histologic and immunohistochemical features examined displayed many statistically significant changes, and they were usually not limited to the tumors that displayed a subjective histologic response. Both tumors with and without a subjectively defined histologic response had significant decreases in mean gland cellularity, the frequency of nucleoli, the number of mitotic figures, and the mean nuclear grade, with acquisition of more abundant eosinophilic cytoplasm, squamous metaplasia, and secretion. This suggests that the histologic characteristics that were chosen to reflect a histologic response based on prior publications are perhaps insensitive when confined solely to subjective interpretation.
We wished to determine whether initial ER and PR would be useful in predicting the probability of a response to MPA. Since more than 90% of tumors were initially ER positive and almost 80% were PR positive, the ability to discriminate by these features is somewhat weakened. But, the initial presence or intensity of ER or PR was not related to subjective histologic response, although lower pre-treatment nuclear grade and lower frequency of Ki-67 positive cells did.
Irrespective of the presence of a histologic response, following MPA therapy, ER, PR and PRB were significantly down-regulated, as were Ki-67 and Bcl-2. However, Casp3 did not change significantly. Mean Ki-67 and Bcl-2 decreases following MPA were greater in histologic responders than non-responders, but not decreases in ER, PR, PRB and Casp3. Saegusa and Okayasu also reported down-regulation in Bcl-2, Ki-67, and PR [21] but not ER following MPA therapy. Dahmoun et al. examined the effects of MPA after two weeks on proliferation and steroid receptor content in endometrioid adenocarcinoma compared to baseline values, and they found decreases in Ki-67 and PR without a significant change in the apoptotic index or Bcl-2 content [22]. In contrast, Yamazawa et al., and Utsunomiya et al. found that initial high content of PR was associated with a complete histologic response, typically following six months of therapy [6, 23]. Unlike almost all other investigators, Utsunomiya et al. did not find significant changes in the steroid receptor content or Ki-67 labeling of tumors following MPA therapy[23]
Our observations of highly significant decreases in mitotic indices, gland cellularity, Ki-67 and Bcl-2 all provide support for the interpretation that MPA acts by decreasing cell proliferation, while the increase in metaplasia, luminal secretory product, suggest that cell differentiation has increased concomitantly. If tumor cell death were an important consequence of progestin therapy, we would have anticipated a significant increase in Casp3 rather than a non-significant decrease. Protocols that combine hormonal and chemotherapy have been proposed for treatment of metastatic endometrial adenocarcinoma. The decrease in measures of proliferation during MPA therapy identified in this study suggests that initial or alternating hormonal therapy may diminish the efficacy of chemotherapeutic agents that require high cell turnover. The observation of down-regulation of PR following progestin therapy prompted earlier studies in which constant tamoxifen (to augment PR) was coupled with alternating medroxyprogesterone acetate (MPA) administration in athymic mice containing subcutaneous human endometrial adenocarcinoma. We found that tamoxifen was equally effective in regenerating PR as estradiol, but it produced significantly less tumor cell growth (24). Based on these observations, the GOG conducted a phase 2 study of women with measurable, advanced or metastatic endometrial carcinoma using a regimen of constant tamoxifen and alternating weeks of oral progestin therapy (25). The response rate of 39% reflects the best response reported for hormonal therapy of endometrial carcinoma. Unfortunately, in both the mouse model and in women, the tumors eventually become refractory to the hormonal therapy (25,26). Further work is needed to identify the mechanisms of resistance to this alternating hormonal therapy, but the concept of designing a regimen of alternating the administration of progestin therapy with chemotherapy while maintaining constant tamoxifen to stimulate both proliferation and PR is appealing (27).
The strengths of this study include the large study population, all of whom had endometrioid carcinomas and received an identical progestin treatment with hysterectomy performed three weeks after initiation of treatment, and requirement for pre and post-treatment tissue for multiple histologic and immunohistochemical assays. Other investigators have identified a median time to response of 12 weeks or 36 weeks [3,14], suggesting that more profound histologic changes might have been observed if a more prolonged course of MPA had been administered. Since most of the women in this study did not wish to delay their surgery, a 3-week interval was chosen since it represents an interval that is frequently observed between the initial visit and the date of hysterectomy. Our ability to examine only a single time point is a limitation of the study, and consequently, we also cannot entirely eliminate the unlikely possibility that a cytotoxic effect might have occurred as an immediate progestin response. A second limitation was our inability to examine the entire endometrium and carcinoma from each patient enrolled on the study, since the responses may have differed in portions of the tumor not sampled or submitted.
Finally, we observed that the histologic response in the tumors differed quantitatively and qualitatively from that of the adjacent benign endometrium, where decidual change accompanied luminal secretion and secretory exhaustion of glands. Decidual change almost never occurred in endometrial stroma adjacent to neoplastic glands, and this suggests that a paracrine effect in which some gland: stromal interaction may be essential for either decidualization or complete tumor cell differentiation to occur. This mechanism may be critical to tumor response and warrants further investigation.
In summary, we were able to address, but not completely answer, the five questions of the study. We found the following: 1) MPA induces a subjectively defined histologic response in more than 60% of endometrioid adenocarcinomas of the uterine corpus; 2) the probability of response is not strongly related to the initial content of PR; 3) MPA down-regulates ER and dramatically down-regulates PR in endometrioid adenocarcinomas; 4) MPA produces histologic and immunohistochemical changes of diminished proliferation and greater cell differentiation, including gland cellularity, nuclear grade, nucleoli, metaplasia, secretion, Ki-67, and Bcl-2; and 5) the data suggests that MPA acts by differentiation and diminished proliferation of neoplastic cells rather than cell killing.
Acknowledgments
This study was supported by National Cancer Institute grants CA 27469 (Gynecologic Oncology Group) and CA 37517 (Gynecologic Oncology Group Statistical and Data Center).
The following GOG member institutions participated in this protocol: University of Iowa Hospitals and Clinics, University of New Mexico, Fox Chase Cancer Center, University of Oklahoma and Case Western Reserve University.
References
- 1.Fiorica JV, Brunetto VL, Hanjani P, et al. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:10–4. doi: 10.1016/j.ygyno.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Whitney CW, Brunetto VL, Zaino RJ, et al. Phase II study of medroxyprogesterone acetate plus tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:4–9. doi: 10.1016/j.ygyno.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Ramirez P, Frumovitz M, Bodurka DC, et al. Hormonal therapy for the managmenet of grade 1 enodmetrial adenocarcinoma: a literature review. Gynecol Oncol. 2004;95:133–138. doi: 10.1016/j.ygyno.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 4.Kim YB, Holschneider CH, Ghosh K, et al. Progestin alone as primary treatment of endometrial carcinoma in premenopausal women. Report of seven cases and review of the literature. Cancer. 1997;79:320–7. doi: 10.1002/(sici)1097-0142(19970115)79:2<320::aid-cncr15>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Minaguchi T, Nakagawa S, Takazawa Y, et al. Combined phospho-Akt and PTEN expressions associated with post-treatment hysterectomy after conservative progestin therapy in complex atypical hyperplasia and stage Ia, G1 adenocarcinoma of the endometrium. Cancer Lett. 2007;248:112–22. doi: 10.1016/j.canlet.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Yamazawa K, Hirai M, Fujito A, et al. Fertility-preserving treatment with progestin, and pathological criteria to predict responses, in young women with endometrial cancer. Hum Reprod. 2007;22:1953–58. doi: 10.1093/humrep/dem088. [DOI] [PubMed] [Google Scholar]
- 7.Niwa K, Tagami K, Lian Z, et al. Outcome of fertility-preserving treatment in young women with endometrial carcinomas. BJOG. 2005;112:317–20. doi: 10.1111/j.1471-0528.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 8.Ushijima K, Yahata H, Yoshikawa H, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25:2798–803. doi: 10.1200/JCO.2006.08.8344. [DOI] [PubMed] [Google Scholar]
- 9.Kaku T, Yoshikawa H, Tsuda H, et al. Conservative therapy for adenocarcinoma and atypical endometrial hyperplasia of the endometrium in young women: central pathologic review and treatment outcome. Cancer Lett. 2001;167:39–48. doi: 10.1016/s0304-3835(01)00462-1. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe J, Watanabe K, Jobo T, et al. Significance of p27 as a predicting marker for medroxyprogesterone acetate therapy against endometrial endometrioid adenocarcinoma. Int J Gynecol Cancer. 2006;16(Suppl 1):452–57. doi: 10.1111/j.1525-1438.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 11.Creasman WT, McCarty KS, Sr, Barton TK, et al. Clinical correlates of estrogen- and progesterone-binding proteins in human endometrial adenocarcinoma. Obstet Gynecol. 1980;55:363–70. doi: 10.1097/00006250-198003000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich CE, Young PC, Stehman FB, et al. Steroid receptors and clinical outcome in patients with adenocarcinoma of the endometrium. Am J Obstet Gynecol. 1988;158:796–807. doi: 10.1016/0002-9378(88)90075-0. [DOI] [PubMed] [Google Scholar]
- 13.Geisinger KR, Marshall RB, Kute TE, et al. Correlation of female sex steroid hormone receptors with histologic and ultrastructural differentiation in adenocarcinoma of the endometrium. Cancer. 1986;58:1506–17. doi: 10.1002/1097-0142(19861001)58:7<1506::aid-cncr2820580722>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler DT, Bristow RE, Kurman RJ. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am J Surg Pathol. 2007;31:988–98. doi: 10.1097/PAS.0b013e31802d68ce. [DOI] [PubMed] [Google Scholar]
- 15.Zaino RJ, Kurman RJ, Diana KL, et al. The utility of the revised International Federation of Gynecology and Obstetrics histologic grading of endometrial adenocarcinoma using a defined nuclear grading system. A Gynecologic Oncology Group study. Cancer. 1995;75:81–6. doi: 10.1002/1097-0142(19950101)75:1<81::aid-cncr2820750114>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 16.Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab. 2011;22:145–52. doi: 10.1016/j.tem.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kistner RW. Histological effects of progestins on hyperplasia and carcinoma in situ of the endometrium. Cancer. 1959;12:1106–22. doi: 10.1002/1097-0142(195911/12)12:6<1106::aid-cncr2820120607>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 18.Varga A, Henriksen E. Clinical and histopathologic evaluation of the effect of 17-alpha-hydroxyprogesterone-17-n-caproate on endometral carcinoma. Obstet Gynecol. 1961;18:658–72. [PubMed] [Google Scholar]
- 19.Kamoi S, Ohaki Y, Mori O, et al. Serial histologic observation of endometrial adenocarcinoma treated with high-dose progestin until complete disappearance of carcinomatous foci--review of more than 25 biopsies from five patients. Int J Gynecol Cancer. 2008;18:1305–14. doi: 10.1111/j.1525-1438.2007.01166.x. [DOI] [PubMed] [Google Scholar]
- 20.Saegusa M, Okayasu I. Progesterone therapy for endometrial carcinoma reduces cell proliferation but does not alter apoptosis. Cancer. 1998;83:111–21. doi: 10.1002/(sici)1097-0142(19980701)83:1<111::aid-cncr15>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Saegusa M, Okayasu I. Down-regulation of bcl-2 expression is closely related to squamous differentiation and progesterone therapy in endometrial carcinomas. J Pathol. 1997;182:429–36. doi: 10.1002/(SICI)1096-9896(199708)182:4<429::AID-PATH872>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 22.Dahmoun M, Bäckström T, Boman K, et al. Apoptosis, proliferation, and hormone receptors in endometrial carcinoma: results depending on methods of analysis. Int J Oncol. 2003;22:115–22. doi: 10.3892/ijo.22.1.115. [DOI] [PubMed] [Google Scholar]
- 23.Utsunomiya H, Suzuki T, Ito K, et al. The correlation between the response to progestogen treatment and the expression of progesterone receptor B and 17beta-hydroxysteroid dehydrogenase type 2 in human endometrial carcinoma. Clin Endocrinol (Oxf) 2003;58:696–703. doi: 10.1046/j.1365-2265.2003.01766.x. [DOI] [PubMed] [Google Scholar]
- 24.Satyaswaroop PG, Zaino RJ, Mortel R. Estrogen-like effects of tamoxifen on human endometrial carcinoma transplanted into nude mice. Cancer Res. 1984;44:4006–10. [PubMed] [Google Scholar]
- 25.Whitney CW, Brunetto VL, Zaino RJ, et al. Phase II study of medroxyprogesterone acetate plus tamoxifen in advanced endometrial carcinoma:. a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:4–9. doi: 10.1016/j.ygyno.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Satyaswaroop PG, Clarke CL, Zaino RJ, et al. Apparent resistance in human endometrial carcinoma during combination treatment with tamoxifen and progestin may result from desensitization following down-regulation of tumor progesterone receptor. Cancer Letters. 1992;62:107–14. doi: 10.1016/0304-3835(92)90180-4. [DOI] [PubMed] [Google Scholar]
- 27.Herzog TJ. What is the clinical value of adding tamoxifen to progestins in the treatment of advanced or recurrent endometrial cancer? Gynecol Oncol. 2004;92:1–3. doi: 10.1016/j.ygyno.2003.11.014. [DOI] [PubMed] [Google Scholar]


