Abstract
Microbial oil is a potential alternative to food/plant-derived biodiesel fuel. Our previous screening studies identified a wide range of oleaginous yeast species, using a defined laboratory medium known to stimulate lipid accumulation. In this study, the ability of these yeasts to grow and accumulate lipids was further investigated in synthetic hydrolysate (SynH) and authentic ammonia fiber expansion (AFEX™)-pretreated corn stover hydrolysate (ACSH). Most yeast strains tested were able to accumulate lipids in SynH, but only a few were able to grow and accumulate lipids in ACSH medium. Cryptococcus humicola UCDFST 10-1004 was able to accumulate as high as 15.5 g/L lipids, out of a total of 36 g/L cellular biomass when grown in ACSH, with a cellular lipid content of 40% of cell dry weight. This lipid production is among the highest reported values for oleaginous yeasts grown in authentic hydrolysate. Pre-culturing in SynH media with xylose as sole carbon source enabled yeasts to assimilate both glucose and xylose more efficiently in the subsequent hydrolysate medium. This study demonstrates that ACSH is a suitable medium for certain oleaginous yeasts to convert lignocellullosic sugars to triacylglycerols for production of biodiesel and other valuable oleochemicals.
Keywords: Lignocellulosic, Cryptococcus humicola, Biodiesel, Energy, Oleochemicals
Introduction
Oleaginous yeasts are capable of accumulating over 20% of their cell mass as intracellular lipids and are considered alternative strategy of producing second generation fuels including biodiesel. Compared to filamentous fungi and algae, yeasts (eukaryotic microorganisms classified in the kingdom Fungi) generally have a higher growth rate and can grow to higher cell density (Li et al. 2007). Lipids produced by yeasts have similar fatty acid profiles to those of vegetable oils. However, the high cost of synthetic culture media makes microbial oils less economically viable (Chen et al. 2009; Huang et al. 2013a; Wiebe et al. 2012). Recent work on the ability of yeasts to grow and utilize wastes materials and accumulate carbon as lipids, have suggested the possibility of producing oleochemicals including biodiesel from lignocellulosic hydrolysates, which are the product of pretreatment followed by enzymatic hydrolysis of lignocellulosic biomass (Huang et al. 2013a; Zhu et al. 2008). Microbial conversion of lignocellullosic sugars to triacylglycerols, and subsequent conversion to biodiesel by trans-esterification with short chain alcohols, is a renewable, sustainable, biodegradable, and non-toxic alternative to petroleum diesel (Ageitos et al. 2011; Atabani et al. 2012; Galafassi et al. 2012; Li et al. 2008b; Rossi et al. 2011; Thiru et al. 2011). Challenges in this technology include identifying or developing yeasts able to tolerate the inhibitors in hydrolysates, and convert the carbohydrates to lipids.
Efforts to find robust oleaginous yeasts for biodiesel production have involved two strategies: (1) screening of wild yeast strains for appropriate properties (Sitepu et al. 2012; Sitepu et al. 2013) and (2) manipulation/modification of specific genes of model yeasts to improve their performance for certain purposes (Shi et al. 2011). The latter has gained much more attention as genetic tools have become readily available, for example the oleaginous model yeast Yarrowia lipolytica has been genetically modified to accumulate more or different lipids (Beopoulos et al. 2009; Beopoulos et al. 2008a; Beopoulos et al. 2008b; NICAUD et al. 2010). These studies, however, have utilized a small number of well studied yeasts strains (Chen et al. 2009). Many other oleaginous yeast strains have not been systematically evaluated on pretreated biomass hydrolysate.
The Phaff Yeast Culture Collection located at the University of California Davis contains more than 7,000 yeast strains belonging to over 800 species, and represents a valuable resource for identifying novel lipid accumulators in addition to the previously known species. From our two previous screening studies alone, we have identified 17 new oleaginous yeast species (Sitepu et al. 2012) (Sitepu et al. 2013), in addition to the 40 oleaginous yeast species previously published (Sitepu et al. 2014). Newly identified oleaginous yeasts can be utilized in future research including genetic manipulation studies.
The lipid-inducing laboratory medium that is used for screening high lipid-producing yeasts is quite different in composition from the complex hydrolysates, which are the main target for future microbial biodiesel production. There are different pretreatment technologies available to deconstruct complex polymers of plant cell walls, including acid hydrolysis, steam explosion, alkaline wet oxidation, and hot water pretreatment (Alvira et al. 2010). AFEX™ is a technology that utilizes both physical (high temperature and pressure) and chemical (ammonia) processes to achieve effective pretreatment. AFEX™ promotes partial cellulose decrystalization, partial hemicellulose depolymerization and reduces the lignin recalcitrance in the treated biomass (Balan et al. 2009). The benefits of AFEX™ over many other pretreatment technologies include the preservation of inherent plant nutrients and the reduced production of inhibitors as byproducts, which eliminates the need for washing steps to remove inhibitors from the pretreated biomass. Washing leaches out essential nutrients for microbial growth.
To date, few studies have been reported that investigated the conversion of lignocellulosic biomass to lipids and/or biodiesel by yeasts. They are limited to a handful of oleaginous yeasts. Some of these studies include, (i) conversion of cassava starch hydrolysate by Rhodosporidium toruloides (Wang et al. 2012) or Rhodotorula mucilaginosa (Li et al. 2010b), (ii) corn cob acid hydrolysate by Trichosporon coremiiform and T. dermatis (Huang et al. 2013b) T. cutaneum (Chen et al. 2012; Gao et al. 2014), (iii) corn stover hydrolysate by T. cutaneum (Liu et al. 2012), (iv) rice straw hydrolysate by T. fermentans (Huang et al. 2009), (v) sugar cane bagasse hydrolysate by T. fermentans (Huang et al. 2012a) or Yarrowia lipolytica (Tsigie et al. 2011), or (vi) wheat straw hydrolysate by five commonly studied oleaginous yeast species (Yu et al. 2011). Lipid yields in these reports range from 3 g/L (Liu et al. 2012), up to 15.8 g/L when fed 123.5 g/L carbon source (Huang et al. 2012a).
The current study is part of a systematic sequential screening approach to investigate lipid accumulation by different yeast strains from the Phaff Yeast Culture Collection. In a previous yeast screening for lipid accumulation using a defined laboratory medium, more than 60% (44 out of 69 strains) of the yeasts tested were able to accumulate over 20% intracellular lipids when grown in a defined laboratory medium (Sitepu et al. 2013). The purpose of the current study was to identify yeasts able to convert carbohydrates in authentic hydrolysates to intracellular lipids.
Twenty-five yeasts that accumulated >30% lipids (Sitepu et al. 2013) were selected for this study (Table 1). In addition, 14 yeast strains which were isolated from Indonesian tropical rain forests were also included, based on their likely oleaginous characters, such as floating in 20% glycerol, belonging to known oleaginous species, or being taxonomically related to known oleaginous species. One known oleaginous species, Trichosporon cutaneum, that is reportedly tolerant to inhibitors, i.e. organic acids (acetic acid, formic acid and levunilic acid) and furans (furfural and 5-HMF) (Chen et al. 2009) was also included in this study, although hydrolysates derived from Ammonia Fiber Expansion (AFEX™) pretreated biomass accumulate less of these inhibitors when compared to hydrolyzates resulting from dilute acid pretreated feedstocks (Chundawat et al. 2010; Jin et al. 2012).
Table 1.
Yeast species, other collection ID, source habitat and their oleaginous characteristic
| No. | Species and UCDFST ID | Other collection ID | Source Habitat | Oleaginous characteristic |
Screening stage |
|---|---|---|---|---|---|
| 1. | Candida intermedia UCDFST 11-472 | FORDACC 939, InaCC 0502 | Leaf litter, Papalia secondary rain forest, Southeast Sulawesi, Indonesia | Buoyant** | IA |
| 2. | Candida aff. kashinagacola UCDFST 12-524 | FORDACC 4051 | Insect tunnel in infested wood, Papalia secondary forest, Southeast Sulawesi, Indonesia | Buoyant** | IA |
| 3. | Candida aff. kazuoi UCDFST 10-784 | FORDACC 627 | Fungus under bark of decaying wood, Papalia secondary rain forest, Southeast Sulawesi, Indonesia | Buoyant** | IA |
| 4. | Candida aff. sagamina UCDFST 10-1002 | FORDACC 577 | Liquid in cup fungus growing on wood | Buoyant** | IA |
| 5. | Candida tenuis UCDFST 10-1070 | FORDACC 558, InaCC 0471 | Leaf litter, Mekongga, Southeast Sulawesi, Indonesia | Buoyant** | IA |
| 6. | Candida aff. tenuis UCDFST 11-461 | FORDACC 1649, InaCC 0507 | Leaf litter, Papalia secondary rain forest, Southeast Sulawesi, Indonesia | Buoyant** | IA |
| 7. | Cryptococcus aff. taibaiensis UCDFST 73-750 | Freycin leaf axil, Lanai, Hawaii, USA | 37.37%* | IA | |
| 8. | Cryptococcus albidus UCDFST 63-203 | Fales hot spring effluent, Mono Lake, California, USA | 33.97% * | IA | |
| 9. | Cryptococcus flavescens UCDFST 12-401 | FORDACC 1648 | Leaf litter, Papalia secondary rain forest, Southeast Sulawesi, Indonesia | Buoyant** | IA |
| 10. | Cryptococcus humicola UCDFST 10-1004 | FORDACC 579 | Surface of a fungal fruiting body, Sulawesi, Indonesia | 35.48%* | IA, IB, IIA |
| 11. | Cryptococcus humicola UCDFST 12-717 | FORDACC 1608 | Leaf litter, Papalia secondary rain forest, Southeast Sulawesi, Indonesia | Buoyant** | IA, IB, IIA |
| 12. | Cryptococcus laurentii UCDFST 12-803 | FORDACC 4420 | Leaf surface of Solanum torvum Swartz. (Solanaceae), Papalia secondary forest, Southeast Sulawesi, Indonesia | Buoyant** | IA, IB, IIA |
| 13. | Cryptococcus magnus UCDFST 10-900 | FORDACC 733 | Insect gut of Cerambycid larva, Papalia secondary rain forest, Southeast Sulawesi, Indonesia | Buoyant** | IA |
| 14. | Cryptococcus ramirezgomezianus UCDFST 54-11.224 | Fruiting body of Pleurotus fungus, California, USA | 40.05%* | IA | |
| 15. | Cryptococcus terreus UCDFST 61-443 | Soil, California, USA | 51.68%* | IA, IB, IIA | |
| 16. | Cryptococcus victoriae UCDFST 10-939 | FORDACC 768 | Insect gut of larva, Papalia secondary rain forest, Southeast Sulawesi, Indonesia | Buoyant** | IA |
| 17. | Cryptococcus wieringae UCDFST 05-544 | Prunus cerasus nectar, Wolfskill Experimental Orchard, Winters, California, USA | 52.65%* | IA | |
| 18. | Kurtzmaniella cleridarum UCDFST 76-729.2 | Mushroom, California coast area, USA | 33.33%* | IA, IB, IIA | |
| 19. | Leucosporidiella creatinivora UCDFST 62-1032 | Exudate of alder tree, California, USA | 48.58%* | IA, IB, IIA | |
| 20. | Lipomyces lipofer UCDFST 78-19 | CBS 944, NRRL Y-11555 | Soil | 51.33%* | IA, IB, IIA |
| 21. | Lipomyces starkeyi UCDFST 78-23 | Opuntia stricta, Yarrawonga, New South Wales, Australia | 40.00%* | IA, IB, IIA | |
| 22. | Rhodosporidium babjevae UCDFST 04-877 | Olive fly (Batrocera oleae) Davis, California, USA | 46.87%* | IA | |
| 23. | Rhodosporidium babjevae UCDFST 05-736 | Olive fly (Batrocera oleae) Davis, California, USA | 43.20%* | IA | |
| 24. | Rhodosporidium babjevae UCDFST 05-775 | Sap on Olea europaea, Winters, California, USA | 65.32%* | IA | |
| 25. | Rhodosporidium babjevae UCDFST 68-916.1 | Insect frass of Alnus sp. (alder), Emory Creek, British Columbia, Canada | 35.00%* | IA | |
| 26. | Rhodosporidium diobovatum UCDFST 08-225 | Obtained from CBS | 39.69%* | IA | |
| 27. | Rhodosporidium kratochvilovae UCDFST 62-121 | Campanula rotundifolia flower, Canada | 39.80%* | IA | |
| 28. | Rhodosporidium paludigenum UCDFST 09-163 | Obtained from CBS | 39.73%* | IA | |
| 29. | Rhodosporidium sphaerocarpum UCDFST 68-43 | Auxotrophic mutant of strain UCDFST 48-23 | 36.60%* | IA | |
| 30. | Rhodosporidium toruloides UCDFST 68-264 | CBS 315 | Air, Tokyo, Japan | 45.67%* | IA |
| 31. | Rhodotorula glutinis UCDFST 06-542 | Olive fly (Batrocera oleae) Winters, California, USA | 58.05%* | IA | |
| 32. | Rhodotorula cf. glutinis UCDFST 05-613 | Olive fly (Batrocera oleae) Davis, California, USA | 40.62%* | IA | |
| 33. | Rhodotorula mucilaginosa UCDFST 10-1102 | FORDACC 564, InaCC 0465 | Leaf litter, Papalia secondary rain forest, Southeast Sulawesi, Indonesia | Reported oleaginous species (Sitepu et al. 2012) | IA |
| 34. | Rhodotorula mucilaginosa UCDFST 40-129 | Soil, California, USA | 32.74%* | IA | |
| 35. | Schwanniomyces vanrijiae var. yarrowii 11–462 | FORDACC 1639, InaCC 0451 | Leaf litter, Papalia secondary rain forest, Southeast Sulawesi, Indonesia | Buoyant** | IA |
| 36. | Sporobolomyces bannaensis UCDFST 11-470 | FORDACC 937, InaCC 0521 | Leaf surface, Papalia secondary rain forest, Southeast Sulawesi, Indonesia | Buoyant** | IA |
| 37. | Tremella enchepala UCDFST 68-887.2 | Cracked bark of Salix sp,. near Prince George, British Columbia, Canada | 41.67%* | IA, IB, IIA | |
| 38. | Trichosporon cutaneum UCDFST 76-64 | NRRL Y-1490, CBS 2466 | Skin lesion | Reported oleaginous genus | IA |
| 39. | Trichosporon guehoae UCDFST 60-59 | Slime flux of a chestnut tree, Winschoten, Netherlands | 37.48%* | IA, IB, IIA |
Note.
Yeast strains were grown in Medium A with 12% glucose without nitrogen source(Sitepu et al. 2013);
When transferred into 20% glycerol, cells float to the surface.
UCDFST: Phaff Yeast Culture Collection, University of California Davis, USA. FORDACC: Forest Microbe Culture Collection, Ministry of Forestry, Bogor, Indonesia. CBS: Centrallbureau voor Schimmelcultures. NRRL: US Department of Agriculture, Agricultural Research Service, Peoria, IL, USA.
Methods
Yeast strains
Yeast strains listed in Table 1 are preserved in 20% glycerol at −80°C in the Phaff Yeast Culture Collection at the University of California Davis (http://phaffcollection.ucdavis.edu). Yeasts were revived by streaking onto potato dextrose agar (PDA, DifcoTm, Sparks, MD, USA), and incubated and maintained at room temperature. Fresh plate cultures of ≤ 7 days old were used throughout the study.
Analytical methods
Microbial dry mass
For screening stage 1A, microbial dry mass was estimated by filtering 2 mL cultures through a pre-dried 0.45µm, 47mm diameter cellulose nitrate membrane filter (Cat. # 7141-204, Whatman®, Piscataway, NJ, USA), which was then dried at 60°C overnight and weighed. Intracellular lipids were evaluated by the Nile Red fluorometric method of (Sitepu et al. 2012).
Nile red assay is a rapid technique for screening large numbers of samples to identify oleaginous yeast candidates; however, this preliminary step should be followed by other analytical methods such as gravimetric analysis to allow the detection of small differences in lipid content. For all other screenings, microbial dry mass was estimated by harvesting a known volume of culture and centrifuging at 3,220 × g for 10 min. The supernatant was decanted, and the pellet was washed twice with 20 mL sterile deionized water. Microbial cell pellets were stored overnight at −80 °C, lyophilized by freeze drying (Freezone® 4.5 L Freeze Dry System Model 7750020, Labconco®, Kansas City, Missouri, USA) at −46 °C at 0.133 mBar for 48 h. The microbial dry cell pellet was weighed to determine cell biomass yield, and stored at −80°C prior to lipid analysis.
Lipid analysis
Lipids were quantified with Nile red assay and/or gravimetric methods described previously (Sitepu et al. 2012; Sitepu et al. 2013)
Reducing sugars and byproduct analyses
To quantify selected metabolites, culture samples were diluted in ultrapure water (1:10, culture: water) and filter sterilized using Multiscreen HTS 96-well filtration system (Cat.# MSHVS4510, Bilerica, MA, USA). Reducing sugars (glucose and xylose) and when applicable, byproducts (acetate, ethanol and glycerol) were analyzed by HPLC with a Biorad Aminex HPX-87H column using the method described previously (Li et al. 2010a).
Screening procedure
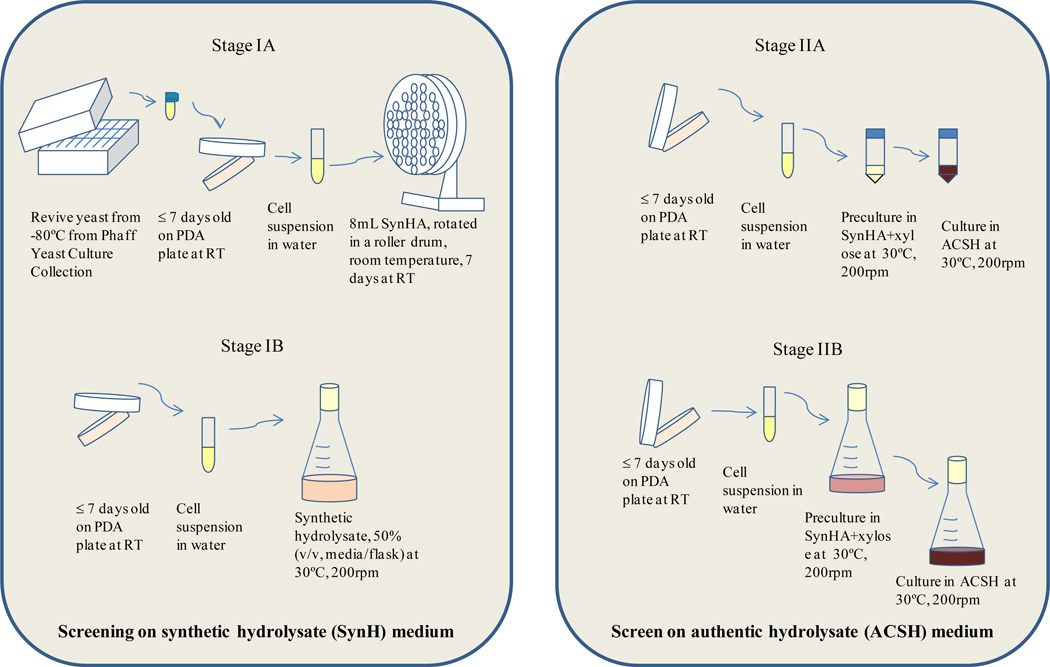
The screening procedure is described below and also depicted in Figure 1.
Figure 1.
Screening scheme of Stage I and Stage II
Screening Stage I: Synthetic corn stover hydrolysate
Screening stage IA
The objective of stage 1A was to screen a large number of yeast strains at test tube culture scale to identify strong candidates for the subsequent shake flask study (stage 1B). The thirty nine (39) yeasts strains listed in Table 1 were used in this study. One loop (1–2 µL) of less than 7 day-old yeast colony was suspended in 5mL sterile ultrapure water (Milli-Q, EMD Millipore Corporation, Billerica, MA, USA) and vortexed to homogenously distribute the cells. One hundred µL of this culture suspension was inoculated into a test tube containing 8 mL of filter-sterilized SynH media. The pH was adjusted to 5.5 with 6 N HCl before filtration. Cultures were incubated in a roller drum at RT. Samples were withdrawn aseptically to obtain relative growth data by measuring optical density at 600 nm (OD600) using a microplate reader (VersaMax™, Molecular Devices, LL., Sunnyvale, California, USA). Cultures were harvested 7 days after inoculation and several measurements were made. Dry cell biomass was measured. Total lipid content was estimated by Nile red assay as described in section 2.2, in duplicate. Nine yeast strains were selected for further analysis based on high cell biomass and intracellular lipid concentration: Cryptococcus humicola UCDFST 10-1004, Cr. laurentii UCDFST 12-803, Cr. humicola UCDFST 12-17, Trichosporon guehoae UCDFST 60-59, Cr. terreus UCDFST 61-443, Tremella enchepala UCDFST 68-887.2, Kurtzmaniella cleridarum UCDFST 76-729.2, Lipomyces starkeyi UCDFST 78-23, and Leucosporidiella creatinivora UCDFST 62-1032 (Table 1 and Fig. 3).
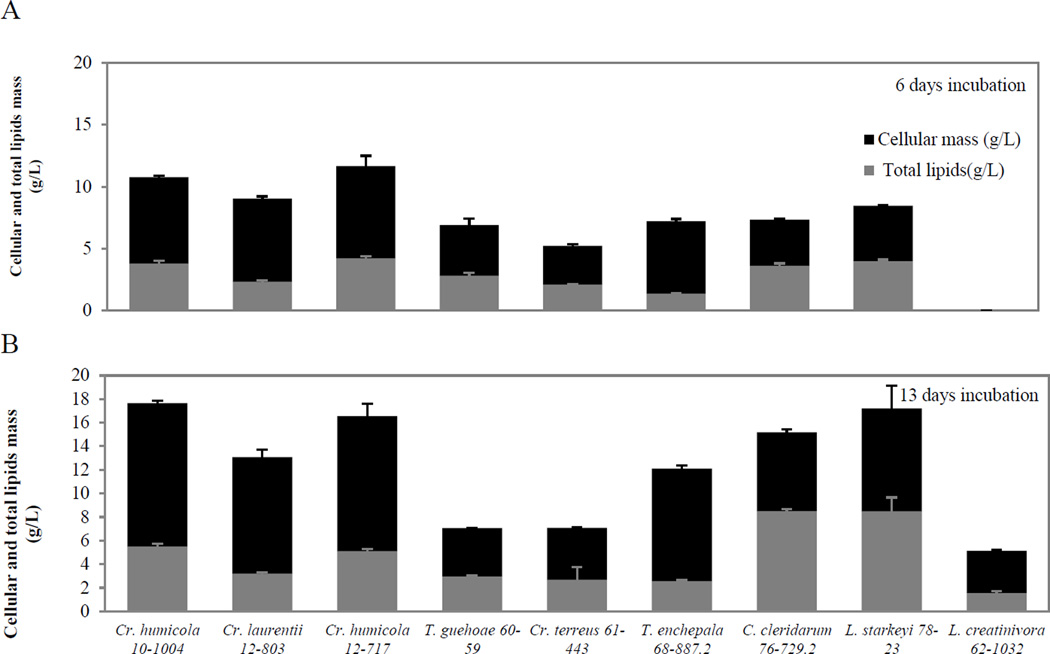
Figure 3.
Cellular mass and total lipid production of nine yeast strains grown in SynH at (A) 6 and (B) 13 days at 30°C, 200rpm. L. creatinivora UDCFST 62-1032 was a slow grower.
Screening stage IB
In this stage, nine oleaginous yeasts from Stage IA were scaled up in 250 mL synthetic AFEX hydrolysate (SynH) culture in duplicate in 500 mL baffled Erlenmeyer flasks, closed with a foam stopper (Cat.# 14-127-40G, Jaece Industries, North Tonawanda, NY, USA). SynH media composition approximates that of authentic AFEX hydrolysate (Table 2, Tang et al. submitted manuscript). Use of baffled flasks and foam stoppers facilitated better aeration of the cultures. They were incubated in a rotary shaker incubator (Series 25, New Brunswick Scientific Co., Inc., Edison, New Jersey, USA) at 200 rpm at 30 °C. Samples were withdrawn aseptically daily for determination of cell density by measuring the OD600. Two destructive samplings were done, at 6 and at 13 days after inoculation. From each flask, three 50 mL cultures were harvested in 50 mL conical tubes, and samples processed as described in section 2.2 for both lipid determinations by Nile red and gravimetric analysis (Sitepu et al. 2012; Sitepu et al. 2013).
Table 2.
Standard composition of synthetic media (SynH) mimicking authentic alkaline pretreated corn stover hydrolysate (ACSH) composition (Tang et al., submitted manuscript)
| No. | Chemical group | Chemical name | Concentration/unit |
|---|---|---|---|
| 1. | Carbon | Glucose | 60 g/L |
| Xylose | 26 g/L | ||
| 2. | Nitrogen | Ammonium sulfate (NH4)2SO4* | 5.23 g/L |
| Peptone** | 4.35 g/L | ||
| 3. | Vitamin* | D-Panthothenic acid sodium salt | 3.01 µM |
| Pyridoxal hydrochloride | 2.14 µM | ||
| Nicotinic acid | 26.78 µM | ||
| Biotin | 0.1 µM | ||
| Thiamine hydrochloride | 0.4 µM | ||
| 4. | Mineral salts | Potassium phosphate monobasic (KH2PO4) | 3319.28 mg/L |
| Potassium phosphate dibasic (K2HPO4) | 415.66 mg/L | ||
| Potassium chloride (KCl) | 4341.80 mg/L | ||
| Magnesium chloride hexahydrate (MgCl2.6H20) | 2449.73 mg/L | ||
| Calcium nitrate tetrahydrate ((CaNO3)2.4H2O) | 711.30 mg/L | ||
| Sodium carbonate anhydrous (Na2CO3) | 574.96 mg/L | ||
| Sodium chloride (NaCl) | 634.08 mg/L | ||
| Manganous chloride tetrahydrate (MnCl2.4H20) | 13.23 mg/L | ||
| Cobalt chloride hexahydrate (CoCl2.6H20) | 0.06 mg/L | ||
| Cupric chloride dihydrate (CuCl2) | 0.27 mg/L | ||
| Zinc chloride (ZnCl2) | 2.53 mg/L | ||
| Ferric chloride hexahydrate (FeCl3.6H20) | 2.69 mg/L | ||
Note. Because the concentrations of the chemicals can vary among authentic hydrolysis batches, an average value is used in the synthetic recipe.
Ammonium sulfate concentration is equivalent to ammonia content in the hydrolysate,
Peptone concentration is equivalent to total amino acid content in the hydrolysate.
Screening stage II: Authentic ACSH
Preparation for authentic AFEX Corn stover hydrolysate
Corn stover with glucan and xylan contents of 38.0% and 23.5%, respectively, was AFEX pretreated as previously described (Balan et al. 2009). AFEX pretreatment conditions applied were 1:1 ammonia to biomass ratio, 0.6 g water per g dry biomass, 140 °C and 30 min. AFEX pretreated corn stover has the same composition as the untreated one. AFEX pretreated corn stover was enzymatically hydrolyzed at 6.0 wt.% glucan loading in an Applikon Ez-Control 5L bioreactor with a total mixture of 4000 g. Enzymes used for hydrolysis included Cellic Ctec3 (Novozymes, Franklinton, NC): 12 mg protein/g glucan (0.022 ml Ctec3/g dry biomass) and Cellic Htec3 (Novozymes, Franklinton, NC): 9 mg protein/g glucan (0.021 ml Htec3/g dry biomass). The protein concentrations in Ctec3 (210.6 mg/ml) and Htec3 (164.6 mg/ml) were estimated by Dairyone (Ithaca, NY) using Kjeldahl method. Enzymatic hydrolysis was conducted at 50°C and pH 4.8 for 96 h. The hydrolysate was then centrifuged and unhydrolyzed solids were removed. The liquid hydrolysate was immediately sterile filtered after centrifugation and stored at 4 °C.
Screening stage IIA: 50 mL Bio-reaction tube
Authentic corn stover hydrolysate (ACSH) was used for growth media in Stage II screening. ACSH batch used in this experiment had 63.2 g/L glucose and 28.9 g/L xylose. The pH of the ACSH was initially 4.6, and was adjusted to 5.5 with 10 M KOH. The same nine yeast strains used in Section 2.2.2 were used, with some modification of the culture conditions. Results obtained by Huang et al. (Huang et al. 2012b) suggested that a 5% ratio of inoculum volume to growth media volume (v/v) would increase cell mass yields. Therefore, one loop of <7-day old yeasts were suspended in 5 mL sterile ultrapure water, and 0.5 mL of this suspension was inoculated into a 50 mL conical bio-reaction tube (Celltreat, Cat.# 229475, Shirley, MA, USA) containing 9.5 mL SynH with 3% xylose as sole carbon source to give 5% (v/v) inoculum concentration and a 20% ratio of media: tube volume. These pre-cultures were incubated at 30 °C at 200 rpm for 2 days. Xylose was used as sole carbon source to induce utilization of both glucose and xylose at faster rates in the subsequent media. Then, 0.5 mL of this seed culture was inoculated into 50mL bio-reaction tubes containing 9.5 mL ACSH media at pH 5.5 in triplicate. Samples were withdrawn aseptically for measuring the OD600 daily until cultures were two to three days past the exponential stage of growth, a total of five days. Nile red and gravimetric assays were performed as detailed previously. At harvesting, 5 mL of the cultures were centrifuged and processed for cell dry weight and lipid determination by gravimetric analysis as described above. From each of the three cultures per strain, duplicate samples were processed for gravimetric assay making a total of up to 6 replicates per strain. Other measurements for cell density, lipid analysis were performed as detailed in section 2.2. Three yeast strains that had the highest cell biomass yield and intracellular lipid content were selected for scale up: C. humicola UCDFST 10-1004 and 12-717, and C. laurentii UCDFST 12-803.
Screening stage IIB: scale up cultures
Based on the previous results, three yeast strains with the highest cell biomass and intracellular lipid content from stage IIA were selected for further study, including Cryptococcus humicola UCDFST 10-1004 and 12-717, and C. laurentii UCDFST 12-803. The yeasts were scaled up in 100 mL growth in authentic hydrolysate (ACSH) in 500 mL baffled Erlenmeyer flasks with foam stoppers, in triplicate. Seed culture and inoculation were performed as described previously. 20 mL samples in triplicate were collected at 7 days, and cell density and reducing sugars were determined. Lipid analysis was performed by Nile red and gravimetric assays as described previously.
Data analysis
When applicable, data were subjected to one-way analysis of variance using Microsoft Office Excel. A p value of less than 0.05 was considered significant. Pairwise significant differences were tested by the Fisher’s LSD-test to group non-significantly different treatment.
Results
Yeast growth and total intracellular lipid accumulation
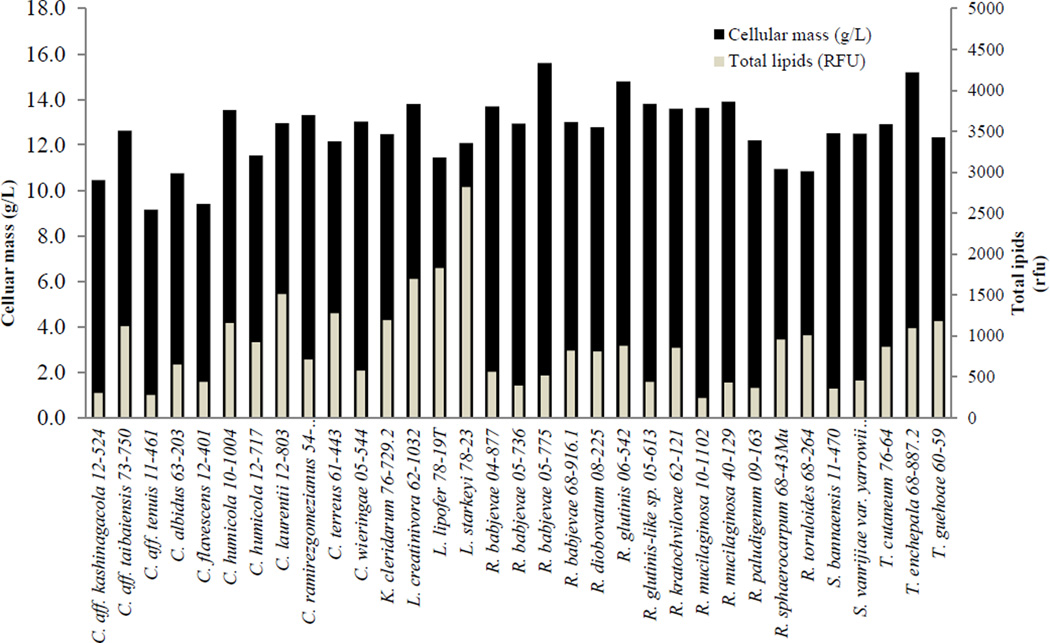
Thirty three (33) out of 39 yeasts tested grew in test tubes containing 8 mL of SynH media (Table 1). Cellular dry mass varied from 9.2 g/L to 15.6 g/L achieved by Candida aff. tenuis UCDFST 11-461 and R. babjevae UCDFST 05-775, respectively. SynH medium is a complex and rich medium that mimics the composition of ACSH with regard to carbon and nitrogen sources, vitamins and mineral salts that are generally needed to support yeast growth (Table 2).
As seen in Figure 2, lipid accumulation varied significantly among the different yeast strains. High cellular mass correlated weakly with high lipid accumulation (r = 0.04), as found in a previous study (Sitepu et al. 2013). Two strains of a known oleaginous species Rhodotorula mucilaginosa strains UCDFST 10-1102 and UCDFST 40-129, for instance, were among the 10 yeasts with the highest cell mass but they were among the ten yeasts with the lowest lipids.
Figure 2.
Cellular mass production as measured by cell dry mass, and total lipid accumulation as measured by Nile Red fluorescence, by 33 yeast strains, 7 days after incubation in test tubes containing 8mL SynH and agitated at room temperature.
In scaled up cultures grown in 250mL SynH batch media (Stage IB), two strains of the same species, Cryptococcus humicola UCDFST 10-1004 and UCDFST 12-717, had the highest microbial cell mass of 10.78 and 11.66 g/L at 6 days and they continued to increase to 17.65 and 16.57 at 13 days. They were significantly different from the other 8 strains (P<0.001). Lipomyces starkeyi strain UCDFST 78-23 had medium cellular mass production at 6 day but increased cellular mass production at later stages of growth. This strain and UCDFST 10-1004 and 12-717, were among the highest producers of cell biomass. Leucosporidiella creatinivora UCDFST 62-1032, however, was a slow grower and consistently had the lowest cellular mass at the two sampling time points (Fig. 3).
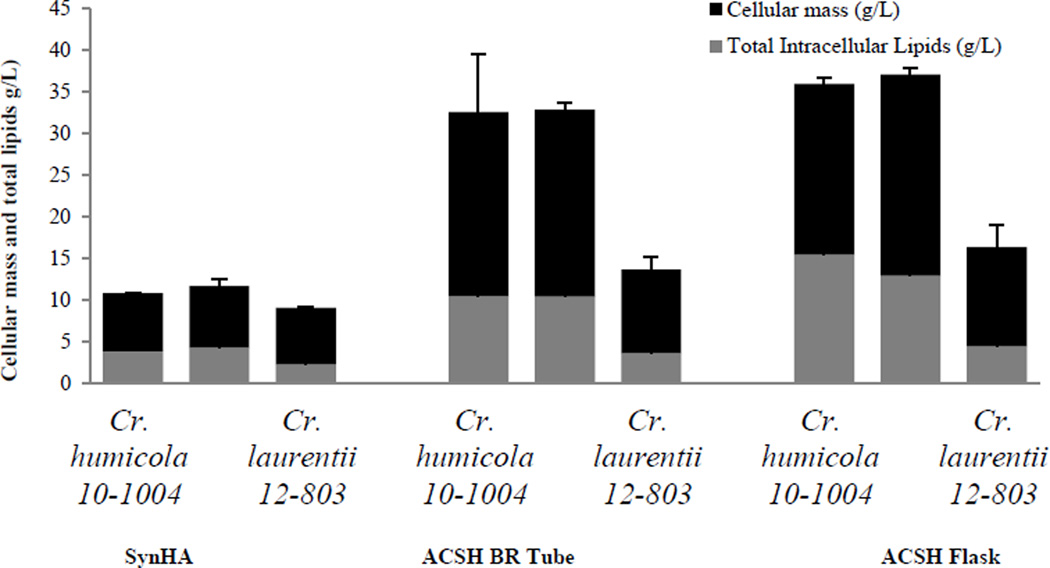
Five of nine yeast strains selected had relatively high cellular mass and total lipid production in the SynH media (Stage IB) but they had poor growth (UCDFST 61-443, and 78-23) or no growth (UCDFST 62-1032, 68-887.2, 76-729.2) in ACSH in bio-reaction tubes (Stage IIA). On the other hand, four oleaginous strains grew well in ACSH as indicated by cell optical density at OD600 (P<0.001) and cellular mass production. They were Cryptococcus humicola UCD-FST 10-1004 and 12-717, C. laurentii 12–803 and Trichosporon guehoae UCDFST 60-59. All, except T. guehoae UCDFST 60-59 that had the lowest growth and lipid content among the four (10.4 g/L and 2.7 g/L for cellular and total lipid mass, respectively) were scaled up in 100mL ACSH media. The two C. humicola strains UCD-FST 10-1004 and 12-717 consistently had the highest cellular mass and total lipids in ACSH in both bio-reaction tubes and shake flask cultures (P<0.001, Fig. 4). In shake flask cultures, the mass and lipids were 35.93 g/L with 15.47 g/L lipids, and 37.05g/L with 12.92 g/L for C. humicola strains UCD-FST 10-1004 and 12-717, respectively.
Figure 4.
Microbial cell and lipid dry mass accumulation by yeasts in different media and culture conditions.
These three yeasts produced much higher cellular biomass and lipid yields when grown in ACSH than in SynH (Fig. 4). Growth and lipid production were more efficient in baffled flasks with foam stoppers containing 20% media, likely due to superior aeration.
pH Effect
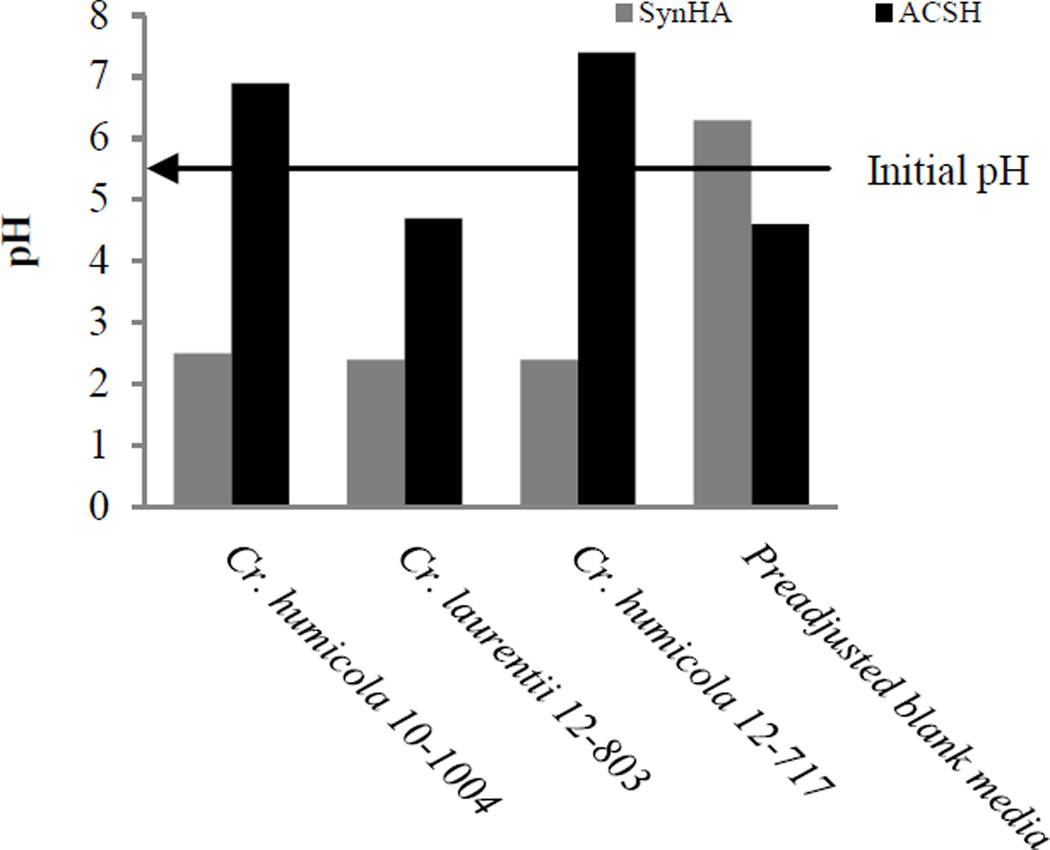
The actual pH of SynH was 6.3, higher than the pH of 4.6 of ACSH. In the hydrolysis, pH of ACSH was adjusted to 4.8 for enzymatic process. ACSH process generated about 2.5 g/L acetate, which was no longer detected at 2 days after inoculation indicating that the yeasts were likely utilized it as a carbon source. While both media (SynH and ACSH) were adjusted to pH 5.5 prior to inoculation, the final pH at harvesting varied. The pH of SynH culture after seven days of incubation ranged from as low as 2.3 to 5.5 in test tube cultures (data not presented) as well as in shake flasks (Fig. 5). Yeasts yielding higher cellular mass generally had lower final culture pH. For example, L. creatinivora strain UCDFST 62-1032 had weak growth at 6 days with pH of 5.6 at 6 days, but the pH dropped to 2.9 at 13 days as the strain produced more cell mass (Fig. 2). No contamination was observed. The ability of yeast to continue their growth in the decreasing extracellular pH in the presence of organic acids may have been due to the ability of yeasts to maintain their intracellular buffering capacity, as observed in other studies (Thomas et al. 2002). On the other hand, the final pH of cultures grown in ACSH at harvesting appeared to be dependent upon the yeast strain (Fig. 2 and 3). Of the three strains that were scaled up, the pH of cultures of C. humicola UCDFST 10-1004 and 12-717 increased from 5.5 to 6.9 and 7.4, respectively, whereas the culture of Cryptococcus laurentii UCDFST 12-803 had a final pH of 4.7. In SynH media, all three yeasts had a final pH of less than 3.0, after 7 days of incubation in the 500 mL baffled flasks. An initial pH of 5.5 and incubation at 30 °C, which were reported previously to be the optimum conditions for fermentation of Saccharomyces cerevisiae 424A(LNH-ST) in ACSH (Lau and Dale 2009), appeared to be also suitable for the yeasts that we tested, as seen in Fig. 2 and 3.
Figure 5.
Final pH of yeast culture in SynH at 6 days and ACSH at 7 days after inoculation.
Sugar utilization
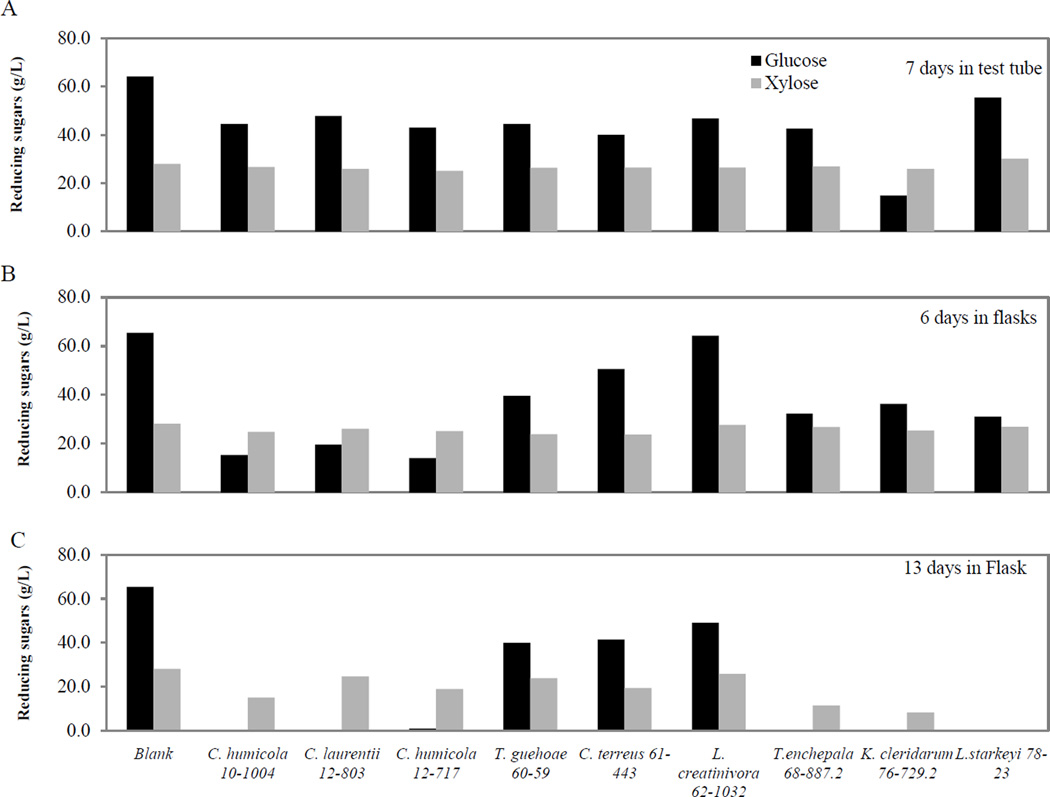
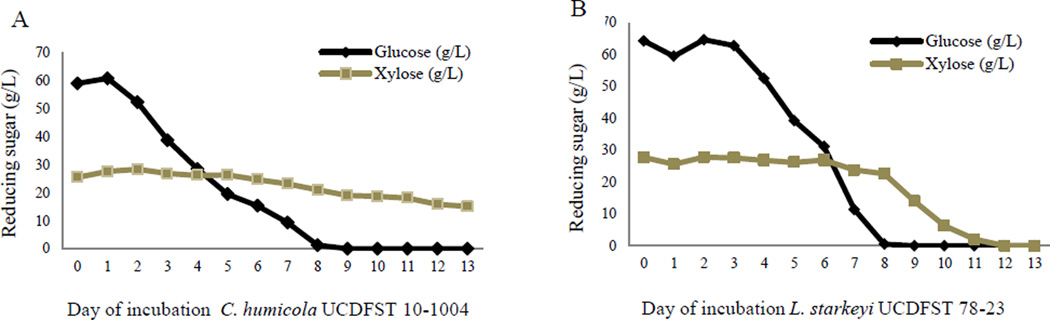
In Stage 1B, cultures were grown in SynH, which contain 60 g/L glucose and 26 g/L xylose Table 2). Without prior pre-culture in xylose as the sole carbon source, the two sugars were not utilized efficiently in the test tube or shake flask cultures at 6 to 7 days (Fig. 6). In test tube cultures (Stage 1A), glucose was partially consumed, and little or none of the xylose was utilized 7 days after inoculation (Fig. 6). In flask cultures (Stage 1B), only L. starkeyi fully consumed both sugars by 13 days after inoculation, which was inefficient for industrial scale where longer incubation time adds to production cost (Fig. 6). Nonetheless, some yeast strains grew and accumulate lipids better than others. The nine yeasts shown in Figure 6 were used in Stage 2 screenings. L. starkeyi strain UCDFST 78-23 was the only strain that completely utilized both sugars although xylose was utilized in the later stage of incubation. Typical trends of sugar utilization are shown for two of the yeasts in Figure 7. An additional step of pre-culturing the yeast in SynH with 3% xylose significantly induced and accelerated efficient utilization of both sugars, as shown in Stage II studies (Fig. 8). All glucose was consumed 4 days after inoculation, and xylose at 5–6 days after inoculation. Pre-culturing significantly improved sugar utilization efficiency for glucose, from 8 days down to 4 days and even more for xylose.
Figure 6.
Residual glucose and xylose by 9 selected yeast strains: (A) in test tubes containing 8 mL SynH; (B) in 500 mL baffled flasks containing 250 mL culture 6 days after inoculation, and (C) 13 days after inoculation
Figure 7.
Typical trend of inefficient xylose utilization in SynH amended with glucose and xylose, without pre-culture in sole xylose medium, shown for two yeast strains: (A) C. humicola UCDFST 10-1004 and (B) L. starkeyi UCDFST 78-23.
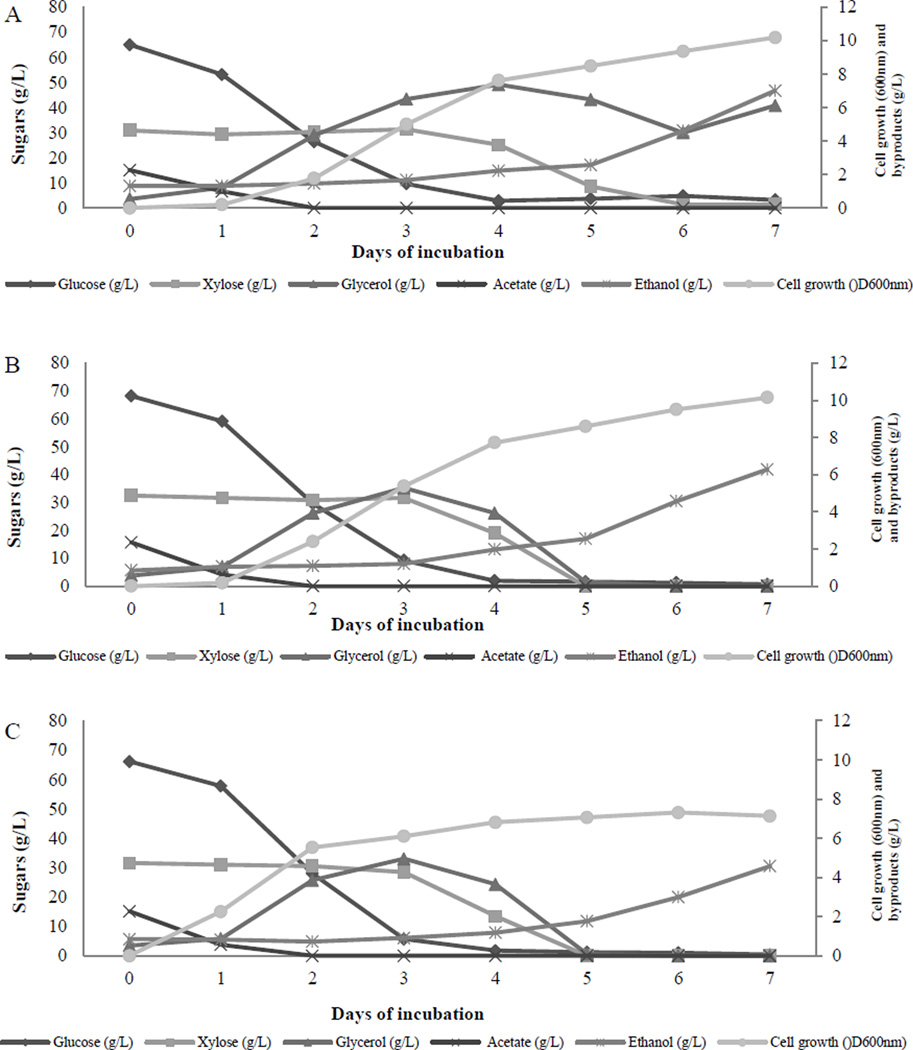
Figure 8.
Cell growth as indicated by optical density at OD600nm, reducing sugars and byproducts of (A) C. humicola strains UCDFST 10-1004, and (B) 12-717, and (C) C. laurentii UCDFST 12-803 in ACSH during 7 days of incubation with pre-culture in xylose as sole carbon source. Circle: cell growth (OD600nm), diamond: glucose (g/L), up triangle: glycerol (g/L), square: acetate g/L, star: ethanol (g/L)
The production of byproducts: ethanol and glycerol
Ethanol was produced as byproduct by all three yeast strains grown in ACSH in flask culture, ranging from 4.6 to 7 g/L culture, 7 days after inoculation in aerobic condition (Fig. 8). We speculated that as the cell density was high at this level, it created a facultative fermentative culture condition which may switch glycolytic pathway to produce acetate and ethanol as the end products. The ability to ferment glucose under oxygen limitation is a common feature for yeast, in fact about 60% (out of about 678 species) are considered fermentative (Rodrigues et al. 2006)
Glycerol production reached its highest level at 3–4 days after incubation and it dropped to zero at later time points for two strains UCDFST 12-717 and 12-803 (Fig. 8). Glycerol production by strain UCDFST 10-1004 was increased from day 6. We speculated that the yeasts may use glycerol in two ways; as backbone to attach the fatty acids to form the triacylglycerol and as a carbon source.
Discussion
Many yeasts tested had poorer growth on ACSH than on SynH and this phenomenon was likely due to the presence of inhibitors in the hydrolysate, such as acetate and other inhibitory compounds (Balan et al. 2009; Lau and Dale 2009) that were not present in SynH. Identification of lipid-accumulating yeasts was preliminary performed using laboratory media (Sitepu et al. 2012; Sitepu et al. 2013). The purpose of this study was to identify oleaginous yeasts that can grow well and accumulate lipids in authentic lignocellulosic hydrolysate. Lipid accumulation which ranged up to 15.5 g/L in shake flask studies in authentic hydrolysate are among the highest reported in the literature, summarized in (Huang et al. 2012a). Reported lipid yields include 15.73 g/L for Trichosporon fermentans grown on sugar cane bagasse hydrolysate (Huang et al. 2012a), 12.3 g/L for T. cutaneum grown on corn cob hydrolysate (Gao et al. 2014), and 11.5 g/L for T. fermentans grown on rice straw hydrolysate. Comparable studies with laboratory media suggest that further increases in yield will be obtained in batch or fed batch studies performed in fermentors (Gong et al. 2012; Zhao et al. 2008).
This study confirms that lipid yields vary depending on the oleaginous yeast species, feedstock types, and cultivation conditions, and that it is feasible to convert sugars in the hydrolysate of lignocellulosic biomass to lipid using oleaginous yeasts.
The conversion of waste materials to lipids has been the subject of research and development since the 1930s (Cohen and Ratledge 2005; Stanier 1946), with major advances and pilot scale production primarily in Germany in the 1940s, such as conversion of whey, molasses and other feedstocks (Woodbine 1959). Interest in use of oleaginous yeasts for production of biodiesel and other oleochemicals has recently expanded due to environmental, energy security and climate concerns (Ageitos et al. 2011; Li et al. 2008a; Rossi et al. 2011). As mentioned in the Introduction, there have been several published studies of conversion of lignocellulosic hydrolysates to lipids using oleaginous yeasts. This work expands the number of species that have been grown on authentic hydrolysates.
Our previous analysis on fatty acid methyl ester (FAME) (Sitepu et al. 2013) showed that the FAME of C. humicola UCDFST 10-1004 comprised primarily of C16 and C18 with 45% C18:1ω9; 28% C16:0; 13% C18:0; 11% C18:2; and 3% other FAME species ranged from C14–C24. Nevertheless, fatty acid profiles may vary with different growth conditions. The fatty acid composition of yeast is similar to vegetable oil, which are primarily C16 and C18 methyl esters. These species of fatty acids have wide applications. Methyl oleate is the main constituent of biodiesel. Palmitic acid and linoleic acid methyl esters are valuable for cosmetics, and stearic acid methyl ester is used for surfactants and softening agents.
The byproduct glycerol, as well as byproducts from cellular biomass after oil extraction, offer additional valuable products from a single process. Production of a suite of co-products from a single culture will aid in development of an economically viable biodiesel production process. The ability of yeast to utilize xylose simultaneously or subsequently is desirable because hemicellulosic xylan (1,4-β-D-heteroxylans) is the second most abundant polysaccharide in lignocellulosic materials (Dekker 1989). This pentose sugar is primarily released along with glucose during enzymatic hydrolysis. The ACSH process produces one third as much xylose as glucose (26 g/L), which is a significant amount of carbon that is wasted if the yeast cannot utilize it. Identification of a yeast strain that is able to utilize both glucose and xylose, and a seed culture method that enhances utilization of both sugars as shown in this study, emphasize the need for continued work in this area. Identification of yeast strains and/or culture conditions that enable utilization of both sugars simultaneously rather than sequentially may be possible with additional research.
Alignment of oleaginous yeasts with a specific lignocellulosic hydrolysate based on ability to utilize the sugars and tolerate the inhibitors present in hydrolysate will further aid in optimization of lipid production.
Acknowledgments
Some yeasts used in this study isolated and identified as part of a collaborative project with the Government of the Republic of Indonesia, funded by Grant Number U01TW008160 from the NIH Fogarty International Center, the NIH Office of Dietary Supplements, the National Science Foundation and the Department of Energy. This project was supported by the USDA Agricultural Food Research Initiative of the National Food and Agriculture, USDA, Grant #35621-04750. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or the National Institutes of Health, the Office of Dietary Supplements, the National Science Foundation, the Department of Energy, or the Department of Agriculture. Atit Kanti and Agustinus Joko Nugroho of LIPI Biology, Indonesia; and Sarah Asih Faulina of FORDA, Ministry of Forestry, Indonesia were thanked for the help with isolation of Indonesia microbes. Erin Cathcart and Silviana Tjahyono of UC Davis are thanked for their technical assistance. The authors thanked anonymous reviewers for providing valuable feedback that significantly improved the quality of the manuscript.
Footnotes
AFEX™ is a trademark of Michigan Biotechnology International (MBI)
Contributor Information
Irnayuli R. Sitepu, Phaff Yeast Culture Collection, Department of Food Science and Technology, University of California, One Shields Avenue, Davis, CA 95616, USA Forestry Research and Development Agency (FORDA), the Ministry of Forestry, Jalan Gunung Batu No.5, Bogor 16610, Indonesia.
Mingjie Jin, Biomass Conversion Research Laboratory, Department of Chemical Engineering and Material Science, Michigan State University, East Lansing, Michigan, USA.
J. Enrique Fernandez, Phaff Yeast Culture Collection, Department of Food Science and Technology, University of California, One Shields Avenue, Davis, CA 95616, USA.
Leonardo da Costa Sousa, Biomass Conversion Research Laboratory, Department of Chemical Engineering and Material Science, Michigan State University, East Lansing, Michigan, USA.
Venkatesh Balan, Biomass Conversion Research Laboratory, Department of Chemical Engineering and Material Science, Michigan State University, East Lansing, Michigan, USA.
Kyria L. Boundy-Mills, Phaff Yeast Culture Collection, Department of Food Science and Technology, University of California, One Shields Avenue, Davis, CA 95616, USA.
References
- Ageitos JM, Vallejo JA, Veiga-Crespo P, Villa TG. Oily yeasts as oleaginous cell factories. Appl Microbiol Biot. 2011;90(4):1219–1227. doi: 10.1007/s00253-011-3200-z. [DOI] [PubMed] [Google Scholar]
- Alvira P, Tomás-Pejó E, Ballesteros M, Negro M. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresource Technology. 2010;101(13):4851–4861. doi: 10.1016/j.biortech.2009.11.093. [DOI] [PubMed] [Google Scholar]
- Atabani A, Silitonga A, Badruddin I, Mahlia T, Masjuki H, Mekhilef S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renewable and Sustainable Energy Reviews. 2012;16:2070–2093. [Google Scholar]
- Balan V, Bals B, Chundawat SS, Marshall D, Dale B. Lignocellulosic Biomass Pretreatment Using AFEX. In: Mielenz JR, editor. Biofuels. Methods in Molecular Biology. Vol. 581. Humana Press; 2009. pp. 61–77. [DOI] [PubMed] [Google Scholar]
- Beopoulos A, Chardot T, Nicaud JM. Yarrowia lipolytica: A model and a tool to understand the mechanisms implicated in lipid accumulation. Biochimie. 2009;91(6):692–696. doi: 10.1016/j.biochi.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Beopoulos A, Mrozova Z, Thevenieau F, Le Dall M-T, Hapala I, Papanikolaou S, Chardot T, Nicaud J-M. Control of lipid accumulation in the yeast Yarrowia lipolytica. Applied and environmental microbiology. 2008a;74(24):7779–7789. doi: 10.1128/AEM.01412-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beopoulos A, Mrozova Z, Thevenieau F, Le Dall MT, Hapala I, Papanikolaou S, Chardot T, Nicaud JM. Control of lipid accumulation in the yeast Yarrowia lipolytica. Appl Environ Microbiol. 2008b;74(24):7779–7789. doi: 10.1128/AEM.01412-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-f, Huang C, Xiong L, Ma L-l. Microbial oil production from corncob acid hydrolysate by Trichosporon cutaneum. Biotechnology letters. 2012;34(6):1025–1028. doi: 10.1007/s10529-012-0869-8. [DOI] [PubMed] [Google Scholar]
- Chen X, Li Z, Zhang X, Hu F, Ryu DD, Bao J. Screening of oleaginous yeast strains tolerant to lignocellulose degradation compounds. Appl Biochem Biotechnol. 2009;159(3):591–604. doi: 10.1007/s12010-008-8491-x. [DOI] [PubMed] [Google Scholar]
- Chundawat SPS, Vismeh R, Sharma LN, Humpula JF, da Costa Sousa L, Chambliss CK, Jones AD, Balan V, Dale BE. Multifaceted characterization of cell wall decomposition products formed during ammonia fiber expansion (AFEX) and dilute acid based pretreatments. Bioresource Technology. 2010;101(21):8429–8438. doi: 10.1016/j.biortech.2010.06.027. doi: http://dx.doi.org/10.1016/j.biortech.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Cohen Z, Ratledge C. Single Cell Oils. Champaign, Illinois: AOCS Press; 2005. [Google Scholar]
- Dekker RF. Plant cell wall polymers. Washington, DC: American Chemical Society; 1989. Biodegradation of the hetero-1, 4-linked xylans; pp. 619–629. [Google Scholar]
- Galafassi S, Cucchetti D, Pizza F, Franzosi G, Bianchi D, Compagno C. Lipid production for second generation biodiesel by the oleaginous yeast Rhodotorula graminis. Bioresour Technol. 2012;111:398–403. doi: 10.1016/j.biortech.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Gao Q, Cui Z, Zhang J, Bao J. Lipid fermentation of corncob residues hydrolysate by oleaginous yeast Trichosporon cutaneum. Bioresource Technology. 2014;152:552–556. doi: 10.1016/j.biortech.2013.11.044. [DOI] [PubMed] [Google Scholar]
- Gong Z, Wang Q, Shen H, Hu C, Jin G, Zongbao K. Co-fermentation of cellobiose and xylose by Lipomyces starkeyi for lipid production. Bioresour Technol. 2012;117:20–24. doi: 10.1016/j.biortech.2012.04.063. [DOI] [PubMed] [Google Scholar]
- Huang C, Chen X-F, Xiong L, Chen X-D, Ma L-L, Chen Y. Single cell oil production from low-cost substrates: The possibility and potential of its industrialization. Biotechnology Advances. 2013a;31:129–139. doi: 10.1016/j.biotechadv.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Huang C, Chen X-F, Xiong L, Yang X-Y, Chen X-D, Ma L-L, Chen Y. Microbial oil production from corncob acid hydrolysate by oleaginous yeast Trichosporon coremiiforme. Biomass and Bioenergy. 2013b;49:273–278. [Google Scholar]
- Huang C, Wu H, Li R-F, Zong M-H. Improving lipid production from bagasse hydrolysate with Trichosporon fermentans by response surface methodology. New Biotechnology. 2012a;29(3):372–378. doi: 10.1016/j.nbt.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Huang C, Wu H, Liu Z, Cai J, Lou W-y, Zong M-h. Effect of organic acids on the growth and lipid accumulation of oleaginous yeast Trichosporon fermentans. Biotechnol Biofuels. 2012b;5(4) doi: 10.1186/1754-6834-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Zong M-H, Wu H, Liu Q-P. Microbial oil production from rice straw hydrolysate by Trichosporon fermentans. Bioresource Technology. 2009;100(19):4535–4538. doi: 10.1016/j.biortech.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Jin M, Gunawan C, Balan V, Dale BE. Consolidated bioprocessing (CBP) of AFEX™-pretreated corn stover for ethanol production using Clostridium phytofermentans at a high solids loading. Biotechnology and Bioengineering. 2012;109(8):1929–1936. doi: 10.1002/bit.24458. [DOI] [PubMed] [Google Scholar]
- Lau MW, Dale BE. Cellulosic ethanol production from AFEX-treated corn stover using Saccharomyces cerevisiae 424A(LNH-ST) Proceedings of the National Academy of Sciences. 2009;106(5):1368–1373. doi: 10.1073/pnas.0812364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B-Z, Balan V, Yuan Y-J, Dale BE. Process optimization to convert forage and sweet sorghum bagasse to ethanol based on ammonia fiber expansion (AFEX) pretreatment. Bioresource technology. 2010a;101(4):1285–1292. doi: 10.1016/j.biortech.2009.09.044. [DOI] [PubMed] [Google Scholar]
- Li M, Liu G-L, Chi Z, Chi Z-M. Single cell oil production from hydrolysate of cassava starch by marine-derived yeast Rhodotorula mucilaginosa TJY15a. Biomass and Bioenergy. 2010b;34(1):101–107. [Google Scholar]
- Li Q, Du W, Dehua L. Perspectives of microbial oils for biodiesel production. Appl Microbiol Biotechnol. 2008a;80:749–756. doi: 10.1007/s00253-008-1625-9. [DOI] [PubMed] [Google Scholar]
- Li Q, Du W, Liu D. Perspectives of microbial oils for biodiesel production. Applied Microbiology and Biotechnology. 2008b;80(5):749–756. doi: 10.1007/s00253-008-1625-9. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhao Z, Bai F. High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme and Microbial Technology. 2007;41(3):312–317. doi: http://dx.doi.org/10.1016/j.enzmictec.2007.02.008. [Google Scholar]
- Liu W, Wang Y, Yu Z, Bao J. Simultaneous saccharification and microbial lipid fermentation of corn stover by oleaginous yeast Trichosporon cutaneum. Bioresour Technol. 2012;118:13–18. doi: 10.1016/j.biortech.2012.05.038. [DOI] [PubMed] [Google Scholar]
- Nicaud J-M, Chardot T, Beopoulos A. New mutant yeast strains capable of accumulating a large of lipids. 2,010,004,141 WO Patent. 2010
- Rodrigues F, Ludovico P, Leão C. Sugar metabolism in yeasts: an overview of aerobic and anaerobic glucose catabolism Biodiversity and ecophysiology of yeasts. Springer; 2006. pp. 101–121. [Google Scholar]
- Rossi M, Amaretti A, Raimondi S, Leonardi A. Biodiesel–feedstocks and processing technologies. InTech, Rijeka; 2011. Getting Lipids for Biodiesel Production from Oleaginous Fungi; pp. 71–92. [Google Scholar]
- Shi S, Valle-Rodriguez J, Siewers V, Nielsen J. Prospects for microbial biodiesel production. Biotechol J. 2011;6:277–285. doi: 10.1002/biot.201000117. [DOI] [PubMed] [Google Scholar]
- Sitepu I, Ignatia L, Franz A, Wong D, Faulina S, Tsui M, Kanti A, Boundy-Mills K. An improved high-throughput Nile red fluorescence assay for estimating intracellular lipids in a variety of yeast species. Journal of Microbiological Methods. 2012;91(2):321–328. doi: 10.1016/j.mimet.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitepu I, Selby T, Lin T, Zhu S, Boundy-Mills K. Carbon source utilization and inhibitor tolerance of 45 oleaginous yeast species. J Ind Microbiol Biotechnol. 2014:1–10. doi: 10.1007/s10295-014-1447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitepu IR, Sestric R, Ignatia L, Levin D, Bruce German J, Gillies LA, Almada LA, Boundy-Mills KL. Manipulation of culture conditions alters lipid content and fatty acid profiles of a wide variety of known and new oleaginous yeasts species. Bioresource Technology. 2013;144:360–369. doi: 10.1016/j.biortech.2013.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Some aspects of microbiological research in Germany BIOS Final Report No 691, Item No 24. London: British Intelligence Objectives Sub-Committee; 1946. [Google Scholar]
- Thiru M, Sankh S, Rangaswamy V. Process for biodiesel production from Cryptococcus curvatus. Bioresour Technol. 2011;102:10436–10440. doi: 10.1016/j.biortech.2011.08.102. [DOI] [PubMed] [Google Scholar]
- Thomas KC, Hynes SH, Ingledew WM. Influence of medium buffering capacity on inhibition of Saccharomyces cerevisiae growth by acetic and lactic acids. Applied and Environmental Microbiology. 2002;68(4):1616–1623. doi: 10.1128/AEM.68.4.1616-1623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigie YA, Wang C-Y, Truong C-T, Ju Y-H. Lipid production from Yarrowia lipolytica Po1g grown in sugarcane bagasse hydrolysate. Bioresource Technology. 2011;102(19):9216–9222. doi: 10.1016/j.biortech.2011.06.047. doi: http://dx.doi.org/10.1016/j.biortech.2011.06.047. [DOI] [PubMed] [Google Scholar]
- Wang Q, Guo F-J, Rong Y-J, Chi Z-M. Lipid production from hydrolysate of cassava starch by Rhodosporidium toruloides 21167 for biodiesel making. Renewable Energy. 2012 [Google Scholar]
- Wiebe MG, Koivuranta K, Penttilä M, Ruohonen L. Lipid production in batch and fed-batch cultures of Rhodosporidium toruloides from 5 and 6 carbon carbohydrates. BMC biotechnology. 2012;12(1):26. doi: 10.1186/1472-6750-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbine M. Microbial Fat: Micro-Organisms as Potential Fat Producers Progress in Industrial Microbiology. 1959:179–245. [Google Scholar]
- Yu X, Zheng Y, Dorgan K, Chen S. Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid. Bioresour Technol. 2011;102:6134–6140. doi: 10.1016/j.biortech.2011.02.081. [DOI] [PubMed] [Google Scholar]
- Zhao X, Kong X, Hua Y, Feng N, Zhao Z. Medium optimization for lipid production through co-fermentation of glucose and xylose by the oleaginous yeast Lipomyces starkeyi. Eur J Lipid Sci Technol. 2008;110:405–412. [Google Scholar]
- Zhu L, Zong M, Wu H. Efficient lipid production with Trichosporon fermentans and its use for biodiesel preparation. Bioresour Technol. 2008;99:7881–7885. doi: 10.1016/j.biortech.2008.02.033. [DOI] [PubMed] [Google Scholar]