Abstract
Background
Host genetic variability has been implicated in chemotherapy-induced peripheral neuropathy (CIPN). A dose-limiting toxicity for chemotherapy agents, CIPN is also a debilitating condition that may progress to chronic neuropathic pain. We utilized a bioinformatics approach, which captures the complexity of intracellular and intercellular interactions, to identify genes for CIPN.
Methods
Using genes pooled from the literature as a starting point, we used Ingenuity Pathway Analysis (IPA) to generate gene networks for CIPN.
Results
We performed IPA core analysis for genes associated with platinum-, taxane- and platinum-taxane–induced neuropathy. We found that IL6, TNF, CXCL8, IL1B and ERK1/2 were the top genes in terms of the number of connections in platinum-induced neuropathy and TP53, MYC, PARP1, P38 MAPK and TNF for combined taxane-platinum–induced neuropathy.
Conclusion
Neurotoxicity is common in cancer patients treated with platinum compounds and anti-microtubule agents and CIPN is one of the debilitating sequela. The bioinformatic approach helped identify genes associated with CIPN in cancer patients.
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a debilitating condition. CIPN is a dose-limiting toxicity for chemotherapy agents, such as oxaliplatin, cisplatin, and platinum [1–4]. Chemotherapeutic agents may cause structural damage to peripheral nerves, which can result in aberrant somatosensory processing by the peripheral and/or central nervous system. The symptoms of CIPN vary depending on the type of chemotherapy administered and which nerve fibers are affected. Unusual sensations (paresthesia), numbness, balance problems or pain may result from chemotherapies that affect the sensory nerve fibers. When motor nerves are affected, patients may report weakness of the muscles in the feet and hands.
Patients who suffer from CIPN have a higher risk (as much as threefold higher) of developing neuropathic pain (NP) [5]. Defined as “pain initiated or caused by primary lesion or dysfunction in the nervous system,” NP occurs in nearly 40 % of patients who experience cancer pain [6, 7]. Patients with NP experience higher pain intensity and less effective control of their pain with conventional analgesia [8]. Further, patients with NP rate their level of pain relief to be significantly lower than those with nociceptive pain (defined as pain caused by activation of primary afferents in somatic or visceral tissues) in response to a single dose of an opioid [8, 9]. Patients with NP report twice as many visits to their health care provider (p = 0.02) and take more prescription (50 % versus 19 %; p = 0.001) and over-the-counter medications (62.5 % versus 45 %; p = 0.08) for pain than those without NP [5].
Published guidelines for the initial treatment of NP include the use of gabapentin, pregabalin, carbamazepine, tricyclic antidepressants, oxycodone, morphine, methadone, tramadol, duloxetine, and venlafaxine [10, 11]. However, placebo-controlled trials have shown that medications such as gabapentin [12] and glutamine [13] have no statistically significant effects on NP. Animal and human studies have been conducted to identify the best ways to treat and manage NP [14–20]. Because CIPN is a risk factor for the development of NP in cancer patients, a better understanding of the potential biological mechanisms underlying CIPN has huge clinical significance.
Host genetic variability has been implicated in many pain conditions, including neuropathy. Each of these studies assessed different therapeutic agents and different genetic mechanisms. However, it is understood that as a complex trait, several genes are implicated in CIPN. Bioinformatics provides tools for using large-scale information to produce comprehensive networks of genes and the underlying biological pathways implicated in a phenotype. Therefore, in this study, we used the Ingenuity Pathway Analysis (IPA), a bioinformatic tool for analyzing biological data, and performed a comprehensive network-based approach to identify genes implicated in neuropathy induced by chemotherapy agents. Compared to traditional regression approaches, network-based approaches can provide a holistic picture that captures the complexity of intracellular and intercellular interactions in diseases [21]. Furthermore, the network-based approaches can identify genes and pathways related to a disease or phenotype, which will lead to a better understanding of the underlying biological mechanisms [22]. Further, networks generated from IPA core analysis may suggest new candidate genes for future studies of CIPN.
Methods
With the goal of identifying a comprehensive list of genes and potentially novel genes associated with CIPN, we first conducted a literature search as described below. Using genes pooled from the literature as a starting point, we used IPA to generate gene networks for CIPN.
Literature review
Using the PubMed database, we performed a comprehensive literature review, limiting our search to human studies and articles published in English before July 2014. The primary purpose of the literature search was to identify genes associated with CIPN in cancer patients. The terms we used were “cancer neuropathy SNP,” “cancer neuropathy SNPs,” “cancer neuropathy gene,” “cancer neuropathy genes,” “cancer neurotoxicity SNP,” “cancer neurotoxicity SNPs,” “cancer neurotoxicity gene” and “cancer neurotoxicity genes.” We then screened the resulting articles based on the title, abstract, and the full text, and excluded duplicate articles. Next, we manually searched the reference lists of the articles identified in our initial search and those in related review articles to identify additional relevant articles (Table 1). From these studies, we retrieved the information about genes harboring or close to the significantly associated genetic variants (SNPs or haplotypes) and included those genes in the IPA. In particular, we included only those genes for IPA analysis that (1) have been replicated in an independent study or meta-analysis, (2) have at least one SNP that reached the genome-wide significance level, or (3) have a known biological functional significance (e.g., multi-drug resistance, drug metabolism, and mediating developmental events in the nervous system). We also summarized the information based on the different chemotherapy agents used for cancer patients.
Table 1.
Number of articles obtained using different search terms
| Search terms | # of articles by PubMed search | # of articles by initial screen | # of articles from references | # of articles included |
|---|---|---|---|---|
| cancer neuropathy SNPs(SNP) | 30 | 20 | 36 | 56 |
| cancer neuropathy genes(gene) | 266 | 1 | 0 | 1 |
| cancer neurotoxicity SNPs(SNP) | 37 | 6 | 0 | 6 |
| cancer neurotoxicity genes(gene) | 349 | 1 | 0 | 1 |
| Total | 682 | 28 | 36 | 64 |
Ingenuity pathway analysis
IPA (Ingenuity® Systems, www.ingenuity.com) is a software that connects a list of molecules in a set of networks based on the scientific information contained in the Ingenuity Knowledge Base of biological interactions and functional annotations from millions of relationships between proteins, genes, complexes, cells, tissues, drugs, and diseases [23, 21]. In the networks, nodes are used to represent molecules (e.g., genes, chemicals, protein families, complexes, microRNA species and biological processes) [24] and lines connecting two molecules are used to represent the relationship between them. Many different types of relationships are considered in the IPA analyses, including activation, binding, causation, chemical-chemical interaction, expression enzyme catalysis, inhibition, biochemical modification, protein-protein binding and transcription.
In this study, we utilized the IPA core analysis function to generate relevant networks that identify additional genes that interact with the genes identified from the literature review (denoted as focus genes in IPA). The IPA core analysis function is a process to create networks on the basis of the focus genes [25]. The working hypothesis for network generation is that the biological function involves locally dense interactions; thus, IPA uses an algorithm to attempt to generate networks that are as densely connected as possible [26]. The network generation process first ranks the focus genes in decreasing order on the basis of triangular connectivity, which measures the number of triangular connections in which a gene functions (or pairs of genes to which a gene is connected). The most connected focus gene (the top ranked gene) is considered to be the starting seed gene. Next, the remaining focus genes that are in the neighborhood of the starting seed gene are added to generate the first seed gene network. A neighborhood is defined as a gene plus the genes exactly one connection away from that gene. Then the second seed gene network is identified from the focus genes that are not included in the first seed gene network. The process continues until all focus genes are represented in a relevant network. Subsequently, all smaller networks are combined to make larger networks by connecting seed gene networks through an additional non-focus gene. If the gene network does not reach the maximum network size (140 genes in this study), IPA will then connect additional genes/networks from its database to any of the genes involved in the gene network. Specifically, given a network, to identify additional genes to be added, IPA gives priority to the genes that have the largest overlap with the existing network and have the least number of neighbors. This property is measured using a metric called specific connectivity, which is calculated by dividing the number of genes in the intersection of the neighborhood and the existing network by the union of the number of genes in the neighborhood and the existing network. The gene with the highest specific connectivity score is included in the existing network. Importantly, the IPA analysis can exclude a focus gene from the resulting network if such a gene is less likely to have connections (i.e., biological relationships) with the network.
The resulting functions/pathways/networks are evaluated using the right-tailed Fisher’s exact test, which provides p values based on the null hypothesis that the association between a set of focus genes and a given function/pathway/network is due to random chance [25]. Specifically, if the final network includes n genes and nf of them are focus genes, the p value is the probability of finding nf or more focus genes in a set of n genes randomly selected from the IPA pre-specified database [26]. A score, which is assessed as -log10(p value), is used to rank the resulting functions/pathways/networks. We used a significance level of <10−5 in our study (score > 5) when selecting networks [21].
We limited the IPA analysis to human studies. In the IPA core analysis, we used the Ingenuity Knowledge Base as the reference set. In order to generate networks in the core analysis, we used the settings of a maximum of 140 genes per network and 25 networks per analysis, because the networks for up to 140 genes allow for the possibility that the same network can include all focus genes [27]. We reported the most interconnected genes in the networks as the key genes of interest, because highly connected molecules (called hubs) are typically associated with biological functions or diseases [22, 24, 21, 26, 27].
Results
Literature review
From our search of the PubMed database, we initially identified 682 articles. After screening the title, abstract and full text, we excluded 654 articles for the following reasons (Table 1): (1) not human studies; (2) not published in English; (3) meta-analysis study, review or letter to the editor; (4) clinical trial studies; (5) not genetic association studies; (6) not neuropathy-related phenotypes studies; (7) not cancer studies; and (8) duplicate articles from different searches. We then manually searched the reference lists from the resulting 28 articles and from related review articles about genetic neuropathy studies, and identified 36 more articles. As a result, we had a total of 64 articles from which we extracted information to identify the focus genes and perform the analyses through IPA.
Table 2 lists the information we retrieved from each of the studies, including the year of publication, first author, ethnicity of patient population, cancer type, sample size, phenotypes, and significant genes. These studies included different cancer sites and patients of different ethnic groups. Neuropathy (or neurotoxicity) in cancer patients is usually induced by the chemotherapy agents used in cancer treatment, such as oxaliplatin, cisplatin, and platinum, and is usually measured according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events or Common Toxicity Criteria.
Table 2.
List of genetic association studies for chemotherapy-induced neuropathy in cancer patients, sorted by publication year and name of first author
| Year | First author | Ethnicity | Cancer type | Sample size | Phenotype | Significant genes |
|---|---|---|---|---|---|---|
| 2003 | Aplenc R [41] | W, AA, H | Acute lymphoblastic leukemia | 533 | Peripheral neuropathy | CYP3A4, CYP3A5 |
| 2004 | Isla D [42] | W | Lung | 62 | Docetaxel-cisplatin-treated neurological | None |
| 2006 | Lecomte T [43] | W | Gastrointestinal solid tumors | 64 | Oxaliplatin-related cumulative neuropathy | GSTP1 |
| 2006 | Sissung TM [44] | W | N/A | 26 | Paclitaxel-induced neuropathy | ABCB1 |
| 2007 | Gamelin L [45] | W | Colon, rectum | 145 | Oxaliplatin-induced neurotoxicity | AGXT |
| 2007 | Marsh S [46] | N/A | Ovarian | 914 | Paclitaxel/docetaxel-induced neuropathy | None |
| 2007 | Oldenburg J [47] | W | Testicular | 238 | Self-reported chemotherapy-induced long-term toxicities | GSTP1 |
| 2007 | Ruzzo A [48] | W | Colorectal | 166 | Oxaliplatin-induced neurotoxicity | GSTP1 |
| 2008 | Keam B [49] | A | Gastric | 73 | Peripheral sensory neuropathy | None |
| 2008 | Pare L [50] | W | Colorectal | 126 | Cumulative oxaliplatin-induced neuropathy | None |
| 2008 | Sissung TM [51] | N/A | Prostate | 73 | Docetaxel-induced neuropathy | ABCB1 |
| 2009 | Argyriou AA [52] | W | Colorectal | 62 | Oxaliplatin-induced peripheral neuropathy | None |
| 2009 | Goekkurt E [53] | W | Gastric | 134 | Neurotoxicity | GSTP1 |
| 2009 | Green H [54] | W | Ovarian | 38 | Sensory/motor neuropathy | None |
| 2009 | Kim HS [55] | A | Epithelial ovarian | 118 | Taxane/platinum- induced neurotoxicity | ERCC1 |
| 2009 | Kweekel DM [56] | W | Colorectal | 91 | Neurotoxicity | None |
| 2009 | Mir O [57] | W | Breast, lung, prostate | 58 | Docetaxel(Taxotere)-induced peripheral neuropathy | GSTP1 |
| 2009 | Seo BG [58] | A | Gastric | 94 | Neuropathy | None |
| 2010 | Antonacopoulou AG [59] | W | Colorectal | 55 | Chronic oxaliplatin-induced peripheral neuropathy | ITGB3 |
| 2010 | Boige V [60] | W | Colorectal | 349 | FOLFOX-induced severe neurologic toxicity | None |
| 2010 | Chen YC [61] | A | Colorectal | 166 | Oxaliplatin-induced chronic cumulative neuropathy | GSTP1 |
| 2010 | Cho HJ [62] | A | Diffuse large B-cell lymphoma | 94 | Chemotherapy-related neurotoxicity | None |
| 2010 | Inada M [63] | A | Colorectal | 51 | Oxaliplatin-induced peripheral neuropathy | ERCC1, GSTP1 |
| 2010 | Kanai M [64] | A | Colorectal | 82 | Early-onset oxaliplatin-induced neuropathy | None |
| 2010 | Khrunin AV [65] | W | Ovarian | 104 | Cisplatin-based neuropathy | GSTM1, GSTM3 |
| 2010 | Li QF [66] | A | Gastric | 92 | Neurological toxicity | GSTP1 |
| 2010 | McLeod HL [67] | W, A, AA, H | Metastatic colorectal | 520 | Diarrhea, vomiting, paresthesia, febrile neutropenia and neutropenia | GSTP1 |
| 2010 | Ofverholm A [68] | W | Breast, ovarian | 36 | Occurrence and degree of neurotoxicity | None |
| 2010 | Rizzo R [69] | W | Breast | 95 | Taxane-induced hypersensitivity and sensory neuropathy | None |
| 2011 | Basso M [70] | W | Colorectal, pancreatic, bile ducts | 40 | Acute oxaliplatin neurotoxicity | SK3 |
| 2011 | Bergmann TK [71] | W | Ovarian | 119 | Sensory neuropathy | None |
| 2011 | Bergmann TK [72] | W | Ovarian | 92 | Sensory neuropathy | None |
| 2011 | Broyl A [73] | W | Multiple myeloma | 369 | Bortezomib/vincristine-induced peripheral neuropathy | RHOBTB2, CPT1C, SOX8, caspase 9, ALOX12, IGF1R, SOD2, MYO5A, MBL2, PPARD, ERCC4, ERCC3, AURKA, MKI67, GLI1, DPYD, ABCC1 |
| 2011 | Cibeira MT [74] | W | Multiple myeloma | 28 | Thalidomide-induced peripheral neuropathy | GSTT1 |
| 2011 | Corthals SL [75] | W | Multiple myeloma | 238 | Bortezomib induced peripheral neuropathy | CYP17A1 |
| 2011 | Favis R [76] | W | Myeloma | 139 | Bortezomib-induced peripheral neuropathy | CTLA4, PSMB1, CTSS, GJE1, DYNC1I1, TCF4 |
| 2011 | Hong J [77] | A | Colorectal | 52 | Sensory neuropathy | GSTP1 |
| 2011 | Johnson DC [78] | W | Multiple myeloma | 1495 | Thalidomide-related peripheral neuropathy | ABCA1, ICAM1, PPARD, SERPINB2, SLC12A6 |
| 2011 | Leskela S [79] | W | Lung, breast, ovary, uterus, head and neck | 118 | Neurotoxicity | CYP2C8, CYP3A5 |
| 2011 | Sucheston LE [80] | W, AA | Breast | 888 | Taxane-induced neurotoxicity | FANCD2 |
| 2012 | Baldwin RM [81] | W, AA, A | Breast | 855 | Paclitaxel induced peripheral sensory neuropathy | FGD4, FZD3, EPHA5 |
| 2012 | Braunagel D [82] | W | Acute myeloid leukemia | 360 | Cytarabine-induced neurotoxicity | NME1 |
| 2012 | Fung C [83] | W, A, AA, H | Testicular germ cell tumor | 137 | Cisplatin-induced neurotoxicity, peripheral neuropathy | None |
| 2012 | Hasmats J [84] | W | Ovarian, lung, carcinoma in uteri/peritoneal/breast | 94 | Paclitaxel/carboplatin-induced neuropathy | ABCA1 |
| 2012 | Hertz DL [85] | W, AA | Breast | 111 | Peripheral neuropathy | CYP2C8 |
| 2012 | Leandro-Garcia LJ [86] | W | Ovary, lung, breast | 214 | Paclitaxel-induced peripheral neuropathy | TUBB2A |
| 2012 | Won HH [87] | A | Colon | 96 | Severe oxaliplatin-induced chronic peripheral neuropathy | TAC1, FOXC1, GMDS, ITGA1, PELO, ACYP2, TSPYL6, DLEU7, BTG4, POU2AF1, CAMK2N1, FARS2, LYRM4 |
| 2013 | Argyriou AA [88] | W | Colorectal | 200 | Oxaliplatin-induced peripheral neuropathy | SCN4A, SCN10A |
| 2013 | Bergmann TK [89] | W | Ovarian | 241 | Paclitaxel induced neuropathy | None |
| 2013 | Cecchin E [90] | W | Colorectal | 144 | Oxaliplatin neurotoxicity | ABCC1, ABCC2 |
| 2013 | de Graan AJ [91] | W | Esophagus, ovary, cervix, endometrial, breast, lung, head/neck | 261 | Paclitaxel-induced neurotoxicity | CYP3A4 |
| 2013 | Hertz DL [92] | W, AA | Breast | 209 | Paclitaxel-induced neuropathy | CYP2C8 |
| 2013 | Kumamoto K [93] | A | Colorectal | 63 | Oxaliplatin-induced sensory peripheral neuropathy | GSTP1, GSTM1 |
| 2013 | Leandro-Garcia LJ [94] | W | Ovary, fallopian tube, peritoneum, lung, uterus, breast | 144 | Paclitaxel induced peripheral sensory neuropathy | EPHA4, EPHA6, EPHA5, XKR4, LIMK2 |
| 2013 | Lee KH [95] | A | Colon | 292 | Sensory neuropathy | XRCC1 |
| 2013 | Liu YP [96] | A | Gastric | 126 | Oxaliplatin-induced neurotoxicity | GSTP1 |
| 2013 | McWhinney-Glass S [97] | N/A | Ovarian | 404 | Platinum/taxane-induced neurotoxicity | SOX10, BCL2, OPRM1, TRPV1 |
| 2013 | Oguri T [98] | A | Colorectal | 70 | Oxaliplatin-induced chronic peripheral neurotoxicity | ACYP2, FARS2, ERCC1, TAC1 |
| 2014 | Abraham JE [99] | W | Breast | 1303 | Taxane-related sensory neuropathy | ABCB1, TUBB2A, CYP2C8, ABCC2, CYP1B1, KIAA0146-PRKD, SLCO1B1, EPHA6 |
| 2014 | Bhojwani D [100] | N/A | Acute lymphoblastic leukemia | 369 | Methotrexate-induced neurotoxicity | ASTN2, PXDC1, IYD |
| 2014 | Custodio A [101] | W | Colon | 206 | Oxaliplatin-induced peripheral neuropathy | CCNH, ABCG2 |
| 2014 | Hertz DL [102] | W, AA, A | Breast | 412 | Paclitaxel-induced peripheral neuropathy | CYP2C8, ABCG1 |
| 2014 | Khrunin AV [103] | W | Ovarian | 104 | Cisplatin-based neurotoxicity | None |
| 2014 | Lee SY [104] | A | Breast | 85 | Paclitaxel and gemcitabine combination chemotherapy neurotoxicity | RRM1 |
W: White; A: Asian; AA: African American; H: Hispanic
In Table 3, we summarize the focus genes from the literature review with respect to neuropathy induced by different chemotherapy agents, including platinum, taxane, platinum/taxane, Bortezomib, bortezomib/vincristine, thalidomide, methotrexate, cytarabine, platinum/fluorouracil, platinum/S-1 (i.e., oral fluoropyrimidine consists of tegafur, 5-chloro-2,4 dihydroxypyrimidine, and potassium oxonate), taxane/gemcitabine, platinum/fluorouracil/leucovorin, platinum/fluorouracil/irinotecan, prednisone/vincristine/methotrexate, platinum/capecitabine, platinum/fluorouracil/irinotecan/leucovorin and rituximab/cyclophosphamide/doxorubicin/vincristine/prednisone. Among the different (or combined) chemotherapy agents, those studied most frequently in relation to drug-induced neuropathy were platinum, taxane and the combination of platinum/taxane, for which our literature search respectively produced 21, 19 and 5 related papers.
Table 3.
Summary of genes associated with chemotherapy agent-specified neuropathy from the literature review. Number of papers for each agent-specified neuropathy, number of genes associated with each agent-specified neuropathy and number of agent-specified neuropathies associated with each gene are shown. For the association between a gene and an agent-specified neuropathy, the number of relating papers is listed
| Agent | P | T | P/T | B | B/V | Th | M | Cyt | P/F | P/S | T/G | P/F/L | P/F/I | Pr/V/M | P/C | P/F/I/L | R/Cyc/D/V/Pr | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genes | # of papers | 21 | 19 | 5 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 |
| (IPA symbols) | # of genes | 26 | 19 | 7 | 7 | 17 | 6 | 3 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 0 | 0 | 0 |
| # of agents | ||||||||||||||||||
| GSTP1 | 6 | 7 | 1 | 1 | 1 | 1 | 1 | |||||||||||
| ERCC1 | 2 | 2 | 1 | |||||||||||||||
| ACYP2 | 1 | 2 | ||||||||||||||||
| FARS2 | 1 | 2 | ||||||||||||||||
| GSTM1 | 1 | 2 | ||||||||||||||||
| TAC1 | 1 | 2 | ||||||||||||||||
| ABCC2 | 2 | 1 | 1 | |||||||||||||||
| ABCC1 | 2 | 1 | ||||||||||||||||
| ABCG2 | 1 | 1 | ||||||||||||||||
| AGXT | 1 | 1 | ||||||||||||||||
| BTG4 | 1 | 1 | ||||||||||||||||
| CAMK2N1 | 1 | 1 | ||||||||||||||||
| CCNH | 1 | 1 | ||||||||||||||||
| DLEU7 | 1 | 1 | ||||||||||||||||
| FOXC1 | 1 | 1 | ||||||||||||||||
| GMDS | 1 | 1 | ||||||||||||||||
| GSTM3 | 1 | 1 | ||||||||||||||||
| ITGA1 | 1 | 1 | ||||||||||||||||
| ITGB3 | 1 | 1 | ||||||||||||||||
| KCNN3 | 1 | 1 | ||||||||||||||||
| LYRM4 | 1 | 1 | ||||||||||||||||
| PELO | 1 | 1 | ||||||||||||||||
| POU2AF1 | 1 | 1 | ||||||||||||||||
| SCN10A | 1 | 1 | ||||||||||||||||
| SCN4A | 1 | 1 | ||||||||||||||||
| TSPYL6 | 1 | 1 | ||||||||||||||||
| CYP2C8 | 2 | 5 | 1 | |||||||||||||||
| ABCB1 | 1 | 3 | ||||||||||||||||
| EPHA5 | 1 | 2 | ||||||||||||||||
| EPHA6 | 1 | 2 | ||||||||||||||||
| TUBB2A | 1 | 2 | ||||||||||||||||
| CYP3A4 | 2 | 1 | 1 | |||||||||||||||
| CYP3A5 | 2 | 1 | 1 | |||||||||||||||
| ABCG1 | 1 | 1 | ||||||||||||||||
| CYP1B1 | 1 | 1 | ||||||||||||||||
| EPHA4 | 1 | 1 | ||||||||||||||||
| FANCD2 | 1 | 1 | ||||||||||||||||
| FGD4 | 1 | 1 | ||||||||||||||||
| FZD3 | 1 | 1 | ||||||||||||||||
| LIMK2 | 1 | 1 | ||||||||||||||||
| SLCO1B1 | 1 | 1 | ||||||||||||||||
| SPIDR | 1 | 1 | ||||||||||||||||
| XKR4 | 1 | 1 | ||||||||||||||||
| ABCA1 | 2 | 1 | 1 | |||||||||||||||
| BCL2 | 1 | 1 | ||||||||||||||||
| OPRM1 | 1 | 1 | ||||||||||||||||
| SOX10 | 1 | 1 | ||||||||||||||||
| TRPV1 | 1 | 1 | ||||||||||||||||
| CTLA4 | 1 | |||||||||||||||||
| CTSS | 1 | |||||||||||||||||
| CYP17A1 | 1 | |||||||||||||||||
| DYNC1I1 | 1 | |||||||||||||||||
| GJC3 | 1 | |||||||||||||||||
| PSMB1 | 1 | |||||||||||||||||
| TCF4 | 1 | |||||||||||||||||
| PPARD | 2 | 1 | ||||||||||||||||
| ALOX12 | 1 | 1 | ||||||||||||||||
| AURKA | 1 | 1 | ||||||||||||||||
| CASP9 | 1 | 1 | ||||||||||||||||
| CPT1C | 1 | 1 | ||||||||||||||||
| DPYD | 1 | 1 | ||||||||||||||||
| ERCC3 | 1 | 1 | ||||||||||||||||
| ERCC4 | 1 | 1 | ||||||||||||||||
| GLI1 | 1 | 1 | ||||||||||||||||
| IGF1R | 1 | 1 | ||||||||||||||||
| MBL2 | 1 | 1 | ||||||||||||||||
| MKI67 | 1 | 1 | ||||||||||||||||
| MYO5A | 1 | 1 | ||||||||||||||||
| RHOBTB2 | 1 | 1 | ||||||||||||||||
| SOD2 | 1 | 1 | ||||||||||||||||
| SOX8 | 1 | 1 | ||||||||||||||||
| GSTT1 | 1 | 1 | ||||||||||||||||
| ICAM1 | 1 | 1 | ||||||||||||||||
| SERPINB2 | 1 | 1 | ||||||||||||||||
| SLC12A6 | 1 | 1 | ||||||||||||||||
| ASTN2 | 1 | 1 | ||||||||||||||||
| IYD | 1 | 1 | ||||||||||||||||
| PXDC1 | 1 | 1 | ||||||||||||||||
| NME1 | 1 | 1 | ||||||||||||||||
| RRM1 | 1 | 1 | ||||||||||||||||
| XRCC1 | 1 | 1 |
P: Platinum; T: Taxane; P/T: Platinum/Taxane; B: Bortezomib; B/V: Bortezomib/Vincristine; Th: Thalidomide; M: Methotrexate; Cyt: Cytarabine; P/F: Platinum/Fluorouracil; P/S: Platinum/S-1; T/G: Taxane/Gemcitabine; P/F/L: Platinum/Fluorouracil/Leucovorin; P/F/I: Platinum/Fluorouracil/Irinotecan; Pr/V/M: Prednisone/Vincristine/Methotrexate; P/C: Platinum/Capecitabine; P/F/I/L: Platinum/Fluorouracil/Irinotecan/Leucovorin; R/Cyc/D/V/Pr: Rituximab/Cyclophosphamide/Doxorubicin/Vincristine/Prednisone
Among the focus genes reported in the articles, GSTP1, CYP2C8 and ABCB1 were studied the most frequently (Table 4). ABCC2 and GSTP1 were associated with both platinum- and taxane-induced neuropathy; CYP2C8 was associated with both taxane- and platinum/taxane-induced neuropathy; and ERCC1 was associated with platinum- and platinum/taxane-induced neuropathy. Besides platinum-, taxane- and platinum/taxane- induced neuropathy, neuropathy induced by other chemotherapy agents were not frequently studied. Therefore, we focused on the genes associated with platinum-, taxane- and platinum/taxane-induced neuropathy in our analyses.
Table 4.
Focus genes* associated with platinum-, taxane-, and platinum/taxane- induced neuropathy, as identified through the literature review
| Platinum-induced neuropathy | Taxane-induced neuropathy | Platinum/Taxane-induced neuropathy |
|---|---|---|
| ABCC1 | ABCB1 | ABCA1 |
| ABCC2 | ABCC2 | BCL2 |
| ABCG2 | ABCG1 | CYP2C8 |
| ACYP2 | CYP1B1 | ERCC1 |
| AGXT | CYP2C8 | OPRM1 |
| BTG4 | CYP3A4 | SOX10 |
| CAMK2N1 | CYP3A5 | TRPV1 |
| CCNH | EPHA4 | |
| DLEU7 | EPHA5 | |
| ERCC1 | EPHA6 | |
| FARS2 | FANCD2 | |
| FOXC1 | FGD4 | |
| GMDS | FZD3 | |
| GSTM1 | GSTP1 | |
| GSTM3 | LIMK2 | |
| GSTP1 | SLCO1B1 | |
| ITGA1 | SPIDR | |
| ITGB3 | TUBB2A | |
| KCNN3 | XKR4 | |
| LYRM4 | ||
| PELO | ||
| POU2AF1 | ||
| SCN10A | ||
| SCN4A | ||
| TAC1 | ||
| TSPYL6 |
*Genes shown to be significant based on the literature
IPA core analysis
We performed the IPA core analysis for the focus genes reported to be associated with platinum-, taxane- and platinum/taxane- induced neuropathy. The significant networks revealed from the IPA core analyses are shown in Figs. 1, 2 and 3 for the focus genes reported to be associated with platinum-, taxane- and platinum/taxane- induced neuropathy, respectively. In the networks, the solid and dashed edges or arrows indicate direct and indirect interactions, respectively. In Table 5, we report the genes that had at least 15 connections (i.e., hubs, suggesting biological importance) in the networks, ranked by the number of connections for each gene.
Fig. 1.
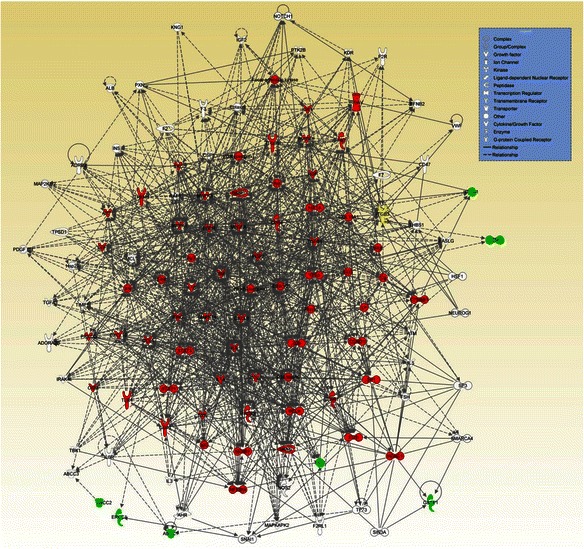
The most significant network (p value = 10−12) generated by IPA core analysis for 26 focus genes associated with platinum-induced neuropathy. Green: focus genes; red: genes with at least 15 connections; yellow: focus genes with at least 15 connections. Dashed and solid lines represent indirect and direct interactions, respectively
Fig. 2.
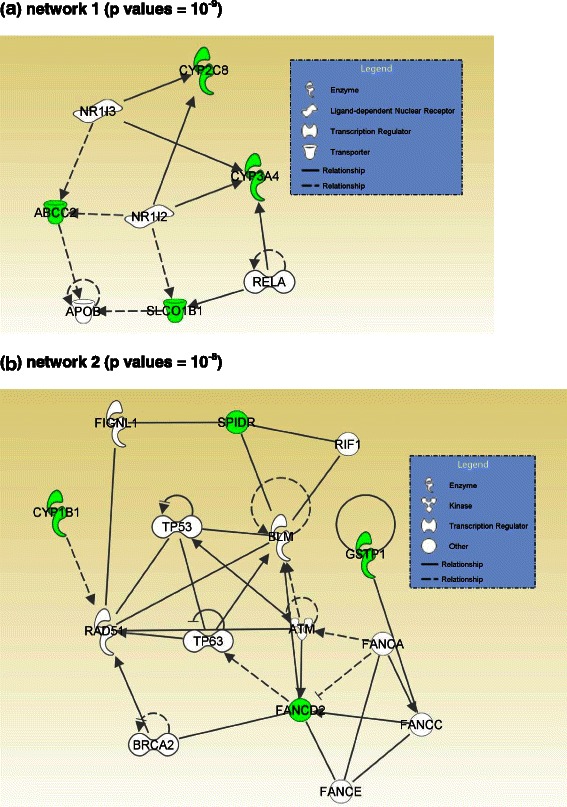
The most significant networks (p values = 10−9 and 10−8) generated by IPA core analysis for 19 focus genes associated with taxane-induced neuropathy. Green: focus genes. Dashed and solid lines represent indirect and direct interactions, respectively. a network 1 (p values = 10−9). b network 2 (p values = 10−8)
Fig. 3.
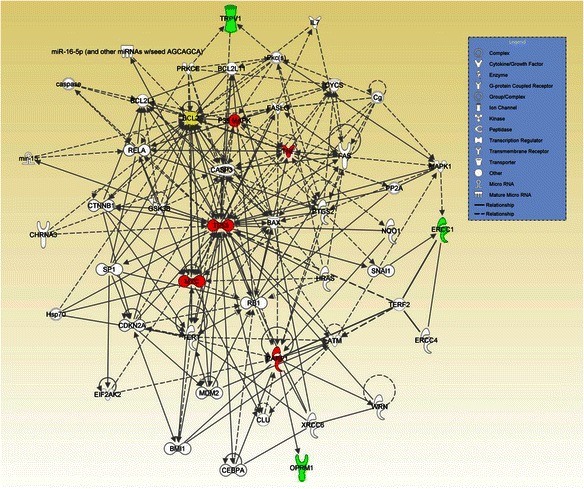
The most significant network (p value = 10−8) generated by IPA core analysis for 7 focus genes associated with platinum/taxane-induced neuropathy. Green: focus genes; red: genes with at least 15 connections; yellow: focus genes with at least 15 connections. Dashed and solid lines represent indirect and direct interactions, respectively
Table 5.
List of genes with at least 15 connections (i.e., hubs*) in the networks, ranked by the number of connections for each gene
| Platinum-induced CIPN | Platinum/taxane-induced CIPN | ||
|---|---|---|---|
| IPA Symbol | # of connections | IPA Symbol | # of connections |
| IL6 | 70 | TP53 | 42 |
| TNF | 69 | BCL2** | 28 |
| CXCL8 | 56 | MYC | 16 |
| IL1B | 55 | PARP1 | 16 |
| ERK1/2 | 54 | P38 MAPK | 15 |
| VEGFA | 52 | TNF | 15 |
| MAPK1 | 51 | ||
| NFkB (complex) | 46 | ||
| P38 MAPK | 45 | ||
| TGFB1 | 43 | ||
| COL18A1 | 42 | ||
| CCL2 | 39 | ||
| IFNG | 38 | ||
| PTGS2 | 37 | ||
| ERK | 34 | ||
| TP53 | 34 | ||
| MAPK3 | 33 | ||
| Akt | 32 | ||
| STAT3 | 30 | ||
| CD3 | 29 | ||
| JUN | 29 | ||
| PI3K (complex) | 29 | ||
| EGFR | 28 | ||
| MMP1 | 28 | ||
| HGF | 27 | ||
| Jnk | 27 | ||
| CCL5 | 26 | ||
| CD40 | 26 | ||
| IL1A | 26 | ||
| ITGB1** | 26 | ||
| MMP2 | 25 | ||
| Cg | 24 | ||
| FN1 | 24 | ||
| RELA | 24 | ||
| TLR4 | 23 | ||
| Vegf | 23 | ||
| CXCL10 | 22 | ||
| EGF | 21 | ||
| ITGB3 | 21 | ||
| MAPK14 | 21 | ||
| NFKBIA | 21 | ||
| SP1 | 21 | ||
| STAT1 | 21 | ||
| AKT1 | 20 | ||
| HIF1A | 20 | ||
| SRC | 20 | ||
| TERT | 20 | ||
| Pkc(s) | 19 | ||
| CTNNB1 | 18 | ||
| Focal adhesion kinase | 18 | ||
| FOS | 18 | ||
| HDAC1 | 18 | ||
| IgG | 18 | ||
| ITGAV | 18 | ||
| NFKB1 | 18 | ||
| CD44 | 17 | ||
| FGF2 | 17 | ||
| Lh | 17 | ||
| MAPK8 | 17 | ||
| SYK | 17 | ||
| Ap1 | 16 | ||
| CCND1 | 16 | ||
| IGF1 | 16 | ||
| PRKCD | 16 | ||
| TREM1 | 16 | ||
| OSM | 15 | ||
*Suggests biological importance
**Focus genes
Platinum-induced neuropathy
The IPA core analysis revealed six networks associated with platinum-induced neuropathy. Using a nominal significance level of 10−5, of the 6 networks, we found only one network to be significant (p value of 10−12; Fig. 1. We note that 66 genes (one focus gene and 65 “novel” genes) out of 121 genes in the network have at least 15 connections (Table 5), suggesting the potential biological importance of these genes in CIPN associated with platinum-based chemotherapy. The gene ITGB3 was the only focus gene in the network, and the top 5 “novel” genes were IL6, TNF, CXCL8, IL1B and ERK1/2.
Taxane-induced neuropathy
The IPA core analysis for taxane-induced neuropathy revealed eight networks, two of which were significant, with p values of 10−9 and 10−8 (Fig. 2). There is no hub in the network generated by the IPA core analysis of the focus genes reported to be associated with taxane-induced neuropathy.
Platinum/taxane-induced neuropathy
The IPA core analysis for platinum/taxane-induced neuropathy identified three networks, one of which was significant, with a p value of 10−8 (Fig. 3). We note that 6 genes (one focus gene and 5 additional “novel” genes) out of 48 genes in the network have at least 15 connections. The gene BCL2 is the only focus gene included in the network that has more than 15 connections. The 5 additional genes that directly or indirectly interact with the corresponding focus genes associated with platinum/taxane-induced neuropathy based on the literature are TP53, MYC, PARP1, P38 MAPK and TNF.
Discussion
In this study, we performed a comprehensive literature review to identify genes implicated in CIPN and then used IPA bioinformatic tools to conduct comprehensive pathway and network analyses of the known genes identified in the literature. Neurotoxicity is common in cancer patients who are treated with platinum compounds and anti-microtubule agents, and the development of CIPN is a potentially debilitating sequela. From the literature review, we found that neuropathy induced by platinum compounds and taxanes (and a combination of these two agents) has been studied most frequently. Neuropathy induced by chemotherapy agents other than platinum, taxane and platinum/taxane combinations has not been adequately studied.
Among the focus genes identified from our literature search, GSTP1, CYP2C8 and ABCB1 were most frequently assessed as candidates for CIPN. From the literature review, we also found that the genomic variations of genes associated with neuropathy induced by platinum versus taxane compounds were different. For example, GSTP1, ERCC1, ACYP2, FARS2, GSTM1 and TAC1 were found to be associated with platinum-induced neuropathy in more than one study but were not associated with taxane-induced neuropathy. On the other hand, CYP2C8, ABCB1, EPHA5, EPHA6 and TUBB2A were found to be associated with taxane-induced neuropathy in more than one study, but not to be associated with platinum-induced neuropathy (Table 3). The overall theme is that these CIPN-associated genes are related to the networks that regulate intracellular drug concentrations (e.g., GSTP1, GSTM1 and ABCB1), response to DNA damage (e.g., ERCC1, FANCD2, BCL2, and SOX10), cellular stress response pathways (e.g., BCL2), inflammation (e.g., ABCC1, ABCC2, ABCG2, ITGA1, ITGB3, TAC1, ABCB1, ABCC2, EPHA4, EPHA6, SLCO1B1, TUBB2A, ABCA1, BCL2, OPRM1 and TRPV1), and neuronal plasticity (e.g., ERCC1 and TAC1).
We performed IPA core analysis for the genes associated with platinum-, taxane- and platinum/taxane-induced neuropathy. We found that IL6, TNF, CXCL8, IL1B and ERK1/2 were the top genes in terms of the number of connections in platinum-induced neuropathy, suggesting either direct or indirect interactions with nervous tissue leading to CIPN after exposure to platinum compounds. It is particularly interesting that studies of pain in cancer patients have shown the importance of cytokine genes [28–37] including IL6, TNF and IL1B polymorphisms. These studies hypothesized that cytokines associated with inflammation or tissue damage modify the activity of nociceptors, which contributes to pain hypersensitivity. Studies also suggest that hyperexcitability in pain transmission neurons may also be caused by proinflammatory cytokines produced by glial cells that respond to inflammation or other cancer-produced cytokines. Substance P and excitatory amino acids released from presynaptic terminals result to an exaggerated pain response [38, 39]. In patients with lung cancer, polymorphisms in TNF and IL6 were significantly associated with pain severity (for TNF, GG = 4.12; GA = 5.38; AA = 5.50; p = 0.04) and with morphine-equivalent daily dose (IL-6, GG = 69.61; GC = 93.6; CC = 181.67; p = 0.004) [36]. An additive effect of mutant alleles in IL1B T-31C (odds ratio = 0.55, 95 % confidence interval = (0.31, 0.97)) was also found to be associated with high intensity of pain, depressed mood and fatigue in lung cancer patients [31].
In addition to the top connections in the networks, the overall biological processes involved in the networks help us to better understand the gene-phenotype association. The IPA core analysis is a process for creating molecule networks on the basis of focus genes, which are genes associated with the phenotypes of interest. Because all the focus and non-focus genes in the network have inter-connected relationships, it provides a list of novel candidate genes associated with the phenotype. The network also provides a clearer picture of the (possibly interacting) genes that might be directly or indirectly associated with chemotherapy-induced peripheral neuropathy. The most significant network generated by IPA core analysis for the focus genes associated with platinum-induced neuropathy (Fig. 1) contains genes for inflammation (multiple interleukins, TNF, IFNG, STAT3, STAT1), DNA damage response (TP53) and cell survival (MAPK, JUN, ERK, NFkB). Network 2, which relates to taxane-induced neuropathy (Fig. 2b), includes many genes that are involved in the DNA damage response. The network related to neuropathy induced by combined platinum and taxane therapy (Fig. 3) resembles Fig. 1 in terms of the cellular functions involved, i.e., inflammation, DNA damage response and cell survival. The major commonality among Figs. 1, 2b and 3 is TP53, which is a central hub in these three networks. Network 1, which relates to taxane-induced neuropathy (Fig. 2a), primarily involves drug metabolizing enzymes and transporter proteins that will affect the intracellular concentration of taxanes. These analyses suggest that genetic variations in the DNA damage response are associated with the risk of developing CIPN, and that taxane-induced neuropathy is also affected by genetic variations that regulate intracellular drug levels while this aspect may not be important for platinum compounds.
This bioinformatic approach to expanding gene networks and identifying connection hubs has limitations. First, many proteins do not interact, while others may connect to major hubs that interact with hundreds of genes and proteins. Therefore, it is believed that the degree of connectivity obeys a power law, which means that the network is scale-free, a desired property. However, we found that the IPA metric/algorithm that generates networks does not guarantee that the resulting networks are scale-free, even though the networks may exhibit certain scale-free behavior in which the major hubs are closely followed by smaller ones that have less connectivity, and the smaller hubs are then followed by other nodes with an even smaller degree of connectivity, and so on (see Figs. 1 and 3). Furthermore, the IPA algorithm that generates networks will not continue if the network reaches the pre-specified maximum network size (i.e., 140 genes), which might rule out many nodes with small degrees of connectivity and impact the scale-free behavior. We employed a widely used log-log plot to investigate whether the networks in Figs. 1 and 3 follow the power law [24, 40]. The log-log plot should appear as a decaying straight line if the network obeys the power law, which was not observed in our plot. Therefore, we cannot conclude that the resulting networks are scale-free.
Further limitations could be that the connections may be specific to certain tissues or physiological contexts that are not applicable to CIPN. Many of the connections have not been demonstrated in neural tissue. Nevertheless, this network analysis identified biological processes that are relevant to the mechanism of neuropathy induced by platinum compounds and taxanes, thus providing the basis for future studies of the genes involved in these biological processes. Our study has not discovered any pathways involved in pain perception. Perhaps, due to the fact that many studies in the literature were done as focused search for SNP associations in a relatively small set of genes in pre-selected pathways, such as glutathione, DNA repair, cell cycle, apoptosis, cell signaling, and metabolism. Whether new gene sequencing technology can discover genetic markers associated with differences in neuropathic pain perception remains to be seen. In conclusion, our study has shown putative genes associated with CIPN. Future studies will include the selection of pharmacogenomic panel tests that will help identify patients at risk for CIPN and the routine incorporation of such panels into clinical practice.
Acknowledgements
This work was supported by National Institutes of Health grant R01DE022891 (CCR and SS), 1R01CA131324 (SS), R25DA026120 (SS), R03CA192197 (JW) and the Cancer Prevention Research Institute of Texas grant RP130123 (SS). This research was also supported in part by the Barnhart Family Distinguished Professorship in Targeted Therapy (SS) and the National Cancer Institute grant CA016672.
Footnotes
Cielito C. Reyes-Gibby and Jian Wang contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All co-authors are justifiably credited with authorship, according to the authorship criteria. Final approval is given by each co-author. In details: CCR and SS conceptualized the study. JW performed the literature review and pathway analysis. JW and CCR drafted the manuscript. SS and SJY provided advice and revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Cielito C. Reyes-Gibby, Email: creyes@mdanderson.org
Jian Wang, Email: jianwang@mdanderson.org.
Sai-Ching J. Yeung, Email: syeung@mdanderson.org
Sanjay Shete, Email: sshete@mdanderson.org.
References
- 1.Kannarkat G, Lasher EE, Schiff D. Neurologic complications of chemotherapy agents. Curr Opin Neurol. 2007;20(6):719–725. doi: 10.1097/WCO.0b013e3282f1a06e. [DOI] [PubMed] [Google Scholar]
- 2.Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15(29):3081–3094. doi: 10.2174/092986708786848569. [DOI] [PubMed] [Google Scholar]
- 3.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249(1):9–17. doi: 10.1007/PL00007853. [DOI] [PubMed] [Google Scholar]
- 4.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44(11):1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Reyes-Gibby CC, Morrow PK, Buzdar A, Shete S. Chemotherapy-induced peripheral neuropathy as a predictor of neuropathic pain in breast cancer patients previously treated with paclitaxel. J Pain : official journal of the American Pain Society. 2009;10(11):1146–1150. doi: 10.1016/j.jpain.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caraceni A, Portenoy RK. An international survey of cancer pain characteristics and syndromes. IASP Task Force on Cancer Pain. International Association for the Study of Pain. Pain. 1999;82(3):263–274. doi: 10.1016/S0304-3959(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 7.Grond S, Radbruch L, Meuser T, Sabatowski R, Loick G, Lehmann KA. Assessment and treatment of neuropathic cancer pain following WHO guidelines. Pain. 1999;79(1):15–20. doi: 10.1016/S0304-3959(98)00138-9. [DOI] [PubMed] [Google Scholar]
- 8.Cherny NI. Opioid analgesics: comparative features and prescribing guidelines. Drugs. 1996;51(5):713–737. doi: 10.2165/00003495-199651050-00002. [DOI] [PubMed] [Google Scholar]
- 9.Cherny NI, Thaler HT, Friedlander-Klar H, Lapin J, Foley KM, Houde R, et al. Opioid responsiveness of cancer pain syndromes caused by neuropathic or nociceptive mechanisms: a combined analysis of controlled, single-dose studies. Neurology. 1994;44(5):857–861. doi: 10.1212/WNL.44.5.857. [DOI] [PubMed] [Google Scholar]
- 10.Attal N, Cruccu G, Haanpaa M, Hansson P, Jensen TS, Nurmikko T, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur jneurol : the official journal of the European Federation of Neurological Societies. 2006;13(11):1153–1169. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 11.Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain research & management : the journal of the Canadian Pain Society = journal de la societe canadienne pour le traitement de la douleur. 2007;12(1):13–21. doi: 10.1155/2007/730785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA, et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3) Cancer. 2007;110(9):2110–2118. doi: 10.1002/cncr.23008. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson SD, Loprinzi CL, Sloan JA, Wilke JL, Novotny PJ, Okuno SH, et al. Glutamine does not prevent paclitaxel-associated myalgias and arthralgias. J Support Oncol. 2003;1(4):274–278. [PubMed] [Google Scholar]
- 14.Xiao W, Naso L, Bennett GJ. Experimental studies of potential analgesics for the treatment of chemotherapy-evoked painful peripheral neuropathies. Pain Med. 2008;9(5):505–517. doi: 10.1111/j.1526-4637.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- 15.Tsavaris N, Kopterides P, Kosmas C, Efthymiou A, Skopelitis H, Dimitrakopoulos A, et al. Gabapentin monotherapy for the treatment of chemotherapy-induced neuropathic pain: a pilot study. Pain Med. 2008;9(8):1209–1216. doi: 10.1111/j.1526-4637.2007.00325.x. [DOI] [PubMed] [Google Scholar]
- 16.Ong EC. Controlled-release oxycodone in the treatment of neuropathic pain of nonmalignant and malignant causes. Oncology. 2008;74(Suppl 1):72–75. doi: 10.1159/000143223. [DOI] [PubMed] [Google Scholar]
- 17.Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109(1–2):150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 18.Flatters SJ, Fox AJ, Dickenson AH. Spinal interleukin-6 (IL-6) inhibits nociceptive transmission following neuropathy. Brain Res. 2003;984(1–2):54–62. doi: 10.1016/S0006-8993(03)03092-0. [DOI] [PubMed] [Google Scholar]
- 19.Jin HW, Flatters SJ, Xiao WH, Mulhern HL, Bennett GJ. Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Exp Neurol. 2008;210(1):229–237. doi: 10.1016/j.expneurol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naguib M, Diaz P, Xu JJ, Astruc-Diaz F, Craig S, Vivas-Mejia P, et al. MDA7: a novel selective agonist for CB2 receptors that prevents allodynia in rat neuropathic pain models. Br J Pharmacol. 2008;155(7):1104–1116. doi: 10.1038/bjp.2008.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muurling T, Stankovic KM. Metabolomic and network analysis of pharmacotherapies for sensorineural hearing loss. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2014;35(1):1–6. doi: 10.1097/MAO.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 22.Barabasi AL, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet. 2011;12(1):56–68. doi: 10.1038/nrg2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingenuity Pathway Analysis software. http://www.ingenuity.com/. Accessed May 2014.
- 24.Jeong H, Tombor B, Albert R, Oltvai ZN, Barabasi AL. The large-scale organization of metabolic networks. Nature. 2000;407(6804):651–654. doi: 10.1038/35036627. [DOI] [PubMed] [Google Scholar]
- 25.Ingenuity Pathways Analysis (IPA) of Large Datasets. http://www.usc.edu/hsc/nml/assets/bioinfo/IPA/Data%20Analysis%20training%20Handouts.pdf. Accessed May 2014.
- 26.IPA Network Generation Algorithm. http://www.ingenuity.com/wp-content/themes/ingenuitytheme/pdf/ipa/IPA-netgen-algorithm-whitepaper.pdf. Accessed May 2014.
- 27.Stamatiou GA, Stankovic KM. A comprehensive network and pathway analysis of human deafness genes. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2013;34(5):961–970. doi: 10.1097/MAO.0b013e3182898272. [DOI] [PubMed] [Google Scholar]
- 28.Doong SH, Dhruva A, Dunn LB, West C, Paul SM, Cooper BA, et al. Associations Between Cytokine Genes and a Symptom Cluster of Pain, Fatigue, Sleep Disturbance, and Depression in Patients Prior to Breast Cancer Surgery. Biol Res Nurs. 2014 doi: 10.1177/1099800414550394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveira A, Dinis-Oliveira RJ, Nogueira A, Goncalves F, Silva P, Vieira C, et al. Interleukin-1beta genotype and circulating levels in cancer patients: metastatic status and pain perception. Clin Biochem. 2014;47(13–14):1209–1213. doi: 10.1016/j.clinbiochem.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Stephens K, Cooper BA, West C, Paul SM, Baggott CR, Merriman JD, et al. Associations between cytokine gene variations and severe persistent breast pain in women following breast cancer surgery. J Pain : official journal of the American Pain Society. 2014;15(2):169–180. doi: 10.1016/j.jpain.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reyes-Gibby CC, Swartz MD, Yu X, Wu X, Yennurajalingam S, Anderson KO, et al. Symptom clusters of pain, depressed mood, and fatigue in lung cancer: assessing the role of cytokine genes. Support care cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2013;21(11):3117–3125. doi: 10.1007/s00520-013-1885-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reyes-Gibby CC, Wang J, Spitz M, Wu X, Yennurajalingam S, Shete S. Genetic variations in interleukin-8 and interleukin-10 are associated with pain, depressed mood, and fatigue in lung cancer patients. J Pain Symptom Manage. 2013;46(2):161–172. doi: 10.1016/j.jpainsymman.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCann B, Miaskowski C, Koetters T, Baggott C, West C, Levine JD, et al. Associations between pro- and anti-inflammatory cytokine genes and breast pain in women prior to breast cancer surgery. J Pain : official journal of the American Pain Society. 2012;13(5):425–437. doi: 10.1016/j.jpain.2011.02.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rausch SM, Gonzalez BD, Clark MM, Patten C, Felten S, Liu H, et al. SNPs in PTGS2 and LTA predict pain and quality of life in long term lung cancer survivors. Lung Cancer. 2012;77(1):217–223. doi: 10.1016/j.lungcan.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyes-Gibby CC, Shete S, Yennurajalingam S, Frazier M, Bruera E, Kurzrock R, et al. Genetic and nongenetic covariates of pain severity in patients with adenocarcinoma of the pancreas: assessing the influence of cytokine genes. J Pain Symptom Manage. 2009;38(6):894–902. doi: 10.1016/j.jpainsymman.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reyes-Gibby CC, El Osta B, Spitz MR, Parsons H, Kurzrock R, Wu X, et al. The influence of tumor necrosis factor-alpha −308 G/A and IL-6 -174 G/C on pain and analgesia response in lung cancer patients receiving supportive care. Cancer epidemiol, biomarkers preve : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(11):3262–3267. doi: 10.1158/1055-9965.EPI-08-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reyes-Gibby CC, Wu X, Spitz M, Kurzrock R, Fisch M, Bruera E, et al. Molecular epidemiology, cancer-related symptoms, and cytokines pathway. Lancet Oncol. 2008;9(8):777–785. doi: 10.1016/S1470-2045(08)70197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257(2):139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 39.Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105(1):83–107. doi: 10.1037/0033-295X.105.1.83. [DOI] [PubMed] [Google Scholar]
- 40.Khanin R, Wit E. How scale-free are biological networks. J comput biol : a journal of computational molecular cell biology. 2006;13(3):810–818. doi: 10.1089/cmb.2006.13.810. [DOI] [PubMed] [Google Scholar]
- 41.Aplenc R, Glatfelter W, Han P, Rappaport E, La M, Cnaan A, et al. CYP3A genotypes and treatment response in paediatric acute lymphoblastic leukaemia. Br J Haematol. 2003;122(2):240–244. doi: 10.1046/j.1365-2141.2003.04430.x. [DOI] [PubMed] [Google Scholar]
- 42.Isla D, Sarries C, Rosell R, Alonso G, Domine M, Taron M, et al. Single nucleotide polymorphisms and outcome in docetaxel-cisplatin-treated advanced non-small-cell lung cancer. Ann oncol: official journal of the European Society for Medical Oncology / ESMO. 2004;15(8):1194–1203. doi: 10.1093/annonc/mdh319. [DOI] [PubMed] [Google Scholar]
- 43.Lecomte T, Landi B, Beaune P, Laurent-Puig P, Loriot MA. Glutathione S-transferase P1 polymorphism (Ile105Val) predicts cumulative neuropathy in patients receiving oxaliplatin-based chemotherapy. Clin cancer res : an official journal of the American Association for Cancer Research. 2006;12(10):3050–3056. doi: 10.1158/1078-0432.CCR-05-2076. [DOI] [PubMed] [Google Scholar]
- 44.Sissung TM, Mross K, Steinberg SM, Behringer D, Figg WD, Sparreboom A, et al. Association of ABCB1 genotypes with paclitaxel-mediated peripheral neuropathy and neutropenia. Eur J Cancer. 2006;42(17):2893–2896. doi: 10.1016/j.ejca.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gamelin L, Capitain O, Morel A, Dumont A, Traore S, le Anne B, et al. Predictive factors of oxaliplatin neurotoxicity: the involvement of the oxalate outcome pathway. Clin cancer res : an official journal of the American Association for Cancer Research. 2007;13(21):6359–6368. doi: 10.1158/1078-0432.CCR-07-0660. [DOI] [PubMed] [Google Scholar]
- 46.Marsh S, Paul J, King CR, Gifford G, McLeod HL, Brown R. Pharmacogenetic assessment of toxicity and outcome after platinum plus taxane chemotherapy in ovarian cancer: the Scottish Randomised Trial in Ovarian Cancer. J clin oncol : official journal of the American Society of Clinical Oncology. 2007;25(29):4528–4535. doi: 10.1200/JCO.2006.10.4752. [DOI] [PubMed] [Google Scholar]
- 47.Oldenburg J, Kraggerud SM, Brydoy M, Cvancarova M, Lothe RA, Fossa SD. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and -M1, a retrospective cross sectional study. J Transl Med. 2007;5:70. doi: 10.1186/1479-5876-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruzzo A, Graziano F, Loupakis F, Rulli E, Canestrari E, Santini D, et al. Pharmacogenetic profiling in patients with advanced colorectal cancer treated with first-line FOLFOX-4 chemotherapy. clin oncol: official journal of the American Society of Clinical Oncology. 2007;25(10):1247–1254. doi: 10.1200/JCO.2006.08.1844. [DOI] [PubMed] [Google Scholar]
- 49.Keam B, Im SA, Han SW, Ham HS, Kim MA, Oh DY, et al. Modified FOLFOX-6 chemotherapy in advanced gastric cancer: Results of phase II study and comprehensive analysis of polymorphisms as a predictive and prognostic marker. BMC Cancer. 2008;8:148. doi: 10.1186/1471-2407-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pare L, Marcuello E, Altes A, del Rio E, Sedano L, Salazar J, et al. Pharmacogenetic prediction of clinical outcome in advanced colorectal cancer patients receiving oxaliplatin/5-fluorouracil as first-line chemotherapy. Br J Cancer. 2008;99(7):1050–1055. doi: 10.1038/sj.bjc.6604671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sissung TM, Baum CE, Deeken J, Price DK, Aragon-Ching J, Steinberg SM, et al. ABCB1 genetic variation influences the toxicity and clinical outcome of patients with androgen-independent prostate cancer treated with docetaxel. Clin cancer res : an official journal of the American Association for Cancer Research. 2008;14(14):4543–4549. doi: 10.1158/1078-0432.CCR-07-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Argyriou AA, Antonacopoulou AG, Scopa CD, Kottorou A, Kominea A, Peroukides S, et al. Liability of the voltage-gated sodium channel gene SCN2A R19K polymorphism to oxaliplatin-induced peripheral neuropathy. Oncology. 2009;77(3–4):254–256. doi: 10.1159/000236049. [DOI] [PubMed] [Google Scholar]
- 53.Goekkurt E, Al-Batran SE, Hartmann JT, Mogck U, Schuch G, Kramer M, et al. Pharmacogenetic analyses of a phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil and leucovorin plus either oxaliplatin or cisplatin: a study of the arbeitsgemeinschaft internistische onkologie. J clin oncol : official journal of the American Society of Clinical Oncology. 2009;27(17):2863–2873. doi: 10.1200/JCO.2008.19.1718. [DOI] [PubMed] [Google Scholar]
- 54.Green H, Soderkvist P, Rosenberg P, Mirghani RA, Rymark P, Lundqvist EA, et al. Pharmacogenetic studies of Paclitaxel in the treatment of ovarian cancer. Basic Clin Pharmacol Toxicol. 2009;104(2):130–137. doi: 10.1111/j.1742-7843.2008.00351.x. [DOI] [PubMed] [Google Scholar]
- 55.Kim HS, Kim MK, Chung HH, Kim JW, Park NH, Song YS, et al. Genetic polymorphisms affecting clinical outcomes in epithelial ovarian cancer patients treated with taxanes and platinum compounds: a Korean population-based study. Gynecol Oncol. 2009;113(2):264–269. doi: 10.1016/j.ygyno.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Kweekel DM, Gelderblom H, Antonini NF, Van der Straaten T, Nortier JW, Punt CJ, et al. Glutathione-S-transferase pi (GSTP1) codon 105 polymorphism is not associated with oxaliplatin efficacy or toxicity in advanced colorectal cancer patients. Eur J Cancer. 2009;45(4):572–578. doi: 10.1016/j.ejca.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 57.Mir O, Alexandre J, Tran A, Durand JP, Pons G, Treluyer JM, et al. Relationship between GSTP1 Ile(105)Val polymorphism and docetaxel-induced peripheral neuropathy: clinical evidence of a role of oxidative stress in taxane toxicity. Anna oncol : official journal of the European Society for Medical Oncology/ESMO. 2009;20(4):736–40. doi:10.1093/annonc/mdn698. [DOI] [PubMed]
- 58.Seo BG, Kwon HC, Oh SY, Lee S, Kim SG, Kim SH, et al. Comprehensive analysis of excision repair complementation group 1, glutathione S-transferase, thymidylate synthase and uridine diphosphate glucuronosyl transferase 1A1 polymorphisms predictive for treatment outcome in patients with advanced gastric cancer treated with FOLFOX or FOLFIRI. Oncol Rep. 2009;22(1):127–136. [PubMed] [Google Scholar]
- 59.Antonacopoulou AG, Argyriou AA, Scopa CD, Kottorou A, Kominea A, Peroukides S, et al. Integrin beta-3 L33P: a new insight into the pathogenesis of chronic oxaliplatin-induced peripheral neuropathy? Eur j neurol : the official journal of the European Federation of Neurological Societies. 2010;17(7):963–968. doi: 10.1111/j.1468-1331.2010.02966.x. [DOI] [PubMed] [Google Scholar]
- 60.Boige V, Mendiboure J, Pignon JP, Loriot MA, Castaing M, Barrois M, et al. Pharmacogenetic assessment of toxicity and outcome in patients with metastatic colorectal cancer treated with LV5FU2, FOLFOX, and FOLFIRI: FFCD 2000–05. J clin oncol : official journal of the American Society of Clinical Oncology. 2010;28(15):2556–2564. doi: 10.1200/JCO.2009.25.2106. [DOI] [PubMed] [Google Scholar]
- 61.Chen YC, Tzeng CH, Chen PM, Lin JK, Lin TC, Chen WS, et al. Influence of GSTP1 I105V polymorphism on cumulative neuropathy and outcome of FOLFOX-4 treatment in Asian patients with colorectal carcinoma. Cancer Sci. 2010;101(2):530–535. doi: 10.1111/j.1349-7006.2009.01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cho HJ, Eom HS, Kim HJ, Kim IS, Lee GW, Kong SY. Glutathione-S-transferase genotypes influence the risk of chemotherapy-related toxicities and prognosis in Korean patients with diffuse large B-cell lymphoma. Cancer Genet Cytogenet. 2010;198(1):40–46. doi: 10.1016/j.cancergencyto.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Inada M, Sato M, Morita S, Kitagawa K, Kawada K, Mitsuma A, et al. Associations between oxaliplatin-induced peripheral neuropathy and polymorphisms of the ERCC1 and GSTP1 genes. Int J Clin Pharmacol Ther. 2010;48(11):729–734. doi: 10.5414/CPP48729. [DOI] [PubMed] [Google Scholar]
- 64.Kanai M, Yoshioka A, Tanaka S, Nagayama S, Matsumoto S, Nishimura T, et al. Associations between glutathione S-transferase pi Ile105Val and glyoxylate aminotransferase Pro11Leu and Ile340Met polymorphisms and early-onset oxaliplatin-induced neuropathy. Cancer Epidemiol. 2010;34(2):189–193. doi: 10.1016/j.canep.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 65.Khrunin AV, Moisseev A, Gorbunova V, Limborska S. Genetic polymorphisms and the efficacy and toxicity of cisplatin-based chemotherapy in ovarian cancer patients. Pharmacogenomics J. 2010;10(1):54–61. doi: 10.1038/tpj.2009.45. [DOI] [PubMed] [Google Scholar]
- 66.Li QF, Yao RY, Liu KW, Lv HY, Jiang T, Liang J. Genetic polymorphism of GSTP1: prediction of clinical outcome to oxaliplatin/5-FU-based chemotherapy in advanced gastric cancer. J Korean Med Sci. 2010;25(6):846–852. doi: 10.3346/jkms.2010.25.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McLeod HL, Sargent DJ, Marsh S, Green EM, King CR, Fuchs CS, et al. Pharmacogenetic predictors of adverse events and response to chemotherapy in metastatic colorectal cancer: results from North American Gastrointestinal Intergroup Trial N9741. J clin oncol : official journal of the American Society of Clinical Oncology. 2010;28(20):3227–3233. doi: 10.1200/JCO.2009.21.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ofverholm A, Einbeigi Z, Manouchehrpour S, Albertsson P, Skrtic S, Enerback C. The ABCB1 3435 T allele does not increase the risk of paclitaxel-induced neurotoxicity. Oncology letters. 2010;1(1):151–154. doi: 10.3892/ol_00000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rizzo R, Spaggiari F, Indelli M, Lelli G, Baricordi OR, Rimessi P, et al. Association of CYP1B1 with hypersensitivity induced by taxane therapy in breast cancer patients. Breast Cancer Res Treat. 2010;124(2):593–598. doi: 10.1007/s10549-010-1034-5. [DOI] [PubMed] [Google Scholar]
- 70.Basso M, Modoni A, Spada D, Cassano A, Schinzari G, Lo Monaco M, et al. Polymorphism of CAG motif of SK3 gene is associated with acute oxaliplatin neurotoxicity. Cancer Chemother Pharmacol. 2011;67(5):1179–1187. doi: 10.1007/s00280-010-1466-y. [DOI] [PubMed] [Google Scholar]
- 71.Bergmann TK, Green H, Brasch-Andersen C, Mirza MR, Herrstedt J, Holund B, et al. Retrospective study of the impact of pharmacogenetic variants on paclitaxel toxicity and survival in patients with ovarian cancer. Eur J Clin Pharmacol. 2011;67(7):693–700. doi: 10.1007/s00228-011-1007-6. [DOI] [PubMed] [Google Scholar]
- 72.Bergmann TK, Brasch-Andersen C, Green H, Mirza MR, Skougaard K, Wihl J, et al. Impact of ABCB1 Variants on Neutrophil Depression: A Pharmacogenomic Study of Paclitaxel in 92 Women with Ovarian Cancer. Basic Clin Pharmacol Toxicol. 2011 doi: 10.1111/j.1742-7843.2011.00802.x. [DOI] [PubMed] [Google Scholar]
- 73.Broyl A, Corthals SL, Jongen JL, van der Holt B, Kuiper R, de Knegt Y, et al. Mechanisms of peripheral neuropathy associated with bortezomib and vincristine in patients with newly diagnosed multiple myeloma: a prospective analysis of data from the HOVON-65/GMMG-HD4 trial. Lancet Oncol. 2010;11(11):1057–1065. doi: 10.1016/S1470-2045(10)70206-0. [DOI] [PubMed] [Google Scholar]
- 74.Cibeira MT, de Larrea CF, Navarro A, Diaz T, Fuster D, Tovar N, et al. Impact on response and survival of DNA repair single nucleotide polymorphisms in relapsed or refractory multiple myeloma patients treated with thalidomide. Leuk Res. 2011;35(9):1178–1183. doi: 10.1016/j.leukres.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 75.Corthals SL, Kuiper R, Johnson DC, Sonneveld P, Hajek R, van der Holt B, et al. Genetic factors underlying the risk of bortezomib induced peripheral neuropathy in multiple myeloma patients. Haematologica. 2011;96(11):1728–1732. doi: 10.3324/haematol.2011.041434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Favis R, Sun Y, van de Velde H, Broderick E, Levey L, Meyers M, et al. Genetic variation associated with bortezomib-induced peripheral neuropathy. Pharmacogenet Genomics. 2011;21(3):121–129. doi: 10.1097/FPC.0b013e3283436b45. [DOI] [PubMed] [Google Scholar]
- 77.Hong J, Han SW, Ham HS, Kim TY, Choi IS, Kim BS, et al. Phase II study of biweekly S-1 and oxaliplatin combination chemotherapy in metastatic colorectal cancer and pharmacogenetic analysis. Cancer Chemother Pharmacol. 2011;67(6):1323–1331. doi: 10.1007/s00280-010-1425-7. [DOI] [PubMed] [Google Scholar]
- 78.Johnson DC, Corthals SL, Walker BA, Ross FM, Gregory WM, Dickens NJ, et al. Genetic factors underlying the risk of thalidomide-related neuropathy in patients with multiple myeloma. J clin oncol : official journal of the American Society of Clinical Oncology. 2011;29(7):797–804. doi: 10.1200/JCO.2010.28.0792. [DOI] [PubMed] [Google Scholar]
- 79.Leskela S, Jara C, Leandro-Garcia LJ, Martinez A, Garcia-Donas J, Hernando S, et al. Polymorphisms in cytochromes P450 2C8 and 3A5 are associated with paclitaxel neurotoxicity. Pharmacogenomics J. 2011;11(2):121–129. doi: 10.1038/tpj.2010.13. [DOI] [PubMed] [Google Scholar]
- 80.Sucheston LE, Zhao H, Yao S, Zirpoli G, Liu S, Barlow WE, et al. Genetic predictors of taxane-induced neurotoxicity in a SWOG phase III intergroup adjuvant breast cancer treatment trial (S0221) Breast Cancer Res Treat. 2011;130(3):993–1002. doi: 10.1007/s10549-011-1671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baldwin RM, Owzar K, Zembutsu H, Chhibber A, Kubo M, Jiang C, et al. A genome-wide association study identifies novel loci for paclitaxel-induced sensory peripheral neuropathy in CALGB 40101. Clin cancer resh : an official journal of the American Association for Cancer Research. 2012;18(18):5099–5109. doi: 10.1158/1078-0432.CCR-12-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Braunagel D, Schaich M, Kramer M, Dransfeld CL, Ehninger G, Mahlknecht U. The T_T genotype within the NME1 promoter single nucleotide polymorphism −835 C/T is associated with an increased risk of cytarabine induced neurotoxicity in patients with acute myeloid leukemia. Leuk Lymphoma. 2012;53(5):952–957. doi: 10.3109/10428194.2011.635862. [DOI] [PubMed] [Google Scholar]
- 83.Fung C, Vaughn DJ, Mitra N, Ciosek SL, Vardhanabhuti S, Nathanson KL, et al. Chemotherapy refractory testicular germ cell tumor is associated with a variant in Armadillo Repeat gene deleted in Velco-Cardio-Facial syndrome (ARVCF) Front Endocrinol. 2012;3:163. doi: 10.3389/fendo.2012.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hasmats J, Kupershmidt I, Rodriguez-Antona C, Su QJ, Khan MS, Jara C, et al. Identification of candidate SNPs for drug induced toxicity from differentially expressed genes in associated tissues. Gene. 2012;506(1):62–68. doi: 10.1016/j.gene.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 85.Hertz DL, Motsinger-Reif AA, Drobish A, Winham SJ, McLeod HL, Carey LA, et al. CYP2C8*3 predicts benefit/risk profile in breast cancer patients receiving neoadjuvant paclitaxel. Breast Cancer Res Treat. 2012;134(1):401–410. doi: 10.1007/s10549-012-2054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leandro-Garcia LJ, Leskela S, Jara C, Green H, Avall-Lundqvist E, Wheeler HE, et al. Regulatory polymorphisms in beta-tubulin IIa are associated with paclitaxel-induced peripheral neuropathy. Clin cancer res : an official journal of the American Association for Cancer Research. 2012;18(16):4441–4448. doi: 10.1158/1078-0432.CCR-12-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Won HH, Lee J, Park JO, Park YS, Lim HY, Kang WK, et al. Polymorphic markers associated with severe oxaliplatin-induced, chronic peripheral neuropathy in colon cancer patients. Cancer. 2012;118(11):2828–2836. doi: 10.1002/cncr.26614. [DOI] [PubMed] [Google Scholar]
- 88.Argyriou AA, Cavaletti G, Antonacopoulou A, Genazzani AA, Briani C, Bruna J, et al. Voltage-gated sodium channel polymorphisms play a pivotal role in the development of oxaliplatin-induced peripheral neurotoxicity: results from a prospective multicenter study. Cancer. 2013;119(19):3570–3577. doi: 10.1002/cncr.28234. [DOI] [PubMed] [Google Scholar]
- 89.Bergmann TK, Vach W, Feddersen S, Eckhoff L, Green H, Herrstedt J, et al. GWAS-based association between RWDD3 and TECTA variants and paclitaxel induced neuropathy could not be confirmed in Scandinavian ovarian cancer patients. Acta Oncol. 2013;52(4):871–874. doi: 10.3109/0284186X.2012.707787. [DOI] [PubMed] [Google Scholar]
- 90.Cecchin E, D'Andrea M, Lonardi S, Zanusso C, Pella N, Errante D, et al. A prospective validation pharmacogenomic study in the adjuvant setting of colorectal cancer patients treated with the 5-fluorouracil/leucovorin/oxaliplatin (FOLFOX4) regimen. Pharmacogenomics J. 2013;13(5):403–409. doi: 10.1038/tpj.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Graan AJ, Elens L, Sprowl JA, Sparreboom A, Friberg LE, van der Holt B, et al. CYP3A4*22 genotype and systemic exposure affect paclitaxel-induced neurotoxicity. Clin cancer res : an official journal of the American Association for Cancer Research. 2013;19(12):3316–3324. doi: 10.1158/1078-0432.CCR-12-3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hertz DL, Roy S, Motsinger-Reif AA, Drobish A, Clark LS, McLeod HL, et al. CYP2C8*3 increases risk of neuropathy in breast cancer patients treated with paclitaxel. Ann oncol: official journal of the European Society for Medical Oncology / ESMO. 2013;24(6):1472–1478. doi: 10.1093/annonc/mdt018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumamoto K, Ishibashi K, Okada N, Tajima Y, Kuwabara K, Kumagai Y, et al. Polymorphisms of GSTP1, ERCC2 and TS-3'UTR are associated with the clinical outcome of mFOLFOX6 in colorectal cancer patients. Oncology letters. 2013;6(3):648–654. doi: 10.3892/ol.2013.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leandro-Garcia LJ, Inglada-Perez L, Pita G, Hjerpe E, Leskela S, Jara C, et al. Genome-wide association study identifies ephrin type A receptors implicated in paclitaxel induced peripheral sensory neuropathy. J Med Genet. 2013;50(9):599–605. doi: 10.1136/jmedgenet-2012-101466. [DOI] [PubMed] [Google Scholar]
- 95.Lee KH, Chang HJ, Han SW, Oh DY, Im SA, Bang YJ, et al. Pharmacogenetic analysis of adjuvant FOLFOX for Korean patients with colon cancer. Cancer Chemother Pharmacol. 2013;71(4):843–851. doi: 10.1007/s00280-013-2075-3. [DOI] [PubMed] [Google Scholar]
- 96.Liu YP, Ling Y, Qi QF, Zhang YP, Zhang CS, Zhu CT, et al. Genetic polymorphisms of ERCC1118, XRCC1399 and GSTP1105 are associated with the clinical outcome of gastric cancer patients receiving oxaliplatinbased adjuvant chemotherapy. Mol med rep. 2013;7(6):1904–1911. doi: 10.3892/mmr.2013.1435. [DOI] [PubMed] [Google Scholar]
- 97.McWhinney-Glass S, Winham SJ, Hertz DL, Yen Revollo J, Paul J, He Y, et al. Cumulative genetic risk predicts platinum/taxane-induced neurotoxicity. Clin cancer res : an official journal of the American Association for Cancer Research. 2013;19(20):5769–5776. doi: 10.1158/1078-0432.CCR-13-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oguri T, Mitsuma A, Inada-Inoue M, Morita S, Shibata T, Shimokata T, et al. Genetic polymorphisms associated with oxaliplatin-induced peripheral neurotoxicity in Japanese patients with colorectal cancer. Int J Clin Pharmacol Ther. 2013;51(6):475–481. doi: 10.5414/CP201851. [DOI] [PubMed] [Google Scholar]
- 99.Abraham JE, Guo Q, Dorling L, Tyrer J, Ingle S, Hardy R, et al. Replication of genetic polymorphisms reported to be associated with taxane-related sensory neuropathy in patients with early breast cancer treated with Paclitaxel. Clin cancer res : an official journal of the American Association for Cancer Research. 2014;20(9):2466–2475. doi: 10.1158/1078-0432.CCR-13-3232. [DOI] [PubMed] [Google Scholar]
- 100.Bhojwani D, Sabin ND, Pei D, Yang JJ, Khan RB, Panetta JC, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J clin oncol : official journal of the American Society of Clinical Oncology. 2014;32(9):949–959. doi: 10.1200/JCO.2013.53.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Custodio A, Moreno-Rubio J, Aparicio J, Gallego-Plazas J, Yaya R, Maurel J, et al. Pharmacogenetic predictors of severe peripheral neuropathy in colon cancer patients treated with oxaliplatin-based adjuvant chemotherapy: a GEMCAD group study. Ann oncol : official journal of the European Society for Medical Oncology/ESMO. 2014;25(2):398–403. doi: 10.1093/annonc/mdt546. [DOI] [PubMed] [Google Scholar]
- 102.Hertz DL, Roy S, Jack J, Motsinger-Reif AA, Drobish A, Clark LS, et al. Genetic heterogeneity beyond CYP2C8*3 does not explain differential sensitivity to paclitaxel-induced neuropathy. Breast Cancer Res Treat. 2014;145(1):245–254. doi: 10.1007/s10549-014-2910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khrunin AV, Khokhrin DV, Moisseev AA, Gorbunova VA, Limborska SA. Pharmacogenomic assessment of cisplatin-based chemotherapy outcomes in ovarian cancer. Pharmacogenomics. 2014;15(3):329–337. doi: 10.2217/pgs.13.237. [DOI] [PubMed] [Google Scholar]
- 104.Lee SY, Im SA, Park YH, Woo SY, Kim S, Choi MK, et al. Genetic polymorphisms of SLC28A3, SLC29A1 and RRM1 predict clinical outcome in patients with metastatic breast cancer receiving gemcitabine plus paclitaxel chemotherapy. Eur J Cancer. 2014;50(4):698–705. doi: 10.1016/j.ejca.2013.11.028. [DOI] [PubMed] [Google Scholar]


