Abstract
Objective:
We hypothesized that subthalamic nucleus (STN) deep brain stimulation (DBS) will improve long-term potentiation (LTP)-like plasticity in motor cortex in Parkinson disease (PD).
Methods:
We studied 8 patients with PD treated with STN-DBS and 9 age-matched healthy controls. Patients with PD were studied in 4 sessions in medication (Med) OFF/stimulator (Stim) OFF, Med-OFF/Stim-ON, Med-ON/Stim-OFF, and Med-ON/Stim-ON states in random order. Motor evoked potential amplitude and cortical silent period duration were measured at baseline before paired associated stimulation (PAS) and at 3 different time intervals (T0, T30, T60) up to 60 minutes after PAS in the abductor pollicis brevis and abductor digiti minimi muscles.
Results:
Motor evoked potential size significantly increased after PAS in controls (+67.7% of baseline at T30) and in patients in the Med-ON/Stim-ON condition (+55.8% of baseline at T30), but not in patients in the Med-OFF/Stim-OFF (−0.4% of baseline at T30), Med-OFF/Stim-ON (+10.3% of baseline at T30), and Med-ON/Stim-OFF conditions (+17.3% of baseline at T30). Cortical silent period duration increased after PAS in controls but not in patients in all test conditions.
Conclusions:
Our findings suggest that STN-DBS together with dopaminergic medications restore LTP-like plasticity in motor cortex in PD. Restoration of cortical plasticity may be one of the mechanisms of how STN-DBS produces clinical benefit.
Motor complications such as motor fluctuations and levodopa-induced dyskinesia (LID) are major sources of disability for patients with Parkinson disease (PD).1 They may be associated with impaired long-term potentiation (LTP) and long-term depression of synaptic plasticity in the corticostriatal synapses of basal ganglia.2 Studies in animal models of PD and in patients with PD undergoing subthalamic nucleus (STN) deep brain stimulation (DBS)3,4 have shown that PD is associated with impaired synaptic plasticity in the striatum and the substantia nigra. Moreover, LID was associated with failure of low-frequency stimulation to depotentiate LTP in an experimental parkinsonian model,5 and abnormal depotentiation was demonstrated in the motor cortex in patients with PD.6
Synaptic plasticity can be investigated by paired associated stimulation (PAS), which is a noninvasive, well-studied experimental design to induce LTP-like changes in the human motor cortex.7 Studies using PAS found that LTP-like plasticity is impaired in the motor cortex and is restored by dopaminergic medication in patients with PD without dyskinesia8–10 but not in patients with LID,8 suggesting that abnormal motor cortical synaptic plasticity is present in PD and may be involved in the pathophysiology of LID.
STN-DBS is increasingly being used to treat moderate to advanced PD.11 However, how STN stimulation improves motor function and decreases LID is not well understood. In the present study, we examined the effects of STN-DBS on LTP-like plasticity in PD. We hypothesized that STN stimulation improves LTP-like plasticity, suggesting that normalization of motor cortical plasticity may be one of the mechanisms of action whereby STN stimulation improves motor function and decreases LID.
METHODS
Participants.
We studied 8 patients with PD (table 1) without severe tremor who had bilateral STN-DBS for at least 6 months and 9 age-matched healthy controls (6 men and 3 women, aged 58.1 ± 5.6 years, range 50–65 years). We applied exclusion criteria of any cognitive impairment, as evaluated by their physicians and intake of antidepressant.
Table 1.
Clinical features of patients with Parkinson disease with bilateral STN-DBS
Patients were studied in 4 sessions on separate days consisting of medication (Med) OFF/stimulation (Stim) OFF, Med-OFF/Stim-ON, Med-ON/Stim-OFF, and Med-ON/Stim-ON in random order. The stimulators were switched off for 20 minutes before the study in Stim-OFF conditions. Patients were off medications at least 12 hours for the Med-OFF conditions and approximately 1 hour after administration of the usual morning dose in the Med-ON conditions. Neurologic examination documented the clinical benefits of medication intake. Amantadine was withheld for 3 days before the experimental session. Each session was conducted at the same time in the morning with the experimenter blinded to medication and DBS states.
Parkinsonism was assessed with the motor section of the Unified Parkinson's Disease Rating Scale, Part III (UPDRS-III) and the Hoehn and Yahr scale in each session and in the preoperative stage (table 1). Dyskinesia was assessed with UPDRS-IV dyskinesia ratings (items 32–35) in pre- and postoperative conditions.
Standard protocol approvals, registrations, and patient consents.
All participants gave written informed consent. The University Health Network Research Ethics Board approved the protocol.
Experimental setup.
Transcranial magnetic stimulation (TMS) was studied with a Magstim 200 stimulator (The Magstim Company, Whitland, UK). A figure-of-8 coil was placed over the motor cortical representation of the abductor pollicis brevis (APB) and over the abductor digiti minimi (ADM) muscle representation for silent period measurement of the ADM muscle. The posterior-anterior current direction for the experiment was used in the brain. Motor evoked potentials (MEPs) at the dominant hand of the controls and more affected side of the patients were recorded.
EMG activities at the contralateral APB and ADM muscles were recorded with surface electrode. The EMG signals were amplified (model 2024F; Intronix Technologies Corporation, Bolton, Canada), filtered with bandpass 20 Hz to 2.5 kHz, and digitized at a rate of 5 kHz (Micro 1401; Cambridge Electronic Design, UK). The signals were monitored with a computer screen and speakers for relaxation of studied muscles.
The median nerve at the wrist was stimulated with a proximally positioned cathode. The stimulus width and intensity were used at 200 microseconds and 300% perceptual threshold.
In the PAS protocol, the interstimulus interval between median nerve stimulation at the wrist and TMS over the contralateral motor cortex was 21.5 milliseconds. The 180 pairs were stimulated at 0.1 Hz for 30 minutes.8
Experimental design.
In each session (healthy control, Med-OFF/Stim-OFF, Med-OFF/Stim-ON, Med-ON/Stim-OFF, and Med-ON/Stim-ON), resting motor threshold (RMT), MEP amplitude, and cortical silent period (CSP) duration were tested before PAS (baseline) and after PAS for 60 minutes (T0, T30, T60) in both APB and ADM muscles.
Further details of the methods, data analysis, and statistical analysis are provided in the supplementary material on the Neurology® Web site at Neurology.org.
RESULTS
The clinical features of the patients studied are shown in table 1. The doses of dopaminergic medications expressed as levodopa equivalent at the time of the study were 640.6 ± 371.8 mg/d compared with presurgery doses of 1,040.5 ± 434.9 mg/d (p = 0.059).
We completed 3 sessions in patient 1 and 2 sessions in patient 4 because they declined to participate in further sessions. Patient 5 did not take any medication and therefore only had Med-OFF/Stim-OFF and Med-OFF/Stim-ON sessions. Patient 5 was studied on the less affected side because tremor in the stimulator off state precluded satisfactory recording on the more affected side. This patient showed almost normalization of LTP-like plasticity with STN-DBS (+42% of baseline at T30 in Stim-ON condition compared with +6% in Stim-OFF condition). UPDRS motor scores were lower in the Med-ON/Stim-ON compared with the Med-OFF/Stim-OFF session (p = 0.001) and Med-ON/Stim-OFF session (p = 0.005). UPDRS dyskinesia scores were lower after STN-DBS surgery compared with before surgery (p = 0.011) (table 1). While all patients had dyskinesia before surgery, 5 of the 8 patients had dyskinesia even after DBS surgery. When we compared these patients with 3 patients who showed no (UPDRS-IV score 0) dyskinesia after surgery (patients 2, 4, and 5), there was no significant difference in PAS response in Med-OFF/Stim-ON and Med-ON/Stim-ON conditions.
Comparisons between patients and healthy controls.
MEP amplitude ratio to baseline.
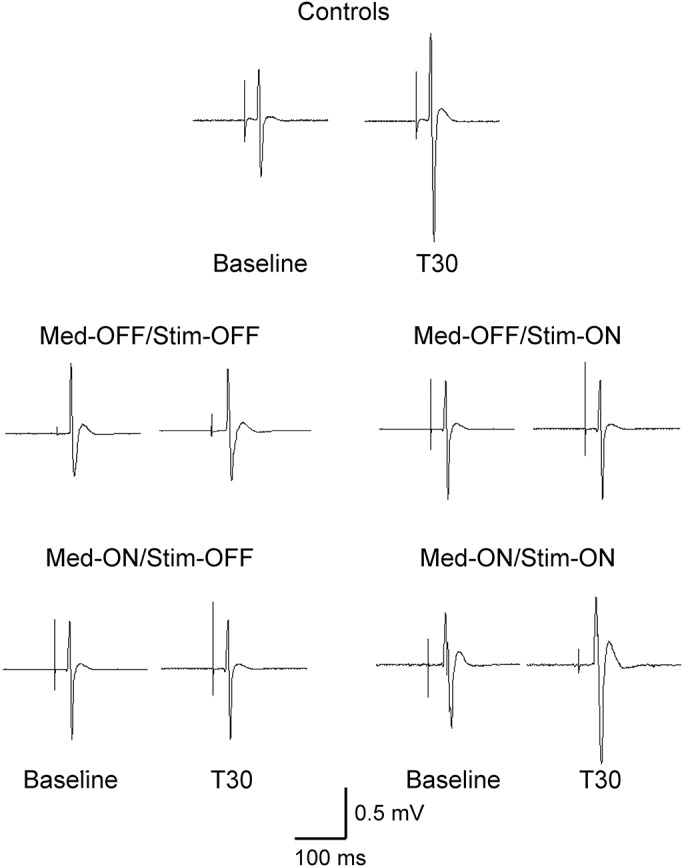
Figure 1 shows examples from a control participant and from a patient. The group data are shown in figure 2A and table e-1. For the APB muscle, analysis of variance (ANOVA) including control participants and patients showed a significant effect of group (F = 5.94, p = 0.001) and time (F = 4.96, p = 0.003) with a nonsignificant time × group interaction (F = 1.78, p = 0.06). Post hoc analysis showed reduced plasticity in the Med-OFF/Stim-OFF (p = 0.002) and Med-OFF/Stim-ON (p = 0.018) groups compared with controls, with a trend for reduced plasticity in the Med-ON/Stim-OFF group compared with controls (p = 0.051). There was no difference between the Med-ON/Stim-ON group and controls (p = 0.851). To further explore the effects of PAS, we performed mixed-model ANOVAs in each group. There was a significant effect of time on MEP amplitude in the controls (F = 4.25, p = 0.012) and in the Med-ON/Stim-ON session (F = 4.78, p = 0.013), whereas the effect of time was not significant in the other sessions. Post hoc analyses showed increased MEP amplitude at T30 and T60 compared with baseline for control participants (+67.7% of baseline at T30, +72.3% at T60) and in the Med-ON/Stim-ON session (+55.8% at T30, +57.0% at T60) (p < 0.05 for all comparisons). There was no significant change in MEP amplitude at all times after PAS compared with baseline for other sessions.
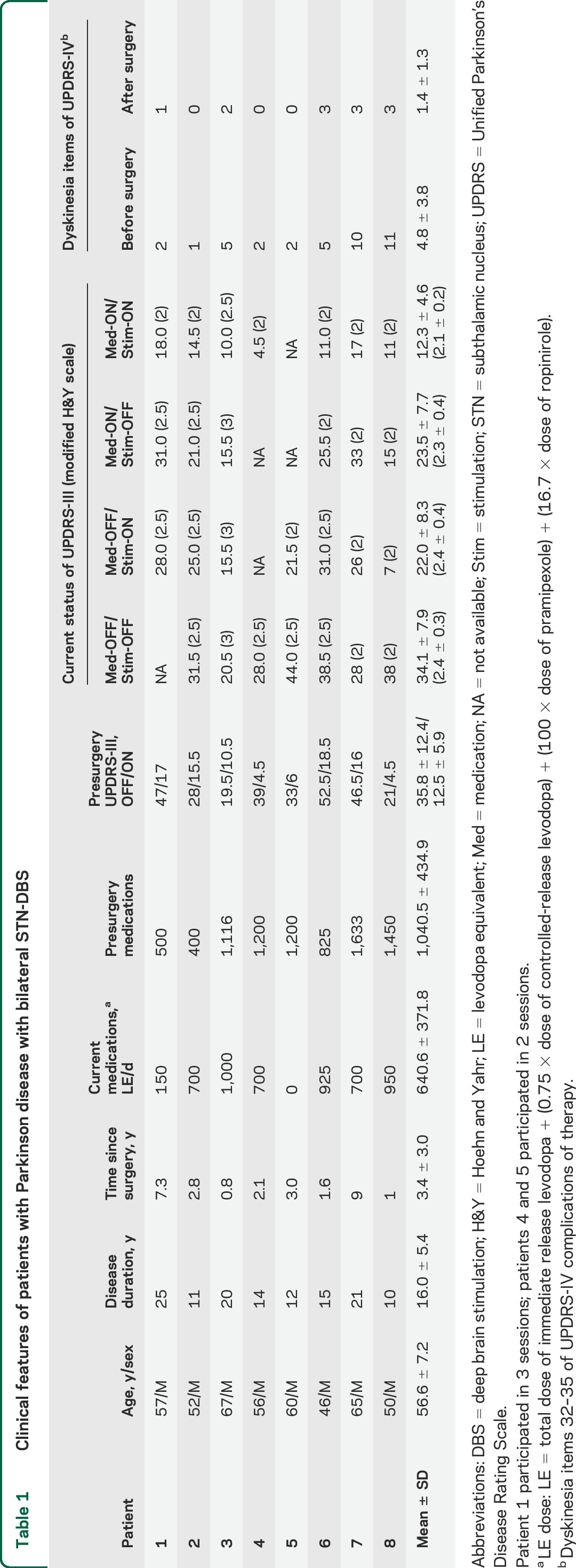
Figure 1. Examples of MEPs of the abductor pollicis brevis muscle from a control participant and a patient with Parkinson disease.
Representative MEPs are averages of 20 trials at baseline and at 30 minutes after PAS in off and on medication and stimulation sessions. There was increased MEP amplitude in the control participant and in the patient in the Med-ON/Stim-ON session, but not in the other sessions after PAS. Med = medication; MEP = motor evoked potential; PAS = paired associated stimulation; Stim = stimulation.
Figure 2. Effect of PAS on MEP amplitudes.
The data are shown as a ratio to the baseline MEP amplitude. Ratios over 1 mean facilitation and ratios below 1 mean inhibition of MEP amplitude. In the abductor pollicis brevis muscle, PAS increased MEP amplitudes in controls and patients in the Med-ON/Stim-ON condition but not in patients in the Med-OFF/Stim-OFF, Med-OFF/Stim-ON, and Med-ON/Stim-OFF conditions (A). PAS did not significantly increase MEP amplitude in the abductor digiti minimi muscle in controls and patients in all the conditions (B). *p < 0.05 by post hoc test compared with baseline. Error bars represent standard error. Med = medication; MEP = motor evoked potential; PAS = paired associated stimulation; Stim = stimulation.
For the ADM muscle, there was a significant effect of group (F = 5.51, p = 0.001) and time (F = 4.59, p = 0.005) without a significant interaction. Post hoc analysis showed no significant difference among groups. Moreover, there was no significant effect of time on MEP amplitude in all groups although there was nonsignificant increase in MEP amplitudes after PAS in control participants (F = 2.75, p = 0.089) and in the Med-ON/Stim-ON session (F = 3.09, p = 0.055) (figure 2B, table e-1).
CSP duration ratio to baseline.
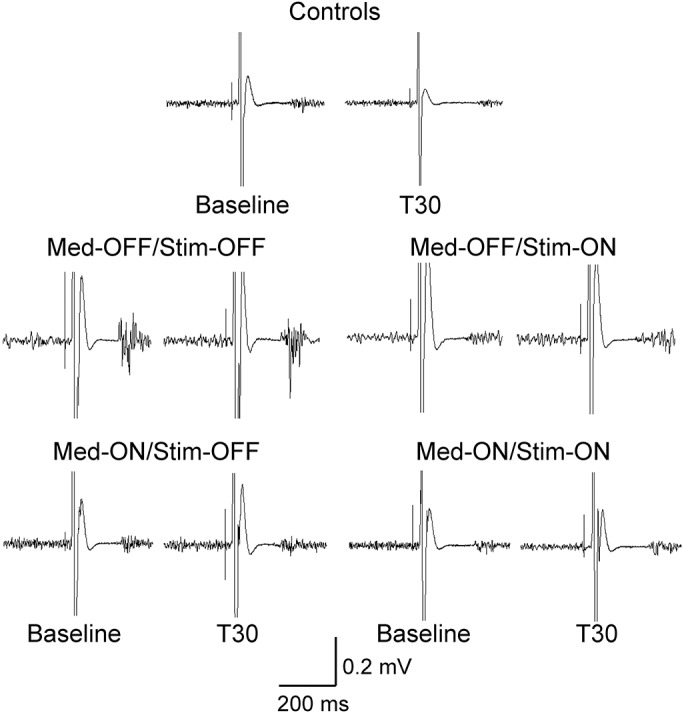
Figure 3 shows examples from a control participant and from a patient, and table e-2 and figure 4 show the group data. For the APB muscle, mixed-model ANOVA showed main effects of group (F = 6.06, p = 0.001), time (F = 2.86, p = 0.041), and time × group interaction (F = 2.16, p = 0.019). In separate ANOVAs for each group, there was a significant effect of time on CSP duration in the control group (F = 4.72, p = 0.01), the Med-OFF/Stim-OFF (F = 3.66, p = 0.024), and the Med-OFF/Stim-ON sessions (F = 3.23, p = 0.047) but not in the other 2 conditions studied. Post hoc analysis showed significantly prolonged CSP duration at T0 (p = 0.011) and nonsignificant prolongation of CSP duration at T30 (p = 0.066) and at T60 (p = 0.184) compared with baseline in control participants but no significance change at all times after PAS in the Med-OFF/Stim-OFF and the Med-OFF/Stim-ON sessions (figure 4A, table e-2).
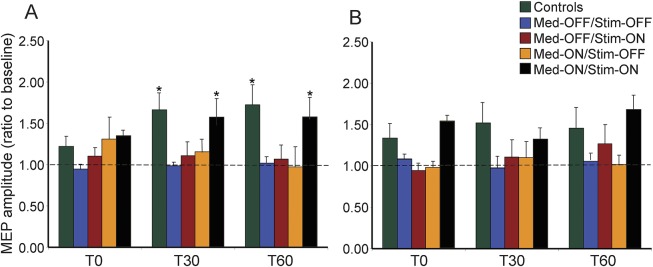
Figure 3. Examples of CSPs from a control participant and a patient with Parkinson disease in the abductor pollicis brevis muscle.
Representative CSP traces are averages of 10 trials at baseline and at 30 minutes after PAS in off and on medication and stimulation sessions. There was increased CSP duration in the control participant but not in the patient in all conditions after PAS. CSP = cortical silent period; Med = medication; PAS = paired associated stimulation; Stim = stimulation.
Figure 4. Effect of PAS on CSP durations.
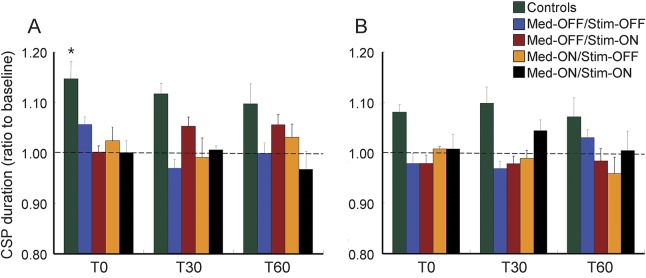
The data are shown as a ratio to the baseline CSP duration. PAS prolonged CSP duration in APB muscle in controls but not in patients with Parkinson disease (A). In the abductor digiti minimi muscle, PAS did not prolong CSP duration in controls and patients (B). *p < 0.05 by post hoc test compared with baseline. Error bars represent standard error. CSP = cortical silent period; Med = medication; PAS = paired associated stimulation; Stim = stimulation.
For the ADM muscle, the effect of group showed significance (F = 3.56, p = 0.015) on CSP duration but the effect of time and time × group interaction was not significant. In separate ANOVAs for each group, there was a significant effect of time in control participants and patients in all sessions studied (figure 4B, table e-2).
Resting motor threshold.
RMT at baseline was similar among the 5 groups in the APB and ADM muscles (controls 36.2% ± 2.8%, Med-OFF/Stim-OFF 41.0% ± 3.4%, Med-OFF/Stim-ON 40.1% ± 2.0%, Med-ON/Stim-OFF 40.7% ± 2.8%, and Med-ON/Stim-ON 40.1% ± 2.1% for the APB muscle). ANOVA showed no significant effect of time or group on RMT in either the APB or ADM muscles.
Background EMG area.
EMG background activity before TMS for measurement of MEP amplitude showed no difference at baseline between controls and patients in all sessions in the APB and ADM muscles (table e-3). The assessment of the CSP in Med-ON/Stim-ON session for the APB muscle at T30 and T60 of patient 2 and of the CSP in Med-OFF/Stim-ON session for the APB muscle at T60 of patient 5 was excluded from the analysis as the majority (>50%) of trials were rejected because of increased background EMG activity.
Effect of STN stimulation and dopaminergic medications on MEP amplitude and CSP duration in patients with PD.
Mixed-model ANOVA for MEP amplitude ratio to baseline for the APB muscles in patients with PD showed higher MEP amplitudes in medication on compared with off (F = 14.96, p = 0.001) and in the stimulation on compared with off (F = 9.06, p = 0.004) conditions, but no significant effects of time (F = 1.72, p = 0.179), medication × stimulation interaction (F = 1.25, p = 0.267), medication × time interaction (F = 1.67, p = 0.179), or stimulation × time interaction (F = 1.36, p = 0.262).
For the ADM muscle, MEP amplitudes were higher in the medication on (F = 5.26, p = 0.025) and stimulation on (F = 14.29, p = 0.001) than in the off conditions and there was a significant medication × stimulation interaction (F = 6.38, p = 0.014). Figure 2B shows that the effects of stimulation were greater in the medication on than the medication off state. The effects of time (F = 1.58, p = 0.214), medication × time interaction (F = 0.73, p = 0.537), and stimulation × time interaction (F = 2.36, p = 0.079) were not significant.
ANOVAs for CSP duration ratio to baseline for the APB and ADM muscles in patient groups showed no significant effect of the main factors and their interactions.
DISCUSSION
We found that patients with advanced PD had impaired LTP-like motor cortical plasticity induced by PAS in Med-OFF/Stim-OFF, Med-OFF/Stim-ON, and Med-ON/Stim-OFF states but plasticity was restored to normal levels in the Med-ON/Stim-ON condition.
Our finding of deficient PAS-induced motor cortex plasticity in the Med-OFF/Stim-OFF session is consistent with previous studies.8–10,12–14 There are several possible mechanisms of how dopamine deficiency leads to reduced motor cortex plasticity in patients with PD who are off medication. These findings may be similar to blockade of LTP in corticostriatal slices from dopamine-denervated rats.15 Experimental studies have demonstrated that dopamine strongly influences striatal LTP and long-term depression.3 In PD, there are prominent synchronized oscillatory activities in the 10- to 35-Hz range in the basal ganglia–thalamocortical loop16 and they may lead to abnormal motor cortex plasticity by disturbing cortical activities necessary to perform appropriate movements.17 Alternatively, there is dopaminergic denervation in the upper layers of the motor and prefrontal cortices in PD.18
Dopaminergic medications did not restore cortical plasticity induced by PAS in the Med-ON/Stim-OFF session although presurgical doses were not used because they might induce significant dyskinesia that would interfere with the study. Thus, the reduction of the dosage of dopaminergic medications after DBS may partly account for this finding but doses used still achieved significant antiparkinsonian effects. Since all our patients had LID (table 1), this finding is similar to our previous observation that LTP-like plasticity induced by PAS was not restored by levodopa administration in patients with PD and dyskinesia.8 In addition, the patients in the current study had more advanced PD than those in the previous study.8 However, it is possible that adequate treatment of PD by any combination of DBS and medication would restore motor cortex plasticity. The impaired PAS-induced plasticity in the Stim-OFF states (either Med-ON or Med-OFF) suggested that the effects of STN stimulation on cortical plasticity did not persist after the stimulation was turned off. This is consistent with the observation that most of the improvement in the cardinal symptoms of PD disappeared in less than half an hour after STN-DBS was switched off.19
STN stimulation alone did not restore cortical plasticity as no significant change in MEP amplitude was induced by PAS in the Med-OFF/Stim-ON session (figure 2). However, the Med-ON/Stim-ON session showed an increase in MEP amplitudes after PAS, comparable to those in controls (figure 2). These findings suggest that dopaminergic medications together with STN-DBS are needed to restore LTP-like plasticity in motor cortex in patients with advanced PD, supporting the suggestion that STN-DBS and dopaminergic medications have synergistic effects in patients with PD.20,21
The ADM muscle showed less increase in MEP amplitude than the APB muscle in control participants, confirming the topographical specificity of PAS reported in previous studies.7 However, mixed ANOVA among patients with PD showed that both medication and stimulation increased the plasticity induced by PAS for this heterotopic muscle, and there were synergistic effects of medication and DBS as demonstrated by the significant medication and stimulation interaction.
Several mechanisms may account for the restoration of cortical plasticity in patients with PD receiving STN stimulation. Previous studies showed that STN-DBS restored deficient cortical inhibition.22 There is evidence from animal models of PD that the effects of STN stimulation are at least partly mediated by short-latency cortical activation from antidromic stimulation of the cortico-subthalamic projection.23,24 This is consistent with cortical evoked potentials studies in patients with PD that demonstrated short-latency cortical activation from STN-DBS.25,26 Moreover, TMS studies showed increased cortical excitability time-locked to STN-DBS at short latency that corresponds to antidromic activation of the cortico-subthalamic pathway and at longer latencies that may involve the pallidal-thalamocortical pathway.26 STN-DBS may also override abnormal synchronized oscillations and disrupted firing patterns in PD and replaces them with more regular firing within the basal ganglia–thalamocortical loop.27,28 Thus, the normalized firing patterns in the basal ganglia may lead to reconditioned plasticity response in the motor cortex through its connections.17
Since PAS involves median nerve stimulation, the somatosensory system and sensorimotor integration likely has a crucial role in the effects of PAS on the human motor cortex. Previous studies found abnormal sensorimotor integration in patients with PD.29 STN stimulation improved short-latency afferent inhibition reduced by dopaminergic medications and normalized long-latency afferent inhibition in the on-medication state.30,31 Of note, there were synergistic effects of medications and STN-DBS on both short- and long-latency afferent inhibition, similar to effects of PAS plasticity.30,31 Thus, the restoration of motor cortex plasticity in patients with PD by STN-DBS and dopaminergic medications may be related to an effect of STN stimulation on sensorimotor integration.
All of the patients had reduction of dyskinesia after STN-DBS (table 1). In the presence of STN-DBS, the restoration of LTP-like cortical plasticity by dopaminergic medications is similar to findings in nondyskinetic patients with PD,8 suggesting that STN-DBS may have a direct role in reducing dyskinesias, an effect proposed by several investigators.32,33 Pathologic plasticity in corticostriatal synaptic connections could result in atypical motor patterns, characteristic features of LID.5,34 Decreased LTP-like plasticity seen in corticostriatal and motor cortical networks may impair the organization of desired motor patterns and leads to subsequent development of LID, which consists of undesired motor patterns.8 Therefore, the effect of STN stimulation on synaptic plasticity in motor cortex may contribute to reduction in dyskinesia. Our findings may be compared to the effects of globus pallidus interna DBS on PAS in patients with dystonia. PAS-induced cortical plasticity is exaggerated in patients with dystonia.35,36 Chronic globus pallidus interna DBS was reported to normalize or even decrease PAS-induced plasticity to below normal levels.37,38
For the APB muscle, the CSP duration was prolonged at T0 compared with baseline in control participants, but there was no change in CSP duration in any of the sessions in patients with PD. This is similar to the findings of a previous study in patients with PD and LID.8 Therefore, STN stimulation did not directly affect the inhibitory intracortical system mediating the CSP.
There are several limitations to the present study. We studied a small number of patients and some patients did not complete all study sessions because the protocol was very demanding for the patients with advanced PD. Nevertheless, we were able to obtain robust effects. Since LID was a main reason for patients to undergo STN-DBS, we did not have patients without LID in the study. Therefore, whether the findings are specifically related to LID requires further study. Because we did not record PAS before DBS surgery, we could not examine the time course of cortical plasticity following STN-DBS implantation. It has been shown that the correction of short- and long-latency afferent inhibition in PD requires long-term STN-DBS.31 Further investigations with longitudinal studies including preoperative assessment and serial studies after surgery in a larger number of patients will reveal more information about how STN-DBS changes cortical plasticity in PD.
We found that cortical plasticity induced by PAS in patients with advanced PD is restored by STN-DBS together with dopaminergic medications. STN-DBS and dopaminergic medications may have synergistic effects on cortical plasticity. Normalization of motor cortex plasticity may be one of the mechanisms of action of STN-DBS.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Kyngha Seok for his able assistance with statistical analysis.
GLOSSARY
- ADM
abductor digiti minimi
- ANOVA
analysis of variance
- APB
abductor pollicis brevis
- CSP
cortical silent period
- DBS
deep brain stimulation
- LID
levodopa-induced dyskinesia
- LTP
long-term potentiation
- Med
medication
- MEP
motor evoked potential
- PAS
paired associated stimulation
- PD
Parkinson disease
- RMT
resting motor threshold
- Stim
stimulation
- STN
subthalamic nucleus
- TMS
transcranial magnetic stimulation
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Sang Jin Kim: data collection and analysis, drafting of manuscript. Kaviraja Udupa: data analysis, editing of manuscript. Zhen Ni: editing of manuscript. Elena Moro: editing of manuscript. Carolyn Gunraj: data collection. Filomena Mazzella: data collection. Andres M. Lozano: editing of manuscript. Mojgan Hodaie: editing of manuscript. Anthony E. Lang: editing of manuscript. Robert Chen: conception, design, data analysis, editing of manuscript.
STUDY FUNDING
This work was funded by the Canadian Institutes of Health Research (CIHR) (MOP 15128).
DISCLOSURE
S. Kim, K. Udupa, and Z. Ni report no disclosures relevant to the manuscript. E. Moro received speaking fees from Medtronic and St. Jude Medical and serves as a consultant for Medtronic and St. Jude Medical. C. Gunraj and F. Mazzella report no disclosures relevant to the manuscript. A. Lozano serves as a consultant for Medtronic, Boston Scientific, St. Jude Medical, and Functional Neuromodulation. M. Hodaie and A. Lang report no disclosures relevant to the manuscript. R. Chen received a research grant from Medtronic. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001;16:448–458. [DOI] [PubMed] [Google Scholar]
- 2.Pisani A, Centonze D, Bernardi G, Calabresi P. Striatal synaptic plasticity: implications for motor learning and Parkinson's disease. Mov Disord 2005;20:395–402. [DOI] [PubMed] [Google Scholar]
- 3.Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci 2007;30:211–219. [DOI] [PubMed] [Google Scholar]
- 4.Prescott IA, Dostrovsky JO, Moro E, Hodaie M, Lozano AM, Hutchison WD. Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson's disease patients. Brain 2009;132:309–318. [DOI] [PubMed] [Google Scholar]
- 5.Picconi B, Centonze D, Hakansson K, et al. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci 2003;6:501–506. [DOI] [PubMed] [Google Scholar]
- 6.Huang YZ, Rothwell JC, Lu CS, Chuang WL, Chen RS. Abnormal bidirectional plasticity-like effects in Parkinson's disease. Brain 2011;134:2312–2320. [DOI] [PubMed] [Google Scholar]
- 7.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 2000;123:572–584. [DOI] [PubMed] [Google Scholar]
- 8.Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R. Motor cortex plasticity in Parkinson's disease and levodopa-induced dyskinesias. Brain 2006;129:1059–1069. [DOI] [PubMed] [Google Scholar]
- 9.Ueki Y, Mima T, Kotb MA, et al. Altered plasticity of the human motor cortex in Parkinson's disease. Ann Neurol 2006;59:60–71. [DOI] [PubMed] [Google Scholar]
- 10.Schwingenschuh P, Ruge D, Edwards MJ, et al. Distinguishing SWEDDs patients with asymmetric resting tremor from Parkinson's disease: a clinical and electrophysiological study. Mov Disord 2010;25:560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med 2013;368:610–622. [DOI] [PubMed] [Google Scholar]
- 12.Kojovic M, Bologna M, Kassavetis P, et al. Functional reorganization of sensorimotor cortex in early Parkinson disease. Neurology 2012;78:1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishore A, Popa T, Balachandran A, et al. Cerebellar sensory processing alterations impact motor cortical plasticity in Parkinson's disease: clues from dyskinetic patients. Cereb Cortex 2014;24:2055–2067. [DOI] [PubMed] [Google Scholar]
- 14.Udupa K, Chen R. Motor cortical plasticity in Parkinson's disease. Front Neurol 2013;4:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centonze D, Gubellini P, Picconi B, Calabresi P, Giacomini P, Bernardi G. Unilateral dopamine denervation blocks corticostriatal LTP. J Neurophysiol 1999;82:3575–3579. [DOI] [PubMed] [Google Scholar]
- 16.Eusebio A, Brown P. Oscillatory activity in the basal ganglia. Parkinsonism Relat Disord 2007;13(suppl 3):S434–S436. [DOI] [PubMed] [Google Scholar]
- 17.Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Mov Disord 2003;18:357–363. [DOI] [PubMed] [Google Scholar]
- 18.Gaspar P, Duyckaerts C, Alvarez C, Javoy-Agid F, Berger B. Alterations of dopaminergic and noradrenergic innervations in motor cortex in Parkinson's disease. Ann Neurol 1991;30:365–374. [DOI] [PubMed] [Google Scholar]
- 19.Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology 2003;60:78–81. [DOI] [PubMed] [Google Scholar]
- 20.Timmermann L, Braun M, Groiss S, et al. Differential effects of levodopa and subthalamic nucleus deep brain stimulation on bradykinesia in Parkinson's disease. Mov Disord 2008;23:218–227. [DOI] [PubMed] [Google Scholar]
- 21.Maurer C, Mergner T, Xie J, Faist M, Pollak P, Lucking CH. Effect of chronic bilateral subthalamic nucleus (STN) stimulation on postural control in Parkinson's disease. Brain 2003;126:1146–1163. [DOI] [PubMed] [Google Scholar]
- 22.Cunic D, Roshan L, Khan FI, Lozano AM, Lang AE, Chen R. Effects of subthalamic nucleus stimulation on motor cortex excitability in Parkinson's disease. Neurology 2002;58:1665–1672. [DOI] [PubMed] [Google Scholar]
- 23.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science 2009;324:354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q, Ke Y, Chan DC, et al. Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron 2012;76:1030–1041. [DOI] [PubMed] [Google Scholar]
- 25.Walker HC, Huang H, Gonzalez CL, et al. Short latency activation of cortex during clinically effective subthalamic deep brain stimulation for Parkinson's disease. Mov Disord 2012;27:864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuriakose R, Saha U, Castillo G, et al. The nature and time course of cortical activation following subthalamic stimulation in Parkinson's disease. Cereb Cortex 2010;20:1926–1936. [DOI] [PubMed] [Google Scholar]
- 27.Birdno MJ, Grill WM. Mechanisms of deep brain stimulation in movement disorders as revealed by changes in stimulus frequency. Neurotherapeutics 2008;5:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vitek JL. Deep brain stimulation: how does it work? Cleve Clin J Med 2008;75(suppl 2):S59–S65. [DOI] [PubMed] [Google Scholar]
- 29.Sailer A, Molnar GF, Paradiso G, Gunraj CA, Lang AE, Chen R. Short and long latency afferent inhibition in Parkinson's disease. Brain 2003;126:1883–1894. [DOI] [PubMed] [Google Scholar]
- 30.Sailer A, Cunic DI, Paradiso GO, et al. Subthalamic nucleus stimulation modulates afferent inhibition in Parkinson disease. Neurology 2007;68:356–363. [DOI] [PubMed] [Google Scholar]
- 31.Wagle Shukla A, Moro E, Gunraj C, et al. Long-term subthalamic nucleus stimulation improves sensorimotor integration and proprioception. J Neurol Neurosurg Psychiatry 2013;84:1020–1028. [DOI] [PubMed] [Google Scholar]
- 32.Figueiras-Mendez R, Marin-Zarza F, Antonio Molina J, et al. Subthalamic nucleus stimulation improves directly levodopa induced dyskinesias in Parkinson's disease. J Neurol Neurosurg Psychiatry 1999;66:549–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moro E, Esselink RJ, Benabid AL, Pollak P. Response to levodopa in parkinsonian patients with bilateral subthalamic nucleus stimulation. Brain 2002;125:2408–2417. [DOI] [PubMed] [Google Scholar]
- 34.Chase TN. Striatal plasticity and extrapyramidal motor dysfunction. Parkinsonism Relat Disord 2004;10:305–313. [DOI] [PubMed] [Google Scholar]
- 35.Quartarone A, Bagnato S, Rizzo V, et al. Abnormal associative plasticity of the human motor cortex in writer's cramp. Brain 2003;126:2586–2596. [DOI] [PubMed] [Google Scholar]
- 36.Weise D, Schramm A, Stefan K, et al. The two sides of associative plasticity in writer's cramp. Brain 2006;129:2709–2721. [DOI] [PubMed] [Google Scholar]
- 37.Tisch S, Rothwell JC, Bhatia KP, et al. Pallidal stimulation modifies after-effects of paired associative stimulation on motor cortex excitability in primary generalised dystonia. Exp Neurol 2007;206:80–85. [DOI] [PubMed] [Google Scholar]
- 38.Ruge D, Tisch S, Hariz MI, et al. Deep brain stimulation effects in dystonia: time course of electrophysiological changes in early treatment. Mov Disord 2011;26:1913–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


