Dyskinesia in Parkinson disease (PD) usually involves the neck, trunk, limbs, and face.1–3 Isolated or predominant respiratory involvement is rarely reported, and can lead to inappropriate cardiopulmonary tests and management. We report successful diagnosis and treatment of such a case for the first time using bilateral subthalamic nucleus deep brain stimulation (STN DBS).
Classification of evidence.
This is a single observation without control (Class IV).
Case report.
A 64-year-old right-handed man with an 8-year history of PD presented with respiratory distress. His PD started with resting tremor, bradykinesia, and rigidity in his right hand, which gradually spread to the other side. His parkinsonism responded well to carbidopa/levodopa, with dosages being gradually escalated to 25/250 mg strength 1 tablet QID, until about 10 months prior to his visit, when he developed apparent respiratory distress, with involuntary rapid, strenuous, and distressful breathing (video clip 1 on the Neurology® Web site at Neurology.org), accompanied by anxiety and mild tremor of the hands. He had these spells several times daily, lasting about 2 hours each time, leading to several emergent visits and hospitalizations for extensive workups, including EKG, stress echocardiogram, pulmonary function, and chest CT pulmonary embolism protocol, but none was revealing.
After carefully checking his diary, we found that the respiratory distress started about 1 hour after 1 tablet of 25/250 mg strength carbidopa/levodopa, which lasted for 2 hours, as also confirmed by our prolonged observation in clinic. We hypothesized that his isolated respiratory distress was peak-dose respiratory dyskinesia, although we also had to rule out other possibilities, such as akathisia, given his anxiety, or nonmotor respiratory dysrhythmia.
To test these hypotheses, we first added an additional tablet of 25/100 mg strength carbidopa/levodopa on each dose, which made his respiratory distress much worse, suggesting dyskinesia as the cause of his respiratory distress. We then reduced the carbidopa/levodopa to 25/100 mg each dose, which led to complete resolution of the respiratory distress but aggravated his parkinsonism (video clip 2).
We then resumed 25/250 mg carbidopa/levodopa every 6 hours QID with additional amantadine 100 mg bid, which did not improve his respiratory symptoms. We therefore reduced carbidopa/levodopa to 25/100 mg strength 2 tablets each dose with amantadine 100 mg BID, which only minimally improved his respiratory symptom, while his parkinsonism was slightly worsened.
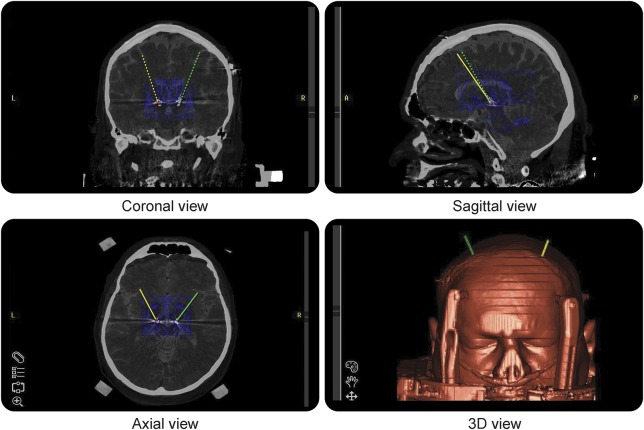
Given the fact that STN DBS could control general dyskinesia and parkinsonism and reduce the levodopa equivalent dose,4 DBS was considered. The patient had 56% improvement on Unified Parkinson's Disease Rating Scale (UPDRS)–III score at levodopa “on” (score 20) compared to “off” state (score 45). Bilateral STN DBS was subsequently successfully placed as described elsewhere,5 with documentation of exact lead placement using intraoperative CT fused with preoperative planning MRI and Schaltenbrand-Wahren atlas (figure). His respiratory dyskinesia and parkinsonism responded well to the stimulation. His dyskinesia was completely controlled on initial settings of left C+/2-2.5 volts/60 μs/130 Hz and right C+/10-1.8 volts/60 μs/130 Hz at 1-month postsurgical visit (video clips 3 and 4), even at his usual medication “on” state, with well-controlled parkinsonism (video clip 4). His respiratory dyskinesia remained completely controlled at his 6-month postsurgical visit on left C+/2-2.7 volts/60 μs/130 Hz and right C+/10-2.8 volts/60 μs/130 Hz and 33% reduction of his presurgical levodopa equivalent dosage. He had UPDRS-III scores of 22 at DBS “on” and medication “off” state, and 14 at DBS “on” and medication “on” state at both visits.
Figure. Localization of the bilateral subthalamic nucleus deep brain stimulation.
Discussion.
Respiratory dyskinesia as part of general dyskinesia was reported and treated with unilateral GPi DBS in one PD case.6 Our case demonstrated a peak-dose dyskinesia solely affecting the respiratory muscles. With careful history taking and medication challenge, we diagnosed it as peak-dose respiratory dyskinesia, rather than akathisia, nonmotor respiratory dysrhythmia, or other cardiopulmonary disease. We further demonstrated for the first time that bilateral STN DBS completely controlled the respiratory dyskinesia even at medication “on” state. Respiration is controlled voluntarily and involuntarily through the motor and premotor cortex, brainstem, and cervical phrenic nucleus and nerve, involving sensors and autonomic system as well.7 It is not clear how DBS controls respiratory dyskinesia, though it most likely involves stimulating the basal ganglia loop (as it does for limb/trunk dyskinesia) and possibly the thalamic-hypothalamic connections and hypothalamic autonomic system as well, thus influencing both voluntary and involuntary respiration.6,7 It is not clear why only the respiratory muscles were involved. We did not perform the needle EMG study to characterize the underlying physiology for fear of complications, such as pneumothorax due to the perforation of the needle through the diaphragm during dyskinesia. This case will help manage this probably underrecognized and undertreated respiratory dyskinesia in patients with PD in the future.
Supplementary Material
Acknowledgments
Acknowledgement: The authors thank the patient for cooperation.
Footnotes
Supplemental data at Neurology.org
Author contributions: Tao Xie: drafting and revising the manuscript for design, content and analysis of data, intraoperative monitoring, DBS programming, and patient care. Rui Guan: drafting and revising the manuscript for content and analysis of data. Julia Staisch: revising the manuscript for content and analysis of data. Vernon Towle: revising the manuscript for content and analysis of data and intraoperative monitoring. Peter Warnke: revising the manuscript for content and analysis of data, performing the surgery.
Study funding: No targeted funding reported.
Disclosure: T. Xie was supported in part by the Michael J. Fox Foundation, NIH, and GE Healthcare for research. R. Guan, J. Staisch, and V. Towle report no disclosures relevant to the manuscript. P. Warnke reports being Associate Editor of the Journal of Neurology, Neurosurgery and Psychiatry. Go to Neurology.org for full disclosures.
References
- 1.Jankovic J. Motor fluctuation and dyskinesia in Parkinson's disease: clinical manifestations. Mov Disord 2005;20(suppl 11):S11–S16. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic J, Nour F. Respiratory dyskinesia in Parkinson's disease. Neurology 1986;36:303–304. [DOI] [PubMed] [Google Scholar]
- 3.Rice JE, Antic R, Thompson PD. Disordered respiration as a levodopa-induced dyskinesia in Parkinson's disease. Mov Disord 2002;17:524–527. [DOI] [PubMed] [Google Scholar]
- 4.Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med 2010;362:2077–2091. [DOI] [PubMed] [Google Scholar]
- 5.Xie T, Kang UJ, Warnke P. Effect of stimulation frequency on immediate freezing of gait in newly activated STN DBS in Parkinson's disease. J Neurol Neurosurg Psychiatry 2012;83:1015–1017. [DOI] [PubMed] [Google Scholar]
- 6.Oyama G, Foote KD, Iyer SS, et al. Unilateral GPi-DBS as a treatment for levodopa-induced respiratory dyskinesia in Parkinson disease. Neurologist 2011;17:282–285. [DOI] [PubMed] [Google Scholar]
- 7.Hyam JA, Kringelbach ML, Silbun PA, et al. The autonomic effects of deep brain stimulation: a therapeutic opportunity. Nat Rev Neurol 2012;8:391–400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.