Abstract
Objective:
To evaluate the appropriateness of dopamine receptor antagonist prescriptions in hospitalized patients with dopamine-requiring diseases after implementation of an automated prescription alert system.
Methods:
We examined dopamine receptor antagonist prescriptions in hospitalized patients with dopamine-requiring diseases and physician response to an automated drug contraindication alert system at Barnes-Jewish Hospital from 2009 to 2013. A detailed review of patient medical records was performed for all alert events generated when a physician prescribed a dopamine receptor antagonist concurrently with a dopamine receptor agonist in hospitalized patients. Two movement disorders neurologists determined the appropriateness of each prescription, based on patient medical history, through consensus. Physician response to alert was compared by indication for the prescription and physician specialty.
Results:
Of 237 orders, 197 (83.1%) prescriptions for dopamine receptor antagonists were considered inappropriate. The prevalence of inappropriate dopamine receptor antagonist prescriptions per levodopa prescriptions was 16.10% (95% confidence interval 9.47, 22.73) in psychiatry, 7.51% (6.16, 8.86) in general medicine, 6.14% (4.49, 7.79) in the surgical specialties, and 0.85% (0.46, 1.25) in the neurologic/neurosurgical specialties. Of the inappropriate prescriptions, 146 (74.1%) were continued despite the alert. The strongest predictor of discontinuation of dopamine receptor antagonist medications was use of the medication to treat nausea or emesis (p < 0.001).
Conclusions:
Despite successfully identifying instances when dopamine antagonists were prescribed to patients with dopamine-requiring diseases, the alert system modestly affected physician prescribing behavior, highlighting the need for improved education of health care providers.
Patients with dopamine-requiring diseases may experience worsening of disease symptoms when given dopamine receptor–blocking medications, and use of these medications should generally be avoided in these patients. Dopamine receptor antagonists, which are commonly prescribed for nausea, emesis, and psychosis, exacerbate these patients' symptoms by blocking the motor effects of intrinsic and pharmacologic dopamine.
The American Academy of Neurology (AAN) has established guidelines for the treatment of common comorbidities of Parkinson disease (PD), such as psychosis. Based on an evaluation of existing literature up to 2006, the AAN recommends clozapine and quetiapine over atypical antipsychotics such as risperidone, olanzapine, and ziprasidone, which are known to have extrapyramidal effects.1–5 In addition, antiemetic medications such as prochlorperazine and metoclopramide should not be administered, as they also exacerbate parkinsonian symptoms.6,7 Avoidance of such antidopaminergic drugs is critical to ensuring good health outcomes in this patient population.
In January 2009, Barnes-Jewish Hospital in St. Louis implemented an automated alert system to notify physicians when they concurrently prescribed a dopamine receptor agonist and dopamine receptor antagonist to an inpatient. The alert was employed to prevent patients with dopamine-requiring disease from receiving dopamine receptor antagonists inappropriately. The alert message contained a warning about potential adverse effects of the combination of the 2 classes of medications. We investigated the effectiveness of the alert system in preventing inappropriate prescriptions of dopamine receptor antagonists to patients with dopamine-requiring disease and factors associated with inappropriate prescriptions.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Washington University School of Medicine Institutional Review Board. A waiver of informed consent was obtained for all study participants.
Participants.
This study was approved by the Washington University Human Research Protection Organization. Alerts triggered in the electronic order entry system by any inpatient clinical service at Barnes-Jewish Hospital from November 2009 through January 2013 were examined. These alerts were triggered by the prescription of a dopamine receptor antagonist for an inpatient who was already prescribed carbidopa-levodopa or vice versa. Dopamine agonists were not included in the alert because of their common use for restless legs syndrome and because nearly all patients with PD will take levodopa within a few years of disease onset. The alerts and names of the patients were collected from electronic archive data, and a detailed review of these patients' electronic medical records was completed. Patient data extracted from records included age, sex, presenting symptoms, final diagnosis for the hospital visit, past medical and surgical history, and dopamine-requiring disease diagnosis. The following information about the dopamine receptor antagonist prescriptions was also gathered: name of the medication, indication, dose, start and stop dates, number of doses given, dates and times the medication was given or attempted to be given, prescribing physician, and clinical service on which the alert was triggered.
Data definitions.
The data were organized in terms of occurrences when a physician ordered a contraindicated medication for a patient with a dopamine-requiring disease. If a physician ordered multiple prescriptions for a dopamine antagonist in succession over a short amount of time (less than a 1-hour period) and an alert was triggered each time, we considered that situation to be one occurrence of the physician ordering the medication. Therefore, multiple alerts for the same medication for the same patient that occurred within 1 hour of the first alert were counted as one patient alert in our analysis. The exception to this rule was when different physicians triggered an alert for the same patient. In these cases, each alert contributed to the final dataset.
To ensure consistent data coding, we adopted the following rules when classifying whether or not an inappropriate prescription was discontinued in response to an alert:
If the dopamine antagonist was discontinued within 24 hours and no dose was given, then the medication was considered discontinued in response to the alert.
If doses of the dopamine antagonist had been given before the alert but none was given after the alert was triggered and the order was discontinued within 24 hours, then the medication was considered discontinued in response to the alert. This rule was devised for situations in which the dopamine receptor antagonist was prescribed first and the alert was triggered with the prescription of carbidopa-levodopa.
If the dopamine antagonist was given or attempted to be given after the alert, then the medication was considered not discontinued in response to the alert.
If the dopamine antagonist was discontinued via patient discharge, then the medication was considered not discontinued in response to the alert.
Determination of “appropriateness.”
Two movement disorders neurologists classified each patient's dopamine antagonist prescription as appropriate or inappropriate. Both neurologists took into account each patient's dopamine-requiring condition, presenting symptoms and final diagnosis for the hospital visit, past medical history, and the indication for the dopamine receptor antagonist prescription. The neurologists evaluated this information independently and were in agreement on 96% of the patients. For the 4% in whom there was initial disagreement, the neurologists discussed the case and reached a consensus. The primary principles used by the neurologists were that the use of dopamine antagonists in patients with parkinsonism was inappropriate but that the use of a dopamine antagonist was appropriate in patients with restless legs syndrome who had acute psychosis or mania. Consistent with the AAN guidelines, the use of clozapine or quetiapine was considered to be acceptable in patients with parkinsonism; these medications were not included in the alert system.
Statistical analysis.
Descriptive data were expressed as total number of occurrences and percentage. Prevalence of inappropriate dopamine receptor antagonist prescriptions for each service was calculated as the number of inappropriate dopamine receptor antagonist orders divided by the total number of levodopa orders from November 2009 through January 2013 for each service with 95% confidence intervals (CIs). Total levodopa orders for each service were factored in to adjust for service size. For alerts in which the prescription was inappropriate, we examined factors associated with physician response to the alert as a dichotomous outcome. Specifically, we used multivariable unconditional logistic regression models suitable for dependent data in Stata (version 11.1, College Station, TX) and report respective odds ratios (ORs) and 95% CIs as a measure of association while accounting for patient sex and age at the time of the alert.
RESULTS
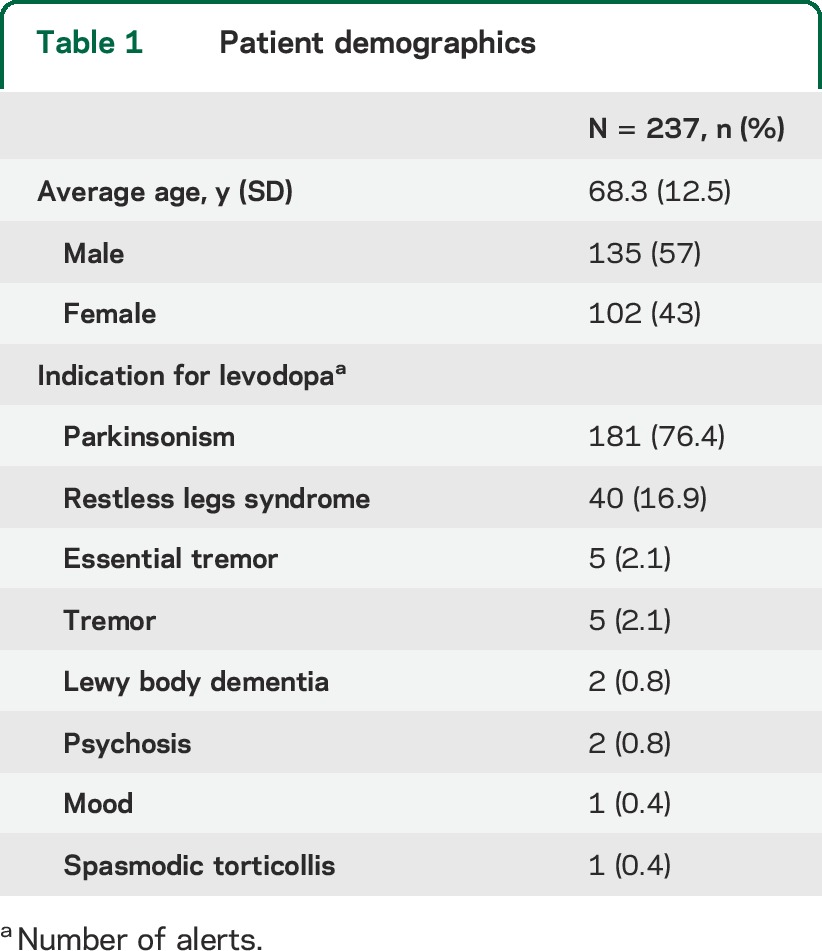
There were 239 alerts for inpatients with dopamine-requiring disease who were prescribed a dopamine antagonist from November 2009 through January 2013. Two alerts were excluded from analysis because the patient records had unclear documentation of the dopamine-requiring disease. The remaining 237 alerts were included in the analysis. Demographics of the study population for the corresponding alerts are in table 1. PD was the most common dopamine-requiring disease (76.4%). Restless legs syndrome was the dopamine-requiring condition for most of the remaining alerts (16.9%).
Table 1.
Patient demographics
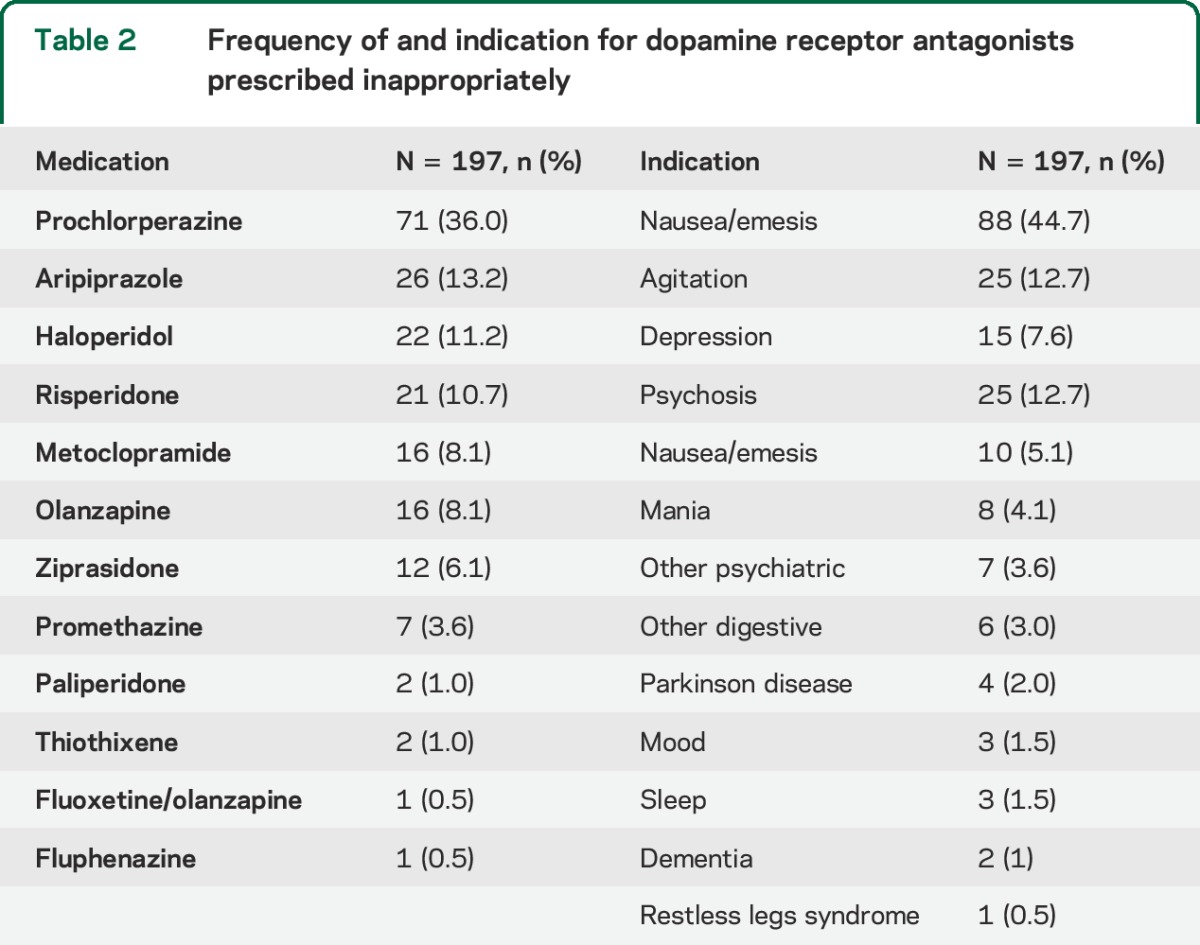
Dopamine receptor antagonist medications triggering an alert and their indication are in table 2. Prochlorperazine was the most common inappropriately prescribed medication and accounted for 71 (36.0%) of the inappropriate prescriptions. Psychiatric symptoms (agitation, depression, psychosis, mania, anxiety, mood, and other psychiatric symptoms) made up 93 (47.2%) of the indications for inappropriate prescriptions. Nausea/emesis was the second most common indication for prescribing a dopamine receptor antagonist (44.7%).
Table 2.
Frequency of and indication for dopamine receptor antagonists prescribed inappropriately
Of the 237 alerts, 197 (83.1%) were for prescriptions for dopamine antagonists that were considered inappropriate. Moreover, when we excluded one patient who accounted for a majority of the appropriate prescriptions, 92.9% of all alerts were for inappropriate prescriptions. Although not statistically significant, there was a suggestion that the percentage of alerts that were inappropriate differed by both time of day (90.0% inappropriate in the day and 96.0% inappropriate in the night) and year. In particular, 95.8% of alerts in the earliest years of the alert system (2009 through 2011) were inappropriate, in contrast to 87.0% thereafter.
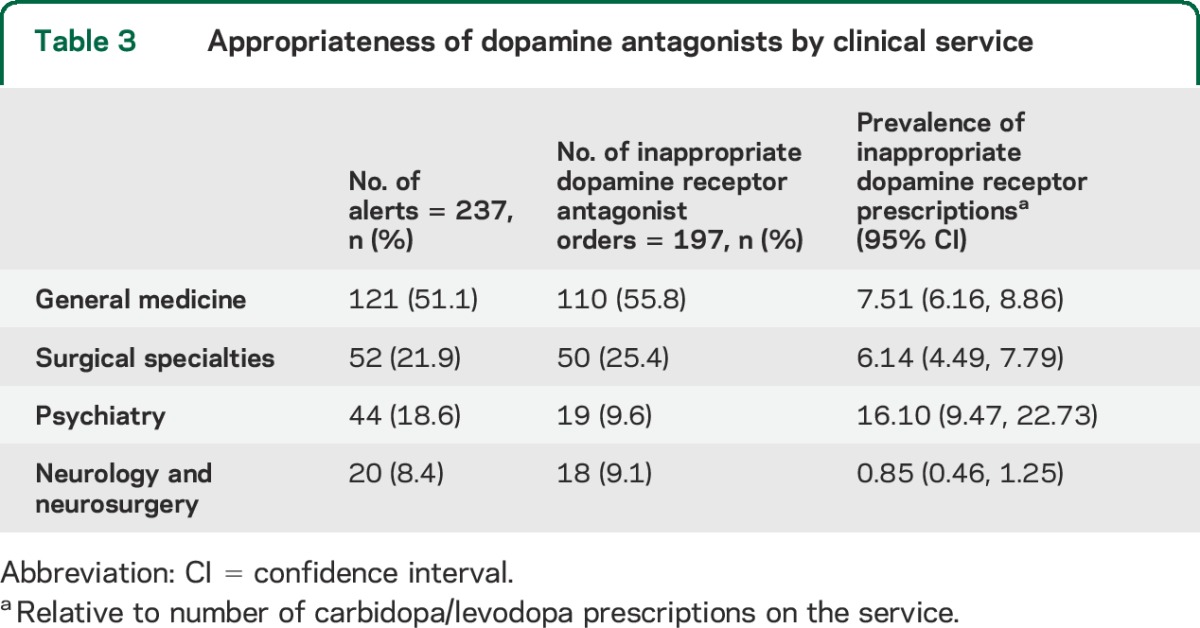
General medicine was responsible for the most, and neurology/neurosurgery the fewest, inappropriate dopamine receptor antagonist orders (table 3). However, when accounting for the number of levodopa prescriptions over the study period by service, psychiatry had the highest prevalence of inappropriate dopamine receptor antagonist prescriptions.
Table 3.
Appropriateness of dopamine antagonists by clinical service
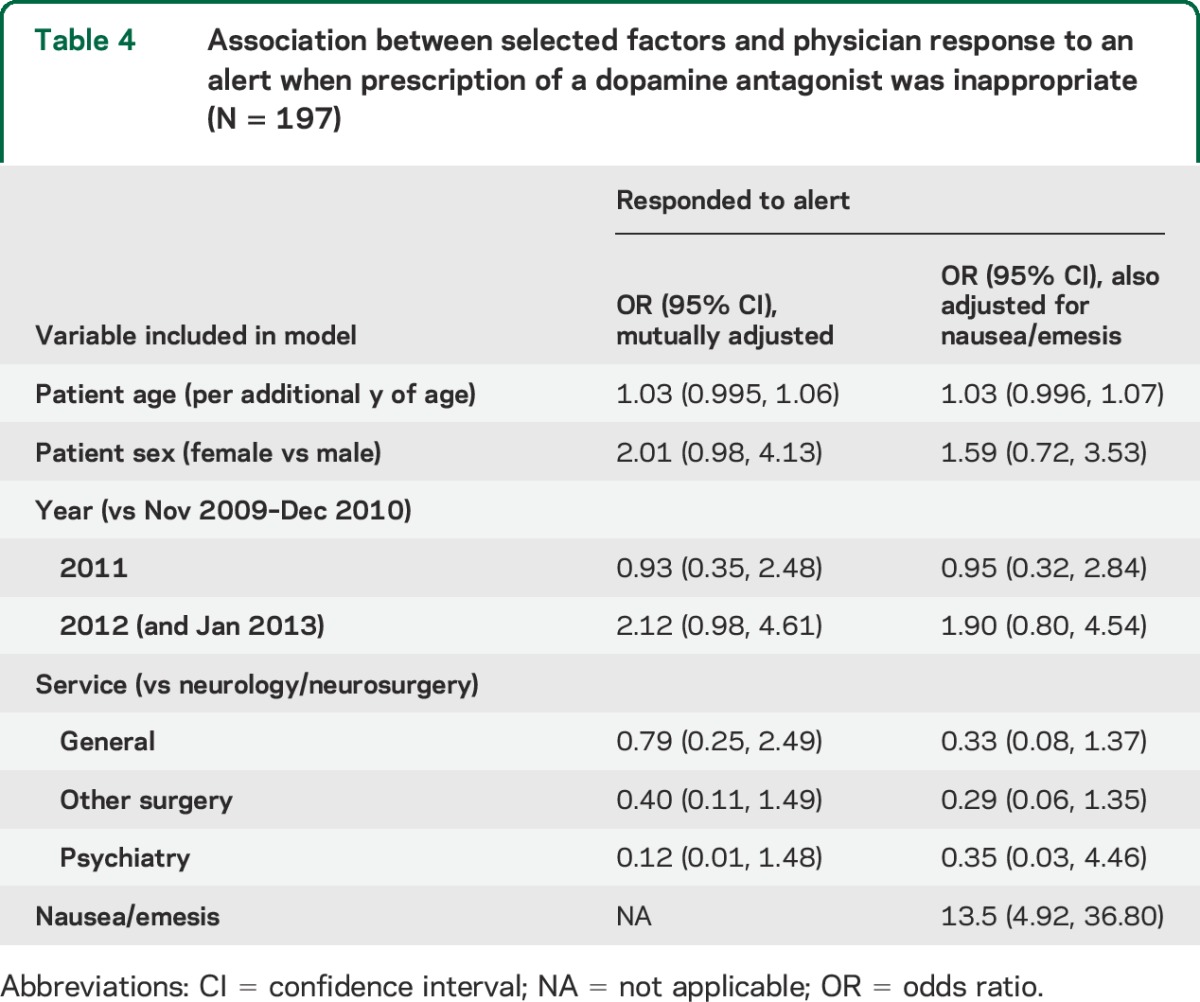
Of the 197 inappropriate prescriptions, only 51 (25.9%) were discontinued in response to the alert. Inappropriate orders were least likely to be stopped on the psychiatry service, with 94.7% of inappropriate orders continued despite the alert. In general medicine, surgical, and neurologic/neurosurgical services, inappropriate orders were continued in 68.2%, 82.0%, and 66.7% of dopamine-requiring patients, respectively. Results from multivariable regression accounting for age, sex, and year of the alert were largely consistent with these descriptive findings (table 4). The strongest predictor of dopamine antagonist discontinuation, i.e., response to the alert, was the use of a dopamine antagonist for nausea/emesis (OR 13.5, 95% CI 4.92–36.80). In general, compared with neurologic services (neurology and neurosurgery), non-neurologic services were associated with continuation of the dopamine antagonist prescription despite the alert (p = 0.098), but there was otherwise little indication of differences across the different service types. Finally, there appeared to be greater responsiveness to alerts in the most recent years compared with the earliest years of the program (OR 1.90, 95% CI 0.80–4.54).
Table 4.
Association between selected factors and physician response to an alert when prescription of a dopamine antagonist was inappropriate (N = 197)
DISCUSSION
The results of this study suggest that the alert system identified instances in which the physicians did not provide the appropriate management of patients with PD and other dopamine-requiring diseases. However, the alert system was not effective in preventing the inappropriate medications from being given, as the majority of orders were continued despite the alert. Neurologic services were more responsive to the alert system and less likely to prescribe dopamine antagonists inappropriately than non-neurologic services. These findings are probably due to greater familiarity with the neurologic diseases and the repercussion of inappropriate dopamine receptor blocking medications in patients with parkinsonism. The strongest predictor of discontinuation of inappropriate dopamine receptor antagonist prescription was use for nausea and emesis. Physician willingness to switch to a different medication likely reflects the availability of ondansetron, which is a well-tolerated alternative that is commonly used in the hospital. The alternative choices for managing psychotic patients with parkinsonism are more complicated and include clozapine, quetiapine, and adjustment of PD medications. Although the system failed to meet our goal of substantially improving the quality of patient care with regard to inappropriate use of dopamine antagonists, the potential decline in inappropriate prescriptions, as well as improvement in responsiveness to the alert over time, suggests that there may be a gradual increase in awareness through repeated exposure to this alert.
Although not directly addressed in our study, alert fatigue may contribute to the high rates of continuing inappropriate medications despite alerts. The alerts examined in this study were likely only a fraction of the alerts that resulted as each patient's medications were entered into the electronic system. When dozens of alerts are triggered from a medication list, physicians can become desensitized to them and override alerts,8 although at least one study found no correlation between drug-drug alert override rates and the number of alerts.9 Other studies have suggested that alert fatigue can result in adverse outcomes.10,11 One alternative to the simple alert that indicates a medication may be contraindicated in a patient taking carbidopa-levodopa would be an alert that also provides alternative medication suggestions. The impact of this type of modified alert could be measured easily through the electronic order entry system and could represent an important patient safety intervention to reduce patient morbidity, length of stay, and cost. Similarly, methods that reduce “interruptive” alerts12 could reduce alert fatigue and improve responsiveness to critical interactions like those that we studied. One possible approach would be prioritization of drug-drug alerts such that only the most important alerts are presented to the provider, using a contextual model to select those alerts that are most clinically relevant.13 Not all possible drug-drug interactions require the same level of provider scrutiny.
There are several potential limitations to this study. Retrospective examination of medical records provides only the medical context for the prescriptions. Physician reasoning was not always apparent in what was written in patient notes. In addition, physicians' responses to the alert were not explicit in the medical records, and our rules for determining their responses had to be based on the time the dopamine antagonist was discontinued and whether doses were given after the alert. We also could not measure patient outcomes or costs since our system does not have an integrated health care record and many patients receive follow-up care outside of the hospital system. Demonstrating that these medication choices led to greater morbidity and cost could accelerate changes in policies and procedures to prevent inappropriate dopamine receptor antagonist prescription. Nevertheless, this study demonstrates the need to rethink strategies for prevention of adverse outcomes for vulnerable hospitalized patients with parkinsonism.
GLOSSARY
- AAN
American Academy of Neurology
- CI
confidence interval
- OR
odds ratio
- PD
Parkinson disease
AUTHOR CONTRIBUTIONS
Marie Morris collected data and prepared the first draft of this manuscript. Allison W. Willis edited the original draft and performed statistical analysis. Susan Searles Nielsen performed statistical analysis and edited this manuscript. Franklin McCann assisted with data collection and edited this manuscript. Angela Birke assisted with data collection and data management and edited the manuscript. Brad A. Racette supervised data collection, oversaw data analysis, and edited this manuscript.
STUDY FUNDING
This study was supported by the National Institute for Environmental Health Sciences (R01ES021488, K24ES017765, P42ES004696, R21ES017504, R01ES021488 to B.A. Racette), the National Institute of Neurological Disorders and Stroke (K23NS081087 to A.W. Willis), the Michael J. Fox Foundation, National Institute of Neurological Disorders and Stroke National Center for Research Resources (NCRR0) and NIH Roadmap for Medical Research Grant Number UL1 RR024992, the American Parkinson Disease Association, the St. Louis Chapter of the American Parkinson Disease Association, and the Brain Biology and Behavior Initiative endowment to University of Pennsylvania.
DISCLOSURE
M. Morris reports no disclosures. A.W. Willis receives government research support from the NIH [KL2 RR024994, K23NS081087] and National Center for Research Resources (NCRR0) and the Brain Biology and Behavior Initiative endowment to University of Pennsylvania. S. Searles Nielsen, F. McCann, and A. Birke report no disclosures. B.A. Racette received research support from Teva (PI), Adamas Pharmaceuticals (PI), Auspex Pharmaceuticals (PI), Eisai (PI), Allergan (PI), Merz Pharmaceuticals GmbH (PI), Pfizer (PI), Civitas Therapeutics (PI), Kyowa Hakko Kinn Pharma (PI), and AbbVie (PI); received government research support from NIH [K24ES017765 (PI), R21ES17504 (PI), R01ES021488 (PI), R01ES021488-02S1 (PI), P42 ES04696 (co-I)]; and received research support from the Michael J. Fox Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Miyasaki JM, Shannon K, Voon V, et al. Practice Parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:996–1002. [DOI] [PubMed] [Google Scholar]
- 2.Goetz CG, Blasucci LM, Leurgans S, Pappert EJ. Olanzapine and clozapine: comparative effects on motor function in hallucinating PD patients. Neurology 2000;55:789–794. [DOI] [PubMed] [Google Scholar]
- 3.Breier A, Sutton VK, Feldman PD, et al. Olanzapine in the treatment of dopamimetic-induced psychosis in patients with Parkinson's disease. Biol Psychiatry 2002;52:438–445. [DOI] [PubMed] [Google Scholar]
- 4.Ondo WG, Levy JK, Vuong KD, Hunter C, Jankovic J. Olanzapine treatment for dopaminergic-induced hallucinations. Mov Disord 2002;17:1031–1035. [DOI] [PubMed] [Google Scholar]
- 5.Weiden PJ, Iqbal N, Mendelowitz AJ, Tandon R, Zimbroff DL, Ross R. Best clinical practice with ziprasidone: update after one year of experience. J Psychiatr Pract 2002;8:81–97. [DOI] [PubMed] [Google Scholar]
- 6.Avorn J, Gurwitz JH, Bohn RL, Mogun H, Monane M, Walker A. Increased incidence of levodopa therapy following metoclopramide use. JAMA 1995;274:1780–1782. [PubMed] [Google Scholar]
- 7.Ganzini L, Casey DE, Hoffman WF, McCall AL. The prevalence of metoclopramide-induced tardive dyskinesia and acute extrapyramidal movement disorders. Arch Intern Med 1993;153:1469–1475. [PubMed] [Google Scholar]
- 8.van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc 2006;13:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryant AD, Fletcher GS, Payne TH. Drug interaction alert override rates in the meaningful use era: no evidence of progress. Appl Clin Inform 2014;5:802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller AM, Boro MS, Korman NE, Davoren JB. Provider and pharmacist responses to warfarin drug-drug interaction alerts: a study of healthcare downstream of CPOE alerts. J Am Med Inform Assoc 2011;18(suppl 1):i45–i50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carspecken CW, Sharek PJ, Longhurst C, Pageler NM. A clinical case of electronic health record drug alert fatigue: consequences for patient outcome. Pediatrics 2013;131:e1970–e1973. [DOI] [PubMed] [Google Scholar]
- 12.Phansalkar S, van der Sijs H, Tucker AD, et al. Drug-drug interactions that should be non-interruptive in order to reduce alert fatigue in electronic health records. J Am Med Inform Assoc 2013;20:489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riedmann D, Jung M, Hackl WO, Stuhlinger W, van der Sijs H, Ammenwerth E. Development of a context model to prioritize drug safety alerts in CPOE systems. BMC Med Inform Decis Mak 2011;11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]


