Introduction
This report reflects the state-of-the-art and future directions of basic and clinical research into biomarkers for Duchenne Muscular Dystrophy (DMD), as discussed during the 204th ENMC workshop in Naarden, from January 24–26, 2014. Biomarkers have been defined as cellular, biochemical, molecular alterations or biological characteristics that are measurable in biological material as indicator of normal biological or pathogenic processes [1]. Biomarkers may be used in differential and early diagnosis, and in monitoring of disease progression, regression, or therapeutic responses. Duchenne Muscular Dystrophy (DMD) is a severe hereditary muscle disorder due dystrophin gene mutations and presenting with variable clinical severity [2]. Recently novel experimental drugs have been developed for DMD and several trials are ongoing, raising the urgent need of having fine tools for measuring trial outcomes as well as for optimizing the selection of eligible patients [3, 4]. The use of clinical parameters measuring muscle strength and function is limited due to their dependence on motivation, large intra-individual variability, lack of linear relationship between the 2 and slow response time [5]. Conversely, molecular biomarkers may show earlier response to treatment and reflect the different pathophysiological aspects of the disease [6]. The use of biomarker panels for the diagnosis, prognosis, and monitoring of DMD (and more in general of rare chronic neuromuscular disorders or NMDs) as well as to guide the choice of therapeutic regimens may significantly improve the current clinical practice, by facilitating the evaluation of emerging therapies in drug trials and their regulatory approval. On the other hand, during the drug development process, the availability of a biomarker able to predict drug response and/or occurrence of adverse events could be of utmost importance and could also reduce the costs of the drug development [7]. Biomarkers can also serve for patient stratification and selection of appropriate subjects for clinical trials. Biomarkers can therefore have a positive impact on the economical load, patients’ care and novel therapies.
Since the field of biomarker development is in a state of flux and given the need for biomarkers for DMD, an ENMC workshop took place in Naarden on the 23–25th of January 2014. It aimed at sharing data and results on biomarker discovery and validation for DMD, as well as to define strategies to implement biomarker discovery and use in DMD clinical trials. 27 people attended, including clinicians, researchers, drug companies, parents association, and an expert associated to EMA.
Workshop Aims
The workshop intended to increase cooperative and harmonised effort in designing common research plans for biomarker discovery and development, and bridging clinical measures and biomarkers research. A comprehensive overview on biomarker research in DMD was presented, highlighting some established and yet unpublished data, and encouraging data sharing and collaboration, which is necessary for validation of biomarker data in larger cohorts. A highly multidisciplinary group attended the workshop. The focus of the workshop was on Duchenne muscular dystrophy including ongoing trials, extremely well defined pathomechanisms, currently available exploratory biomarkers as a prototype for this field. Nevertheless the output of this workshop is expected to be useful for other NMDs. This collaborative EU and USA workshop aimed at establishing a large cooperation in order to increase critical mass, patient data and bio-samples to allow robust biomarker validation.
The following sessions were focused on: 1) the need for additional biomarkers for DMD, 2) strategies to identify and employ biomarkers with focus on novel high throughput technologies, 3) known biomarkers, 4) outcome measures in DMD and how biomarkers may be surrogates for outcome measures, 5) role of modifying factors in DMD, 6) planning the future for biomarker identification. This last session was organised in concurrent roundtables in order to concretise the output of the workshop in future actions. The key topics considered to set up collaborations were registries and data sharing, biomarkers in clinical trials and integration with the clinical outcome measures and EMA requirement for biomarker regulation.
Session 1: The need for biomarkers
Alessandra Ferlini (Ferrara University) introduced the characteristics and meaning of biomarkers by defining key terms: analytical validity (accurate measure of specific parameter), clinical validity (finely measuring disease characteristics with bidirectional correlation), stability (not influenced by e.g. exercise, diet etc.), clinical utility (likelihood of improvement), feasibility (including the concept of repeatability which confers robustness to biomarkers, time and cost effective issues.
There are two main types of biomarker categories:
Diagnostic (disease diagnosis, progression, disease stratification, disease screening, disease mechanisms)
Therapeutic (pharmacokinetic, pharmacodynamic, prognostic, surrogate endpoints for therapy monitoring, safety, efficacy).
Biomarkers are important in all NMDs since these are progressive diseases, requiring chronic treatment. Clinical outcome measures certainly serve as a gold standard for monitoring disease course and trial outcomes. However, in paediatric NMDs clinical outcomes may have insufficient sensitivity to detect small changes/benefit occurring during the usually short clinical trials.
The task of biomarker discovery requires considerable effort and may be targeted, relying on candidate pathways, or may be less biased using targeted “omics” approaches. The two approaches are not mutually exclusive, as both can be combined [8]. Following the discovery phase, validation in large homogeneous patient cohorts is mandatory in order to provide robustness to the data. If a biomarker becomes validated, regulation and development processes (generally carried out by companies) will drive the biomarker into the translational field via qualification by Regulatory authorities and approval. Ferlini also summarised aims and goals of the BIO-NMD project European Union funded project in which collaborative studies focused on DMD biomarker identification were performed (www.bio-nmd.eu).
Considering the state-of-the-art of DMD as well as the current therapeutic options, the urgent questions for biomarker search revolve around the following points:
analyzing information flow (defining common strategies, pipelines, methods);
validating and translating biomarkers into clinical practice which necessitates sufficiently powered translational cohorts. As DMD is a rare disease, international cooperative efforts are needed;
methods to share results and define homogeneous guidelines for the biomarker mechanistic flowchart.
Alessandra Ferlini listed the main workshop milestones which are:
data sharing (current state of the art in EU and USA)
identifying planned/ongoing studies
defining biomarker discovery modalities (harmonization needed)
defining models for biomarker validation and development
defining main tasks for clinical translation
biomarker prioritization to facilitate regulatory validation
She also introduced the Biomarker working group at IRDIRC and its main aims, some of these synergistically overlap to those of the ENMC workshop (see at www.irdirc.org).
Afrodite Lourbakos (Prosensa Therapeutics) presented the biomarker requirements from the industry perspective, which are based on three main processes: method validation for measuring a biomarker, biomarker qualification process (exploratory, probable validated biomarker) along with the drug development process. It is important for industry to consider the following points: a) is a biomarker plausible based on current scientific knowledge, b) can it be measured as far as technology allows and meaningful results that are reliable and can be interpreted are obtained, c) is it ethical to justify the samples required from patients.
Inclusion of biomarkers in clinical protocols is important yet the primary efficacy objective is the clinical outcome, as we aim to treat a disease and biomarkers can be useful as secondary endpoints. The Clinical Protocol which is reviewed by Ethics committees along with the informed consent that patient signs defines which biomarkers are measured in a study, and clinical protocol amendments need to be approved by the ethics committee for the measurement of newly discovered/proposed biomarkers.
Analysis of clinical samples performed under GCLP is described in an analytical plan, which aligned with the clinical protocol, and analysis done by operators with qualification records under the guidance of a quality assurance department.
There are well known sample collection issues: defining the most appropriate samples (invasive or non-invasive), the number and frequency of sample collection, informed consent status, the setting (clinical trial or research), training or support of staff, shipment and storage of samples, infrastructure, and sample quality. Processing samples is based on different methods (quantitative, qualitative) and correspondent validation procedures, which are described by FDA and EMA guidelines.
Biomarkers can be studied prospectively (f.i. predictive and pharmacodynamics biomarkers in placebo controlled trials) or retrospectively (as for exploratory biomarkers in completed, randomized trials). In prospective studies Analytical Plans providing analytical details (cutoffs, statistical methods, etc.) interpretational guidelines (level of statistical significance, magnitude of effect, etc.) are needed [9]. Since natural history studies are important in the identification and qualification process of biomarkers, Prosensa is conducting a study with 250 DMD patients for 3 years (currently 80 subjects enrolled for 1 year) with focus on disease progression and serum biomarkers.
The general discussion touched on informed consent documents, including the acknowledgement that some existing studies may permit use of samples for biomarker discovery if for example samples are stored in approved biobanks. In the future, clinical trials in DMD should allow for biomaterials to be used for biomarker discovery. This will require specific agreements with pharmaceutical companies as well as ethical issues to be considered with adequate informed consents to be signed by patients.
Giuseppe Novelli (TorVergata University, Rome) illustrated the biomarker requirements from the regulatory perspective. The aims are harmonising the procedure for biomarker interpretation and application of technical guidelines and requirements for product registration (across EMA, FDA, Japanese regulatory authorities). Meetings and conferences such as ICH (International Conference on Harmonization) are instrumental for this. Working parties are formed and the guidelines already produced. EMA pays lots of attention on pharmacogenomics with pharmacogenomics working party providing recommendations. EMA has focused on pharmacogenomics, personalized medicine and biomarker qualification. Genomic biomarkers do not expand to proteomics and metabolomics but genomic biomarker rules are likely to be translated to rules for proteomics and metabolomics. Recent attention has been paid on personalized medicine and biomarker qualifications by:1) dedicated biomarker qualification procedures; 2) developing of regulatory standardized procedure; 3) agreement on what is needed to confirm that a biomarker is specific, sensitive, reproducible and can be used for regulatory decision making.
There are differences between pharmacogenomics (related to both DNA and RNA) and pharmacogenetics (which applies to DNA markers only) biomarkers. Genomic biomarkers need to be scientifically robust, clinically relevant, and practical to implement. The qualification pilot process requires usually at least 200 days and if successful is released to public consultation in around 2 months.
A voluntary exploratory data submission is now possible and modalities are reported [10]. EMA has formed an Innovation task force (ITF), which organises briefing meetings, it also includes scientific and legal competences. There is a specific Forum to discuss and dialogue with applicants before submission of scientific data (preparatory activities).
Briefing meetings are meant to complement and reinforce full applications. In the current phase of biomarker development for DMD, briefing meetings with the ITF are probably the more appropriate way to communicate with EMA.
Future Directions
Currently, 20% of all drug applications contain some aspects of personalized medicine. For 13 drugs, it is now mandatory to perform a genetic test before the prescription of the drug to the patient (EMA guidelines). Costs of precision medicine based on personal whole genome sequencing will be certainly reduced [11]. and this omics profiling might become routine in biomarker studies. This approach opens to the incidental findings, due to the application of high throughput genetic analyses. Therefore the cooperation between academia and industry is important for analyzing incidental findings and biomarker research in general.
Elizabeth Vroom (United Parent Project Muscular Dystrophy, The Netherland) presented the biomarker requirements from the patients’ perspective.
Parents and patients feel the need of the development and validation of biomarkers in order to speed up drug development. According to the EMA website, it is expected that the use of biomarkers in research will contribute to faster public access to new medicines*. So patients/parents feel the need of reliable BIOMARKER to get access to treatments.
Parents realize trials that only consider clinical endpoints may need to be long to allow assessment of clinical efficacy of the tested drug. Families want trials to be as short and safe as possible, preferable using non-invasive measurements and giving answers for all stages of the disease. Global harmonisation of regulatory perspective on the use of biomarkers is needed.
There is a lot of confusion in the Duchenne Community about the role of different biomarkers in drug development. The difference between ‘biomarker’ and ‘surrogate endpoint’ (type of biomarker that has been validated to be used as primary/secondary endpoint) is not always clear and sometimes hard to understand. More specific the restoration of dystrophin during a clinical trial is seen by several parents as enough evidence to approve a drug.
Education of patients/parents is needed, as well as more consistent use of terminology by professionals. A lay document to clarify the role of biomarkers was reported [12]. UPPMD document has been generated as a spin-off activity of this workshop (http://exonskipping.eu/wp-content/uploads/2014/02/UPPMDBiomarkerExplanation.pdf). (see in *http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/general/general_content_000349.jsp)
Session 2 : Set up of biomarkers studies
Hanns Lochmuller (Newcastle University) illustrated the issues related to registries, collection and samples sharing in DMD. Addressing the translational pathway is the major focus of the TREAT-NMD Alliance. Biomarker research is a new topic for the 3-year work plan of the Alliance. TREAT-NMD has facilitated the set-up and standardisation of patient registries for clinical trials, as genetic data is necessary for patient recruitment. DMD patient registries grew exponentially over the last 7 years, and in 2014 they hold information on more than 10,000 DMD patients in 40 countries [13]. The registries represent a research tool for standards of care, trial readiness, and biomarker discovery. Recent analysis of the registry data revealed a change in DMD natural history with introduction of interventions and treatments, new standards of care, and increased survival, but also differences between countries (e.g. treatment with corticosteroids). Different standards of care clearly correlate with outcomes, such as ambulation, steroid users are 5 times more likely to walk at age between 12 and 17 years. Biomarker research needs to take these changes and differences into account. Several lessons were learned from BIO-NMD: with dedicated clinical personnel many DMD serum samples were collected and deposited in biobanks for future use. However, there are only few longitudinal samples. Sample collection through standardized SOP is essential, and informed consent should include multiple purposes rather than just one experiment or project. Good clinical phenotyping is essential and needs manpower, and the phenotype dataset should be restricted to the most relevant fields. Samples from all comers should be collected as part of the clinical routine rather than cherry picking patients with specific features. Existing infrastructure should be used for sample collection (ongoing clinical trials, natural history studies, biobanks, registries) as much as possible. Biomarker research is an important part of new EC-funded research consortia such as Neuromics and RD-Connect under the auspices of the IRDIRC (www.treat-nmd.eu; www.rd-connect.eu; www.rd-neuromics.eu; www.irdirc.org). The following topics were identified by workshop participants in relation to biomarker research: availability of normal control samples, especially from children; the use of plasma or serum for blood biomarkers; further standardization of clinical items; data sharing.
Afrodite Lourbakos (Prosensa Therapeutics) offered an overview on biomarker studies in clinical trials. The ideal situation for a biomarker in clinical trial would be that we target the disease cause and monitor the causal protein (dystrophin), but in practice we should look at a whole spectrum of DMD pathology. The therapeutic pathway for antisense oligonucleotides (AONs) effect is known [3]. A number of markers are currently monitored in AON clinical trials: a) imaging biomarkers (Magnetic resonance imaging, MRI, and Positron emission tomography, PET); b) molecular biomarkers (DNA, RNA, proteins, lipids, sugars, metabolites); c) pharmacokinetic as AON concentration in plasma and muscle; d) pharmacodynamic (skipped transcript, dystrophin rescue); e) disease biomarkers (miRNA profile, inflammatory and fibrosis markers, serum CK, MMP9); f) pharmacogenetics (SNPs); g) safety biomarkers as ALT, thromobocytes, urine metabolites.
Patient variation in AON trial response may be due to deletion type (to confirm proper diagnosis), patient genetics (e.g. SNPs impacting on progression, or severity or treatment response) and patient disease severity and progression. Creatine kinase in serum is routinely used for DMD diagnosis. CK decreases with disease progression and age (above 5–6 years of age). CK declines yearly between 8.7% and 18% with high intra-individual variation overtime [14, 15]. CK was measured in drisapersen trials and was specified in clinical protocols as secondary efficacy endpoint, with primary efficacy endpoint being 6MWD. CK assay data were presented from 3 drisapersen studies. Table 1 shows the CK analysis in subjects treated with 6mg/kg drisapersen, in the three different clinical trials. In conclusion, albeit CK reduction is interesting (since there is a difference between treatment and placebo) CK results need to be correlated to other biomarkers. Further examination is ongoing to evaluate changes in CK per subject in sub-population in these Prosensa studies (changes related to age, clinical outcome, other biomarkers, imaging, ambulant vs non/ambulant).
Table 1.
Table for CK analysis in subjects treated with 6mg/kg drisapersen.
The CK analysis results in DMD patients treated with 6mg/kg drisapersen are shown. In the Clinical Study DMD114117 (phase 2, weekly vs intermittent vs placebo, 53 subjects) the CK profile shows a trend towards a CK reduction in both trials arms with drisapersen compared to placebo. In the Clinical Study DMD114044 (phase 3, treatment vs control, 186 subjects) the CK profile in treated and placebo patients (Pivotal study) till week 48 shows a large and significant decrease. In the Clinical Study DMD 114876 (phase 2, 3 or 6 mg/kg vs placebo, 51 subjects) CK is decreasing in 6mg/kg treated patients.
| Trial | Visit (week) | CK Treatment difference (IU/L) | p-value |
|---|---|---|---|
| DMD114876 | 24 | −1058 | 0.534 |
| DMD114117 | 49 | −1736 | 0.303 |
| DMD114044 | 48 | −4045 | <0.001 |
In the general discussion, the pathophysiological meaning of CK was questioned, since CK reflects different parameters as necrosis, muscle leakiness (sarcolemma integrity) and muscle-intrinsic parameters [15] Therefore the decrease of CK after drisapersen, but also documented in ataluren and gentamycin trials [16] may non-specifically reflect membrane stabilization.
Alessandra Ferlini (Ferrara University) introduced the genomic technologies for biomarker discovery. She reported on a profilomics approach, that means defining the omics (as exome, transcriptome and proteome) profile in the same patient/individual, in DMD patients to identify biomarkers for corticosteroid response. Corticosteroids are the only proven efficacious drug for ameliorating DMD phenotype. Among DMD patients, responders and low responders are, however, well described [17]. Considering the known side effects of corticosteroid therapy, identifying poorly responsive patients is a key point. Data analysis is ongoing. The novelty of this study is discovery biomarkers by integrating multiple omics strategies in order to identify biomarkers supported by multiple evidences. The bottleneck of omics analysis is adopting adequate statistical tools. Chiara Scapoli (University of Ferrara) has set up novel tools to confer more statistic power to small patient numbers when using omics data.
The general discussion focused on the meaning of omics data and SNPs analysis. DMD boys with different corticosteroid response show a profoundly different proteomics profile. This data supports the possibility to use proteomic data (in skeletal muscle) in early disease stage for predicting steroid response.
The profilomics data suggest that the muscle homeostasis is longer maintained in steroid responders and the drug response might already be predictable at time of diagnosis, based on the signature in the biopsy. Pharmacogenetics SNPs possibly involved in steroid response might be also implicated in disease progression.
Cristina Al-Khalili- Szigyarto (KTH Institute, Stockholm) introduced the issue of biomarker technologies on protein metabolites.
When studying proteomics, different protein parameters should be taken into account, namely a) tissue distribution; b) subcellular distribution; c) abundance; d) modification patterns; e) interaction patterns.
Many platforms use antibodies (abs) that constitute a valuable resource for proteomics studies. The Human Protein Atlas program has a large antigen (protein epitope Signature Tags - prEST) and an antibody collection generated in-house. Both the antibodies and the prETSs are valuable reagents for various proteomics methods. The program aims to create a large repository of antibodies and has generated up to date 21984 ab targeting 16621 gene products (www.proteinatlas.org).
The antibodies are used for protein expression profiling in tissues, cells and body fluids using Western blot, protein arrays, immunohistochemical staining of tissues and immunofluorescence microscopy. Since the specificity of antibodies is context and application dependent, to increase confidence, annotation of protein expression is based on experimental results obtained with paired antibodies. Validation of protein expression can be subsequently further scrutinized by comparison to non-antibody based experimental evidence or theoretical predictions (Uniprot, ENSEMBL, CCSD and literature). Protein expression can be validated by analysis of transcript performed by RNA-seq.
Antibodies combined with the bead-based array technology allow analysis of body fluids using only small volumes of serum/plasma. This approach and generated data was recently published [18].
In the general discussion the genetic and environmental factors possibly influencing protein expression were debated. Among these, some proteins and their interaction with antibodies may be age-dependent, considering protein abundance.
Yetrib Hathout (Childrens National Hospital, Washington) presented data on mass spectrometry methods for discovery of serum surrogate biomarkers in DMD in particular a novel technique based on targeted mass spectrometry (MS) to accurately quantify levels of dystrophin protein in skeletal muscle extracts. The technique was found to be reproducible and linear over 5 orders of magnitude [19]. The technique was tested to measure levels of restored dystrophin in mdx mice injected with morpholino drug (PMO). There were differences in the amount of restored dystrophin between muscles and between mice. Good correlation between dystrophin quantified by mass spectrometry and immunohistochemistry was observed. The correlation between mass spectrometry and western blot was weaker. He also presented data on serum protein biomarkers discovery in mdx mouse model and cross-comparison with DMD patients. Using SILAC mouse strategy he was able to identify and quantify 355 proteins in sera samples of mdx and wild type mice of which 27 potential markers associated with dystrophin deficiency. Elevated biomarkers (21 proteins) were mostly of muscle origin and included myofibrillar proteins, glycolytic enzymes, transporter proteins and many other muscle specific proteins. Decreased proteins were mostly of extracellular origin involved in extracellular matrix remodelling. Similar biomarkers were confirmed by mass spectrometry in sera of DMD patients. Levels of several of these potential biomarkers correlated with disease progression in both mdx mouse and in serum samples of DMD patients but decreased with age in similar manner as CK.
The general discussion was focused on the precision and sensitivity of the MS methods to detect proteins, and the challenges to identify specific dystrophin isoforms or truncated shorter isoforms, either in tissues and cell cultures.
Elena Schwartz (Ariadne DX, USA) overviewed the interpretation of biomarker studies. She presented an Integrated Translational Approach to Developing Surrogate Biomarkers for Clinical Trials in DMD. Three issues were discussed: 1) discovery of DMD-Associated Biomarker Candidates, 2) data integration and 3) Biomarker Prioritization.
Developing a Neuromuscular Disease (NMD) Knowledgebase: Ariadne Diagnostics was a member of the EU-funded consortium, Bio-NMD. A large amount of biomarker data was generated by the project members; including single nucleotide polymorphisms (SNPs), altered gene and protein expression. Using sophisticated bioinformatics tools and algorithms, all data was overlaid with existing information regarding known protein-protein interactions to create an NMD-Knowledgebase of cellular and molecular pathways affected by DMD. This Knowledgebase has a series of nine cellular pathways. These include: Calcium Overload, Oxidative Stress, Inflammation & Fibrosis, Muscle Function & Contraction, Skeletal Muscle Remodelling, MSTN (myostatin) – IGF1 Crosstalk, Glucose Metabolism, Glycogen Metabolism, Tricarboxylic Acid Cycle. The calcium overload pathway is of particular interest given that it is known to lead to apoptosis, fibrosis, muscle cell differentiation and regeneration.
Ariadne-Dx team presented data obtained in the BIO-NMD project using advanced bioinformatics analyses to identify and prioritize easily assayable biomarkers from vast amount of “omics” data collected. Top 100 DMD-associated genes were identified based on the prioritization scheme.
Summarising the data presented, Ariadne has mapped the major DMD-associated/affected disease pathways, and identified a broad collection of DMD-associated biomarkers [20]. The approach based on identified pathways may indicate novel drug targets to reduce/control the damaging effects of dystrophin loss.
Session 3- Known biomarkers in DMD
Elena Pegoraro (University of Padua) illustrated the data on osteopontin (SPP1) as biomarker as a collaborative project between University of Padova and Childrens Hospital (CINRG) in Washington. A discovery phase was based on SNPs located in genes involved in muscle size, strength, response to training, inflammation and metabolism (19 candidate SNPs); and differential expression profiling experiments in mild vs severe DMD (47 candidate SNPs). 106 patients from Padova (no siblings in the cohort) and 156 from CINRG were considered for validation. Steroid regimen in the two cohorts was variable. SNP rs28357094 in SPP1 promoter was genotyped and a dominant model was used. A significant correlation between rs28357094 genotype and muscle strength decline using all muscle phenotypes was reported with the T allele associated to a better muscle performance. Survival analysis for age at loss of ambulation confirmed the advantage for the T carrying patients. However no covariate analysis including the DMD mutation was done.
An Italian multicenter longitudinal study with 1 year follow-up was done to evaluate if the stratification for SPP1 rs28357094 [21]
The hypothesis is that SP1 drives SPP1 transcription in patients carrying the T allele, where in the G carrying patients the transcription is driven by glucocorticoids and results in a higher OPN expression level. Preliminary results in DMD muscle culture showed that deflazacort treatment decreases OPN expression in both myoblasts and myotubes in DMD carrying T allele, whereas deflazacort does not decrease OPN in myotubes in G allele carrying patients. It is conceivable that the higher OPN level may exacerbate pro-inflammatory cascade and maybe detrimental for muscle.
The implications of SPP1 as genetic modifiers in disease severity and steroid treatment were discussed.
Kevin Flanigan (Nationwide Children’s Hospital) presented data regarding the LTBP4 gene as a modifier for DMD, using data from the United Dystrophinopathy Project gathered from 7 centers in the US [22] LTBP4 encodes latent TGFβ binding protein 4, and was identified as a modifier in a genome wide scan in mouse models of muscular dystrophy [23]. Among a cohort of 874 phenotyped, dystrophinopathy patients (including 674 diagnosed with DMD, 160 with Becker muscular dystrophy [BMD], and 45 with intermediate muscular dystrophy [IMD]), 254 were identified who had lost ambulation before age 20 years, regardless of the clinical classification. In the human LTBP4 gene, four coding region non-synonymous SNPs define two common haplotypes, referred to as VTTT and IAAM. Although no association with SPP1 was seen in this cohort, those DMD patients homozygous for IAAM had prolonged ambulation, and the finding was more pronounced in steroid-treated patients (to age 12.5 ± 3.3 years, versus 10.7 ± 2.1 years at loss of ambulation) than in steroid-naïve patients (11.2 ± 2.7 vs. 9.8 ± 2.0 years) [24]. Consistent with the hypothesis a protective effect from this haplotype, patient fibroblasts homozygous for IAAM fibroblasts demonstrate reduced TGFβ signalling compared to those with a VTTT allele. These results suggest that the effect of LTBP4 genotype should be considered as a pre-defined post-hoc analysis in clinical trials.
David Israeli (Genethon, Paris) presented miRNA as biomarkers for DMD. The deregulation of a number of miRNAs in serum and plasma samples of DMD patients and animal models has recently been demonstrated by a number of groups. These were collectively termed the “dystromiRs”. A collaborative study of Genethon and the Institute of Myology focused on mouse models for various muscular dystrophies. This study identified disease-specific serum miRNA profiles and the dysregulation, in common for degenerative muscular dystrophies, of the dystromiRs miR-1, miR-133a, miR-133b, miR-193b, miR-206 and miR-378 [25]. A recent multicentric large-scale study was focused on the GRMD dog model for DMD. High throughput sequencing and RT qPCR technologies were employed for the profiling of hundreds of serum samples, and resulted in the identification of three distinct groups of deregulated miRNAs. The deregulation of certain miRNAs was evaluated further in the mdx mouse model and in cohorts of DMD and BMD patients. In addition to the previously identified dystromiRs (mentioned above), in GRMD dog serum the deregulation of miR-95 was found. This miRNA, present in primate and dogs but not in rodent, is transcribed from an intron of the ABLIM2 gene, which encodes for a skeletal muscle enriched muscle-actin interacting protein.
The DLK1-Dio3 genomic locus miRNAs is one of the largest miRNA clusters in the human genome. DLK1-Dio3 miRNAs are upregulated in regenerating muscle in the mouse and are deregulated in hypertrophic hind limb muscle of the Callipyge sheep. In GRMD dogs the entire miRNA cluster is highly upregulated in the serum. The cardiac-enriched miRNA group includes miR-208a, miR-208b and miR-499 that were identified upregulated in the serum in GRMD dogs. Of these miR-208b and miR-499 are expressed in both skeletal and cardiac muscle while miR-208a expression is thought to be heart-specific. No correlations were found between a set of cardiac functional parameters and serum levels of these three cardiac enriched miRNAs in a study of a cohort of one-year old GRMD dogs. Evidence was presented for temporal large fluctuations in miR-208a expression level in GRMD sera. It was hypothesized that these fluctuations result from succession of discrete and transient episodes of cardiomyocyte degeneration. Upregulation of the cardiac-enriched miRNAs was confirmed in DMD patients (Jeanson-Leh et al., submitted). A study of a cohort of BMD patients confirmed serum upregulation of miR-206, but none of the other dystromiRs. An upregulation was found in this cohort of miR-499, which was more discriminative than miR-206 in a subgroup of the older patients in this cohort (Wahbi et al., submitted).
Pietro Spitali (from LUMC, Leiden) presented the data on dystrophin transcript quantification. Exon skipping and stop codon read-through are among the most promising and advanced therapeutic approaches for DMD. Since both target dystrophin transcript it is important to understand how much target is available for correction. So far standard operating procedures are not available for transcript quantification in patients with DMD. It is known from literature that the method used to quantify exon skipping is sensitive to the number of amplification cycles used, that the dystrophin transcript is lowly expressed with 1000–2000 copies per ng of mRNA meaning less than 1 copy per cell (or nucleus). Differences among muscles represent an extra layer of complexity since both dystrophin mRNA and protein levels have been reported to be different among muscle groups and especially higher in heart compared to the other muscles.
Detailed analysis of the dystrophin transcript in mdx mice and Becker patients showed reduced transcript levels towards the 3′ end compared to healthy controls [26]. Interestingly the degree of transcript imbalance significantly correlated with the dystrophin amounts in BMD patients. The cause of transcript instability was not identified yet but exon skipping (up to 30%) was not able to correct for transcript instability in mdx mice gastrocnemius muscles.
General discussion focused on the importance of a standardization of the protocol for exon skipping quantification.
Peter ‘t Hoen (LUMC, Leiden) reported on MMP9 as a serum biomarker for disease progression.
MMP-9 is currently the most advanced protein biomarker for disease progression and response to therapy, since it has been evaluated in >300 patients from three medical centres, with longitudinal data from >60 patients [27]. MMP-9 was initially selected because its mRNA was elevated in DMD muscles in a range of expression profiling studies, it is a known biomarker in other diseases with inflammatory component (such as rheumatoid arthritis) [28], and there is a robust ELISA system available for quantification.
MMP9 is involved in extracellular matrix breakdown and tissue remodelling. Dis-balance with tissue inhibitors of MMPs (TIMPs) may lead to fibrosis. MMP9 overexpression aggravates the muscle phenotype of dystrophin-deficient animals [29, 30].
Cross-sectional studies demonstrated higher MMP9 serum levels compared to healthy controls, despite considerable variation among cohorts. Technical validation of the findings was obtained with gelatin zymography and antibody arrays as alternative technologies. MMP9 serum levels increased significantly over time in two longitudinal cohorts, with follow-up on up to 5 time points over 5 years. Serum MMP-9 is most advanced as biomarker but more work is needed for qualification and approval as therapeutic biomarker. Ongoing measurements in a range of clinical trials for DMD are supporting the utility of MMP-9 as a biomarker for therapeutic response. An association of MMP9 serum levels with clinical outcome would further strengthen its utility.
Christina Al-Khalili Szigyarto (KTH, Stockholm) illustrated data on biomarker discovery in serum/plasma by immunoassays. Biomarker discovery is dependent on several aspects regarding: i)selection of samples relevant for the hypothesis; ii) adequate representation of the disease; iii) selection of appropriate control samples (age-matched); iv) large samples collections; v) high quality of samples; vi) accompanying patient data (harmonized).
Proteomics research on rare disorders is often impeded by the availability samples. In comparison to other disorders the number and the volume of samples are limited. These disadvantages can be circumvented by the use of different blood sample types e.g. both serum and plasma. Using multiplexed antibody suspension bead arrays for proteomic profiling of serum and plasma samples, biomarker candidates were identified based on concordances in the analysis from 4 cohorts [19]. The analysis was performed in small volumes of samples making it suitable for analysis of body fluids such as serum and/or plasma.
The majority of the proteins with differential expression profiles were proteins associated with muscle function and mitochondrial proteins. The targets contribution to the separation of DMD, BMD and controls was achieved by using a panel of 4 proteins CA3, ETFA, MYL3 and MDH2 as markers.
The results are supported by previous reports in mdx and chicken which indicate that CA3 is a more appropriate marker than CK due to a more specific expression pattern.
Kay Ohlendieck (National University of Ireland) showed data on profiling proteomics in skeletal muscle. The presentation provided an overview of the mass spectrometry-based proteomics of skeletal and cardiac muscle, using fluorescence two-dimensional difference in-gel electrophoresis (2D-DIGE) and label-free mass spectrometry. A comparative overview of key proteomic findings was presented with respect to disuse-related muscular atrophy, myotonia, muscular dystrophy and natural muscle aging. Proteins were categorized according to major biological functions, such as excitation-contraction coupling, the contraction-relaxation cycle, ion handing, muscle metabolism and the cellular stress response. In contrast to other neuromuscular disorders or the natural aging process, which exhibit often unilateral shifts in metabolic pathways and/or the contractile apparatus, the proteomic profiling of DMD has not revealed clear tendencies of switches in entire protein families, but a more generally perturbed protein expression pattern. However, individual proteins exhibit considerable changes in their abundance in dystrophin-deficient muscles, including certain glycolytic enzymes, mitochondrial enzymes, cytosolic calcium-binding proteins, calcium-regulatory proteins of the sarcoplasmic reticulum and many molecular chaperones [31, 32]. The proteomic characterization of the dystrophin-glycoprotein complex was carried out by studying total muscle extracts, crude microsomal fractions, highly purified sarcolemma preparations and isolated dystrophin complex. For the mass spectrometric analysis of isolated sarcolemma vesicles and the dystrophin-glycoprotein complex was used. This approach could clearly label individual protein bands that represent full-length dystrophin isoform and its associated glycoproteins and identify them by mass spectrometry. In summary, the comparative proteomic profiling of dystrophic tissue extracts has identified a large number of changes in the abundance of distinct muscle proteins. Some of these proteins may be useful as future biomarker candidates to improve diagnostic, prognostic and/or therapeutic
The general discussion focused on various technical aspects of proteomic analysis. Also the comparison between human and mouse data were discussed as well the different proteomic pattern in mdx skeletal muscle and heart, possibly due to the differing subcellular localization of the dystrophin-glycoprotein complex, since, while the muscle dystrophin complex is restricted to the sarcolemma, the cardiac complex is also located in the transverse tubules. It was also agreed that verification of proteomic findings should be always carried out by both immunofluorescence and immunoblotting analysis.
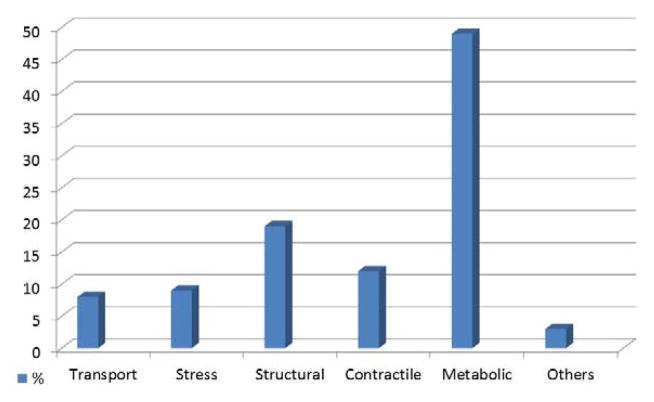
Cecilia Gelfi (University of Milan) reported results on DMD vs BMD proteomic profiling in skeletal muscle. Although the genetic basis of DMD and BMD are well resolved, the cellular mechanisms associated to physiopathology remain largely unknown. Secondary mechanisms may play important role contributing to muscle loss as non-mechanical processes. Studies on mdx mouse, and on dystrophic dog model suggested that alteration in signal transduction pathways are significant factors contributing to the disease. She studied biopsies of 15 DMD (1–8 years old) and 15 BMD (1–11 years old) provided by the Telethon bank and 30 healthy control subjects (20–40 years old). The proteomic study was based on 2D-DIGE followed by protein identification by MALDI MS/MS and ESI MS/MS. 75 spots were identified as differentially expressed between DMD and BMD. According to Group Ontology functional classes, the most represented classes were metabolic and contractile proteins (Figure 1). In particular metabolic proteins deregulated were less abundant in DMD patients. Contractile proteins were also less abundant in DMD patients, whereas proteins involved in regeneration and cytoskeletal remodelling were more abundant. Some trends were however opposite between DMD and BMD taken controls as reference. Major differences were observed in proteins regulating metabolism: BMD were characterized by a relatively spared muscle metabolic capacity compared to DMD. In particular, in BMD, proteins involved in lipid utilization were more abundant whereas enzymes regulating lipid synthesis were downregulated.
Figure 1.
Diagram showing the most relevant Gene Onthology functional classes deregulated between DMD and BMD patients, resulting from 2D-DIGE experiments. Biopsies of 15 DMD and 15 BMD aged matched patients were provided by the Telethon bank. Each sample was run in triplicate. One-way ANOVA coupled to Tukey’s multiple group comparison test were applied, the significance level was set at p<0.01. Proteins identified as significantly deregulated in DMD vs BMD muscles, were classified into 5 major categories. A large proportion of these proteins belong to muscle contraction, muscle development, cytoskeletal rearrangement, metabolism, stress response and transport.
Kevin Flanigan (Nationwide Children’s Hospital) reviewed the experience of the Biochemical Outcome Measures (BOM) group, formed as an international effort to assess methods for standardizing quantification of dystrophin levels with a goal toward use in clinical trials. Dystrophin quantification – whether by immunocytochemistry, immunofluorescence, or western blot, can be technically challenging. Open questions include what is the most important biological readout: overall dystrophin, or the number of dystrophin positive fibers?
Following discussions at a 2009 EMA/TREAT-NMD workshop [33], in which lack of harmonization in of quantification methods for dystrophin was highlighted the BOM group was formed.
Following a series of refinements of the techniques used, five laboratories took part in a multicenter validation effort: University College London, the Flanigan laboratory at Nationwide Children’s Hospital, Newcastle University, the Institute of Myology (Paris), and Prosensa (Leiden). All agreed to use only one of two preselected methods for immunofluorescence [34, 35], and three of the laboratories quantified dystrophin using both. The results were promising from the point of view of reproducibility, and are now part of a submitted manuscript (Anthony et al., under revision). There was good inter- and intra-lab variability, and very good correlation between the two published methods. The immunofluorescence results correlated with the western blot performed in each lab.
The consensus opinion of the BOM was that these results show that good inter-laboratory concordance in dystrophin quantification can be obtained through the use of standardized protocols making use of published and peer-reviewed methods. While further validation in a larger population of patients will be helpful, the group considers that western blot and immunofluorescence give complementary information, and combined analysis is likely the best approach. The group noted that the inter-lab reliability of counting dystrophin positive fibers has not yet been assessed, but remains challenging as it currently relies on a qualitative rather than quantitative call of what is a positive fiber. In all cases, the consensus remains that for studies of agents with mechanisms of action that are supposed to increase dystrophin expression or localization, comparisons to pre-treatment biopsies remains critical, as methods of absolute quantification of dystrophin are not yet practical. Discussion also centered difficulties in standardizing control tissue, due to variations in expression between individuals and even between muscles.
Session 4 – Clinical biomarkers in DMD
Eugenio Mercuri (Catholic University, Rome) presented the clinical outcome measures and deep phenotyping in DMD. New concepts in outcome measures: need for identifying measures that fit into a conceptual framework for the disease, well validated with other measures, and suitable for multicenter studies. In the last years increasing pressure from regulatory agencies and advocacy groups to identify clinically meaningful measures was made. At a recent meeting with EMA it has been discussed that the 6MTW, a measure of fatigue and endurance that is currently used as the primary outcome measure in the most clinical trials, should be used in combination with secondary measures, such as the North Star Ambulatory Assessment, that reflects activities of daily living. Over the last few years there has been an effort by the community to combine the experience on natural history data collected by different networks. Reliability studies, cross sectional studies, natural history studies and validation for 6MWT and NSAA are present (discussed with EMA and FDA last year). The clinical meaningfulness of the suggested measures, and in particular of the suggestion that 30 m on the 6MWT should be considered as an important results, has been discussed using different approaches : i) statistical methods (Craig Mc Donald work important for this) [36]ii) correlation with other measures: 30 meters difference are clinically meaningful, since when DMD boys were subdivided according to 30m intervals, there was a clear reduction of the risk of losing ambulation within 2 years for each interval of 30 m considered [37] Natural history studies have also suggested that it is possible to identify subgroups of DMD boys with different trajectories. DMD boys younger than 7 years tend to have better scores when reassessed after one year at variance with the older ones in whom there is an obvious slope of deterioration. The same applies to boys who are able to walk more than 350 meters at baseline compared to those walking less than 350 meters. Other possible variables, such as different mutation groups have also been considered. Preliminary results were also shown on the use of the Performance of Upper limb scale (PUL) [38] that has been recently validated and for which the first longitudinal data are becoming available Other promising exploratory clinical markers, such as muscle MRI, actymyo and myotools have also been discussed.
Glenn Walter (UF College of Medicine and the National High Magnetic Field Laboratory, Gainesville, Florida, USA) presented a summary of magnetic resonance (MR) techniques as outcome measures. This included an overview of the current use of MRI and MR spectroscopy as a non-invasive biomarker for disease progression in DMD. A description of the use of T1 based MRI scoring of muscle involvement and MR measures of muscle size, quantitative T2, and quantitative muscle composition (fat fraction) was presented. All of these methods have been used to discriminate between unaffected controls and DMD muscles. The results from a large natural history study were presented which utilizes MR measures to track longitudinal changes in disease progression in DMD. It was found that muscle T2 is elevated in all DMD age groups, while elevated muscle fat fraction is not always seen in the youngest DMD subjects (5–7 years old). These early changes in muscle T2 may represent increased muscle inflammation prior to fatty tissue infiltration, as indicated based on the differences observed in young DMD subjects which are steroid naïve. Progressive increases in fatty tissue deposition within one year in the soleus and vastus lateralis muscles were observed in all DMD age groups (5–12 years old) but not in the unaffected controls. It was shown that Dixon imaging allows for high spatial resolution imaging of fat fraction, and can be used to create fat fraction maps that allow for the quantitative measurement of muscle heterogeneity. Quantitative T2, Dixon imaging and 1H-MRS could all detect disease progressive within 3-month intervals and were used to determine individual muscle specific disease progression. The individual trajectories for disease progression revealed a large range of disease progression rates between DMD subjects and between individual lower and upper leg muscles.
Thomas Voit (Institute of Myology, Paris) provided a summary on DMD pathophysiology. In a collaborative effort between the Institute of Myology, Paris, and Généthon, Evry, France, a large prospective biomarker study is being carried out supported by Advance Diagnostics for New therapeutic Approaches (ADNA) and AFM. This study includes the serum and urine samples of an American and a European DMD cohort (>100 patients/cohort aged 3 to 20 years) plus an age-matched European control cohort. Both the American and the European cohort contain, in addition to cross-sectional samples, longitudinal samples from steroid-naïve DMD patients and follow-up samples after induction of steroid treatment. Different–omic approaches are carried out with the goal to identify biomarkers but also to obtain new clues to the underlying pathophysiology of DMD. The ultimate goal of these studies is to monitor disease evolution and treatment effects. A first search aimed to identify altered protein levels in serum of DMD patients and was carried out under the leadership of Fédor Svinartchouk (Institute of Myology, Paris). This approach used mass spectrometry and analysed serum after depletion of the most abundant serum proteins such as albumin, which tend to mask less abundant but informative proteins. This approach identified 27 abnormally up-regulated proteins in DMD serum and one down-regulated protein. The most up-regulated protein in DMD serum was myomesin 3. The myomesin protein forms dimers which constitute a ‘molecular spring’ conferring elasticity and connecting directly titin to myosin at the M-line of the sarcomere [39]. Myomesin 3 was first detected as up-regulated in pooled sera of young DMD (3–8y), young treated DMD (3–8y), and old DMD (13–20y) patients. This was subsequently confirmed by Western blot analysis using myomesin 3-specific antibodies in 146 individual DMD sera across the 3–20y age range. Myomesin 3 was absent from corresponding normal control sera. In order to test if abnormal myomesin 3 excretion into serum is more generally linked to dystrophin absence the protein was investigated in mdx mouse and GRMD dog sera. Similar abnormal serum levels of myomesin 3 were detected in these dystrophin-deficient animal models. To test further if other forms of muscular dystrophy, which are linked to a pathomechanism disrupting the plasma membrane costameric framework also present this abnormality a limited number of sera from sarcoglycan-deficient LGMD patients were studied, and the same abnormality was detected. To explore if myomesin 3 could be used as a marker to follow the effects of a therapeutic approach, the α-sarcoglycan-deficient mouse model was chosen because the therapeutic effect of alpha-sarcoglycan replacement was known [40]. These studies were carried out by Isabelle Richard at Généthon. Gene replacement therapy by AAV8-α-SG at a dose of 1X10e11 vg partially, and by a dose of 5X10e11 almost completely corrected abnormal myomesin 3 excretion into α-SG ko mouse serum. In functional terms the decrease of myomesin 3 levels after partial or near-complete α-SG gene rescue correlated strongly (R2>0.7) with force as measured by escape test (Alban Vignaud) in contrast to CK levels, which only showed a poor correlation (R2= 0.25). At the same time physical exercise such as downhill running in mdx mouse did not lead to a significant increase of myomesin 3 excretion into serum. These data indicate that myomesin 3 may be a potent biomarker to monitor gene replacement approaches in muscular dystrophies, which are associated to abnormal myomesin 3 serum excretion. Voit also reported published data on biomarker search in urine in DMD [41].
Session 5 – Complexity of finding biomarkers in DMD: modifying factors
Elizabeth McNally described genome wide strategies to look for pathways that alter the outcome of muscular dystrophy, especially the cardiac function. Using a mouse model of limb girdle muscular dystrophy type 2C, which shares similar pathology to DMD, an intercross approach was used. This approach previously identified Ltbp4, which has been subsequently replicated in human DMD. A new genetic screen focused on identifying cardiopulmonary modifiers by examining how multiple cardiac and muscle traits varied across the intercross cohort. Specific attention was on echocardiography parameters including cardiac dimensions and function. These parameters are thought to be reflective of the timeline of disease that occurs in DMD and other forms of NMD. A QTL that modifies cardiopulmonary function was identified on chromosome 11. Anxa6 was identified on chromosome 11 as the modifier gene [42]. An alternate anxa6 transcript was identified in the severe D2 strain, which causes a cryptic splice site and a truncated protein of 32 kD protein detected in heart and abdominal muscles.
Electroporation studies showed that annexin A6 is recruited to the site of damage after laser injury of the sarcolemma. The presence of the truncated annexin A6 inhibits resealing. Whether ANXA6 modifies human muscular dystrophy is not yet known.
Valeria Ricotti summarized the efficacy of early steroid in DMD. The North Star UK clinical network encompasses longitudinally prospectively collected clinical data on DMD boys treated in accordance to the standards of care. Since 2004, 17 UK neuromuscular centres currently follow about 600 DMD patients with a mean age of 6.5 years for initiation of glucocorticoid therapy. It was previously reported [43] that DMD boys reach their maximum motor function between 6–7 years of age, when they start declining, with an overall median loss of ambulation at 13 years (95% CI 12.1–13.5). Boys on intermittent prednisolone lost ambulation earlier at 12 years of age, while on a daily regimen they lost ambulation at 14.5 years of age. Side effects profiling of the two regimens were previously reported [43]. In this ongoing study, it was further explored if starting steroids before 5 years of age may have a positive impact on motor function. In a multilevel model Ricotti compared the total NorthStar Ambulatory Assessment score of 36 boys who started glucorticoids before age 5 (mean age 4.4) with 139 boys who started steroids after 5 years of age (mean age 5.9). It was observed that boys who started glucocorticoids before 5 gained additional 1.6 units per year (p=0.05). Furthermore, when comparing the mean total NSAA score at age 7 between the two groups, the early starters had a total score of 28/34 compared to 2/34 of the boys who started steroids between 5 and 6.5 years (p=0.003). Longitudinal data beyond age 7 remains sparse, as only in the very recent years clinical practise has shifted towards an earlier initiation of therapy; therefore the long term benefits and side effects of starting steroids at an earlier phase of the disorder requires further evaluation.
In conclusion, when investigating for disease modifiers, not only glucocorticoids regimens but also age at starting steroids is an important variable to consider.
Thomas Voit presented the ageing as factor influencing DMD and biomarkers discovery. Ageing in normal skeletal muscle is a complex process which results in loss of muscle mass and strength and has been called sarcopenia, in the elderly. A reduced regenerative capacity, decreased protein synthesis, increased apoptosis, but also metabolic factors like insulin resistance contribute to muscle loss. Here what is known about these factors in DMD was reviewed with a perspective of using such information for biomarker generation. The number of satellite cells decreases between 20 and 80 years of age in a linear fashion in all muscles (except for cricopharyngeal muscle) [44, 45]. In vitro, replicative senescence of human myoblasts is reached at a telomere length of around 8–9kb. An alternative arrest (i.e. not through telomere shortening) can occur in the human (in contrast to the mouse) through activation of the p16 pathway [46]. This pathway gets activated through oxidative stress and inflammation, relevant mechanisms in DMD. The early failure of regeneration in DMD is a key feature of the disease. If myoblasts are isolated from DMD muscle (most existing data is from limb muscles, notably quadriceps femoris) telomeres are dramatically shorter in muscular dystrophy, in particular in DMD. Normal myoblasts of a newborn when cultivated in vitro would undergo 55–65 divisions, 30 divisions at the age of 9 years, and 19 divisions at the age of 26 years. In DMD, myoblasts from a 7 year-old boy would undergo 19 divisions, at 11 years 14 divisions, and at 14 years 5 divisions. This means that at 11 years the number of divisions is as low as that observed in myoblasts isolated from 80 year-old normal people, and at 14 years it is much lower than what is ever observed even in normal elderly individuals. Exhaustion of the satellite pool through ongoing necrosis and regeneration, but also other factors such as fibrosis and a hostile microenvironment may be contributing factors. In particular, maintenance of the satellite cell pool is dependent on asymmetric division where one daughter cell differentiates into a myonucleus and one daughter cell returns to a quiescent state in the satellite cell niche. That external factors influence the satellite cell niche and modify the regenerative capacity in vivo and during ageing had been shown by the group of Rando [47, 48] Similarly, previous work from our laboratory had shown that the total protein secretion of myoblasts in vitro declines dramatically with age [44].
He reported the identification of soluble factors, which contribute to the microenvironment of skeletal muscle and the cell to cell signalling involved. A combination of multiplex immunoassay, LC-MS/MS and 2D gel-MS was used to identify the secreted proteome in serum-free grown myoblast cultures. These studies identified 257 soluble secreted proteins and 666 proteins without secretion signal. These could be attributed by further fractionation and electron microscopy to proteins secreted via exosomes and microparticles, these two representing independent secretion pathways of skeletal muscle [49]. Both exosomes and microparticles were shown to carry, in addition to proteins, different small RNAs. In addition, myoblast-derived exosomes as well as microparticles were shown to deliver their cargo to neighbouring cells such as fibroblasts, and with different delivery characteristics for exosomes versus microparticles. A follow-up study showed that dystrophin deficiency in vitro leads to severe disturbance of vesicle associated protein secretion [50]. Therefore, correction of in vitro protein secretion could be envisaged as a quality control biomarker for various dystrophin correction or replacement approaches. Applying the combination approach of iTrac, 2 D –MS and antibody mapping to the characterization of the ageing secretome, 98 proteins were found altered in the senescent secretome, 55 downregulated proteins and 38 upregulated. The three methods gave complementary results with only 1 protein detected altered by all three (TIMP 1, upregulated (unpublished results). Bioinformatics modelling ascribed key alterations to homeostatic processes like IGF signalling and proteases and their inhibitors (unpublished results). Work characterizing the secretome in ageing DMD muscle is under way. Number of satellite cells decreases with age in biceps and masseter and other muscles (except pharyngeal). Telomeres become shorter with age and myoblasts purified from muscle double less depending on the age of the donor. It is still not known whether there is a correlation between the aging degree of the cells and different muscle groups. Satellite cells undergo asymmetric division and after the division the cell that remains quiescent migrates back to the cell niche. This capability is reduced over time. The presence of myofibers plays a positive role in the capability to maintain this property.
Francesco Muntoni reported on the report of the EMA meeting on AON therapy. In June 2013 follow up meeting of a meeting coordinated by TREAT-NMD in 2010. This second meeting was organised with EMA providing a general advise on specific issues, but it was not an official meeting in which the comments of the participants represent a specific position of EMA. The meeting was structured into different parts in which feedback on clinical and biochemical outcome measures was sought. Regarding the clinical outcome measures, the discussion focused on 1) young children/neonates; 2) ambulatory patients; 3) non ambulatory patients. Regarding the first point, a number of recent experiences in which young cohorts of young DMD children had been assessed were reviewed [51, 38].
The conclusion is that a number of functional tools allow to reliably detecting motor difficulties after the age of 3 in DMD and could therefore potentially be used as endpoints in clinical trials.
One issue discussed at the meeting with EMA relates to whether data from a positive study in an older patient population (such as the one in current clinical trials) could be transposed to younger DMD boys; the feedback was that while the rationale for the use of an intervention in a younger population can be appreciated, it will be challenging to assume that the same treatment will have an effect in a younger DMD population, hence extrapolate efficacy to a younger population without a specific study. This will for example make a neonatal study complex and lengthy to be performed. The availability of clinical outcome measures in children from 3 years onwards could however be used to design an appropriate clinical study in this target population. Regarding the issues of functional outcome measures in the ambulant population, the meeting with EMA clearly demonstrated the complex relationship in DMD between strength and function and why it is not rationale to use both strength and function as co-primary endpoints. Craig McDonald presented extensive data in this respect, showing that co-primary endpoints could dilute an efficacy effect as they do not correlate linearly.
While a lot of correlative data was presented at the meeting with EMA on correlation of 6MWT with other functional measures, disability and clinically meaningful changes for the patients, there is still some reluctance from regulators in accepting this measure as the only measure of clinical efficacy. The use of functional scale as secondary endpoint that should show a positive trend in the same direction as the 6MWT was discussed. The coherent behaviour in other secondary outcome, including biochemical outcome measures (such as dystrophin in muscle biopsy in dystrophin restoration studies) was also stressed.
The issues for the non-ambulant patient population is similar as for the early young DMD population, i.e. no direct extrapolation is likely, without data from a study to confirm that a treatment will also be effective in this population.
The regulatory authority feedback was very helpful as it is important investigators understand on one hand the regulatory requirements for drug approval for indications that are not specifically those addressed by current phase III studies; and at the other hand for the regulators to have a better insight on the specific issues that dealing with DMD individuals at different stages of their conditions determines.
Muntoni finally presented data from clinical decline in DMD and intermediate DMD/BMD patients in whom low levels of dystrophin expression were correlated to clinical outcome (loss of ambulation and 6 monthly decline in North Star scores). These data are encouraging as they suggest that also modest levels of dystrophin (<10%) do provide a clinical benefit to patients.
In order to better summarise both workshop outputs and future directions, 3 breakout sessions were organised.
Breakout session report: recommendations and future directions
BREAKOUT SESSION 1 –Existing DMD biomarkers (lead by Cristina Al-Khalili Szigyarto)
The aim of the session was to compile the list of currently most promising genetic and progression biomarkers. The most promising biomarkers are CK, dystrophin, MRI and MMP9. In addition, several promising biomarkers have been reported on, but remain to be further studied and validated before used in clinical context.
Statement of dystrophin as a biomarker
The true value of dystrophin as a biomarker requires redefining the underlying requirements on biomarkers and the purpose of its usage. Recent technological development allows quantification of dystrophin over a wide range detecting as little as 5% changes relative to normal amount. Two aspects have been raised that makes dystrophin difficult to use as a surrogate end point biomarker. The pathophysiological alterations experienced by muscular dystrophy patients, can not be reverted and restoration of dystrophin upon treatment concerns only existing muscle tissue and does not contribute to regeneration of muscles already replaced by connective tissue and fat. The second aspect concerns the correlation with clinical outcome measures like the 6min walk. However, quantification of dystrophin is particularly important for the rare exon skipping trials due to the need for mechanistic proof of the drug. Thus, dystrophin clearly has at the moment an important role as a pharmacodynamic biomarker, and more work will be required to assess if dystrophin could also be used as a surrogate biomarker or not [12].
Quantification of dystrophin is only representative for the analysed muscle and does not provide information about the overall muscle mass. Samples are retrieved from different muscle types at different sites making comparison and interpretation of results difficult to conclude. In comparison to dystrophin, MRI can provide a more representative estimation of muscle damage and muscle restoration. In order to facilitate the use of dystrophin as pharmacodymanic biomarker it was considered important:
To selecting the most representative muscle type for analysis
To standardize protocols for the analysis and interpretation of the results
To prepare a white paper
Transcript and exon skipping quantification is also important. As for protein analysis SOPs are needed. Secondary biomarkers are needed for disease progression (as CK and MMP-9), prognostics and patient stratification (miR-206 and vimentin) and other exploratory biomarkers. Deeper understanding of these markers is required for their qualification as biomarkers. In order to speed up the qualification process needs for qualification of biomarkers were considered:
comparison of BMD – IMD – DMD for patient stratification and understanding of pathophysiological differences,
collaboration between platforms regarding sample retrieval, handling, preparation, and analysis has to be consolidated,
due to limited sample availability Phase 2 clinical trial samples should be accessible coupling MRI with molecular biomarker profiles.
Dystrophin quantification and MRI are the most developed biomarkers but secondary biomarkers are still needed. To increase the pace for biomarker validation samples and data should be shared (e.g knowledge database for biomarkers in NMD), samples should be shared and biomarkers prioritized for samples from clinical trials and biobanks.
BREAKOUT SESSION 2- Defining necessary actions for combination of biomarker data across cohorts (lead by Pietro Spitali)
The aims of this session were : i) defining the interaction between existing modifiers (SPP1 and LTBP4) ; ii) listing cardiac biomarkers ; iii) discussing issues to harmonize phenotypes ; iv) samples needed for biomarker validation studies.
Partners agreed to share data on SPP1 and LTBP4 SNPs in order to have enough power to test interaction between genetic modifiers and between SNPs and steroid treatment. It was also agreed to study genetic modifiers in BMD patients carrying similar mutations to reduce the variation introduced by the mutation as much as possible. To do this, BMD patients with the most common mutations - del 45–47 and del 45–48 – will be selected in all participating centres. It was estimated that at least 100 patients are needed to have sufficient statistical power.
It was proposed to test Annexin6 variants in DMD. Issues were identified in certain definitions such as ambulation loss or in corticosteroid treatment. It was suggested that prospectively it could be decided to define ambulation loss when patients are not able to walk more than 10 m. The definition of corticosteroid users was also debated, since a unique definition is still lacking.
Partners agreed about a EU-USA (or even wider) inventory of all serum and plasma samples available for DMD patients in order to facilitate access to samples and validate candidate biomarkers. It was suggested that partners who are collecting samples during clinical trials could register to TREAT-NMD to build up a repository. The collected samples could be made available under ethical rules and agreements.
BREAKOUT SESSION 3 Identifying issues that we want to make clear to the regulatory authorities regarding biomarker qualification for RD, including DMD (lead by Eugenio Mercuri, Giuseppe Novelli and Annemieke Aartsma-Rus)
During this session the ideal requirements of surrogate endpoints were discussed. Ideally, they should be minimally invasive (e.g. MRI, serum or urine), be measurable in a standardized way and of course correlate with or be predictive of clinical benefit. It is important to take the requirements of the regulators for biomarker qualification into account at an early stage of discovery.
After biomarker identification, validation is needed in well-defined cohorts. For this natural history studies would be ideal, because this involves patients who undergo functional testing in a standardized way and who are seen regularly, so longitudinal samples (serum or urine) are available and biomarker levels can be linked to function. It is crucial that consent of patients in these trials (and also placebo-controlled trials) is flexible enough to allow using of these samples for validation of surrogate endpoints.
Since serum samples collected in these well controlled trials are so valuable, the recommendation would be harmonize and coordinate efforts for collection and storage of these samples. Also, a committee involving scientists, clinical network representatives and patient representatives should be installed to on the one hand act as gate keepers of these valuable samples, but also facilitate distribution of samples to scientists with promising candidate biomarkers.
Future outlook
Time clearly indicates that biomarkers are vital in Duchenne muscular dystrophy as well as in all rare diseases. The workshop has provided a wide, multidisciplinary and interactive view on biomarker research and clinical application. The breakout sessions were useful to concretize the future applications and collaborations. The outcomes of the workshop articulate in a variety of collaborative actions.
The main collaborative plans that have been agreed are:
study population: establishing a EU-USA collaboration by sharing data/samples/methods to maximise success in the biomarkers identification and validation, harmonising phenotype definition
list of currently existing biomarker for DMD and defining dystrophin as a biomarker and how to standardise its measurement
setting up a biomarkers database for DMD (facilitated by Parents Associations)
defining issues that need to be clarified with EMA to approaching biomarkers regulation and translational in trials.
ENMC Biomarker Meeting Lay Report (the view of Parents Associations, cured by Elizabeth Vroom and Sharon Hesterly)
There is an urgent need for effective therapies for Duchenne muscular dystrophy, a fatal progressive neuromuscular disease. A clinical trial that takes one or two years (as is now being discussed) to measure a functional outcome represents very real loss for those participants on placebo or who do not meet eligibility requirements. Although the pipeline for potential treatments is full, the ability to test these treatments efficiently must be improved, and the use of biomarkers is essential to improving the efficiency of trials. The presentations at this meeting made it clear that significant preliminary work has been done to establish various categories of biomarkers for Duchenne, from markers of therapeutic effect to markers of pharmacokinetic or pharmacodynamic activity. It was also clear that the level of complexity involved in developing and validating a biomarker is not insignificant. With these observations in mind, current efforts underway in Duchenne lend themselves very well to a centralized consortium approach so that prospective biomarkers can be prioritized and validated in a systemic way. As representatives of patient organizations we would like to work with the academic community, industry and regulators to identify a way forward that provides the right incentives for a more coordinated approach.
Workshop participants
Annemieke Aartsma-Rus (LUMC, NL and Newcastle University UK)
Alessandra Ferlini (Ferrara University, Italy)
Kevin Flanigan (Nationwide Children’s Hospital, Columbus OH, USA)
Hanns Lochmuller (Newcastle University, UK)
Peter-Bram ‘t Hoen (LUMC, NL)
Francesco Muntoni (UCL Institute of Child Health & Great Ormond Street Hospital, London, UK)
Elena Pegoraro (University of Padova, Italy)
Yetrib Hathout (Childrens National Hospital, Washington USA)
Cristina Al-Khalili Szigyarto (KTH School of Biotechnology, Stockholm, Sweden)
Giuseppe Novelli (University of Rome, Italy)
Sharon Hesterly (PPMD, USA)
Gisèle Bonne (Institute de Myologie, Paris, France)
Ed Kaye (Sarepta Therapeutics, Camebridge MA, USA)
Afrodite Loubarkos (Prosensa Therapeutics, Leiden, NL)
Glenn Walter (UF College of Medicine, Gainesville, FL, USA)
Elizabeth McNelly (University of Chicago, USA)
Eugenio Mercuri (Rome University, Italy)
Judith van Deutekom (Prosensa Therapeutics, Leiden, NL)
Kay Ohlendieck (National University of Ireland)
Elena Schwartz (Ariadne Genomics, Rockville, USA)
David Israeli (Genethon, Evry, France)
Valeria Ricotti (UCL, London, UK)
Cecilia Gelfi (University of Milan, Italy)
Pietro Spitali (LUMC, Leiden, NL)
Thomas Voit (Institute de Myologie, Paris, France)
Elizabeth Vroom (Dutch Duchenne Parent Project and UPPMD)
Victor Dubowitz (ENMC honorary member)
Acknowledgments
This workshop was made possible by the financial support of the European Neuromuscular Centre (ENMC) and its main sponsors: Association Française contre les Myopathies (France), Deutsche Gesellschaft für Muskelkranke (Germany), Muscular Dystrophy Campaign (UK), Muskelsvindfonden (Denmark), Prinses Beatrix Spierfonds (The Netherlands), Schweizerische Stiftung für die Erforschung der Muskelkrankheiten (Switzerland), Telethon Foundation (Italy), Spierziekten Nederland (The Netherlands) and Associated member: Finnish Neuromuscular Association (Finland).
Work by the authors was supported by the European Community Framework Programme 7 grant agreement no. 241665 (BIO-NMD), no. 305444 (RD-Connect) and no. 305121 (Neuromics).
References
- 1.Novelli G, Ciccacci C, Borgiani P, et al. Genetic tests and genomic biomarkers: regulation, qualification and validation. Clinical Case in Methabolism. 2008 May;5(2):149–54. [PMC free article] [PubMed] [Google Scholar]
- 2.Muntoni F, Torelli S, Ferlini A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2003 Dec;2(12):731–40. doi: 10.1016/s1474-4422(03)00585-4. Review. [DOI] [PubMed] [Google Scholar]
- 3.Arechavala-Gomeza V, Anthony K, Morgan J, et al. Antisense oligonucleotide-mediated exon skipping for Duchenne muscular dystrophy: progress and challenges. Curr Gene Ther. 2012;12:152–60. doi: 10.2174/156652312800840621. Review. [DOI] [PubMed] [Google Scholar]
- 4.Mendell JR, Rodino-Klapac L, Sahenk Z, et al. Gene therapy for muscular dystrophy: lessons learned and path forward. Neurosci Lett. 2012 Oct 11;527(2):90–9. doi: 10.1016/j.neulet.2012.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercuri E, Mayhew A, Muntoni F, et al. TREAT-NMD Neuromuscular Network. Towards harmonisation of outcome measures for DMD and SMA within TREAT-NMD; report of three expert workshops: TREAT-NMD/ENMC workshop on outcome measures, 12th–13th May 2007, Naarden, The Netherlands; TREAT-NMD workshop on outcome measures in experimental trials for DMD, 30th June–1st July 2007, Naarden, The Netherlands; conjoint Institute of Myology TREAT-NMD meeting on physical activity monitoring in neuromuscular disorders, 11th July 2007, Paris, France. Neuromuscul Disord. 2008 Nov;18(11):894–903. doi: 10.1016/j.nmd.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Russell C, Rahman A, Mohammed AR. Application of genomics, proteomics and metabolomics in drug discovery, development and clinic. Ther Deliv. 2013 Mar;4(3):395–413. doi: 10.4155/tde.13.4. [DOI] [PubMed] [Google Scholar]
- 7.Bai JP, Barrett JS, Burckart GJ, et al. Strategic Biomarkers for Drug Development in Treating Rare Diseases and Diseases in Neonates and Infants. AAPS J. 2013 Jan 19; doi: 10.1208/s12248-013-9452-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scotton C, Passarelli C, Neri M, et al. Biomarkers in rare neuromuscular diseases. Exp Cell Res. 2013 Dec 31; doi: 10.1016/j.yexcr.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Buyse M, Sargent DJ, Grothey A, et al. Biomarkers and surrogate end points--the challenge of statistical validation. Nat Rev Clin Oncol. 2010 Jun;7(6):309–17. doi: 10.1038/nrclinonc.2010.43. [DOI] [PubMed] [Google Scholar]
- 10.Goodsaid FM, Amur S, Aubrecht J, et al. Voluntary exploratory data submissions to the US FDA and the EMA: experience and impact. Nat Rev Drug Discov. 2010 Jun;9(6):435–45. doi: 10.1038/nrd3116. [DOI] [PubMed] [Google Scholar]
- 11.Hollebecque A, Massard C, Soria JC. Implementing precision medicine initiatives in the clinic: a new paradigm in drug development. Curr Opin Oncol. 2014 May;26(3):340–6. doi: 10.1097/CCO.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 12.Aartsma-Rus A, Ferlini A, Vroom E. Biomarkers and surrogate endpoints in Duchenne: Meeting report. Neuromuscul Disord. 2014 Aug;24(8):743–5. doi: 10.1016/j.nmd.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Bladen CL, et al. The TREAT-NMD Duchenne muscular dystrophy registries: conception, design, and utilization by industry and academia. Hum Mutat. 2013 Nov;34(11):1449–57. doi: 10.1002/humu.22390. [DOI] [PubMed] [Google Scholar]
- 14.Sun SC, Peng YS, He JB, et al. Changes of serum creatine kinase levels in children with Duchenne muscular dystrophy. Zhongguo Dang Dai Er Ke Za Zhi. 2008 [PubMed] [Google Scholar]
- 15.Zatz M, Rapaport D, Vainzof M, et al. Serum creatine-kinase (CK) and pyruvate-kinase (PK) activities in Duchenne (DMD) as compared with Becker (BMD) muscular dystrophy. J Neurol Sci. 1991 Apr;102(2):190–6. doi: 10.1016/0022-510x(91)90068-i. [DOI] [PubMed] [Google Scholar]
- 16.Malik V, Rodino-Klapac LR, Viollet L, et al. Gentamicin-induced readthrough of stop codons in Duchenne muscular dystrophy. Ann Neurol. 2010 Jun;67(6):771–80. doi: 10.1002/ana.22024. [DOI] [PubMed] [Google Scholar]
- 17.Flanigan KM. The muscular dystrophies. Semin Neurol. 2012 Jul;32(3):255–63. doi: 10.1055/s-0032-1329199. [DOI] [PubMed] [Google Scholar]
- 18.Ayoglu Burcu, Chaouch Amina, Lochmüller Hanns, et al. Affinity proteomics within rare diseases: A BIO-NMD study for blood biomarkers of muscular dystrophies. EMBO Mol Med. 2014 Jun 11;6(7):918–36. doi: 10.15252/emmm.201303724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown KJ, Marathi R, Fiorillo AA, et al. Accurate Quantitation of Protein in Human Skeletal Muscle Using Mass Spectrometry. J Bioanal Biomed. 2012 Dec 18;(Suppl 7) doi: 10.4172/1948-593X.S7-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotelnikova E, Shkrob MA, Pyatnitskiy MA, et al. Novel approach to meta-analysis of microarray datasets reveals muscle remodeling-related drug targets and biomarkers in Duchenne muscular dystrophy. PLoS Comput Biol. 2012 Feb;8(2) doi: 10.1371/journal.pcbi.1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bello L, Piva L, Barp A, et al. Importance of SPP1 genotype as a covariate in clinical trials in Duchenne muscular dystrophy. Neurology. 2012 Jul 10;79(2):159–62. doi: 10.1212/WNL.0b013e31825f04ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flanigan KM, Dunn DM, von Niederhausern A, Weiss RB, et al. United Dystrophinopathy Project Consortium. Mutational spectrum of DMD mutations in dystrophinopathy patients: application of modern diagnostic techniques to a large cohort. Hum Mutat. 2009 Dec;30(12):1657–66. doi: 10.1002/humu.21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heydemann A, Ceco E, Lim JE, et al. Latent TGF-beta-binding protein 4 modifies muscular dystrophy in mice. Ann Neurol. 2013 Apr;73(4):481–8. doi: 10.1172/JCI39845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flanigan KM, Ceco E, Lamar KM, et al. United Dystrophinopathy Project. LTBP4 genotype predicts age of ambulatory loss in Duchenne muscular dystrophy. Ann Neurol. 2013 Apr;73(4):481–8. doi: 10.1002/ana.23819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vignier N, Amor F, Fogel P, et al. Distinctive Serum miRNA Profile in Mouse Models of Striated Muscular Pathologies. PLoS One. 2013;8(2):e55281. doi: 10.1371/journal.pone.0055281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spitali J, van den Bergen C, Verhaart IE, et al. Aartsma-Rus, “DMD transcript imbalance determines dystrophin levels”. FASEB J. 2013 Aug; doi: 10.1096/fj.13-232025. [DOI] [PubMed] [Google Scholar]
- 27.Nadarajah VD, van Putten M, Chaouch A, et al. Serum matrix metalloproteinase-9 (MMP-9) as a biomarker for monitoring disease progression in Duchenne muscular dystrophy (DMD) Neuromuscul Disord. 2011 Aug;21(8):569–78. doi: 10.1016/j.nmd.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Klimiuk PA, Sierakowski S, Latosiewicz R, et al. Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in different histological variants of rheumatoid synovitis. Rheumatology (Oxford) 2002 Jan;41(1):78–87. doi: 10.1093/rheumatology/41.1.78. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Mittal A, Makonchuk DY, et al. Matrix metalloproteinase-9 inhibition ameliorates pathogenesis and improves skeletal muscle regeneration in muscular dystrophy. Hum Mol Genet. 2009 Jul 15;18(14):2584–98. doi: 10.1093/hmg/ddp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hindi SM, Shin J, Ogura Y, et al. Matrix metalloproteinase-9 inhibition improves proliferation and engraftment of myogenic cells in dystrophic muscle of mdx mice. PLoS One. 2013 Aug 15;8(8):e72121. doi: 10.1371/journal.pone.0072121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinkmeier H, Ohlendieck K. Chaperoning heat shock proteins: proteomic analysis and relevance for normal and dystrophin-deficient muscle. Proteomics Clin Appl. 2014 Jun 3; doi: 10.1002/prca.201400015. [DOI] [PubMed] [Google Scholar]
- 32.Holland A, Ohlendieck K. Proteomic profiling of the dystrophin-deficient mdx phenocopy of dystrophinopathy-associated cardiomyopathy. Biomed Res Int. 2014;2014:246195. doi: 10.1155/2014/246195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muntoni F Meeting Steering Committee and TREAT-NMD Network. The development of antisense oligonucleotide therapies for Duchenne muscular dystrophy: report on a TREAT-NMD workshop hosted by the European Medicines Agency (EMA), on September 25th 2009. Neuromuscul Disord. 2010 May;20(5):355–62. doi: 10.1016/j.nmd.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Arechavala-Gomeza V, Kinali M, Feng L, et al. Immunohistological intensity measurements as a tool to assess sarcolemma-associated protein expression. Neuropathol Appl Neurobiol. 2010 Jun;36(4):265–74. doi: 10.1111/j.1365-2990.2009.01056.x. [DOI] [PubMed] [Google Scholar]
- 35.Taylor LE1, Kaminoh YJ, Rodesch CK, et al. Quantification of dystrophin immunofluorescence in dystrophinopathy muscle specimens. Neuropathol Appl Neurobiol. 2012 Oct;38(6):591–601. doi: 10.1111/j.1365-2990.2012.01250.x. [DOI] [PubMed] [Google Scholar]
- 36.McDonald CM, Henricson EK, Abresch RT, et al. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve. 2013 Sep;48(3):343–56. doi: 10.1002/mus.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazzone ES, Pane M, Sormani MP, et al. 24 month longitudinal data in ambulant boys with Duchenne muscular dystrophy. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0052512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pane M, Scalise R, Berardinelli A, et al. Early neurodevelopmental assessment in Duchenne muscular dystrophy. Neuromuscul Disord. 2013 Jun;23(6):451–5. doi: 10.1016/j.nmd.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Pinotsis N, Chatziefthimiou SD, Berkemeier F, et al. Superhelical architecture of the myosin filament-linking protein myomesin with unusual elastic properties. PLoS Biol. 2012 Feb;10(2) doi: 10.1371/journal.pbio.1001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fougerousse F, Bartoli M, Poupiot J, et al. Phenotypic correction of alpha-sarcoglycan deficiency by intra-arterial injection of a muscle-specific serotype 1 rAAV vector. Mol Ther. 2007 Jan;15(1):53–61. doi: 10.1038/sj.mt.6300022. [DOI] [PubMed] [Google Scholar]
- 41.Rouillon J, Zocevic A, Leger T, et al. Proteomics profiling of urine reveals specific titin fragments as biomarkers of Duchenne muscular dystrophy. Neuromuscul Disord. 2014 Jul;24(7):563–73. doi: 10.1016/j.nmd.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Swaggart KA1, Demonbreun AR, Vo AH, et al. Annexin A6 modifies muscular dystrophy by mediating sarcolemmal repair. Proc Natl Acad Sci U S A. 2014 Apr 22;111(16):6004–9. doi: 10.1073/pnas.1324242111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricotti V, Ridout DA, Scott E, et al. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry. 2013;84:698–705. doi: 10.1136/jnnp-2012-303902. [DOI] [PubMed] [Google Scholar]
- 44.Bigot A, Jacquemin V, Debacq-Chainiaux F, et al. Replicative aging down-regulates the myogenic regulatory factors in human myoblasts. Biol Cell. 2008 Mar;100(3):189–99. doi: 10.1042/BC20070085. [DOI] [PubMed] [Google Scholar]
- 45.Gidaro T, Negroni E, Perié S, et al. Atrophy, fibrosis, and increased PAX7-positive cells in pharyngeal muscles of oculopharyngeal muscular dystrophy patients. J Neuropathol Exp Neurol. 2013 Mar;72(3):234–43. doi: 10.1097/NEN.0b013e3182854c07. [DOI] [PubMed] [Google Scholar]
- 46.Zhu CH, Mouly V, Cooper RN, et al. Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell. 2007 Aug;6(4):515–23. doi: 10.1111/j.1474-9726.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 47.Conboy IM, Conboy MJ, Wagers AJ, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005 Feb 17;433(7027):760–4. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 48.Carlson ME, Conboy IM. Loss of stem cell regenerative capacity within aged niches. Aging Cell. 2007 Jun;6(3):371–82. doi: 10.1111/j.1474-9726.2007.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Bihan MC, Bigot A, Jensen SS, et al. In-depth analysis of the secretome identifies three major independent secretory pathways in differentiating human myoblasts. J Proteomics. 2012 Dec 21;77:344–56. doi: 10.1016/j.jprot.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Duguez S, Duddy W, Johnston H, et al. Dystrophin deficiency leads to disturbance of LAMP1-vesicle-associated protein secretion. Cell Mol Life Sci. 2013 Jun;70(12):2159–74. doi: 10.1007/s00018-012-1248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Connolly AM, Florence JM, Cradock MM, et al. MDA DMD Clinical Research NetworkMotor and cognitive assessment of infants and young boys with Duchenne Muscular Dystrophy: results from the Muscular Dystrophy Association DMD Clinical Research Network. Neuromuscul Disord. 2013 Jul;23(7):529–39. doi: 10.1016/j.nmd.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]