Abstract
Super-refractory status epilepticus is a life-threatening condition. Resistance to benzodiazepine and barbiturate treatment for this disorder is thought to be due to internalization of synaptic γ-aminobutyric acid (GABA)A receptors, and withdrawal of benzodiazepines and barbiturates during treatment often triggers seizure recurrence. The neurosteroid allopregnanolone acts as a positive allosteric modulator of synaptic and extrasynaptic GABAA receptors. Here we describe the use of allopregnanolone in 2 pediatric patients with super-refractory status epilepticus. This treatment allowed the general anesthetic infusions to be weaned with resolution of status epilepticus. This is the first report of allopregnanolone use to treat status epilepticus in children.
Super-refractory status epilepticus (SRSE), or seizures continuing for >24 hours despite general anesthesia, is a neurologic emergency with high morbidity and mortality.1 SRSE is often managed with benzodiazepines and barbiturates or general anesthesia, but treatment is limited by side effects and pharmacoresistance.2
Resistance to benzodiazepines is thought to be due to internalization of synaptic, but not extrasynaptic, γ-aminobutyric acid (GABA)A receptors.3 The neurosteroid allopregnanolone is a metabolite of progesterone, and has been proposed as a novel treatment for status epilepticus (SE).4,5 Allopregnanolone acts as a positive allosteric modulator of synaptic and extrasynaptic GABAA receptors, and terminates benzodiazepine-refractory SE in animal models.6 The potentiating effect of allopregnanolone on extrasynaptic GABAA receptors enhances tonic inhibition.7
We previously reported the treatment of new onset SRSE with allopregnanolone in a 23-year-old man.8 Here we describe the first use of allopregnanolone to treat SRSE in 2 children.
Patients and Methods
Patient 1
Patient 1 is a healthy 11-year-old girl who presented to another hospital (day 0 in Fig 1A) in SE. She was positive for antithyroglobulin, anti–Gad-65, and antimicrosomal antibodies. She was treated with 6 days of intravenous (IV) methylprednisolone, plasmapheresis (5 exchanges over 5 days), IV immunoglobulin (IVIG; 2g/kg), and rituximab (375mg/m2). Convulsive and nonconvulsive seizures were treated with multiple IV antiseizure agents, including continuous infusions of pentobarbital and propofol. She received maintenance doses of phenytoin, levetiracetam, and phenobarbital. Two attempts to reduce burst suppression resulted in breakthrough seizures. On hospital day (HD) 16 she was transferred to our hospital. At the time of transfer she was being treated with 4 antiseizure agents and was in pentobarbital-induced burst suppression.
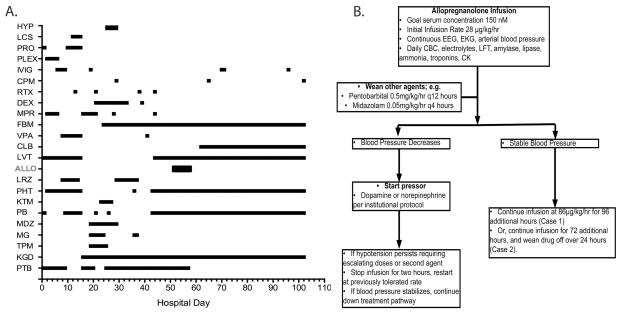
Fig. 1.
(A) Antiseizure and immunomodulatory medications used for Patient 1 by day of hospitalization. Day 0 = admission date. (B) Summary of allopregnanolone infusion protocol used for Patients 1 and 2 with hemodynamic and laboratory monitoring. ALLO = allopregnanolone; CBC = complete blood count; CK = creatine kinase; CLB = clobazam; CPM = cyclophosphamide; DEX = dexamethasone; EEG = electroencephalogram; EKG = electrocardiogram; FBM = felbamate; HYP = mild hypothermia; IVIG = intravenous immunoglobulin; KGD = ketogenic diet; KTM = ketamine; LCS = lacosamide; LFT = liver function testing; LRZ =lorazepam ; LVT = levetiracetam; MDZ = midazolam; MG = magnesium; MPR = methylprednisolone; PB = phenobarbital; PHT = phenytoin; PLEX = plasmapheresis; PRO = propofol; PTB = pentobarbital; RTX = rituximab; TPM = topiramate; VPA = valproate<zaq;2>.
Treatment for SRSE was continued with a combination of the ketogenic diet, additional IV methylprednisolone, and continued pentobarbital (day 16, see Fig 1A). Subsequent other therapies included magnesium infusion, mild hypothermia, ketamine, and repeated immunotherapy with IVIG, steroids, cyclophosphamide, and rituximab (see Fig 1A). Continuous electroencephalographic (cEEG) monitoring was used to confirm the presence of burst suppression, and to monitor the response to reduction in the rate of pentobarbital infusion. On HDs 19, 21, 37, and 45 (see Fig 1A) the pentobarbital rate was slowly reduced in the presence of midazolam, other agents including the ketogenic diet, and felbamate. The felbamate level on HD 41 was 19.0μg/ml (normal range = 30–50). β-OH-butyrate levels on HD 27 and 69 were 1.49 and 3.17mmol/l (normal range = 0.04–0.18).
Despite appropriate drug levels and doses (felbamate, 60mg/kg/day; phenobarbital, levels = 44–90μg/ml; phenytoin, level = 16.8μg/ml, levetiracetam, dose = 40mg/kg/day; and ketogenic diet, ratio of 4.5:1), multiple attempts to wean the pentobarbital resulted in recurrence of electrographic and clinical seizures. While weaning pentobarbital, prolonged video EEG monitoring was implemented in epochs of 12 to 24 hours. A mixture of primarily clinically apparent, as well as rare electrographic-only seizures were detected. These seizures were associated with a rhythmic theta-alpha focal discharge with diffuse bilateral spread. Clinical seizures consisted of a mixture of staring, eye fluttering, eye deviation, and rare focal motor convulsions. Each seizure lasted from 2 to 5 minutes and resolved spontaneously. Seizures increased in frequency up to 10 per hour before weaning was stopped and burst suppression was reinitiated.
On HD 52, after nearly continuous infusions of pentobarbital, midazolam, and ketamine, we received US Food and Drug Administration (FDA) approval for the emergency use of allopregnanolone (3α-hydroxy-5α-pregnan-20-one) IV solution (0.5mg/ml in 0.9% NaCl with 6% Captisol [Ligand Pharmaceuticals, La Jolla, CA], manufactured at the University of California, Davis). The goal of therapy was to enable weaning from pentobarbital. Allopregnanolone was infused over 5 days (see Fig 1B), after which pentobarbital sedation was weaned and discontinued. There were no hemodynamic or metabolic derangements referable to the allopregnanolone infusion. SE did not recur after the allopregnanolone infusion, and over the remainder of the hospitalization she only had intermittent seizures 1 to 2 times per week that either were self-limited or responded to intranasal midazolam. She was transferred for inpatient rehabilitation, regained her ability to walk, and is now back at home, continuing to show cognitive improvement, reading, doing arithmetic, and playing the piano.
Patient 2
The second patient is a 2-year-old girl with speech delay and epilepsy diagnosed 2 months earlier who presented with SE associated with a febrile illness. No infectious agents were identified. Convulsive and nonconvulsive seizures were treated with increasing doses of IV levetiracetam and phenobarbital with cEEG monitoring. On HD 3, high-dose midazolam and propofol infusions were added. Seizure frequency was not affected by a trial of pyridoxine followed by 5 days of IV methylprednisolone while continuing maintenance dosing of phenobarbital (10mg/kg/day; plasma concentration = 45 to >80μg/ml), midazolam (0.3mg/kg/h), and levetiracetam (105mg/kg/day). Pentobarbital infusion produced sustained burst suppression on HD 9. Two attempts to wean midazolam during pentobarbital treatment while continuing all other medications resulted in electrographic seizure recurrence. During the pentobarbital infusion, she developed hypotension requiring vasopressors, an ileus, and persistent urinary retention.
The majority of seizures were electrographic and varied in duration from seconds to minutes. When midazolam or pentobarbital infusion rates were decreased, seizures occurred up to 16 times per hour. Many arose from the right temporal region, starting in beta frequencies, evolving to slower frequencies, and ending with around 3 per second sharps. None of these seizures had a clinical correlation. Other electrographic seizures started in the right frontal region with 10- to 12Hz activity, followed by an increase in amplitude and decrease in frequency over the duration of the seizure, which occasionally involved the entire right hemisphere. Left temporal seizures were characterized by an abrupt onset of rhythmic 2- to 3Hz waveforms occurring maximally over the left midtemporal region.
Emergency use of allopregnanolone was approved by the FDA on HD 15. The goal of therapy was to enable weaning from pentobarbital and midazolam infusions, with secondary effects of discontinuing vasopressor support and restoring bowel function. Allopregnanolone was infused according to the protocol in Figure 1B, and tapered off between hours 96 and 120 as a precaution for seizure recurrence. The midazolam infusion was titrated off over the first 24 hours, followed by tapering pentobarbital from 5.0 to 0.5mg/kg/h over 72 hours. Twice daily rufinamide dosing started at hour 48, and enteral lorazepam was started at hour 96. An electrographic seizure occurred after the allopregnanolone infusion ended, and pentobarbital was adjusted to 1mg/kg/h with suppression of all seizures. The patient continued on lower doses of pentobarbital (0.5–1mg/kg/h) for 12 additional days. As the midazolam and pentobarbital were decreasing, the patient’s blood pressure recovered, vasopressors were discontinued, and the ileus resolved. The child was transferred to inpatient rehabilitation, regained milestones, and is now able to walk and speak. The etiology for her seizures remains unknown.
Allopregnanolone Dosing and Toxicity Monitoring
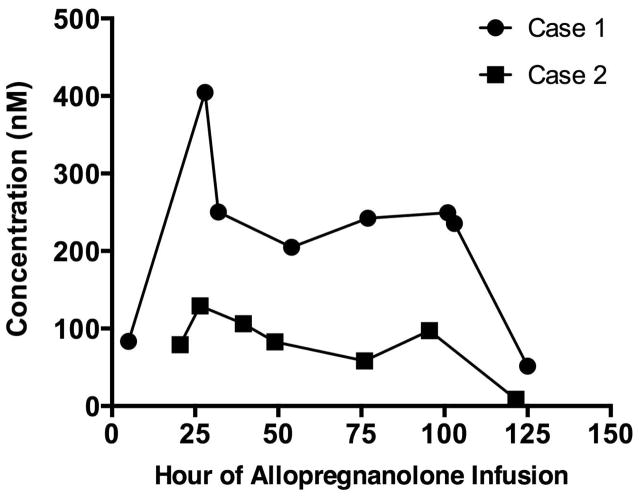
Both patients were treated with a continuous infusion of allopregnanolone using a similar dosing schedule combined with physiologic and laboratory monitoring (see Fig 1B). The target infusion rate of 86μg/kg/h was determined by pharmacokinetic modeling to result in a steady-state level of 150nM.9 Because allopregnanolone has not previously been administered to children, the infusion rate was increased gradually over the first 24 hours. Then, to achieve the target rapidly, a single bolus of 86μg/kg was administered at 24 hours. In hindsight, this was not needed given the peak serum level achieved (404.7nmol) at hour 28. For Patient 1, the same infusion rate was continued until hour 120, when it was discontinued. For Patient 2, the infusion was tapered from hour 96 to hour 120. In both cases, the objective of treatment was to enable the withdrawal of pentobarbital and/or midazolam without the recurrence of seizures. There were no adverse drug effects detected by any of the laboratory tests used (data not shown).
In Patient 1, plasma levels above the goal 150nM were achieved after the bolus at 24 hours (Fig 2), and remained above this level for the duration of treatment. In Patient 2, plasma levels were slightly below this target yet achieved the same therapeutic goal.
Fig. 2.
Allopreganolone plasma concentrations in both patients during allopregnanolone infusion.
AQ1: AU: In second paragraph of Acknowledgment, please add “Dr” where appropriate.
AQ2: AU: “LFT = liver function testing” and “MPR = methylprednisolone” okay in Fig 1 legend?
Discussion
Here we report the first 2 uses of allopregnanolone infusion in the treatment of pediatric SRSE. Treatment allowed the withdrawal of general anesthetic infusions (pentobarbital, or pentobarbital and midazolam), which had been required to prevent the recurrence of clinical and electrographic seizures. Physiologic and laboratory monitoring showed no adverse effects of drug treatment. Withdrawal of other antiseizure agents also resulted in resolution of other complications of their use (hypotension, ileus, urinary retention). Importantly in both cases, there had been multiple unsuccessful attempts to wean barbiturates or benzodiazepines and other antiseizure drugs. Determining whether allopregnanolone was instrumental in achieving this response or the response was due to the cumulative effect of the preceding and concomitant treatments will require further study.
Mortality in refractory SE (RSE), SE resistant to 2 antiseizure agents, can be as high as 35%. Among survivors, there are high rates of subsequent epilepsy and severe neurologic impairment.1 Guidelines for the treatment of RSE recommend that following appropriate treatment with benzodiazepines and antiseizure medications such as valproate, phenytoin, or phenobarbital, practitioners should use continuous infusions of anticonvulsant general anesthetics such as midazolam, propofol, or pentobarbital.10,11 This was the approach followed in both these cases.
Before treatment with allopregnanolone, we used a range of high-dose antiseizure, immune-modulating, and metabolic therapies. This is consistent with common practices in the management of RSE.1 A recent survey showed good agreement on initial treatment of SE, but patient age does appear to impact medication decisions.10 Variable combinations of general anesthetic agents, immunomodulation, epilepsy surgery, electroconvulsive therapy, hypothermia, and ketogenic diet as treatment for SRSE have all been reported, and our management approach reflects the lack of data on a optimal treatment for RSE.11
The dose of allopregnanolone we used was empirical and based on the maximum levels permitted by the FDA. Brain levels have been found to be comparable to plasma concentrations (unpublished observations). If this was true for our patients, then the concentrations available at brain GABAA receptors were likely several-fold the effective concentrations for positive modulation of these receptors.12 The mean steady-state plasma level in Patient 2 (86nm) between hours 39.5 and 95.5 of allopregnanolone infusion was lower than the goal of 150nm. This is likely due to greater drug clearance observed at age 2 years compared to adults.13
There is precedent for the use of neurosteroids to treat epilepsy. Progesterone, the precursor to allopregnanolone, has shown efficacy in the treatment of catamenial epilepsy.4 Ganaxolone, a synthetic analog of allopregnanolone, has been studied in clinical trials for both refractory focal epilepsy and infantile spasms, with preliminary evidence of clinical benefit.14,15 A clinical trial of allopregnanolone is investigating its effect in traumatic brain injury.16 By acting on extrasynaptic GABAA receptors, allopregnanolone has the potential to treat RSE, where treatment resistance is believed to be due to internalization and inactivity of synaptic GABAA receptors. Neurosteroids, including allopregnanolone, are a promising treatment for epilepsy and RSE that may overcome resistance to benzodiazepines and barbiturates. In addition, they may facilitate the withdrawal of these agents by preventing rebound seizures, a key problem in the treatment of SRSE.
Acknowledgments
This work was supported by grants to M.A.R. from the Department of Defense Congressionally Directed Medical Research Programs (W81XWH-09-1-0746) and NIH National Institute of Neurological Disorders and Stroke (NS079202).
We thank H. Vaitkevicius for discussions, G. Bauer for the allopregnanolone intravenous formulation manufacturing, C.-Y. Wu for plasma allopregnanolone analyses, and Sage Therapeutics (Cambridge, MA) for permitting the use of Captisol. M.A.R. provided the allopregnanolone intravenous solution.
Footnotes
Authorship
M.S.W. and E.B. conceived the project, collected data, and drafted the manuscript. J.E.N. conceived the project, collected data, and revised the drafted manuscript. M.G., J.G., and C.M.S. revised the drafted manuscript. C.C. collected data and revised the drafted manuscript. S.K. and M.A.R. collected and analyzed data and revised the drafted manuscript. All authors contributed to the current version of the article including either conception, data analysis, or editing.
Potential Conflicts of Interest
J.E.N. coinvestigator, Department of Defense–sponsored clinical trial of allopregnanolone in traumatic brain injury; unpaid consultancy, Sage Therapeutics. S.K.: stock option, Sage Therapeutics; employee of Sage Therapeutics, which is conducting an ongoing clinical trial of allopregnanolone for the treatment of SRSE in adults. M.A.R.: advisory board, equity, Sage Therapeutics; principal investigator, Department of Defense–sponsored clinical trial of allopregnanolone in traumatic brain injury. M.S.W.: unpaid consultancy, Sage Therapeutics. The allopregnanolone intravenous formulation was manufactured by the University of California, Davis and was provided free of charge to the treating physicians. The material was not provided by Sage Therapeutics and was not part of a Sage-sponsored clinical trial. Sage Therapeutics is currently developing the formulation of allopregnanolone used in this report for treatment of adults with SRSE and is the sponsor of a clinical trial of this drug in adults with SRSE. None of the authors is a patent holder for relevant inventions.<zbmrule>
References
- 1.Ferlisi M, Shorvon S. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. 2012;135(pt 8):2314–2328. doi: 10.1093/brain/aws091. [DOI] [PubMed] [Google Scholar]
- 2.Hocker S, Wijdicks EF, Rabinstein AA. Refractory status epilepticus: new insights in presentation, treatment, and outcome. Neurol Res. 2013;35:163–168. doi: 10.1179/1743132812Y.0000000128. [DOI] [PubMed] [Google Scholar]
- 3.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy DS, Rogawski MA. Neurosteroids—endogenous regulators of seizure susceptibility and role in the treatment of epilepsy. In: Noebels JL, Avoli M, Rogawski MA, et al., editors. Jasper’s basic mechanisms of the epilepsies contemporary neurology series 80. New York, NY: Oxford University Press; 2012. pp. 984–1002. [Google Scholar]
- 5.Kokate TG, Cohen AL, Karp E, et al. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35:1049–1056. doi: 10.1016/s0028-3908(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 6.Rogawski MA, Loya CM, Reddy K, et al. Neuroactive steroids for the treatment of status epilepticus. Epilepsia. 2013;54 (suppl 6):93–98. doi: 10.1111/epi.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brickley SG, Mody I. Extrasynaptic GABAA receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaitkevicius H, Ng M, Moura L, et al. Successful allopregnanolone treatment of new onset refractory status epilepticus (NORSE) syndrome: first in man experience. Epilepsia. 2013;54(suppl 6):106–124. [Google Scholar]
- 9.Timby E, Balgård M, Nyberg S, et al. Pharmacokinetic and behavioral effects of allopregnanolone in healthy women. Psychopharmacology (Berl) 2006;186:414–424. doi: 10.1007/s00213-005-0148-7. [DOI] [PubMed] [Google Scholar]
- 10.Riviello JJ, Jr, Claassen J, LaRoche SM, et al. Treatment of status epilepticus: an international survey of experts. Neurocrit Care. 2013;18:193–200. doi: 10.1007/s12028-012-9790-1. [DOI] [PubMed] [Google Scholar]
- 11.Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. 134(pt 10):2802–2818. doi: 10.1093/brain/awr215. 201. [DOI] [PubMed] [Google Scholar]
- 12.Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- 13.Ginsberg G, Hattis D, Sonawane B, et al. Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol Sci. 2002;66:185–200. doi: 10.1093/toxsci/66.2.185. [DOI] [PubMed] [Google Scholar]
- 14.Bialer M, Johannessen SI, Levy RH, et al. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI) Epilepsy Res. 2013;103:2–30. doi: 10.1016/j.eplepsyres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Kerrigan JF, Shields WD, Nelson TY, et al. Ganaxolone for treating intractable infantile spasms: a multicenter, open-label, add-on trial. Epilepsy Res. 2000;42:133–139. doi: 10.1016/s0920-1211(00)00170-4. [DOI] [PubMed] [Google Scholar]
- 16.University of California, Davis. [Accessed September 3, 2014];Allopregnanolone for the treatment of traumatic brain injury. Available at: http://clinicaltrials.gov/ct2/show/NCT01673828.