Abstract
Objectives
Glycated haemoglobin A1c (HbA1c) measurement is recommended as an alternative to fasting plasma glucose (FPG) for the diagnosis of pre-diabetes and type 2 diabetes. However, evidence suggests discordance between HbA1c and FPG. In this study we examine a range of metabolic risk features, pro-inflammatory cytokines, acute-phase response proteins, coagulation factors and white blood cell counts to determine which assay more accurately identifies individuals at increased cardiometabolic risk.
Materials and Methods
This was a cross-sectional study involving a random sample of 2,047 men and women aged 46-73 years. Binary and multinomial logistic regression were employed to examine risk feature associations with pre-diabetes [either HbA1c levels 5.7-6.4% (39-46 mmol/mol) or impaired FPG levels 5.6-6.9 mmol/l] and type 2 diabetes [either HbA1c levels >6.5% (>48 mmol/mol) or FPG levels >7.0 mmol/l]. Receiver operating characteristic curve analysis was used to evaluate the ability of HbA1c to discriminate pre-diabetes and diabetes defined by FPG.
Results
Stronger associations with diabetes-related phenotypes were observed in pre-diabetic subjects diagnosed by FPG compared to those detected by HbA1c. Individuals with type 2 diabetes exhibited cardiometabolic profiles that were broadly similar according to diagnosis by either assay. Pre-diabetic participants classified by both assays displayed a more pro-inflammatory, pro-atherogenic, hypertensive and insulin resistant profile. Odds ratios of having three or more metabolic syndrome features were also noticeably increased (OR: 4.0, 95% CI: 2.8-5.8) when compared to subjects diagnosed by either HbA1c (OR: 1.4, 95% CI: 1.2-1.8) or FPG (OR: 3.0, 95% CI: 1.7-5.1) separately.
Conclusions
In middle-aged Caucasian-Europeans, HbA1c alone is a poor indicator of cardiometabolic risk but is suitable for diagnosing diabetes. Combined use of HbA1c and FPG may be of additional benefit for detecting individuals at highest odds of type 2 diabetes development.
Introduction
The prevalence of type 2 diabetes, a chronic disease which causes significant mortality, has increased considerably in world populations, representing a major public health issue [1]. Diabetes is associated with a clustering of cardiometabolic features including obesity, dyslipidaemia, hypertension, insulin resistance, chronic low-grade inflammation [2, 3], and may lead to severe cardiovascular complications [4].
Pre-diabetes, a condition defined by glycaemic profiles that are higher than normal but which do not meet thresholds for diabetes, is a strong risk factor for type 2 diabetes and related complications [5]. The American Diabetes Association (ADA) classifies type 2 diabetes as a fasting plasma glucose (FPG) level ≥7.0 mmol/l and pre-diabetes as impaired FPG levels between 5.6–6.9 mmol/l [2]. In 2009 the International Expert Committee recommended glycated haemoglobin A1c (HbA1c) as an alternative marker [6], and in 2010 the ADA introduced HbA1c cut-points of ≥6.5% (≥48 mmol/mol) for diabetes diagnosis and between 5.7–6.4% (39–46 mmol/mol) as a criterion to identify individuals at a high-risk state of developing diabetes [2]. Perceived benefits of the use of HbA1c measurement, over FPG, include greater pre-analytical stability, lower biological variability and that the assay may be performed in non-fasting blood samples [7, 8]. However, use of HbA1c as a screening tool has been controversial, with research showing discordance between HbA1c and FPG [9–12], and several studies suggesting that factors such as age or ethnicity may influence diagnostic performance [13–15].
The aim of this study was to compare the metabolic profiles in subjects with pre-diabetes and type 2 diabetes, using ADA-recommended HbA1c and FPG diagnostic thresholds, in a random sample of 2,047 middle-aged men and women. In particular, we examined a range of diabetes risk factors, metabolic syndrome (MetS) features, pro-inflammatory cytokines, acute-phase response proteins, coagulation factors and white blood cell (WBC) counts to determine which assay more accurately identifies individuals at increased cardiometabolic risk.
Materials and Methods
Study population
The Cork and Kerry Diabetes and Heart Disease Study (Phase II) was a single centre, cross-sectional study conducted between 2010 and 2011. A random sample was recruited from a large primary care centre in Mitchelstown, County Cork, Ireland. The Livinghealth Clinic serves a population of approximately 20,000 Caucasian-European subjects, with a mix of urban and rural residents. Stratified sampling was employed to recruit equal numbers of men and women from all registered attending patients in the 46–73 year age group. In total, 3,807 potential participants were selected from the practice list. Following the exclusion of duplicates, deaths, and subjects incapable of consenting or attending appointment, 3,051 were invited to participate in the study and of these, 2,047 (49.2% male) completed the questionnaire and physical examination components of the baseline assessment (response rate: 67.1%). The status of non-responders included individuals refusing to participate (59.4%) and those who did not reply (40.6%). Male subjects accounted for 53.7% of non-responders while 43.5% (vs. 42.8% of responders) were >60 years of age. Details regarding the study design, sampling procedures and methods of data collection have been reported previously [16].
Ethics committee approval conforming to the Declaration of Helsinki was obtained from the Clinical Research Ethics Committee of University College Cork. A letter signed by the contact GP in the clinic was sent out to all selected participants with a reply slip indicating acceptance or refusal. All subjects gave signed informed consent, including permission to use their data for research purposes.
Clinical and laboratory procedures
All study participants attended the clinic in the morning after an overnight fast and blood samples were taken on arrival. Data on age, gender, family diabetes history, physician-diagnosed type 2 diabetes and prescription (Rx) medication use were gathered through a self-completed General Health Questionnaire. Triglyceride and high density lipoprotein cholesterol (HDL-C) levels were measured by Cork University Hospital Biochemistry Laboratory on Olympus 5400 biochemistry analysers with Olympus reagents using standardised procedures and fresh samples (Olympus Diagnostica GmbH, Hamburg, Germany). Fasting glucose concentrations were determined using a glucose hexokinase assay (Olympus Life and Material Science Europa Ltd., Lismeehan, Co. Clare, Ireland) and HbA1c levels were measured in the haematology laboratory on an automated high-pressure liquid chromatography instrument Tosoh G7 [Tosoh HLC-723 (G7), Tosoh Europe N.V, Tessenderlo, Belgium]. Serum insulin, c-reactive protein (CRP), tumour necrosis factor alpha (TNF-α), interleukin 6 (IL-6), adiponectin, leptin, resistin and plasminogen activator inhibitor-1 (PAI-1) were assessed using a biochip array system (Evidence Investigator; Randox Laboratories, UK). Complement component 3 (C3) was measured by immunoturbidimetric assay (RX Daytona; Randox Laboratories). White blood cell counts were determined by flow cytometry technology as part of a full blood count.
Three independent measurements of systolic and diastolic blood pressure (BP) were obtained with the subject in a seated position using an Omron M7 digital sphygmomanometer (Omron Healthcare Co. Ltd., Japan). The mean of the second and third readings was considered to be a subject’s BP. The weight and height of each participant were measured to the nearest 0.1 kg and 0.1 cm respectively. Portable electronic Tanita WB-100MA weighing scales (Tanita Corporation, IL, USA) were placed on a firm, flat surface and were calibrated weekly to ensure accuracy. Height was measured using a portable Seca Leicester height/length stadiometer (Seca, Birmingham, UK) and body mass index (BMI) was calculated as weight divided by the square of height. A BMI ≥30 kg/m2 was classified as obese. Waist circumference (WC) was measured between the lowest rib and iliac crest on bare skin. Subjects were instructed to breathe in, and then out, and to hold their breath while measurement was made to the nearest 0.1 cm using a Seca 200 measuring tape. Two independent measurements of WC were taken and the mean of the two was used in analysis. Central obesity was defined as a WC level ≥102 cm for males and ≥88 cm for females.
Classification of biochemical and blood pressure measurements
Lipid, lipoprotein and BP measurements were categorised according to National Cholesterol Education Program Adult Treatment Panel III (NCEP: ATP III) guidelines [17]. Abnormal metabolic risks were defined as high triglycerides ≥1.7 mmol/l and low HDL-C (<1.03 mmol/l in males or <1.29 mmol/l in females). Dyslipidaemia was determined according to both high triglyceride and low HDL-C levels. Elevated BP was classified as systolic BP ≥130 mmHg and/or diastolic BP ≥85 mmHg or Rx anti-hypertensive medication use. High serum insulin was defined as a level equal to or above the 75th percentile in the study sample. Metabolic syndrome was determined according to a modified version of the NCEP: ATP III criterion, substituting serum insulin 75th percentile for impaired FPG. Three or more MetS features (≥3 MetS) was characterised as any combination of the following: obesity defined by WC, high triglyceride levels, low HDL-C, elevated BP and high insulin concentrations. According to ADA guidelines, pre-diabetes was classified as elevated HbA1c levels between 5.7–6.4% (39–46 mmol/mol) or impaired FPG levels between 5.6–6.9 mmol/l. Type 2 diabetes was defined as HbA1c ≥6.5% (≥48 mmol/mol) or FPG ≥7.0 mmol/l [2]. As internationally recognised risk cut-points for the examined biomarkers have not been established, we classified inflammation and raised immune activation as a level above the study population median for each biomarker (C3, CRP, IL-6, TNF-α, leptin, resistin, PAI-1 and WBC) with the exception of adiponectin (below median level).
Statistical analysis
Descriptive characteristics were examined according to diagnosis of pre-diabetes and type 2 diabetes. Categorical features are presented as percentages and continuous variables are displayed as a mean (plus or minus one standard deviation) or a median and interquartile range for skewed data. Binary logistic regression was used to explore diabetes-related risk factor and inflammatory biomarker relationships with pre-diabetes (compared to normoglycaemic subjects) and type 2 diabetes (compared to individuals without diabetes) defined using HbA1c and FPG diagnostic cut-points. Models examining metabolic feature associations with pre-diabetes excluded patients with type 2 diabetes indicated by either HbA1c or FPG, a physician diagnosis or Rx diabetes medication use. Risk feature relationships with pre-diabetes (either HbA1c alone, FPG alone or dual categorisation by both HbA1c and FPG) were further evaluated using multinomial logistic regression. Subjects classified as normoglycaemic by both assays were used as the reference category.
The ability of HbA1c to discriminate pre-diabetes (defined by impaired FPG) and type 2 diabetes (defined by FPG levels ≥7.0 mmol/l) was assessed using receiver operating characteristic curve (ROC) analysis. The area under the curve (AUC) provides a scale from 0.5 to 1.0 (with 0.5 representing random chance and 1.0 indicating perfect discrimination) by which to compare the ability of a marker to detect a positive result [18]. The diagnostic properties of different HbA1c thresholds were contrasted by determining sensitivity and false positive rates (FPR). Levels of agreement between diagnostic methods were ascertained using Cohen’s kappa coefficient (K).
Primary data analysis was conducted using IBM SPSS Statistics Version 20 (IBM Corp., Armonk, NY, USA) for Windows. Confidence intervals for prevalence proportions were calculated using the VasserStats statistical website [19]. For all analyses, a P value (two-tailed) of less than 0.05 was considered to indicate statistical significance. Assay results for HbA1c and FPG were available for 1,995 (97.5%) and 1,994 (97.4%) subjects. Participants missing either HbA1c or FPG data were excluded from multinomial and ROC analyses. Low-level missing values were found within most independent variables. Sensitivity analysis indicated a similar percentage of missing data according to either HbA1c or FPG pre-diabetes and diabetes classifications. Missing independent variable data were thus assumed to be ignorable and missing at random.
Results
Descriptive characteristics
Characteristics of the study population according to pre-diabetes and type 2 diabetes classifications are presented in Table 1. The prevalence of pre-diabetes was 49.1% (95% CI: 46.9%-51.3%) by elevated HbA1c and 11.5% (95% CI: 10.2%-13.0%) by impaired FPG. Subjects categorised as pre-diabetic using HbA1c had lower BMI and WC levels, lower triglyceride and insulin concentrations, higher HDL-C levels, were less hypertensive, and a greater proportion were female when compared to individuals with pre-diabetes defined by FPG.
Table 1. Characteristics of the study population according to pre-diabetes and type 2 diabetes status.
| Feature | Full cohort | Pre-diabetes 1 | Type 2 diabetes 2 | ||
|---|---|---|---|---|---|
| HbA1c | FPG | HbA1c | FPG | ||
| (N = 2047) | (N = 980) | (N = 230) | (N = 146) | (N = 85) | |
| Male | 1008 (49.2) | 441 (45.0) | 150 (65.2) | 95 (65.1) | 59 (69.4) |
| Age | 59.0 (55.0–64.0) | 60.0 (55.0–64.0) | 61.0 (56.0–65.0) | 60.0 (57.0–65.0) | 61.0 (56.5–64.5) |
| Age ≥60 | 981 (47.9) | 510 (52.0) | 125 (54.3) | 83 (56.8) | 51 (60.0) |
| Diagnosed diabetes | 101 (4.9) | - | - | 73 (50.0) | 51 (60.0) |
| On Rx for diabetes | 78 (3.8) | - | - | 60 (41.1) | 41 (48.2) |
| On Rx for hypertension | 584 (28.5) | 307 (31.3) | 98 (42.6) | 81 (55.5) | 48 (56.5) |
| On Rx for cholesterol | 711 (34.7) | 385 (39.3) | 93 (40.4) | 88 (60.3) | 49 (57.6) |
| BMI (kg/m2) | 28.60 ± 4.7 | 28.80 ± 4.7 | 30.45 ± 5.2 | 32.17 ± 5.5 | 31.81 ± 5.5 |
| BMI ≥30 | 668 (32.7) | 345 (35.2) | 109 (47.4) | 85 (58.2) | 49 (57.6) |
| WC (cm) | 97.04 ± 13.2 | 97.08 ± 12.9 | 102.44 ± 12.8 | 107.91 ± 13.7 | 108.52 ± 13.9 |
| WC (HIGH) | 1119 (54.8) | 562 (57.4) | 150 (65.2) | 119 (81.5) | 66 (77.6) |
| Family diabetes history | 390 (19.1) | 176 (18.0) | 46 (20.0) | 62 (42.5) | 41 (48.2) |
| Triglycerides (mmol/l) | 1.22 (0.9–1.7) | 1.23 (0.9–1.7) | 1.41 (1.0–2.0) | 1.58 (1.2–2.3) | 1.68 (1.2–2.3) |
| Triglycerides ≥1.7 | 490 (24.6) | 230 (23.8) | 85 (37.9) | 65 (45.5) | 40 (48.8) |
| HDL-C (mmol/l) | 1.45 ± 0.4 | 1.45 ± 0.4 | 1.32 ± 0.3 | 1.17 ± 0.3 | 1.17 ± 0.4 |
| HDL-C (LOW) | 353 (17.6) | 165 (17.0) | 59 (26.1) | 66 (45.2) | 35 (41.2) |
| Dyslipidaemia | 168 (8.4) | 78 (8.0) | 32 (14.0) | 37 (25.3) | 22 (25.9) |
| Systolic BP (mmHg) | 129.60 ± 16.8 | 130.10 ± 16.1 | 134.78 ± 15.5 | 134.19 ± 17.3 | 136.24 ± 17.4 |
| Diastolic BP (mmHg) | 80.12 ± 9.7 | 80.24 ± 9.6 | 82.25 ± 9.1 | 79.50 ± 10.3 | 80.72 ± 10.5 |
| BP ≥130/85 | 1045 (51.3) | 521 (53.4) | 155 (67.7) | 89 (61.4) | 56 (66.7) |
| HbA1c (%) | 5.7 (5.5–6.0) | 5.9 (5.7–6.0) | 5.8 (5.6–6.1) | 7.0 (6.7–8.1) | 7.6 (6.8–9.0) |
| HbA1c (mmol/mol) | 39 (37–42) | 41 (39–42) | 40 (38–43) | 53 (50–65) | 60 (51–75) |
| FPG (mmol/l) | 4.90 (4.7–5.4) | 5.00 (4.7–5.3) | 5.80 (5.7–6.1) | 6.90 (6.0–9.0) | 8.50 (7.6–10.8) |
| Insulin (μU/ml) | 8.65 (5.3–14.1) | 8.98 (4.6–11.8) | 12.67 (7.4–19.5) | 18.27 (10.6–31.9) | 19.21 (12.1–30.9) |
| Insulin 75th percentile | 497 (25.0) | 238 (24.6) | 98 (43.2) | 94 (65.7) | 59 (70.2) |
| ≥3 MetS features 3 | 606 (29.6) | 298 (30.4) | 112 (48.7) | 103 (70.5) | 63 (74.1) |
| C3 (mg/dl) | 135.92 ± 24.7 | 138.85 ± 24.5 | 141.41 ± 25.8 | 148.13 ± 28.6 | 149.20 ± 24.9 |
| CRP (ng/ml) | 1.35 (1.0–2.3) | 1.43 (1.0–2.4) | 1.38 (1.0–2.3) | 1.79 (1.1–3.2) | 1.91 (1.2–3.0) |
| IL-6 (pg/ml) | 1.81 (1.2–2.9) | 1.91 (1.3–3.0) | 2.02 (1.5–3.0) | 2.92 (1.7–4.8) | 2.83 (1.8–4.6) |
| TNF-α (pg/ml) | 5.97 (4.9–7.3) | 6.02 (5.0–7.3) | 5.94 (4.9–7.5) | 6.99 (5.5–8.3) | 7.09 (5.6–8.1) |
| Adiponectin (ng/ml) | 4.75 (2.9–7.5) | 4.92 (3.1–7.5) | 3.63 (2.4–5.6) | 2.82 (1.7–4.6) | 2.73 (1.9–4.7) |
| Leptin (ng/ml) | 1.95 (1.1–3.1) | 2.09 (1.3–3.5) | 2.06 (1.3–3.8) | 2.28 (1.3–3.9) | 2.09 (1.1–3.4) |
| Resistin (ng/ml) | 5.07 (3.9–6.7) | 4.93 (3.8–6.6) | 4.89 (3.7–6.7) | 6.15 (4.6–7.3) | 5.53 (4.5–7.3) |
| PAI-1 (ng/ml) | 27.38 ± 12.6 | 27.87 ± 12.0 | 29.56 ± 13.2 | 31.35 ± 15.9 | 30.03 ± 11.0 |
| WBC (109/l) | 6.00 ± 1.9 | 6.12 ± 2.1 | 6.33 ± 1.72 | 7.39 ± 2.4 | 7.21 ± 1.9 |
Mean and ± standard deviation are shown for continuous variables. Age, triglycerides, HbA1c, FPG, insulin, CRP, IL-6, TNF-α, adiponectin, leptin and resistin are shown as a median (interquartile range). Numbers and % (in brackets) for categorical variables will vary in different analyses as some variables have missing values.
1Pre-diabetes: HbA1c levels 5.7–6.4% (39–46 mmol/mol) or FPG levels 5.6–6.9 mmol/l.
2Type 2 diabetes: HbA1c ≥6.5% (≥48 mmol/mol) or FPG ≥7.0 mmol/l.
3MetS features: WC (HIGH), triglycerides ≥1.7, HDL-C (LOW), BP ≥130/85 or Rx and insulin 75th percentile.
Logistic regression
In binary logistic regression analyses (Table 2), associations between commonly assessed diabetes risk factors and pre-diabetes were stronger in subjects diagnosed by FPG. Odds ratios for pre-diabetes indicated by HbA1c were non-significant for having a family diabetes history and elevated triglyceride levels, while there was a three-fold increased likelihood (OR: 3.0, 95% CI: 2.2–3.9) of having ≥3 MetS features in participants identified by FPG compared to an odds ratio of 1.6 (95% CI: 1.3–2.0) in pre-diabetes by HbA1c. In contrast, metabolic risk factor relationships with type 2 diabetes were generally comparable according to diagnosis by either assay, with odds ratios of having ≥3 MetS features being 6.1 (95% CI: 4.2–8.8) and 6.8 (95% CI: 4.1–11.2) for subjects diagnosed by HbA1c and FPG respectively. Regardless of definition, patients with pre-diabetes and type 2 diabetes displayed a chronic pro-inflammatory profile as characterised by elevated C3, IL-6, WBC levels and reduced adiponectin concentrations.
Table 2. Odds ratios (95% CI) of having risk factors according to diagnosis of pre-diabetes and type 2 diabetes by HbA1c or FPG.
| Feature | Odds ratios (95% CI) 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-diabetes compared to normoglycaemia 2 | Type 2 diabetes compared to no diabetes 3 | |||||||
| HbA1c | P value | FPG | P value | HbA1c | P value | FPG | P value | |
| Male | 0.8 (0.6–0.9) | <0.001 | 2.3 (1.7–3.0) | <0.001 | 2.0 (1.4–2.9) | <0.001 | 2.5 (1.5–3.9) | <0.001 |
| Age ≥60 | 1.6 (1.3–1.9) | <0.001 | 1.4 (1.1–1.9) | 0.011 | 1.5 (1.1–2.2) | 0.018 | 1.7 (1.1–2.7) | 0.017 |
| Family diabetes history | 1.2 (0.9–1.5) | 0.182 | 1.4 (1.0–2.1) | 0.043 | 4.1 (2.9–5.9) | <0.001 | 5.2 (3.3–8.1) | <0.001 |
| BMI ≥30 | 1.8 (1.4–2.2) | <0.001 | 2.2 (1.7–3.0) | <0.001 | 3.1 (2.2–4.3) | <0.001 | 2.8 (1.8–4.4) | <0.001 |
| WC (HIGH) | 1.5 (1.2–1.9) | 0.001 | 2.0 (1.4–3.1) | 0.001 | 5.4 (2.5–11.8) | <0.001 | 7.4 (2.3–23.5) | 0.001 |
| Triglycerides ≥1.7 | 1.2 (0.9–1.5) | 0.134 | 2.1 (1.5–2.8) | <0.001 | 2.5 (1.8–3.6) | <0.001 | 2.8 (1.8–4.4) | <0.001 |
| HDL-C (LOW) | 1.4 (1.1–1.8) | 0.018 | 2.3 (1.7–3.3) | <0.001 | 4.6 (3.2–6.6) | <0.001 | 3.6 (2.3–5.7) | <0.001 |
| Dyslipidaemia | 1.6 (1.1–2.4) | 0.019 | 2.6 (1.7–4.1) | <0.001 | 4.3 (2.8–6.5) | <0.001 | 4.1 (2.4–6.9) | <0.001 |
| BP ≥130/85 or Rx | 1.4 (1.2–1.7) | <0.001 | 2.5 (1.8–3.5) | <0.001 | 3.0 (1.9–4.8) | <0.001 | 4.4 (2.2–8.6) | <0.001 |
| Insulin 75th percentile | 1.6 (1.3–2.0) | <0.001 | 3.1 (2.3–4.2) | <0.001 | 6.5 (4.5–9.4) | <0.001 | 7.2 (4.4–11.7) | <0.001 |
| ≥3 MetS features 4 | 1.6 (1.3–2.0) | <0.001 | 3.0 (2.2–3.9) | <0.001 | 6.1 (4.2–8.8) | <0.001 | 6.8 (4.1–11.2) | <0.001 |
| C3 5 | 1.8 (1.5–2.2) | <0.001 | 1.4 (1.0–1.8) | 0.032 | 3.3 (2.2–4.9) | <0.001 | 3.1 (1.9–5.0) | <0.001 |
| CRP 5 | 1.4 (1.1–1.7) | 0.001 | 1.2 (0.9–1.5) | 0.293 | 1.5 (1.1–2.2) | 0.02 | 1.6 (1.0–2.6) | 0.032 |
| IL-6 5 | 1.6 (1.3–1.9) | <0.001 | 1.5 (1.1–2.0) | 0.005 | 2.8 (1.9–4.1) | <0.001 | 2.8 (1.7–4.6) | <0.001 |
| TNF-α 5 | 1.2 (1.0–1.4) | 0.078 | 1.0 (0.7–1.3) | 0.738 | 2.3 (1.6–3.3) | <0.001 | 2.7 (1.6–4.4) | <0.001 |
| Adiponectin 5 | 1.4 (1.1–1.7) | 0.004 | 2.0 (1.4–2.7) | <0.001 | 4.0 (2.5–6.2) | <0.001 | 3.2 (1.8–5.6) | <0.001 |
| Leptin 5 | 1.5 (1.2–1.8) | <0.001 | 1.4 (1.1–1.9) | 0.014 | 1.5 (1.0–2.1) | 0.026 | 1.2 (0.8–1.8) | 0.48 |
| Resistin 5 | 0.9 (0.8–1.1) | 0.305 | 0.9 (0.7–1.2) | 0.391 | 2.4 (1.7–3.5) | <0.001 | 1.8 (1.1–2.8) | 0.012 |
| PAI-1 5 | 1.3 (1.1–1.6) | 0.005 | 1.3 (1.0–1.7) | 0.108 | 1.5 (1.0–2.1) | 0.028 | 1.5 (1.0–2.4) | 0.078 |
| WBC 5 | 1.7 (1.4–2.1) | <0.001 | 1.6 (1.2–2.1) | 0.001 | 3.4 (2.3–5.0) | <0.001 | 3.3 (2.0–5.5) | <0.001 |
1Binary logistic regression. Gender adjusted for age (continuous), age ≥60 adjusted for gender, all other variables adjusted for age (continuous) and gender.
2Pre-diabetes: HbA1c ≥5.7% (≥39 mmol/mol) or FPG ≥5.6 mmol/l, models exclude subjects with type 2 diabetes: HbA1c ≥6.5% (≥48 mmol/mol) or FPG ≥7.0 mmol/l or physician diagnosis or Rx diabetes medication use.
3Models exclude 24 subjects that indicated a physician diagnosis or Rx diabetes medication use but who did not have positive HbA1c or FPG test results.
4MetS features: WC (HIGH), triglycerides ≥1.7, HDL-C (LOW), BP ≥130/85 or Rx and insulin 75th percentile.
5Threshold: above median level in the study population except adiponectin (below median level).
The results from multinomial regression models exploring risk factor relationships with pre-diabetes classified by HbA1c alone, FPG alone, or by both HbA1c and FPG together are displayed in Table 3. Odds ratios for obesity, elevated BP, increased insulin concentrations and MetS were higher in participants classified by both assays, with four-fold increased odds (OR: 4.0, 95% CI: 2.8–5.8) of having ≥3 MetS features, compared to either HbA1c (OR: 1.4, 95% CI: 1.2–1.8) or FPG (OR: 3.0, 95% CI: 1.7–5.1) alone. Stronger associations with markers of inflammation were also observed in subjects identified by both criteria.
Table 3. Odds ratios (95% CI) of having risk factors according to diagnosis of pre-diabetes 1 by HbA1c alone, FPG alone, or by both HbA1c and FPG together.
| Feature | Odds ratios (95% CI) 2 | |||||
|---|---|---|---|---|---|---|
| HbA1c alone | P value | FPG alone | P value | HbA1c & FPG | P value | |
| (N = 814) | (N = 62) | (N = 162) | ||||
| Male | 0.8 (0.6–0.9) | 0.006 | 3.3 (1.8–5.9) | <0.001 | 1.6 (1.2–2.3) | 0.005 |
| Age ≥60 | 1.6 (1.3–1.9) | <0.001 | 1.4 (0.8–2.3) | 0.251 | 2.0 (1.4–2.8) | <0.001 |
| Family diabetes history | 1.1 (0.8–1.4) | 0.474 | 1.2 (0.6–2.4) | 0.651 | 1.7 (1.1–2.6) | 0.013 |
| BMI ≥30 | 1.6 (1.3–2.0) | <0.001 | 1.7 (1.0–3.0) | 0.051 | 3.4 (2.4–4.9) | <0.001 |
| WC (HIGH) | 1.4 (1.2–1.8) | <0.001 | 2.0 (1.2–3.4) | 0.011 | 2.6 (1.8–3.7) | <0.001 |
| Triglycerides ≥1.7 | 1.2 (0.9–1.5) | 0.267 | 2.5 (1.4–4.3) | 0.001 | 2.3 (1.4–4.3) | <0.001 |
| HDL-C (LOW) | 1.3 (1.0–1.7) | 0.095 | 2.5 (1.3–4.7) | 0.004 | 2.8 (1.8–4.3) | <0.001 |
| Dyslipidaemia | 1.6 (1.0–2.5) | 0.041 | 3.5 (1.6–7.8) | 0.002 | 3.5 (2.0–6.2) | <0.001 |
| BP ≥130/85 or Rx | 1.3 (1.0–1.6) | 0.012 | 2.2 (1.2–3.9) | 0.009 | 3.3 (2.2–5.1) | <0.001 |
| Insulin 75th percentile | 1.5 (1.2–2.0) | 0.002 | 3.4 (2.0–5.9) | <0.001 | 4.1 (2.8–5.9) | <0.001 |
| ≥3 MetS features 3 | 1.4 (1.2–1.8) | 0.003 | 3.0 (1.7–5.1) | <0.001 | 4.0 (2.8–5.8) | <0.001 |
| C3 4 | 1.8 (1.5–2.3) | <0.001 | 1.4 (0.9–2.4) | 0.17 | 2.2 (1.5–3.1) | <0.001 |
| CRP 4 | 1.4 (1.1–1.7) | 0.002 | 1.1 (0.7–2.0) | 0.640 | 1.5 (1.1–2.2) | 0.017 |
| IL-6 4 | 1.5 (1.2–1.9) | <0.001 | 1.4 (0.8–2.4) | 0.212 | 2.1 (1.5–3.0) | <0.001 |
| TNF-α 4 | 1.2 (1.0–1.5) | 0.096 | 0.8 (0.5–1.4) | 0.524 | 1.1 (0.8–1.6) | 0.446 |
| Adiponectin 4 | 1.3 (1.0–1.6) | 0.043 | 1.3 (0.7–2.3) | 0.373 | 2.6 (1.8–3.9) | <0.001 |
| Leptin 4 | 1.4 (1.2–1.8) | <0.001 | 1.3 (0.8–2.2) | 0.345 | 2.0 (1.4–2.9) | <0.001 |
| Resistin 4 | 1.0 (0.8–1.2) | 0.626 | 1.3 (0.7–2.1) | 0.389 | 0.8 (0.5–1.1) | 0.139 |
| PAI-1 4 | 1.3 (1.1–1.6) | 0.008 | 1.4 (0.8–2.4) | 0.2 | 1.6 (1.1–2.2) | 0.014 |
| WBC 4 | 1.6 (1.3–2.0) | <0.001 | 1.3 (0.7–2.2) | 0.371 | 2.6 (1.8–3.7) | <0.001 |
1Pre-diabetes: HbA1c ≥5.7% (≥39 mmol/mol) or FPG ≥5.6 mmol/l, models exclude subjects with type 2 diabetes: HbA1c ≥6.5% (≥48 mmol/mol) or FPG ≥7.0 mmol/l or physician diagnosis or Rx diabetes medication use.
2Multinomial logistic regression, reference category: normoglycaemia by both HbA1c and FPG. Gender adjusted for age (continuous), age ≥60 adjusted for gender, all other variables adjusted for age (continuous) and gender.
3MetS features: WC (HIGH), triglycerides ≥1.7, HDL-C (LOW), BP ≥130/85 or Rx and insulin 75th percentile.
4Threshold: above median level in the study population except adiponectin (below median level).
ROC analysis
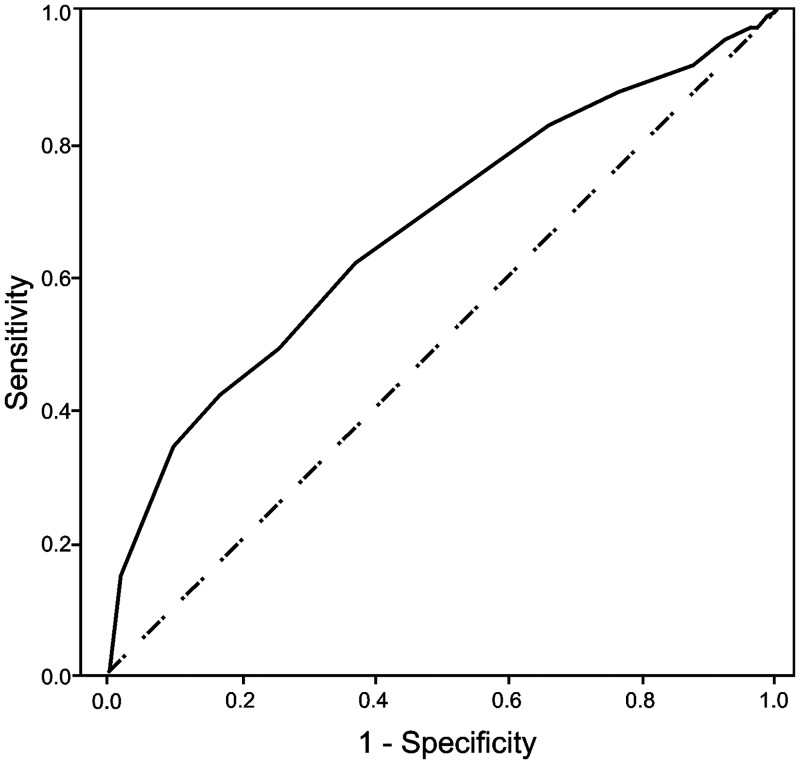
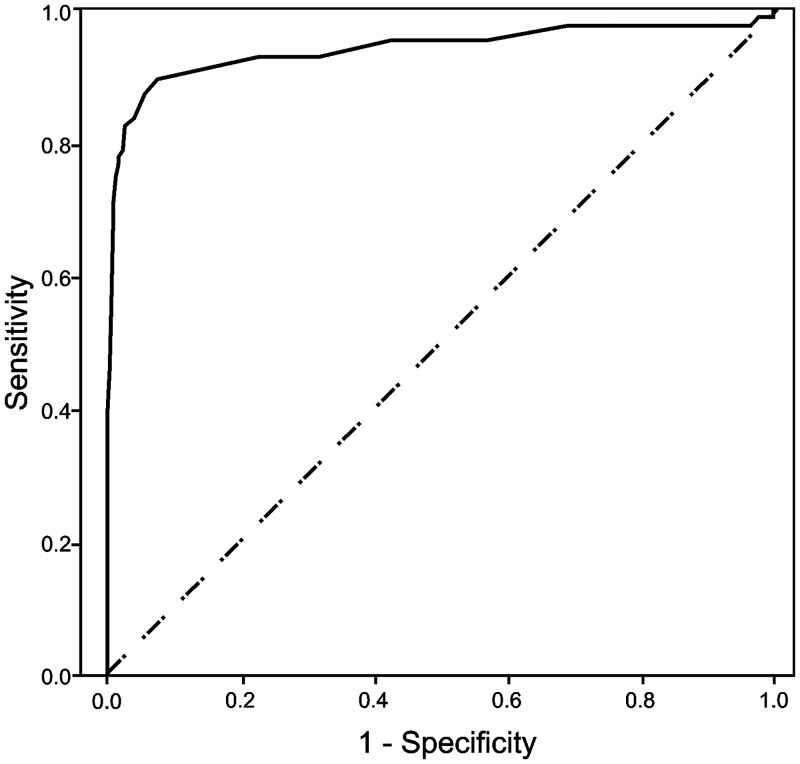
Receiver operating characteristic curves for HbA1c to detect pre-diabetes and type 2 diabetes are shown in Figs 1 and 2. The ability of HbA1c to discriminate pre-diabetes characterised by impaired FPG was low (AUC: 0.668, 95% CI: 0.627–0.710). The HbA1c ≥5.7% (≥39 mmol/mol) pre-diabetes threshold demonstrated marginal sensitivity (72%) and a high FPR (52%). The level of agreement between both diagnostic methods was also poor (K: 0.084). Discriminatory capacity for type 2 diabetes defined by FPG ≥7.0 mmol/l was high (AUC: 0.941, 95% CI: 0.902–0.980). Sensitivity, FPR and kappa for the ADA-recommended HbA1c ≥6.5% (≥48 mmol/mol) cut-off were 84%, 4% and 0.60 respectively.
Fig 1. Receiver operating characteristic curve for HbA1c to discriminate subjects with pre-diabetes.
The figure shows an ROC curve for HbA1c (continuous) to discriminate subjects with pre-diabetes (impaired FPG ≥5.6 mmol/l). The area under the curve value was AUC: 0.668, (95% CI: 0.627–0.710).
Fig 2. Receiver operating characteristic curve for HbA1c to discriminate subjects with type 2 diabetes.
The figure shows an ROC curve for HbA1c (continuous) to discriminate subjects with type 2 diabetes (FPG ≥7.0 mmol/l). The area under the curve value was AUC: 0.941, (95% CI: 0.902–0.980).
Discussion
In this study of 2,047 middle-aged Caucasian-European men and women we show that subjects with HbA1c levels 5.7–6.4% (39–46 mmol/mol) or FPG levels 5.6–6.9 mmol/l may exhibit different cardiometabolic profiles. Stronger relationships with diabetes-related risk features were found using impaired FPG compared to elevated HbA1c to diagnose pre-diabetes. Conversely, the metabolic profiles of patients with type 2 diabetes, defined by either HbA1c ≥6.5% (≥48 mmol/mol) or FPG ≥7.0 mmol/l concentrations, were broadly similar. In addition, it was noted that associations with risk factors and inflammatory markers were higher in pre-diabetic individuals classified by both assays. These results suggest that a combination of both criteria may be useful for detecting subjects at increased cardiometabolic risk.
Noticeably, within this population, a higher percentage of patients were identified as having pre-diabetes by HbA1c (49.1% vs. 11.5% for FPG). A higher prevalence of pre-diabetes by HbA1c in a United Kingdom cohort (N = 8,696) was also noted by Mostafa et al. [20], who reported a prevalence of 44.9% in participants diagnosed by HbA1c compared to 16.2% in subjects detected by an oral glucose tolerance test (OGTT). Similar findings were determined using FPG as the glucose-based criterion. Our results are also consistent with those reported in a recent Chinese study (N = 2,318) and from research examining a Palestinian Arab population (N = 1,370). Du et al. [21] and Kharroubi et al. [22] found reasonable or moderate concordance between HbA1c and FPG for type 2 diabetes, but a higher prevalence by HbA1c and limited overlap for pre-diabetes using ADA-designated thresholds.
However, our results contrast with findings reported in the United States by the Insulin Resistance Atherosclerosis Study (N = 855), which found a higher prevalence of pre-diabetes by FPG (31.1% vs. 10.6% for HbA1c) [23]. Similarly, research utilising data from the National Health and Nutrition Examination Survey (1999–2006) found the prevalence of pre-diabetes in a sample of 7,029 adults to be 28.2% and 12.6% using FPG and HbA1c respectively [24]. Possible reasons for observed prevalence disparities between HbA1c and FPG may include age, gender or ethnic differences in examined populations [10, 14, 15]. In addition, as glucose continues to be metabolized in blood cells even after sampling, discrepancies may be due to biochemical analysis intervals within different studies [7, 22].
Longitudinal research has suggested that combined use of HbA1c and FPG may be beneficial for identifying high-risk subjects. In two Asian studies, Inoue et al. [25] and Heianza et al. [26] demonstrated hazard ratios for type 2 diabetes to be greater for subjects classified by both assays when compared to those diagnosed by either HbA1c or FPG separately. Findings from the Kansai Healthcare Study showed that joint use of both methods improved predictive ability [27]. In ROC analysis, AUCs for models including both HbA1c and FPG were larger than those for HbA1c (0.853 vs. 0.771; P<0.001) or FPG (0.853 vs. 0.818; P<0.001) alone. Recent research by Lipska et al. also revealed that addition of HbA1c to a model with impaired FPG improved discrimination and calibration [28]. The results from the present study imply that the mechanism for this association is that individuals with diabetes-related phenotypes are more accurately identified using combined criteria.
Established risk factors for type 2 diabetes include obesity, raised triglyceride and low HDL-C levels, hypertension and insulin resistance [29]. In particular, subjects with a combination of these features have been shown to have a five-fold increased risk of developing diabetes [30]. Cardiovascular diseases, and in particular obesity-related type 2 diabetes, are also characterised by a low-grade but chronic inflammatory state [31, 32]. This may be reflected in an increased production of pro-inflammatory cytokines and also in higher levels of acute-phase response proteins, coagulation factors, macrophages and immune cells and lower levels of adiponectin, the anti-inflammatory adipokine [32, 33].
In our study it was noted that pre-diabetic individuals categorised by both assays demonstrated a stronger association with cardiometabolic feature clustering and displayed a more pro-inflammatory, pro-atherogenic, hypertensive and insulin resistant profile. Though few prospective studies have comprehensively identified features related to pre-diabetes development, it has been suggested that risk factors for pre-diabetes mirror those for type 2 diabetes [34]. Consequently, on the basis of the similar risk profiles noted in this study between pre-diabetes (defined using both HbA1c and FPG) and type 2 diabetes (classified by either method), these findings also indicate that combined use of both assays may be clinically useful for detecting individuals at highest odds of developing diabetes.
Although HbA1c has long been used as a marker for glycaemic control, its diagnostic performance for type 2 diabetes is still questioned [35–37]. Though a more expensive assay, when compared with FPG, HbA1c has advantages including convenience, greater pre-analytical stability, lower biological variability and increasing international standardisation [7, 37]. Moreover, HbA1c has been shown to correlate with cardiovascular disease and all-cause mortality [38]. However, as diabetes is clinically defined by elevated blood glucose, and not by glycation of proteins, there is concern that using HbA1c to classify type 2 diabetes may lead to major changes in the pathophysiological paradigm that defines the condition [7]. Although a report from the United States inferred that diagnosis by HbA1c, rather than FPG, would not significantly alter type 2 diabetes prevalence, and that categorisation would remain unchanged in as many as 97.7% of subjects [39], evidence is still equivocal [40].
Notably, within our sample, a higher prevalence of diabetes was determined using HbA1c (7.3%, 95% CI: 6.3%-8.6%) compared to FPG (4.3%, 95% CI: 3.5%-5.2%). However, a similar type 2 diabetes prevalence rate in middle-aged Irish adults, defined by HbA1c, was recently reported using data from the nationally representative 2007 Survey of Lifestyle, Attitudes and Nutrition (7.1%, 95% CI: 5.2%-9.0%) [40, 41]. It was also noted that diabetic subjects identified by HbA1c or FPG within the present study displayed markedly similar cardiometabolic profiles. In addition, HbA1c demonstrated high predictive ability for type 2 diabetes diagnosed by FPG ≥7.0 mmol/l levels. Conversely, HbA1c showed poor discriminatory capacity for pre-diabetes defined by impaired FPG.
As HbA1c reflects long-term glycaemic exposure, including postprandial glucose spikes, rather than the acute dysglycaemia indicated by FPG, it is rational to assume that each assay may identify different individuals. Our results suggest that HbA1c may provide greater sensitivity for diagnosing type 2 diabetes within this sample. However, the limited overlap and substantially varied cardiometabolic profiles in subjects diagnosed with pre-diabetes, by either HbA1c or FPG, imply that HbA1c alone may lack specificity to accurately detect individuals at risk of diabetes development. It was also noted that metabolic risk profiles in pre-diabetic subjects, classified by impaired FPG levels only, were also considerably increased. This indicates that a percentage of high-risk individuals would be missed if HbA1c was employed as a sole diagnostic criterion.
This study has several strengths, including a high participation rate (67%). As far as we are aware, ours is the first to compare pre-diabetes and type 2 diabetes prevalence, defined using both HbA1c and FPG criteria, in a middle-aged Irish population. Additionally, few studies have compared a broad range of metabolic risk features and biomarkers with pre-diabetes and type 2 diabetes diagnosed by both assays. Our results are of potential clinical significance in terms of screening and the use of HbA1c as a method for diagnosing diabetes and determining cardiometabolic risk. Accurate estimates of progression rates to type 2 diabetes are needed for efficient allocation of resources and to optimise public health prevention strategies [42]. Importantly, our findings indicate that caution should be taken with regard to how risk is defined as inexact methods may overestimate future diabetes burden [43, 44].
Notwithstanding these strengths, several limitations can be identified. These include single measurements of HbA1c and FPG and that we did not have OGTT results as a comparison test. Although use of a third assay would have allowed a more thorough evaluation of HbA1c and FPG, as discussed by Bonora et al. [7] comparisons between diagnostic methods for pre-diabetes and type 2 diabetes are ambiguous, as a true gold standard test is unavailable. Also, cross-sectional data precludes examination of temporal relationships. Consequently, though results from our research suggest associations between variables, they do not demonstrate an ability to predict type 2 diabetes or future cardiovascular events.
Equally of concern is that our data were derived from a single primary care based sample. Although results from the Cork and Kerry Diabetes and Heart Disease Study demonstrate prevalence rates for obesity and cardiovascular outcomes similar to those observed in other nationally representative Irish studies [40, 41, 45], the possibility that this sample is not representative of the source population must be acknowledged. However, previous research suggests that approximately 98% of Irish adults are registered with a GP and that, even in the absence of a universal patient registration system, it is possible to perform population-based epidemiological studies that are representative using these methods [46]. In addition, Ireland presents a generally ethnically homogeneous population [47]. Thus, the associations we observed between cardiometabolic features and HbA1c and FPG may be comparable to other middle-aged Irish adults. As random sampling of subjects and the use of validated methods for data collection ensured internal sample validity, it is equally possible that the relationships described may be generalisable to a similar middle-aged, Caucasian-European population. Nevertheless, future studies utilising longitudinal data in different samples will be needed to confirm these findings. In particular, it will be necessary to determine whether risk stratification, using both assays, is clinically useful as a method for predicting type 2 diabetes.
Conclusions
In summary, our results suggest that in middle-aged Caucasian-Europeans, when using ADA-recommended cut-points, HbA1c alone is a poor indicator of diabetes risk, but is appropriate for type 2 diabetes diagnosis. Furthermore, combined use of HbA1c and FPG identifies subjects at substantially increased cardiometabolic risk. Although the efficacy and cost-effectiveness of routine screening for diabetes in primary care has not been established [48–50], in light of the increasing prevalence of type 2 diabetes worldwide, there is a need to identify high-risk subjects. Dual screening, utilising both HbA1c and FPG, may provide a more accurate method for predicting cardiometabolic events. Earlier diagnosis could enable earlier targeted interventions or therapies, thus attenuating development of type 2 diabetes and associated cardiovascular complications.
Supporting Information
(ZIP)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a research grant from the Irish Health Research Board (reference HRC/2007/13). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53. [DOI] [PubMed] [Google Scholar]
- 2. Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. 10.2337/dc13-S067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calle M, Fernandez M. Inflammation and type 2 diabetes. Diabetes & metabolism. 2012;38(3):183–91. [DOI] [PubMed] [Google Scholar]
- 4. Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and cardiovascular disease a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–46. [DOI] [PubMed] [Google Scholar]
- 5. Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. The Lancet. 2012;379(9833):2279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gillett MJ. International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes: Diabetes Care 2009; 32 (7): 1327–1334. The Clinical Biochemist Reviews. 2009;30(4):197. [PMC free article] [PubMed] [Google Scholar]
- 7. Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care. 2011;34(Supplement 2):S184–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Church D, Simmons D. More evidence of the problems of using HbA1c for diagnosing diabetes? The known knowns, the known unknowns and the unknown unknowns. Journal of internal medicine. 2014;276(2):171–3. 10.1111/joim.12200 [DOI] [PubMed] [Google Scholar]
- 9. Saukkonen T, Cederberg H, Jokelainen J, Laakso M, Härkönen P, Keinänen-Kiukaanniemi S, et al. Limited Overlap Between Intermediate Hyperglycemia as Defined by A1C 5.7–6.4%, Impaired Fasting Glucose, and Impaired Glucose Tolerance. Diabetes Care. 2011;34(10):2314–6. 10.2337/dc11-0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim JH, Shin JH, Lee HJ, Kim SY, Bae HY. Discordance between HbA1c and fasting plasma glucose criteria for diabetes screening is associated with obesity and old age in Korean individuals. Diabetes research and clinical practice. 2011;94(2):e27–e9. 10.1016/j.diabres.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 11. Marini MA, Succurro E, Castaldo E, Cufone S, Arturi F, Sciacqua A, et al. Cardiometabolic risk profiles and carotid atherosclerosis in individuals with prediabetes identified by fasting glucose, postchallenge glucose, and hemoglobin A1c criteria. Diabetes Care. 2012;35(5):1144–9. 10.2337/dc11-2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rathmann W, Kowall B, Tamayo T, Giani G, Holle R, Thorand B, et al. Hemoglobin A1c and glucose criteria identify different subjects as having type 2 diabetes in middle-aged and older populations: The KORA S4/F4 Study. Annals of Medicine. 2012;44(2):170–7. 10.3109/07853890.2010.531759 [DOI] [PubMed] [Google Scholar]
- 13. Lipska KJ, De Rekeneire N, Van Ness PH, Johnson KC, Kanaya A, Koster A, et al. Identifying dysglycemic states in older adults: implications of the emerging use of hemoglobin A1c. Journal of Clinical Endocrinology & Metabolism. 2010;95(12):5289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inoue M, Inoue K, Akimoto K. Effects of Age and Sex in the Diagnosis of Type 2 Diabetes Using Glycated Haemoglobin in Japan: The Yuport Medical Checkup Centre Study. PloS one. 2012;7(7):e40375 10.1371/journal.pone.0040375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wolffenbuttel BHR, Herman WH, Gross JL, Dharmalingam M, Honghua H J, Hardin DS. Ethnic Differences in Glycemic Markers in Patients With Type 2 Diabetes. Diabetes Care. 2013;36(10):2931–6. 10.2337/dc12-2711 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kearney PM, Harrington JM, Mc Carthy VJ, Fitzgerald AP, Perry IJ. Cohort Profile: The Cork and Kerry Diabetes and Heart Disease Study. Int J Epidemiol. 2013;42(5):1253–62. Epub 2012/09/18. 10.1093/ije/dys131 . [DOI] [PubMed] [Google Scholar]
- 17. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C. Definition of metabolic syndrome report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on scientific issues related to definition. Circulation. 2004;109(3):433–8. [DOI] [PubMed] [Google Scholar]
- 18. Peat J, Barton B. Medical statistics: A guide to data analysis and critical appraisal: BMJ Books; 2008. [Google Scholar]
- 19.Lowry R. VassarStats. The confidence interval of a proportion. Available: http://www.vassarstats.net/prop1.html.2012 December 2012. Available from: http://www.vassarstats.net/prop1.html.
- 20. Mostafa SA, Khunti K, Srinivasan BT, Webb D, Gray LJ, Davies MJ. The potential impact and optimal cut-points of using glycated haemoglobin, HbA1c, to detect people with impaired glucose regulation in a UK multi-ethnic cohort. Diabetes research and clinical practice. 2010;90(1):100 10.1016/j.diabres.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 21. Du TT, Yin P, Zhang JH, Zhang D, Shi W, Yu XF. Comparison of the performance of HbA1c and fasting plasma glucose in identifying dysglycaemic status in Chinese high‐risk subjects. Clinical and Experimental Pharmacology and Physiology. 2013;40(2):63–8. 10.1111/1440-1681.12038 [DOI] [PubMed] [Google Scholar]
- 22. Kharroubi AT, Darwish HM, Al-Halaweh AIA, Khammash UM. Evaluation of glycated hemoglobin (HbA1c) for diagnosing type 2 diabetes and prediabetes among Palestinian Arab population. PloS one. 2014;9(2):e88123 10.1371/journal.pone.0088123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lorenzo C, Wagenknecht LE, Hanley AJ, Rewers MJ, Karter AJ, Haffner SM. A1C Between 5.7 and 6.4% as a Marker for Identifying Pre-Diabetes, Insulin Sensitivity and Secretion, and Cardiovascular Risk Factors The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care. 2010;33(9):2104–9. 10.2337/dc10-0679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mann DM, Carson AP, Shimbo D, Fonseca V, Fox CS, Muntner P. Impact of A1C screening criterion on the diagnosis of pre-diabetes among U.S. adults. Diabetes Care. 2010;33(10):2190–5. Epub 2010/07/16. 10.2337/dc10-0752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inoue K, Matsumoto M, Akimoto K. Fasting plasma glucose and HbA1c as risk factors for type 2 diabetes. Diabetic Medicine. 2008;25(10):1157–63. 10.1111/j.1464-5491.2008.02572.x [DOI] [PubMed] [Google Scholar]
- 26. Heianza Y, Hara S, Arase Y, Saito K, Fujiwara K, Tsuji H, et al. HbA1c 5.7–6.4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet. 2011;378(9786):147–55. Epub 2011/06/28. 10.1016/s0140-6736(11)60472-8 . [DOI] [PubMed] [Google Scholar]
- 27. Sato KK, Hayashi T, Harita N, Yoneda T, Nakamura Y, Endo G, et al. Combined measurement of fasting plasma glucose and A1C is effective for the prediction of type 2 diabetes the Kansai Healthcare Study. Diabetes Care. 2009;32(4):644–6. 10.2337/dc08-1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lipska KJ, Inzucchi SE, Van Ness PH, Gill TM, Kanaya A, Strotmeyer ES, et al. Elevated HbA1c and Fasting Plasma Glucose in Predicting Diabetes Incidence Among Older Adults Are two better than one? Diabetes Care. 2013;36(12):3923–9. 10.2337/dc12-2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alberti K, Zimmet P, Shaw J. Metabolic syndrome—a new world‐wide definition. A Consensus Statement from the International Diabetes Federation. Diabetic Medicine. 2006;23(5):469–80. [DOI] [PubMed] [Google Scholar]
- 30. Stern MP, Williams K, González-Villalpando C, Hunt KJ, Haffner SM. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care. 2004;27(11):2676–81. [DOI] [PubMed] [Google Scholar]
- 31. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. [DOI] [PubMed] [Google Scholar]
- 32. Phillips CM, Perry IJ. Does Inflammation Determine Metabolic Health Status in Obese and Nonobese Adults? The Journal of Clinical Endocrinology & Metabolism. 2013;98(10):E1610–E9. [DOI] [PubMed] [Google Scholar]
- 33. Van Greevenbroek M, Schalkwijk C, Stehouwer C. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: causes and consequences. Neth J Med. 2013;71(4):174–87. [PubMed] [Google Scholar]
- 34. Twigg SM, Kamp MC, Davis TM, Neylon EK, Flack JR. Prediabetes: a position statement from the Australian Diabetes Society and Australian Diabetes Educators Association. Medical journal of Australia. 2007;186(9):461 [DOI] [PubMed] [Google Scholar]
- 35. Lapolla A, Mosca A, Fedele D. The general use of glycated haemoglobin for the diagnosis of diabetes and other categories of glucose intolerance: still a long way to go. Nutrition, Metabolism and Cardiovascular Diseases. 2011;21(7):467–75. 10.1016/j.numecd.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 36. Olson DE, Rhee MK, Herrick K, Ziemer DC, Twombly JG, Phillips LS. Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes Care. 2010;33(10):2184–9. 10.2337/dc10-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cohen RM, Haggerty S, Herman WH. HbA1c for the diagnosis of diabetes and prediabetes: is it time for a mid-course correction? Journal of Clinical Endocrinology & Metabolism. 2010;95(12):5203–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khaw K-T, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Annals of Internal Medicine. 2004;141(6):413–20. [DOI] [PubMed] [Google Scholar]
- 39. Carson AP, Reynolds K, Fonseca VA, Muntner P. Comparison of A1C and fasting glucose criteria to diagnose diabetes among US adults. Diabetes Care. 2010;33(1):95–7. 10.2337/dc09-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Connor JM, Millar SR, Buckley CM, Kearney PM, Perry IJ. The Prevalence and Determinants of Undiagnosed and Diagnosed Type 2 Diabetes in Middle-Aged Irish Adults. PloS one. 2013;8(11):e80504 10.1371/journal.pone.0080504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Balanda KP, Buckley CM, Barron SJ, Fahy LE, Madden JM, Harrington JM, et al. Prevalence of Diabetes in the Republic of Ireland: Results from the National Health Survey (SLAN) 2007. PloS one. 2013;8(10):e78406 Epub 2013/10/23. 10.1371/journal.pone.0078406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morris D, Khunti K, Achana F, Srinivasan B, Gray L, Davies M, et al. Progression rates from HbA1c 6.0–6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia. 2013;56(7):1489–93. 10.1007/s00125-013-2902-4 [DOI] [PubMed] [Google Scholar]
- 43. Mainous AG, Tanner RJ, Baker R, Zayas CE, Harle CA. Prevalence of prediabetes in England from 2003 to 2011: population-based, cross-sectional study. BMJ open. 2014;4(6):e005002 10.1136/bmjopen-2014-005002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yudkin JS, Montori VM. The epidemic of pre-diabetes: the medicine and the politics. Bmj. 2014;349:g4485 10.1136/bmj.g4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leahy S, Nolan A, O'Connell J, Kenny RA. Obesity in an ageing society: implications for health, physical function and health service utilisation. Dublin: TCD; 2014. [Google Scholar]
- 46. Hinchion R, Sheehan J, Perry I. Primary care research: patient registration. Ir Med J. 2002;95(8):249- [PubMed] [Google Scholar]
- 47. Cronin S, Berger S, Ding J, Schymick JC, Washecka N, Hernandez DG, et al. A genome-wide association study of sporadic ALS in a homogenous Irish population. Human molecular genetics. 2008;17(5):768–74. [DOI] [PubMed] [Google Scholar]
- 48. Wareham NJ, Griffin SJ. Should we screen for type 2 diabetes? Evaluation against National Screening Committee criteria. BMJ: British Medical Journal. 2001;322(7292):986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khunti K, Davies M. Should we screen for type 2 diabetes: Yes. BMJ: British Medical Journal. 2012;345. [DOI] [PubMed] [Google Scholar]
- 50.Organization WH. Screening for type 2 diabetes: report of a World Health Organization and International Diabetes Federation meeting: World Health Organization; 2003.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.