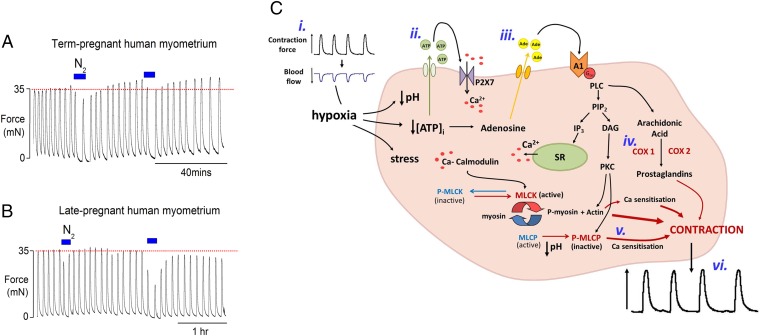
Significance
Parturition requires strong uterine contractions. A longstanding enigma has been how contractions strengthen despite also causing transient ischemia as they occlude uterine blood vessels. Here we demonstrate a hitherto undescribed mechanism whereby in an adaptation at labor, brief hypoxia in uterine muscle, stimulates the contractile activity. We have named this hypoxia-induced force increase, or HIFI. We identify the underlying mechanism, which involves adenosine and prostaglandin and a rise in intracellular calcium, and show it is present in animal and human uterus, but only close to delivery. We speculate that aberrations in this powerful mechanism could underlie contractions being triggered too early (preterm labor) or if HIFI is deficient, weak contractions, and thus poor and unsuccessful term labors.
Keywords: myometrium, smooth muscle, pH, hypoxia, hypoxic preconditioning
Abstract
For successful birth, contractions need to become progressively stronger. The underlying mechanisms are unknown, however. We have found that a novel mechanism, hypoxia-induced force increase (HIFI), is switched on selectively, at term, and is essential to strengthening contractions. HIFI is initiated as contractions cyclically reduce blood flow and produce repeated hypoxic stresses, with associated metabolic and transcriptomic changes. The increases in contractility are a long-lasting, oxytocin-independent, intrinsic mechanism present only in the full-term pregnant uterus. HIFI is inhibited by adenosine receptor antagonism and blockade of cyclooxygenase-2 signaling, and partially reproduced by brief episodes of acidic (but not alkalotic) pH. HIFI explains how labor can progress despite paradoxical metabolic challenge, and provides a new mechanistic target for the 1 in 10 women suffering dysfunctional labor because of poor contractions.
Pulsatile releases of oxytocin are important for labor, but as oxytocin knockout mice deliver normally (1), other mechanisms for increasing contractile strength must occur. A puzzling feature of labor is that contractions become progressively stronger as the myometrium (uterine muscle) experiences repetitive metabolic stress from hypoxia. This reaction occurs because as contractions develop they briefly compress the uterine blood vessels (2, 3). Transient decreases of oxygenation, pH, and ATP, all of which if sustained can decrease contractile activity, occur in vivo with each contraction (3–5). Hypoxia regulates a large number of genes, including those governing metabolism and function in many tissues, and changes in genes associated with hypoxia have been identified as key findings in transcriptomic studies of poorly laboring women (6, 7). Chaemsaithong et al. and Mittal et al. confirmed changes in hypoxia-inducible factor 1a (HIF1a) using RT-PCR, as well as overexpression of cyclooxygenase-2 (COX2) in myometrium and endothelial NOS, which is important for blood vessel dilation. There is, however, no empirical evidence showing how such changes could be important to successful labors, and no existing mechanism linking hypoxia to an increase in contractions. In contrast, studies of hypoxia in myometrium and other smooth muscles show it to decrease contractility (8, 9).
Of note, however, are studies demonstrating ischemic tolerance as an adaptive response initiated by several exposures to a stressor of mild severity, from which resistance to ischemia is markedly increased. This is known as hypoxic or ischemic preconditioning, and has been best investigated in cardiac muscle (10) and brain (11), where it is considered to be a powerful mechanism for limiting ischemic damage. Given that physiological decreases in oxygenation are part of the normal process of labor (4, 12), this finding therefore raised some important questions: What is the effect of brief but repetitive periods of hypoxia on uterine contractility? And, do cycles of brief hypoxia initiate and maintain the progressive augmentation of contractility needed for labor?
Results
Repetitive Hypoxia Causes Persistent Augmentation of Uterine Contractility: Hypoxia-Induced Force Increase.
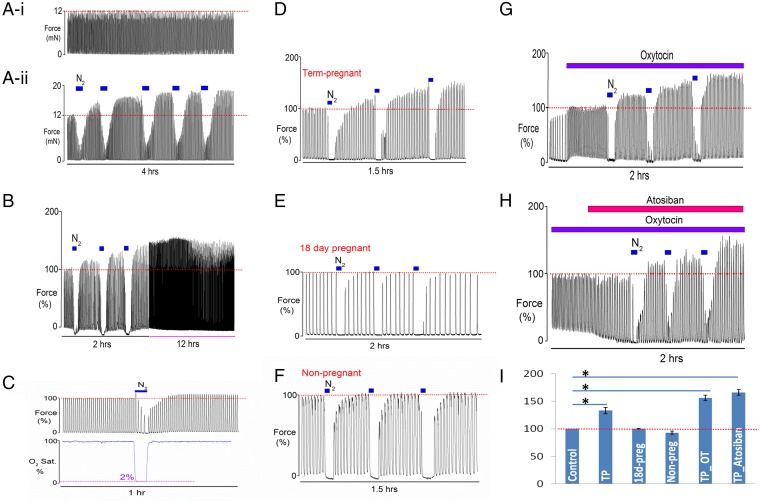
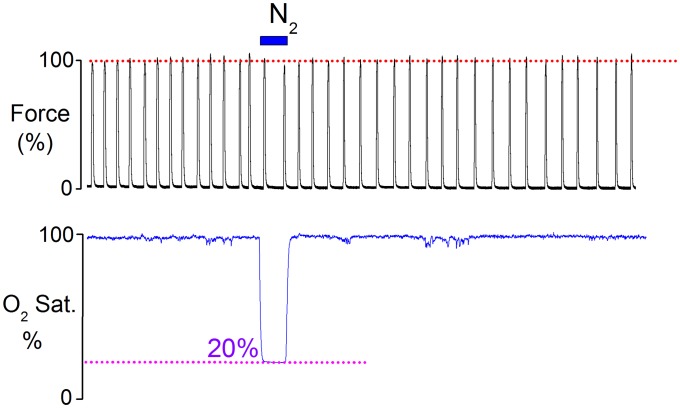
Spontaneous contractile activity can be observed in small uterine strips isolated from the rat. These contractions are steady and regular and this rhythmic muscular activity can be recorded for many hours when tissue is superfused with buffered physiological solution and bubbled with oxygen at 37 °C. A typical recording of spontaneous uterine contractions from term-pregnant (day 22) myometrium is shown in Fig. 1 A, i. In a paired experiment, a second strip of myometrium had five periods of 5 min of hypoxia (produced by bubbling the solution with 100% N2), which reduced the oxygen saturation to around 2%, as seen in Fig. 1C. Consistent with earlier studies, we found that during hypoxia uterine activity was decreased or abolished (8, 13). However, we found that repeating the hypoxia in brief subsequent episodes consistently increased the amplitude of spontaneous uterine contractions during normoxia (Fig. 1 A, ii) (n= 8). We found that 75% of the tested term-pregnant uterine strips reached the maximum level of force increase after three transient hypoxic episodes, and 25% of strips reached their maximum after the fourth episode.
Fig. 1.
The effect of brief hypoxic episodes in rat myometrium, HIFI. (A) Paired data (i) control uterine contractions and (ii) multiple brief hypoxic episodes (Nitrogen, N2) produce increased contractility (HIFI). (B) Increased contractility produced by hypoxia is maintained for many hours. (C) The protocol produced 2% oxygen saturation. (D–F) Brief application of three hypoxic episodes to (D) term-pregnant (TP), (E) 18-d pregnant, and (F) nonpregnant myometrium. (G and H) The effect of brief hypoxic episodes (G) in the presence of oxytocin (OT) and (H) in the presence of the oxytocin receptor antagonist, atosiban. Note that the effects of HIFI are independent of oxytocin. (I) Mean force amplitude after the third hypoxic episode at different gestational stage. *P < 0.05, in comparison with the control. Statistical analysis was performed using Bonferroni one-way ANOVA. All error bars show mean ± SEM.
This augmentation of contractility persisted for up to 12 h (n = 6) (see Fig. 1B for an example). These data demonstrate that hypoxia has a priming effect on myometrium leading to maintained increases in contraction strength, which we have called hypoxia-induced force increase (HIFI).
HIFI Is a Specific Adaptation of Term-Pregnant Myometrium.
Hypoxic episodes were found to produce HIFI in the uterus at term. Fig. 1 D–F and I demonstrate the effects of the same brief (5 min) repeated hypoxia protocol, on day 22, (term-pregnant, Fig. 1D), day 18 pregnant (Fig. 1E), and nonpregnant (Fig. 1F) rat myometrium. Only in term-pregnant rats was the myometrium seen to increase contractility in response to hypoxia, with the amplitude of contractions increasing significantly to 133 ± 6% after the third hypoxic episode, compared with initial values (n = 12) (see Fig. S1 and Tables S1–S3 for absolute values of force and effects on other parameters of contraction). No significant increase in amplitude occurred with this protocol in myometrium from nonpregnant (n = 6) or 18-d pregnant myometrium (n = 9). It can also be seen that at these times the tissue takes progressively longer to recover from the hypoxic episodes. These data show that HIFI switched myometrial activity selectively only in full-term myometrium. We also undertook a subset of experiments in tissue from term-pregnant rats, where the pO2 level was reduced to only 10% (n = 5). Increases in force were again found but these were not as large as those seen with 2% pO2. Thus, the amplitude of force only increased to 121 ± 5%.
Fig. S1.
The effect of transient hypoxic episodes on uterine contractility. The last contraction in the control period before any hypoxia (black line) overlaid with the last contraction during the third recovery period after the third hypoxic episode (red line). Note the amplitudes have been normalized to allow comparisons of rate of fall of force in control and HIFI .There were no differences in rise times or relaxation rates.
Table S1.
A summary comparing the average force amplitude during recovery periods (R1–R4) in experimental strips and matched with the exact time in the paired controls from the same rat (n = 5 each)
| Control (mN) | R1 (mN) | R2 (mN) | R3 (mN) | R4 (mN) | |
| Control | 12 ± 1.4 | 11 ± 0.8 | 11 ± 0.7 | 11 ± 0.8 | 11 ± 0.9 |
| HIFI | 12 ± 1.2 | 13 ± 0.7 | 15 ± 1.1 | 16 ± 1 | 17 ± 0.6 |
| Significance | — | P < 0.05 | P < 0.05 | P < 0.05 | P < 0.05 |
Table S2.
A summary of the mean frequency (the number of contractions in 10 min) that was determined in paired control and matched with the exact time during recovery periods (R1–R4) in HIFI samples (n = 5)
| Frequency | Control | R1 | R2 | R3 | R4 |
| Control | 12 ± 1 | 11 ± 0.9 | 10 ± 1.1 | 10 ± 1 | 9 ± 0.7 |
| HIFI | 12 ± 2 | 10 ± 1.5 | 9 ± 0.7 | 8 ± 0.8 | 7 ± 1 |
Table S3.
A summary of the mean duration of contraction (in minutes) that was measured in paired control and during recovery periods (R1–R4) in HIFI samples (n = 5)
| Duration (m) | Control | R1 | R2 | R3 | R4 |
| Control | 0.3 ± 0.11 | 0.3 ± 0.04 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.05 |
| HIFI | 0.3 ± 0.02 | 0.2 ± 0.1 | 0.3 ± 0.01 | 0.3 ± 0.03 | 0.3 ± 0.1 |
HIFI Is Not Dependent on Oxytocin.
Because oxytocin is a powerful stimulator of uterine contractions, it was important to determine if HIFI can be demonstrated on top of oxytocin stimulation, and if oxytocin is required for HIFI. Addition of oxytocin to the perfusate increased uterine activity as expected (14). Compared with spontaneous activity, in the presence of oxytocin the effects of transient hypoxia on the amplitude of uterine contractions were more pronounced, as shown in Fig. 1G (typical of 10 others). Compared with the amplitude of force under control oxytocin conditions (100%), amplitude increased to 156 ± 5% in term-pregnant myometrium after the third hypoxic episode (Fig. 1G). These data show that HIFI will be present under the normal physiological milieu of labor and will produce functionally significant effects on contraction strength in labor. Although oxytocin clearly stimulates uterine contractions, the data on spontaneous contractions (Fig. 1 A and B) indicates that it is not necessary for the effects of repetitive hypoxia to manifest. We tested this directly, by blocking oxytocin receptors with the selective antagonist atosiban, As shown in Fig. 1H, atosiban did not abolish the increases in force amplitude after hypoxic episodes (n = 4).
Repetitive Transient Acidic pH Episodes Increase Force.
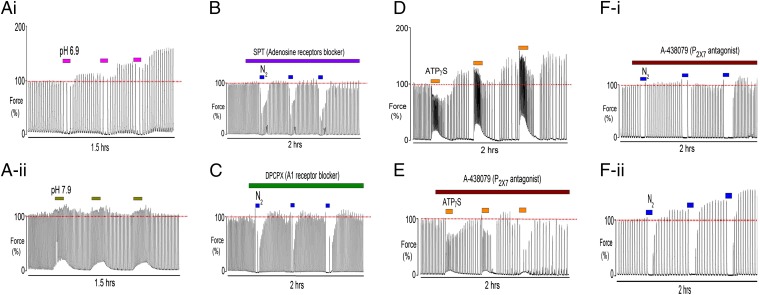
In vivo the ischemia associated with uterine contractions not only reduces oxygenation, it also produces acidification (12) and changes levels of adenosine metabolites, especially ATP (15). Evidence from other tissues (16, 17) points to extracellular acidosis being part of the protective mechanism of ischemic tolerance. In the experiments shown in Fig. 1, the external pH did not change from 7.4 because of buffering with Hepes. We therefore tested whether acidification also contributes to the mechanism of increasing contractility with hypoxia, by superfusing the uterus with repetitive brief episodes of acidic (6.9) external pH. As expected from our previous work (5, 13), during the acidification uterine contractions decreased, especially in frequency, and there was a small decline in baseline tension (Fig. 2 A, i). However, after each episode of acidification the magnitude of the contractions increased in a sustained manner. These increases were qualitatively similar to those produced by hypoxia; force amplitude increased significantly to 131 ± 4% (P < 0.05) after the third acidotic episode compared with initial values (n = 8). In contrast, making alkaline changes to external pH to 7.9 produced the expected stimulation of force and baseline tension (18), but these changes were not sustained upon return to control (7.4) solution (n = 7), and there was no significant steady-state increase in force amplitude (paired data) (Fig. 2 A, ii). In a subset of experiments we combined hypoxia and external acidification to 6.9. This protocol produced a larger mean increase in force than that with hypoxia or acidification alone (141 ± 7 after the third hypoxic/acidotic episode compared with the initial value, n = 5). Thus, in vivo it is likely that external acidification, as well as oxytocin, will synergize with HIFI.
Fig. 2.
The effects of pH, adenosine and ATP on the mechanism of HIFI in term-pregnant rat myometrium. Repetitive brief episodes of (A-i) acidic (6.9) and (A-ii) alkalotic (7.9) external pH on myometrial contractility. Note that acidotic episodes could mimic the effects of hypoxia. Blocking the adenosine receptors with (B) nonspecific adenosine receptor blocker, 8-SPT and (C) with the specific A1 antagonist DPCPX, abolished the effects of hypoxic priming on contractions. (D) Three brief applications of ATPγS increased uterine contractile activity and (E) blocking the P2X7 receptors with A-438079 abolished the rebound increase in force induced by ATPγS episodes. When P2X7 receptors were blocked, little effect of hypoxia occurred with brief hypoxic episodes as in F-i compared with paired control (F-ii).
The Mechanism of HIFI Is via A1 Adenosine Receptor and P2X7 Signaling Pathways.
Increased adenosine as a consequence of hypoxia is recognized as one of the major metabolic changes with hypoxia, and adenosine-stimulating extracellular receptors have also been shown to be a key mechanism for producing preconditioning in cardiac muscle (19, 20). We therefore investigated the effects in myometrium of a nonspecific adenosine receptor antagonist, 8-(p-sulfophenyl) theophylline (8-SPT) and the specific A1 adenosine receptor antagonist, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX). Repeated episodes of hypoxia produced no increase in contractions during normoxia when 8-SPT or DPCPX were present (Fig. 2 B and C; n = 7 and 4, respectively). Both antagonists had no effect on contractions in normoxic control conditions (Fig. 3C).
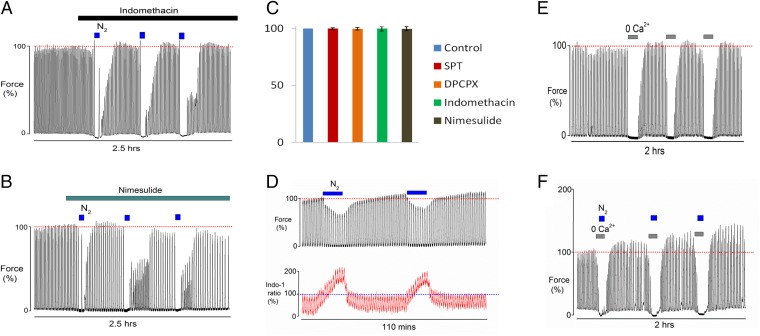
Fig. 3.
The effect of COX and calcium on the mechanisms of HIFI in term-pregnant rat myometrium. Blocking prostaglandin biosynthesis with (A) indomethacin (cyclo-oxgenase inhibitor) or (B) nimesulide (specific COX2 inhibitor) significantly abolished the hypoxic priming induced by brief episodes of hypoxia. (C) Mean force amplitude measured 1 h after the application of drugs showing no significant fall of force because of drugs. (D) Simultaneous measurements of force and Ca2+ (Indo-1 fluorescence) during repeated hypoxia, show the gradually and significantly increased force amplitude was not accompanied by increases in the accompanying calcium transient (note: in these experiments the oxygen saturation was around 5%). Repeated episodes of 0-calcium in the (E) absence and (F) presence of hypoxia. Note that Ca2+ entry is not involved in the mechanisms of HIFI.
In response to hypoxia cells release ATP to the extracellular fluid (15), where it activates purinergic receptors. This activation has also been suggested to be part of the mechanism activating preconditioning. We therefore investigated if ATP in the absence of hypoxia could stimulate myometrial contractions. The effects of three applications of ATPγS (a nonhydrolysable analog) are shown in Fig. 2D. In term-pregnant rat myometrium, ATPγS greatly increased the frequency of contractions. Upon its removal from the perfusate a significant and maintained increase in uterine force amplitude occurred (n = 6, 143 ± 4% measured after the third application of ATPγS). Recent work has suggested that ATP-induced uterine activity in myometrium occurs mainly via binding to P2X7 receptors (21). To determine if the P2X7 receptors mediated the increase in force amplitude during ATP applications and recoveries, a selective antagonist (A-438079) was applied and the hypoxic episodes or ATPγS repeated. We found ATP-increased uterine activity was greatly reduced or abolished with A-438079, as shown in Fig. 2E. Furthermore in paired data (n = 6), blocking P2X7 receptors with A-438079 abolished the hypoxia-induced force increases (Fig. 2 F, i), whereas normal HIFI responses occurred in controls (Fig. 2 F, ii).
COX2 and Intracellular Ca2+ Are Downstream Targets of HIFI.
A major pathway through which adenosine and ATP increase contractility is to increase Ca2+ entry and stimulate prostaglandin production. Prostaglandin F2α (PGF2α), like oxytocin, is a major stimulator of uterine activity in labor (22). Hypoxia has been shown to up-regulate COX2 and increase prostaglandin production in smooth muscle and other tissues (23, 24), and this has been hypothesized to contribute to labor (25). We therefore investigated the effects of inhibiting prostaglandin biosynthesis on HIFI by blocking the cyclo-oxygenases. The effects of the COX1 and COX2 inhibitor, indomethacin, or the relatively specific COX2 inhibitor, nimesulide, are shown in Fig. 3 A and B (n = 5 and 6), respectively. It is clear that both inhibitors abolished HIFI. Neither drug affected control contractions (Fig. 3C). Given the importance of changes in Ca2+ to signaling pathways and contractile activity, we next investigated if the repetitive episodes that increased contractility were associated with increased intracellular Ca2+ or changes in Ca2+ sensitivity of the myofilaments.
Using the fluorescent Ca2+ sensitive indicator, Indo-1, while simultaneously measuring contractions, the effects of hypoxia were investigated (8). Fig. 3D shows the effect of brief hypoxia on myometrial Ca2+ signaling (n = 5). These experiments were performed in a modified bath and perfusion system compatible with fluorescent imaging. The oxygen bath tension was around 5%, as opposed to 2% in the previous experiments (see Methods for details), and thus the HIFI response is not as marked. Consistent with previous studies (26), there was a marked increase in basal Ca2+ levels during hypoxia, which was not accompanied by increased baseline force (Fig. 3D). As indicated by the horizontal lines in Fig. 3D, force clearly increases following the second hypoxic episode but there is no accompanying rise in the Indo-1 ratio (Tables S4 and S5). Our data can be taken to suggest that HIFI affects Ca2+ sensitivity rather than increasing Ca2+ entry, probably through inhibiting myosin light-chain phosphatase (27) or actin-mediated mechanisms (26) (Fig. 4C). Such a Ca2+-sensitizing pathway has been proposed for PGF2α-induced contraction in myometrium (22). To investigate if Ca2+ entry is required during the hypoxic period to initiate HIFI, we used a protocol whereby Ca2+ entry was blocked (zero-external Ca2+ with 1 mM EGTA) and determined the consequences for the contractile response; repeated episodes of zero-external Ca2+ in the presence of 100% O2 or 100% N2 were conducted. As expected (28), we found that removal of Ca2+ from the perfusate abolished contractions (Fig. 3E). Upon readmission of external Ca2+, force rapidly recovered, usually with a small but brief rebound increase in amplitude (Fig. 3E) (n = 7). However, the same protocol, but with application of 5-min hypoxia, caused the same abolition of uterine contractions, but upon reoxygenation and readmission of external Ca2+, force recovered significantly (159 ± 9%) with greater and maintained force amplitude compared with initial value (Fig. 3F) (n = 10). Thus, Ca2+ entry is not required for generating the hypoxia-induced augmentation of uterine contractility.
Table S4.
A summary of mean raw peak for indo-1 ratio that was determined during control and recovery periods (R1–R2) after the repeated episodes of 5-min hypoxia (n = 5)
| Peak of Indo-1 ratio for Ca | Control | R1 | R2 |
| HIFI | 0.046 ± 0.005 | 0.043 ± 0.006 | 0.045 ± 0.007 |
Table S5.
A summary of mean force amplitude that was determined during control and recovery periods (R1–R2) after the repeated episodes of 5-min hypoxia (n = 5) parallel with measurement of Indo-1 ratio
| Force amplitude | Control | R1 | R2 |
| HIFI | 100% | 102 ± 3% | 111 ± 3% |
Fig. 4.
The effect of repeated episodes of hypoxia on human myometrium and the possible mechanism of HIFI. Application of repeated brief hypoxia on (A) term-pregnant laboring human myometrium and (B) pregnant human myometrium earlier in gestation (around 38 wk). Note the rebound increase in force amplitude with normoxia in myometrium at term. (C) Scheme to show mechanisms leading to increased contraction following hypoxic episodes. (i) Contractions occlude uterine blood vessels, producing hypoxia, which results in oxidative stress, decreased pH and [ATP]. (ii) ATP effluxes from the myocyte and stimulates purinergic receptors, particularly P2X7, maintaining intracellular [Ca]. (iii) ATP hydrolysis leads to increased adenosine which stimulates its receptors, especially A1 type. (iv) The ensuing signaling cascade leads to PKC production as well as prostaglandin production and (v) inhibition of myosin light-chain phosphatase via phosphorylation (P-MLCP) and Ca sensitization of the myofilaments. This, in addition to other Ca sensitization mechanisms, including actin-mediated pathways, leads to increased force (vi). COX, cyclo-oxygenases; DAG, diacyl glycerol; IP3, inositol-Tris-phosphate; PIP2, phosphoinositide-bisphosphate; PLC, phopspholipase C; SR, sarcoplasmic reticulum.
HIFI Is Present in Term Human Myometrium.
The above data were obtained on rat myometrium to study HIFI under well-defined conditions and with minimal variation in for example weight, gestational stage, parity, and also because the high frequency of contractions allows for many maneuvers and paired experiments to be undertaken. Given the possible importance of these findings to human labor, we have tested our key finding (i.e., brief repetitive hypoxic periods can lead to increased contractility upon reoxygenation) in human myometrium. Fig. 4 A and B, shows data taken from myometrium of women having Caesarean sections, either at term (n = 6, of whom 3 were in labor) or having a Caesarean section electively and therefore not in labor and earlier in pregnancy (38–39 wk gestation, n = 6). Only in tissues at term was there an increased contractility in normoxia following hypoxia (Fig. 4A); force amplitude significantly increased to 112 ± 2% after the first hypoxic episode relative to the initial control (100%). In nonlaboring women, there was no significant increase in contractility following hypoxia. Fig. 4B shows data typical of six biopsies. Thus, HIFI is selectively present in laboring or full-term, human myometrium.
Discussion
Our work clearly demonstrates that transient hypoxic episodes lead to a gestation-specific, on-going, and maintained increase in myometrial contractile activity, which we have termed hypoxia-induced force increase, or HIFI. Hypoxia is a ubiquitous regulator of a large number of genes, including those governing metabolism and function, with hypoxia-induced transcription factors mediating many of these responses (29). Hypoxic preconditioning, whereby an initial series of hypoxic stimuli produce subsequent hypoxic tolerance, is recognized as a protective mechanism in several tissues (11, 30). The preconditioning triggers a panoply of endogenous adaptive mechanisms and gene transcriptions. It has been suggested that redox signaling is responsible for the conversion of ischemic damage and induction of death signals into preconditioning survival signals (31). It remains to be seen if this is the case in the myometrium. Even in well-studied organs, such as the heart and brain, there is debate as to exact mechanisms underlying preconditioning. We find that the unique setting of labor in biology, where a sustained rise in contraction strength is required for delivery of live offspring, in the face of persistent metabolic challenge initiated by hypoxia, has led to an effective increase in contractile drive in the myometrium. Thus, in term-myometrium a novel tissue response to hypoxia has been uncovered.
Given the range of pathways affected by hypoxia (Fig. 4C), it is to be expected that in myometrium several metabolic factors have been found to contribute and coalesce to bring about an increase in force. The responding genes in the uterus are likely to be similar to those responding to hypoxia in other tissues, and specifically genes associated with hypoxia have been a key finding of studies of the myometrial transcriptome in women laboring poorly because of weak contractions, although the mechanistic relation to contractions was unknown (6). In another molecular study of women with an arrest of descent during parturition, 400 genes were found altered compared with women laboring normally (7). Of note, these authors highlighted changes not just in HIF1α but also those associated with prostaglandin synthase and muscle function.
Hypoxia and ischemia are also associated with dysfunction of the microvasculature, including an increase in permeability (32), which will also affect the extracellular fluid surrounding the myocytes. Our data suggest that brief hypoxic episodes stimulate increased adenosine and ATP release into the acidic extracellular milieu. The increased biosynthesis of prostaglandins, and possibly Ca2+ sensitization also resulting from these changes, culminates in increased uterine contractions. Dougherty et al. (33) have recently shown that PKC activation increases Ca2+ sensitivity of permeabilized lymphatic muscle via myosin light chain 20 phosphorylation-dependent and -independent mechanisms, and this may contribute to the sensitization in myometrium. It is also likely that actin dependent mechanisms of Ca2+ sensitization are evoked (34, 35).
Physiologically, oxytocin is also likely to enhance the HIFI-producing mechanism. These in vitro effects of hypoxia are also found in vivo, following occlusion of uterine blood flow. An increase of force (intrauterine pressure) of 130–140% on reperfusion was reported and lasted for over 30 min. The significance of these data were not appreciated at the time (4, 12). Our protocols (and in vivo studies) were developed to clearly demonstrate the effects of repetitive hypoxia and investigate its mechanism. We were able to show increases of amplitude of around 50% over the course of around an hour. During normal labor, where significant falls in oxygenation may last for only a minute, it is expected that these effects develop more gradually but increment with every contraction.
Myometrial genes and proteins are differentially expressed in pregnancy and labor (36–38). The question therefore arises: Is there evidence for changes in genes or proteins associated with the pathways we are implicating, beyond those addressed above?
Of note here is that the inflammatory transcription factor NF-κB, which modulates COX expression, has been shown to increase (39). Mouse models with mutations in components of steroid and other hormonal synthesis pathways, including phospholipase A2 and the PGF2α receptor, exhibit dystocia or a delay in labor onset [see Kimura et al. (40)], consistent with a role in HIFI. From our work and the data from transcriptomic studies of poor labors, which reported over 40 differentially expressed genes, it may be speculated that failures or delays in this intrinsic mechanism will contribute to the 10% of labors that fail to progress because of poor contractions, leaving an unplanned Caesarean section as the only delivery mode to save the baby.
Methods
Animals and Human Uterine Samples.
Experiments were performed on intact uterine tissue from nonpregnant (175–200 g), late-pregnant (18 d), and term-pregnant (22-d gestation) Wistar rats, humanely killed by cervical dislocation under CO2 anesthesia in accordance with the United Kingdom Home Office guidelines. The uteri were removed and small strips (1–2 mm × 10 mm, width × length) of longitudinal smooth muscle were dissected and mounted in an organ bath containing Hepes-buffered physiological salt solution (154 mM NaCl, 5.6 mM KCl, 1.2 mM MgSO4, 7.8 mM glucose, 10.9 mM Hepes, and 2.0 mM CaCl2), pH 7.4 at 37 °C, and bubbled with 100% O2. This oxygenation was used to ensure equilibration throughout the tissue under control conditions and to allow comparison with previous studies. Some experiments were performed with 20% oxygen to provide data on nonsupraphysiological levels of O2. These five experiments showed there was no effect on force when oxygen was decreased from 100% to 20%. An example trace is shown in Fig. S2. Biopsies of human myometrium were obtained from women undergoing Caesarean section, either electively before full term (week 38–39) and therefore before labor (n = 6), or at term, electively because of breech presentation (40 wk, n = 3) or in labor (n = 3, section for fetal distress). All women provided written informed consent and ethical approval was sought and granted by the Local Research Ethics Committee (REC Ref: 10/H1002/49) and by the Research and Development Director of Liverpool Women’s NHS Foundation Trust, Liverpool, United Kingdom.
Fig. S2.
The effect of lowering the O2 concentration to 20% on uterine contraction. Note that decreasing the O2 from 100% to 20% did not affect the force upon reoxygenation.
Experimental Protocol.
Uterine strips were stretched to a standard resting tension of 10 mN and allowed to equilibrate for at least 30–60 min before any maneuvers were performed. Any strips not producing regular contractions were discarded. Many experiments were paired so that test and control data from an adjacent strip were investigated simultaneously. After steady spontaneous contractions were obtained, the effect of repeated episodes of transient hypoxia was investigated by replacing the oxygen in the chamber bath with 100% N2 for 5–10 min, separated by periods of reoxygenation of 30 min (Fig. S3). In some experiments, strips were stimulated with 5 nM oxytocin throughout. To determine the degree of hypoxia (%O2) achieved, a fiber optic oxygen microsensor (World Precision Instruments) was inserted into the bath close to the contracting uterine strips to measure the pO2 in the bathing solution throughout the entire experiment (Fig. 1C).
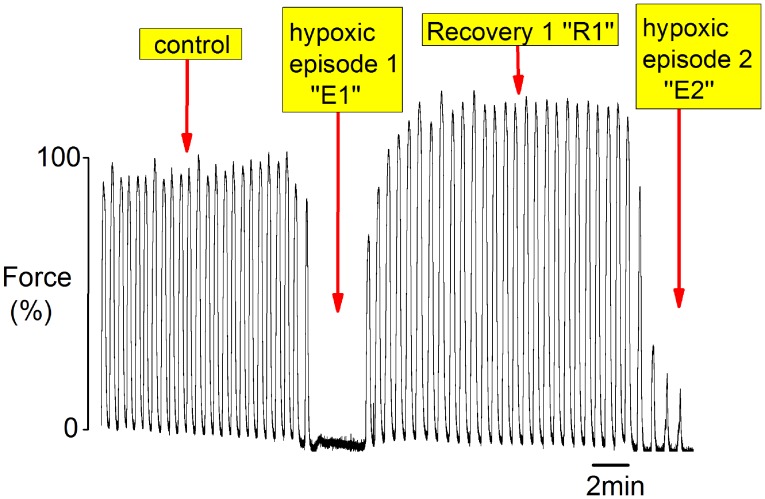
Fig. S3.
Example of protocol and terms used during periods of hypoxia and recovery. After steady contractions were obtained (control), the tissue was challenged with transient hypoxic episodes (E) by decreasing the O2 from 100% to 2% followed by recovery period (R) where O2 was returned to 100% O2.
Solutions.
After establishing the hypoxic protocol on spontaneous contractions (Fig. 1 A–F), subsequent experiments in term-pregnant myometrium, (except for those with ATPγS) were performed in the presence of oxytocin in the superfusate (5 nM). Experiments in 0-Ca2+ were performed with CaCl2 omitted from the solution and 1 mM EGTA added. External pH was altered either by raising it from 7.4 to 7.9 or lowering it to 6.9 by addition of strong acid or base to the superfusing solution. The nonspecific adenosine receptor inhibitor 8-SPT and specific A1 adenosine receptor inhibitor DPCPX, were used at 50 µM and 200 nM, respectively. Indomethacin was used to block cyclo-oxygenases COX1 and COX2 and used at 10 µM, and nimesulide (5 µM) was used to specifically inhibit COX2. ATPγS was used at 1 mM and A-438079 (hydrochloride hydrate) was used at 5 μM to selectively inhibit P2X7 receptors. All reagents were added directly to the perfusate and obtained from Sigma.
Measurements of Simultaneous Force and Calcium.
To determine the role of Ca2+ in the mechanism of hypoxia-induced force increase, we simultaneously recorded myometrial contractility and changes in intracellular calcium transients with repeated episodes of 10-min hypoxia. Small myometrial strips (5 mm × 1 mm × 2 mm) absent of endometrium were dissected and loaded with the fluorescent calcium indicator, Indo-1 acetoxymethyl ester (Indo-1AM, Molecular Probes) for 3 h at room temperature, as previously described (18). Strips were then placed into a 1-mL chamber bath situated above an inverted microscope (Nikon Diaphot, World Precision Instruments) and viewed with 20× power fluor objective lens. The strips were attached at one end to a fixed hook and the other end to a force transducer, were stretched to a resting tension of 2 mN and allowed to equilibrate for at least 30–45 min. The bath was continually superfused with the salt solution (pH 7.4) at a rate of 1 mL/min at 37 °C. To ensure a similar degree of hypoxia was achieved inside the 1-mL chamber bath the perfusate was directly bubbled with N2 gas with the addition of sodium hydrosulfite (0.1 mM) to scavenge oxygen before superfusing the chamber. In addition, N2 was also bubbled directly into the bath using a fine needle inserted into the bathing chamber. Thus, in this experimental set-up, hypoxia was induced by superfusing the tissue with the hypoxic solution and simultaneous bubbling of N2 into the bath chamber for at least 10 min. Measurements of pO2 showed values of 5% were obtained in these experiments.
Statistical Analysis.
Force amplitude of contraction was analyzed during control and treatment periods using Origin Pro Software (Origin Lab, v8.1). Contractile activity during the final 10 min under normoxia preceding the first maneuver was calculated and taken as 100% control. The final 10 min of each recovery period following each hypoxic episode was then analyzed and expressed as a percentage of this control. Data are presented as mean ± SEM using Student’s t-test for comparing two groups or Bonferroni one-way ANOVA for comparing more than two groups as appropriate. P < 0.05 was accepted as statistically significant. n is the number of uterine strips, one from each animal or woman, where appropriate.
Acknowledgments
We thank Dr. Andras Tóth for helpful initial discussion. This research was supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University (M.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1503497112/-/DCSupplemental.
References
- 1.Nishimori K, et al. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci USA. 1996;93(21):11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brar HS, Platt LD, DeVore GR, Horenstein J, Medearis AL. Qualitative assessment of maternal uterine and fetal umbilical artery blood flow and resistance in laboring patients by Doppler velocimetry. Am J Obstet Gynecol. 1988;158(4):952–956. doi: 10.1016/0002-9378(88)90100-7. [DOI] [PubMed] [Google Scholar]
- 3.Larcombe-McDouall J, Buttell N, Harrison N, Wray S. In vivo pH and metabolite changes during a single contraction in rat uterine smooth muscle. J Physiol. 1999;518(Pt 3):783–790. doi: 10.1111/j.1469-7793.1999.0783p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison N, Larcombe-McDouall JB, Wray S. A 31P NMR investigation into the effects of repeated vascular occlusion on uterine metabolites, intracellular pH and force, in vivo. NMR Biomed. 1995;8(1):28–32. doi: 10.1002/nbm.1940080107. [DOI] [PubMed] [Google Scholar]
- 5.Parratt J, Taggart M, Wray S. Abolition of contractions in the myometrium by acidification in vitro. Lancet. 1994;344(8924):717–718. doi: 10.1016/s0140-6736(94)92209-8. [DOI] [PubMed] [Google Scholar]
- 6.Chaemsaithong P, et al. Characterization of the myometrial transcriptome in women with an arrest of dilatation during labor. J Perinat Med. 2013;41(6):665–681. doi: 10.1515/jpm-2013-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittal P, et al. A molecular signature of an arrest of descent in human parturition. Am J Obstet Gynelcol. 2011;204(2):177.e15-33. doi: 10.1016/j.ajog.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monir-Bishty E, Pierce SJ, Kupittayanant S, Shmygol A, Wray S. The effects of metabolic inhibition on intracellular calcium and contractility of human myometrium. BJOG. 2003;110(12):1050–1056. [PubMed] [Google Scholar]
- 9.Kim NN, et al. Altered contractility of rabbit penile corpus cavernosum smooth muscle by hypoxia. J Urol. 1996;155(2):772–778. [PubMed] [Google Scholar]
- 10.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 11.Barone FC, et al. Ischemic preconditioning and brain tolerance: Temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29(9):1937–1950, discussion 1950–1951. doi: 10.1161/01.str.29.9.1937. [DOI] [PubMed] [Google Scholar]
- 12.Harrison N, Larcombe-McDouall JB, Earley L, Wray S. An in vivo study of the effects of ischaemia on uterine contraction, intracellular pH and metabolites in the rat. J Physiol. 1994;476(2):349–354. doi: 10.1113/jphysiol.1994.sp020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wray S. The effects of metabolic inhibition on uterine metabolism and intracellular pH in the rat. J Physiol. 1990;423(1):411–423. doi: 10.1113/jphysiol.1990.sp018030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arrowsmith S, Wray S. Oxytocin: Its mechanism of action and receptor signalling in the myometrium. J Neuroendocrinol. 2014;26(6):356–369. doi: 10.1111/jne.12154. [DOI] [PubMed] [Google Scholar]
- 15.Gourine AV, Llaudet E, Dale N, Spyer KM. Release of ATP in the ventral medulla during hypoxia in rats: Role in hypoxic ventilatory response. J Neurosci. 2005;25(5):1211–1218. doi: 10.1523/JNEUROSCI.3763-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundmark JA, Trueblood N, Wang LF, Ramasamy R, Schaefer S. Repetitive acidosis protects the ischemic heart: Implications for mechanisms in preconditioned hearts. J Mol Cell Cardiol. 1999;31(4):907–917. doi: 10.1006/jmcc.1998.0931. [DOI] [PubMed] [Google Scholar]
- 17.Flacke J-P, Kumar S, Kostin S, Reusch HP, Ladilov Y. Acidic preconditioning protects endothelial cells against apoptosis through p38- and Akt-dependent Bcl-xL overexpression. Apoptosis. 2009;14(1):90–96. doi: 10.1007/s10495-008-0287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce SJ, Kupittayanant S, Shmygol T, Wray S. The effects of pH change on Ca(++) signaling and force in pregnant human myometrium. Am J Obstet Gynecol. 2003;188(4):1031–1038. doi: 10.1067/mob.2003.229. [DOI] [PubMed] [Google Scholar]
- 19.Germack R, Dickenson JM. Adenosine triggers preconditioning through MEK/ERK1/2 signalling pathway during hypoxia/reoxygenation in neonatal rat cardiomyocytes. J Mol Cell Cardiol. 2005;39(3):429–442. doi: 10.1016/j.yjmcc.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Ninomiya H, et al. Complementary role of extracellular ATP and adenosine in ischemic preconditioning in the rat heart. Am J Physiol Heart Circ Physiol. 2002;282(5):H1810–H1820. doi: 10.1152/ajpheart.00760.2001. [DOI] [PubMed] [Google Scholar]
- 21.Miyoshi H, Yamaoka K, Urabe S, Kudo Y. ATP-induced currents carried through P2X7 receptor in rat myometrial cells. Reprod Sci. 2012;19(12):1285–1291. doi: 10.1177/1933719112450333. [DOI] [PubMed] [Google Scholar]
- 22.Woodcock NA, Taylor CW, Thornton S. Prostaglandin F2α increases the sensitivity of the contractile proteins to Ca2+ in human myometrium. Am J Obstet Gynecol. 2006;195(5):1404–1406. doi: 10.1016/j.ajog.2006.03.099. [DOI] [PubMed] [Google Scholar]
- 23.Lim W, et al. Glucocorticoids suppress hypoxia-induced COX-2 and hypoxia inducible factor-1α expression through the induction of glucocorticoid-induced leucine zipper. Br J Pharmacol. 2014;171(3):735–745. doi: 10.1111/bph.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camacho M, et al. Hypoxia upregulates PGI-synthase and increases PGI₂ release in human vascular cells exposed to inflammatory stimuli. J Lipid Res. 2011;52(4):720–731. doi: 10.1194/jlr.M011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batra S, Bengtsson LP. Mechanism for increased production of prostaglandins in labour. Lancet. 1976;1(7970):1164–1165. doi: 10.1016/s0140-6736(76)91547-6. [DOI] [PubMed] [Google Scholar]
- 26.Taggart MJ, Menice CB, Morgan KG, Wray S. Effect of metabolic inhibition on intracellular Ca2+, phosphorylation of myosin regulatory light chain and force in rat smooth muscle. J Physiol. 1997;499(Pt 2):485–496. doi: 10.1113/jphysiol.1997.sp021943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83(4):1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 28.Wray S, et al. Calcium signaling and uterine contractility. J Soc Gynecol Investig. 2003;10(5):252–264. doi: 10.1016/s1071-5576(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 29.Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch. 2005;450(6):363–371. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- 30.Lasley RD, Anderson GM, Mentzer RM., Jr Ischaemic and hypoxic preconditioning enhance postischaemic recovery of function in the rat heart. Cardiovasc Res. 1993;27(4):565–570. doi: 10.1093/cvr/27.4.565. [DOI] [PubMed] [Google Scholar]
- 31.Das DK, Maulik N. Cardiac genomic response following preconditioning stimulus. Cardiovasc Res. 2006;70(2):254–263. doi: 10.1016/j.cardiores.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Eltzschig HK, Eckle T. Ischemia and reperfusion—From mechanism to translation. Nat Med. 2011;17(11):1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dougherty PJ, et al. PKC activation increases Ca²⁺ sensitivity of permeabilized lymphatic muscle via myosin light chain 20 phosphorylation-dependent and -independent mechanisms. Am J Physiol Heart Circ Physiol. 2014;306(5):H674–H683. doi: 10.1152/ajpheart.00732.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taggart MJ, Morgan KG. Regulation of the uterine contractile apparatus and cytoskeleton. Semin Cell Dev Biol. 2007;18(3):296–304. doi: 10.1016/j.semcdb.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuo M, Reardon S, Ikebe M, Kitazawa T. A novel mechanism for the Ca(2+)-sensitizing effect of protein kinase C on vascular smooth muscle: Inhibition of myosin light chain phosphatase. J Gen Physiol. 1994;104(2):265–286. doi: 10.1085/jgp.104.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breuiller-Fouche M, Charpigny G, Germain G. Functional genomics of the pregnant uterus: From expectations to reality, a compilation of studies in the myometrium. BMC Pregnancy Childbirth. 2007;7(Suppl 1):S4. doi: 10.1186/1471-2393-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breuiller-Fouche M, Germain G. Gene and protein expression in the myometrium in pregnancy and labor. Reproduction. 2006;131(5):837–850. doi: 10.1530/rep.1.00725. [DOI] [PubMed] [Google Scholar]
- 38.Esplin MS, et al. The use of cDNA microarray to identify differentially expressed labor-associated genes within the human myometrium during labor. Am J Obstet Gynecol. 2005;193(2):404–413. doi: 10.1016/j.ajog.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 39.Capece A, Vasieva O, Meher S, Alfirevic Z, Alfirevic A. Pathway analysis of genetic factors associated with spontaneous preterm birth and pre-labor preterm rupture of membranes. PLoS One. 2014;9(9):e108578. doi: 10.1371/journal.pone.0108578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura T, et al. What knockout mice can tell us about parturition. Rev Reprod. 1999;4(2):73–80. doi: 10.1530/ror.0.0040073. [DOI] [PubMed] [Google Scholar]