Significance
G-quadruplexes (G4) are four-stranded nucleic acid structures implicated in important biological processes. Interaction between G4 and proteins is essential for the cellular functions of G4. The DEAH (Asp-Glu-Ala-His) box RNA helicase associated with AU-rich element (RHAU) (also named DHX36 or G4R1) specifically binds and unwinds G4 structures. The structure of an 18-aa peptide, identified as the G4-binding domain of RHAU, was solved in the complex with a G4 by NMR spectroscopy. The structure of the complex explains how RHAU specifically recognizes G4 structures and suggests a strategy for G4 recognition by proteins.
Keywords: G-quadruplex, RHAU helicase, DHX36, DEAH-box family, NMR
Abstract
Four-stranded nucleic acid structures called G-quadruplexes have been associated with important cellular processes, which should require G-quadruplex–protein interaction. However, the structural basis for specific G-quadruplex recognition by proteins has not been understood. The DEAH (Asp-Glu-Ala-His) box RNA helicase associated with AU-rich element (RHAU) (also named DHX36 or G4R1) specifically binds to and resolves parallel-stranded G-quadruplexes. Here we identified an 18-amino acid G-quadruplex-binding domain of RHAU and determined the structure of this peptide bound to a parallel DNA G-quadruplex. Our structure explains how RHAU specifically recognizes parallel G-quadruplexes. The peptide covers a terminal guanine base tetrad (G-tetrad), and clamps the G-quadruplex using three-anchor-point electrostatic interactions between three positively charged amino acids and negatively charged phosphate groups. This binding mode is strikingly similar to that of most ligands selected for specific G-quadruplex targeting. Binding to an exposed G-tetrad represents a simple and efficient way to specifically target G-quadruplex structures.
G-quadruplex (G4) is a four-stranded structure of nucleic acids, consisting of a G⋅G⋅G⋅G tetrad (G-tetrad) core and loops (1–5). G4 structures are highly diverse, with different possible relative strand orientations in the G-tetrad core. In a parallel G4 four strands are oriented in the same direction, while in a nonparallel G4 (i) three strands are oriented in the same direction and one in the opposite direction or (ii) two strands are oriented in one direction and the other two in the opposite direction (6).
Potential G4-forming sequences are prevalent in many important regions of the human genome (7). G4s have been observed in human cells (8, 9) and are associated with essential cellular processes such as replication, recombination, transcription, and translation (7, 10, 11). Interaction with proteins is required for the cellular functions of G4s, and proteins that interact with G4s have been increasingly identified (12). For example, helicases, such as BLM (13), FANCJ (14), PIF1 (15), and RHAU (16), have been shown to bind and unwind G4 structures.
Structural studies on G4–protein binding have been attempted by different groups. Computational studies showed that a G4 could fit into a protein cavity of HIV-integrase (17) and RecA protein (18). In an NMR-guided simulation, a nucleophosmin domain was docked into a G4 groove (19). Limited available high-resolution structures of G4–protein complexes did not show direct contacts between the proteins and the G4 core; the binding was either dependent on a loop (20, 21) or a duplex–quadruplex junction formation (21, 22). Recently, NMR and simulation studies reported on a complex, where two antiprion peptide molecules bound at the two ends of an RNA G4 stack, driven by water entropy gain (23). However, from the studies reported to date, the structural basis for specific G4 recognition by proteins has not been fully understood.
RNA helicase associated with AU-rich element (RHAU), a helicase of the DEAH (Asp-Glu-Ala-His) box family, has been shown to tightly bind and resolve G4 structures with high specificity (16). RHAU is associated with different functions, including the formation of stress granules and interchromatin granule clusters, and the degradation of urokinase plasmonigen activator mRNA (24). RHAU was found to associate with telomerase through binding to the G4 formed at the 5′ end of the telomerase RNA (25). The 53-amino acid fragment (from residue 53 to residue 105) of RHAU (termed Rhau53 in this paper; Table 1) binds specifically to G4s. A 13-amino acid RHAU-specific motif (RSM) (from residue 54 to residue 66) has been identified to be essential for the binding (26). Small-angle X-ray scattering (SAXS) data suggested that Rhau53 binds at an end of a G4 (27).
Table 1.
Peptide sequences used in this study
| Name | Sequences (Nterm–Cterm) | Binding* |
| Rhau53 | SMHPGHLKGREIGMWYAKKQGQKNKEAERQERAVVHMDERREEQIVQLLNSVQAK | + |
| Rhau29 | HPGHLKGREIGMWYAKKQGQKNKEAERQE | + |
| Rhau23 | HPGHLKGREIGMWYAKKQGQKNK | + |
| Rhau20 | HPGHLKGREIGMWYAKKQGQ | + |
| Rhau18′ | HPGHLKGREIGMWYAKKQ | + |
| Rhau18 | SMHPGHLKGREIGMWYAKKQ | + |
| Rhau16 | HPGHLKGREIGMWYAK | + |
| Rhau14 | HPGHLKGREIGMWY | – |
| Rhau12 | HPGHLKGREIGM | – |
| Rhau5 | HPGHL | – |
| Rhau9 | KGREIGMWY | – |
| Rhau15′ | KGREIGMWYAKKQGQ | – |
| Rhau20m1 | HPLHLKLREILMWYAKKQGQ | – |
| Rhau20m2 | HPGHLKGREIGMAAAKKQGQ | – |
Nonbinding with T95-2T is considered when the dissociation constant (Kd) is larger than 100 μM. Mutated residues are underlined. Extra residues from the expression constructs (see Methods) are in italic.
Here we report the NMR solution structure of a complex formed between a G4 and a peptide from the N-terminal region of RHAU, revealing general principles for specific recognition of G4s by RHAU.
Results and Discussion
Rhau53 Binds Preferentially to Parallel G4.
Binding of Rhau53 with different DNA and RNA sequences (Table 2) was tested by gel electrophoresis experiments. The data show that Rhau53 binds to the parallel G4 formed by the T95-2T sequence (28) (Fig. 1A) but not the nonparallel G4 formed by the human telomeric sequence Htelo1 (29) (Fig. 1B). However, under molecular crowding condition, Htelo1 shows an additional upper band (Fig. 1C) corresponding to the parallel G4 form (30, 31), which binds Rhau53 (Fig. 1C). Similar results were observed for different human telomeric G4 conformations (SI Appendix, Fig. S1). Quantitative gel analysis (SI Appendix, Fig. S2) shows that the binding affinity of Rhau53 to parallel G4s formed by either T95-2T or Htelo1 (dissociation constant, Kd ≈ 1 µM) is about 100-fold higher than to the nonparallel G4 of Htelo1 (Kd ≈ 100 μM) (Fig. 1D).
Table 2.
DNA and RNA sequences used in this study
| Name | Sequences (5′–3′) | Structure* | Binding† |
| TERC18 | GGGUUGCGGAGGGUGGGC | parallel G4 RNA (35) | + |
| T95-2T‡ | TTGGGTGGGTGGGTGGGT | parallel G4 DNA (28) | + |
| Pu24T | TGAGGGTGGTGAGGGTGGGGA | parallel G4 DNA (36) | + |
| CEB1 | AGGGGGGAGGGAGGGTGG | parallel G4 DNA (37) | + |
| CEB25 | AAGGGTGGGTGTAAGTGTGGGTGGGT | parallel G4 DNA (38) | + |
| 93del | GGGGTGGGAGGAGGGT | parallel G4 DNA (17) | + |
| HT§ | TTGGGTTAGGGTTAGGGTTAGGGA | nonparallel G4 DNA (39) | – |
| Htelo1§ | TAGGGTTAGGGTTAGGGTTAGGG | nonparallel G4 DNA (29, 39) | – |
| Htelo2§ | TAGGGTTAGGGTTAGGGTTAGGGTT | nonparallel G4 DNA (29, 40) | – |
| Htelo3# | GGGTTAGGGTTAGGGTTAGGGT | nonparallel G4 DNA (41) | – |
| Htelo4§ | AGGGCTAGGGCTAGGGCTAGGG | nonparallel G4 DNA (42) | – |
| DX1 | CGCGAATTCGCG | duplex DNA (43) | – |
| DX2 | ATCTGAGAATCAGAT | duplex hairpin DNA (44) | – |
Structure formation was monitored by NMR.
Nonbinding with Rhau53 is considered when the dissociation constant (Kd) is larger than 100 μM.
TAMRA attached to the 5′ end.
FAM attached to the 5′ end.
FAM attached to the 3′ end.
Fig. 1.

Native gel electrophoresis of DNA in the absence and presence of Rhau53: (A) T95-2T, (B) Htelo1, and (C) Htelo1 under crowding condition. Concentration of DNA and Rhau53 was 50–100 nM and 500–1,000 nM, respectively. Filled and open triangle symbols represent the parallel and nonparalel G4 form of Htelo1, respectively. (D) Gel binding analysis for T95-2T (asterisk), Htelo1 (open triangle), and Htelo1 under crowding condition (filled triangle).
Assays on different nucleic acid conformations (SI Appendix, Fig. S3) show that Rhau53 binds to various parallel DNA and RNA G4s, but not nonparallel DNA G4s and duplexes (SI Appendix, Fig. S4). This conclusion is supported by an NMR titration experiment, which shows that upon addition of Rhau53 in a mixture of a parallel and a nonparallel G4, only peaks belonging to the parallel form are perturbed (SI Appendix, Fig. S5).
We next focus our study of RHAU–G4 interaction using T95-2T, a simple model of a parallel G4, which forms a three-layer propeller-type G4 with three single-residue loops (28).
Minimal G4 Binding Domain of RHAU.
Backbone 15N, 13C, and 1H resonances were assigned for Rhau53 in the free form and in complex with an excess of T95-2T (Fig. 2A). Upon complex formation, the chemical shifts of the first 21 amino acids of Rhau53 were largely perturbed, while no significant changes were observed for the rest of the sequence (Fig. 2B and SI Appendix, Fig. S6), suggesting that the G4 binding of Rhau53 is limited to the first 21 amino acids. Binding assays (SI Appendix, Fig. S7) were performed between T95-2T and peptides of different lengths (Table 1). The 16- and 18-amino acid peptides termed Rhau16 and Rhau18, respectively, containing the RSM motif, were the shortest peptides capable of binding to T95-2T. Note that Rhau14 containing the RSM motif with only one additional residue do not bind G4. NMR spectra also show the binding of T95-2T with Rhau16, Rhau18, and Rhau53 (SI Appendix, Fig. S8). Rhau18 and Rhau53 exhibit similar spectral changes upon DNA binding (SI Appendix, Figs. S8 and S9). Like Rhau53, Rhau18 binds specifically to parallel G4, but not to nonparallel G4 and duplex DNA, as observed by gel electrophoresis (SI Appendix, Fig. S10).
Fig. 2.
(A) 15N-1H correlation spectra of Rhau53 free (black) and bound to T95-2T (red). The assignments are shown for the first 21 amino acids. Boxes represent peaks observed at a lower threshold. (B) Chemical shift variation, ∆δ = [(δ1H)2 + (δ15N)2]1/2, plotted as a function of residue number. (Inset) NMR solution structure of Rhau18.
NMR Solution Structure of Rhau18.
The solution structure of Rhau18 (see Table 3 for statistics) was determined by using standard triple-resonance NMR techniques (see Methods). It presents an L-shaped structure (Fig. 2B), containing an α-helix spanning from Gly5 to Ala17 with a hydrophobic core that comprises Leu7, Ile12, and Trp15 (SI Appendix, Fig. S11).
Table 3.
Statistics of the computed structure of Rhau18
| Restraints and structure statistics | Value |
| NMR restraints | |
| Distance restraints | |
| Intraresidue | 280 |
| Interresidue | 29 |
| Torsion angle restraints | |
| Phi | 12 |
| Psi | 12 |
| Structure statistics | |
| NOE violations | |
| Numbers (>0.2 Å) | 0.20 ± 0.63 |
| Deviations from standard geometry | |
| Bond length, Å | 0.005 ± 0.000 |
| Bond angle, ° | 0.810 ± 0.017 |
| Impropers, ° | 0.933 ± 0.071 |
| Pairwise all heavy atom rmsd values, Å | |
| Backbone heavy atoms* | 0.621 ± 0.106 |
| All heavy atoms* | 1.110 ± 0.159 |
| Ramachandran plot analysis† | |
| Most favorable region, % | 85.7 |
| Additional allowed region, % | 14.3 |
| Disallowed region, % | 0.0 |
Except amino acids S1, M2, K19, and Q20.
Analysis using the PROCHECK program (www.ebi.ac.uk/thornton-srv/software/PROCHECK/).
NMR Solution Structure of Rhau18–G4 Complex.
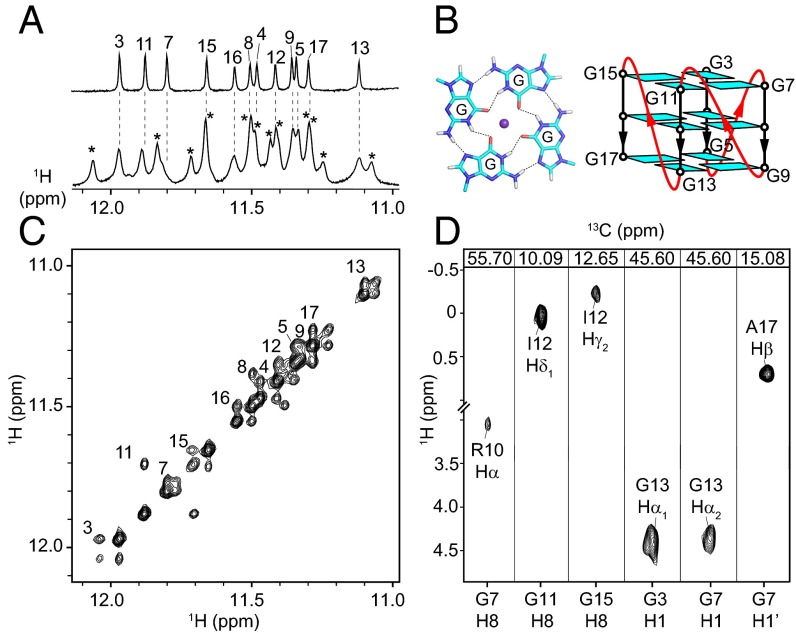
Addition of Rhau18 into T95-2T (at the 1:0.5 DNA/peptide ratio) resulted in the appearance of a new set of peaks in the DNA imino proton spectrum (Fig. 3A). Signals belonging to DNA in the Rhau18–T95-2T complex were assigned through chemical exchange (Fig. 3C) with those of the previously assigned free T95-2T (28). NMR data indicated that T95-2T maintained its three-layered propeller-type parallel G4 upon protein binding (Fig. 3B).
Fig. 3.
(A) Imino proton spectra of T95-2T free (Top) and in the presence of Rhau18 (Bottom). Imino protons in the complex are marked by asterisks. (B) G-tetrad and folding topology of T95-2T. (C) NOESY spectrum (mixing time, 700 ms; temperature, 37 °C) showing exchange peaks between the free and bound form of T95-2T, labeled with the residue number. (D) NOE strip plots showing the intermolecular cross-peaks between the peptide and DNA.
A complex between 15N, 13C-labeled Rhau18, and an excess of unlabeled T95-2T was formed. The 15N, 13C, and 1H resonances for Rhau18 in the complex were assigned using standard triple-resonance experiments (SI Appendix, Fig. S12). Forty-seven intermolecular NOEs cross-peaks were identified between Rhau18 and T95-2T (Fig. 3D and SI Appendix, Fig. S13).
In the solution structure of the Rhau18–T95-2T complex (Fig. 4; see Table 4 for statistics), both Rhau18 and T95-2T retained their overall initial structures (SI Appendix, Fig. S14). Rhau18 binds to the 5′-end G-tetrad of T95-2T, projecting the first two thymine bases outward (Fig. 4). The N-terminal residues of Rhau18 are oriented away from T95-2T, while the C-termimal residues were found to be close to a medium groove of DNA (Fig. 4C). The top G-tetrad is almost entirely covered by the peptide (Fig. 4D). The CH/π- and CH3/π-stacking interactions are observed between peptide residues (Gly9, Ile12, Gly13, and Ala17) and DNA guanine bases (G3, G7, G11, and G15) of the 5′-end G-tetrad (Fig. 4E and SI Appendix, Fig. S15). The positively charged side-chains of Lys8, Arg10, and Lys19 are each located above a groove; electrostatic interactions are favored by the close proximity between these residues and the phosphate groups of G16, G4, and G8/G12, respectively (Fig. 4F). It is noted that the peptide residues involved in maintaining the L-shaped conformation of Rhau18 (Pro4, Leu7, and Trp15) or directly contacting the G-tetrad core (Gly9, Ile12, Gly13, and Ala17) are among the most conserved residues across various species (26), while the positively charged residues (Lys8, Arg10, and Lys18/19) are conserved in higher organisms (26).
Fig. 4.
NMR solution structure of the Rhau18–T95-2T complex. (A) Side and (B) top view of the 10 superimposed lowest-energy structures. (C) Side- and (D) top-view surface representation of the complex. (E) Details of the intermolecular interactions between peptide residues (G9, I12, G13, and A17) and DNA guanine bases of the 5′-end G-tetrad. (F) Details of the intermolecular interactions between peptide positively charged side-chains of K8, R10, and K19 and the DNA phosphate backbone. Rhau18, red; guanines, cyan; thymines, orange; DNA backbone, gray; O4′ atoms, yellow.
Table 4.
Statistics of the computed structure of the Rhau18-T95-2T complex
| Restraints and structure statistics | Value |
| NMR restraints | |
| Peptide restraints | |
| Distance restraints | |
| Intraresidue | 115 |
| Interresidue | 75 |
| Torsion angle restraints | |
| Phi | 10 |
| Psi | 10 |
| DNA restraints | 287 |
| DNA–peptide restraints | 47 |
| Structure statistics | |
| NOE violations | |
| Numbers (>0.2 Å) | 0.00 ± 0.00 |
| Deviations from standard geometry | |
| Bond length, Å | 0.002 ± 0.000 |
| Bond angle, ° | 0.580 ± 0.004 |
| Impropers, ° | 0.303 ± 0.003 |
| Pairwise all heavy atom rmsd values (Å) for the peptide | |
| Backbone heavy atoms* | 1.438 ± 0.320 |
| All heavy atoms* | 1.597 ± 0.121 |
| Ramachandran plot analysis† | |
| Most favorable region, % | 62.1 |
| Additional allowed region, % | 33.6 |
| Disallowed region, % | 4.3 |
Except amino acids S1, M2, and H3 of Rhau18 and thymine bases of T95-2T.
Analysis using the PROCHECK program (www.ebi.ac.uk/thornton-srv/software/PROCHECK/).
Impact of RHAU Peptide Mutants on G4 Binding.
Binding between T95-2T and different peptide mutants was tested by gel electrophoresis (SI Appendix, Fig. S7). Mutations tested previously (26) and here (Rhau20m1, Table 1) on residues Gly9, Ile12, and Gly13 were shown to be detrimental to the G4 binding, consistent with these residues providing specific stacking interactions with the terminal G-tetrad. Substitutions of aromatic residues Trp15 and Tyr16 (Rhau20m2, Table 1), involved in maintaining the L-shaped conformation of Rhau18, by an alanine also inhibited the G4 binding. In contrast, the G4 binding was still observed for a construct containing mutations at the charged residues Lys8 and Arg10. Although the effect of these mutations on the affinity of the complex was not quantified (26), our structure suggests that these charged residues, which are appropriately positioned above the G4 backbone, should contribute in stabilizing the complex by electrostatic interactions.
Binding of Second Peptide Molecule.
At the 1:2 DNA/peptide ratio, NMR proton spectrum shows one set of peaks for DNA and two for the peptide (SI Appendix, Fig. S16A), consistent with the formation of a second complex containing two peptide molecules and one DNA molecule. Imino protons of DNA in the new complex were unambiguously assigned using site-specific 15N-labeled samples (SI Appendix, Fig. S16C). Highly similar intermolecular NOE patterns between the two peptides and DNA (Fig. 5A) suggest the same mode of interaction. NOE data indicate that the second peptide molecule binds at the bottom 3′-end G-tetrad of T95-2T (Fig. 5). The binding at the 3′ end occurs at a higher peptide concentration than the binding at the 5′ end, indicating a lower affinity for the 3′-end bottom site (SI Appendix, Fig. S16). This difference in affinities might be related to different arrangements of the backbone and orientations of the sugars between the 5′- and 3′-end G-tetrads (32). In principle, the full-length RHAU should be able to recognize both the 5′ and 3′ end; however, it remains to be established whether the protein can bind the two ends simultaneously and how this binding assists in resolving G4 structures.
Fig. 5.
(A) NOESY spectrum (mixing time, 350 ms) of the Rhau18–T95-2T complex with more than 2-fold of Rhau18, showing intermolecular NOEs between different protons of Rhau18 and guanines imino protons of T95-2T at the two terminal G-tetrads (labeled in red for the top 5′-end G-tetrad and blue for the bottom 3′-end G-tetrad). Unresolved protons of the two peptide molecules are marked with asterisks. (B) Model for binding between T95-2T and two Rhau18 molecules. Peptide residues (I12, G13, K8, R10, and K19) involved in the interactions with DNA are highlighted. The model was generated based on the NOE data and symmetry consideration.
Exposed G-Tetrad Is Determinant for G4-Specific Recognition by RHAU.
The size and shape of the RHAU binding motif match well with the unique geometry of a G-tetrad. The peptide covers the exposed hydrophobic G-tetrad and clamps the G4 structure using three-anchor-point electrostatic interactions (between three positively charged amino acids and negatively charged phosphate groups). Our structure explains how RHAU specifically recognizes different parallel G4s based on their G-tetrad accessibility at the 5′ end (e.g., T95-2T, CEB25, and PU24T) or 3′ end (e.g., T95-2T, TERC18, CEB1, and 93del) (Table 2 and SI Appendix, Fig. S3). In nonparallel G4s, G-tetrads are generally covered by different types of loops, which would sterically hinder protein binding. RHAU–G4 recognition might be more directly associated to an exposed G-tetrad rather than a specific G4 topology (parallel vs. nonparallel). It should be noted that a left-handed parallel G4 structure with well-covered terminal G-tetrads has been recently reported (33); on the other hand, the formation of a nonparallel G4 with exposed G-tetrad might be possible.
The G4 binding mode of RHAU is strikingly similar to that of most small molecules selected for specific G4 binding (34), which occur through stacking interactions on the terminal G-tetrads and electrostatic interactions with the phosphate backbone. However, as opposed to RHAU, many small molecules do not distinguish between different G4 conformations, probably due to their small sizes allowing them to be accommodated on a terminal G-tetrad even in the presence of loops.
Binding to an exposed G-tetrad represents a simple and efficient way to specifically target G4 structures – a strategy that could also be adopted by other G4-binding proteins. Based on this work, the binding domain can be modified to modulate the affinity and specificity to parallel G4s, and possibly also other G4 conformations. The G4 binding motif can be engineered in other proteins to achieve G4-specific functions, such as regulation of gene expression.
Methods
Sample Preparation.
Unlabeled and 4% site-specific 15N-labeled DNA and RNA oligonucleotides were chemically synthesized on an Applied Biosystems Inc. (ABI) 394 DNA/RNA synthesizer. UV absorbance at 260 nm was used to determine the oligonuleotide concentrations. Carboxytetramethylrhodamine (TAMRA)/Carboxyfluorescein (FAM)-labeled DNA sequences were purchased from Integrated DNA Technologies or AITBiotech. Synthetic peptides were purchased from the Peptide Synthesis Core Facility, School of Biological Sciences, Nanyang Technological University. Detailed procedures for the protein expression and purification are described in SI Appendix.
Gel Electrophoresis.
All of the polyacrylamide native gels were run using 10–20% of acrylamide (37.5:1 acrylamide/bis ratio) at 4 °C or 25 °C, 90–120 V, for 50–120 min. Fluorescence-labeled DNAs were visualized using UV transilluminator. Unlabeled DNAs were visualized using UV shadowing. All of the gel images were analyzed using ImageJ software.
NMR Spectroscopy and Structure Calculation.
NMR experiments were performed on 600-MHz and 700-MHz Bruker spectrometers at 15 °C, 25 °C, or 37 °C. Concentrations of the free and bound proteins were typically between 0.1 mM and 1 mM, in 10–70 mM KCl, 10–20 mM potassium phosphate (pH 6.5) and 5–10% (vol/vol) D2O. Detailed procedures for resonances assignments and structure calculation are described in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by Singapore Ministry of Education Academic Research Fund Tier 3 (MOE2012-T3-1-001) and Tier 2 (MOE2012-T2-1-102), and grants from Nanyang Technological University (to A.T.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.N. is a guest editor invited by the Editorial Board.
Data deposition: Atomic coordinates of Rhau18 and Rhau18-T95-2T complex have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 2N16 and 2N21).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1422605112/-/DCSupplemental.
References
- 1.Gellert M, Lipsett MN, Davies DR. Helix formation by guanylic acid. Proc Natl Acad Sci USA. 1962;48(12):2013–2018. doi: 10.1073/pnas.48.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sen D, Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988;334(6180):364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- 3.Smith FW, Feigon J. Quadruplex structure of Oxytricha telomeric DNA oligonucleotides. Nature. 1992;356(6365):164–168. doi: 10.1038/356164a0. [DOI] [PubMed] [Google Scholar]
- 4.Laughlan G, et al. The high-resolution crystal structure of a parallel-stranded guanine tetraplex. Science. 1994;265(5171):520–524. doi: 10.1126/science.8036494. [DOI] [PubMed] [Google Scholar]
- 5.Parkinson GN, Lee MP, Neidle S. Crystal structure of parallel quadruplexes from human telomeric DNA. Nature. 2002;417(6891):876–880. doi: 10.1038/nature755. [DOI] [PubMed] [Google Scholar]
- 6.Patel DJ, Phan AT, Kuryavyi V. Human telomere, oncogenic promoter and 5′-UTR G-quadruplexes: Diverse higher order DNA and RNA targets for cancer therapeutics. Nucleic Acids Res. 2007;35(22):7429–7455. doi: 10.1093/nar/gkm711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maizels N, Gray LT. The G4 genome. PLoS Genet. 2013;9(4):e1003468. doi: 10.1371/journal.pgen.1003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biffi G, Tannahill D, McCafferty J, Balasubramanian S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat Chem. 2013;5(3):182–186. doi: 10.1038/nchem.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson A, et al. Detection of G-quadruplex DNA in mammalian cells. Nucleic Acids Res. 2014;42(2):860–869. doi: 10.1093/nar/gkt957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cahoon LA, Seifert HS. An alternative DNA structure is necessary for pilin antigenic variation in Neisseria gonorrhoeae. Science. 2009;325(5941):764–767. doi: 10.1126/science.1175653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumari S, Bugaut A, Huppert JL, Balasubramanian S. An RNA G-quadruplex in the 5′ UTR of the NRAS proto-oncogene modulates translation. Nat Chem Biol. 2007;3(4):218–221. doi: 10.1038/nchembio864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oganesian L, Bryan TM. Physiological relevance of telomeric G-quadruplex formation: A potential drug target. BioEssays. 2007;29(2):155–165. doi: 10.1002/bies.20523. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Karow JK, Hickson ID, Maizels N. The Bloom’s syndrome helicase unwinds G4 DNA. J Biol Chem. 1998;273(42):27587–27592. doi: 10.1074/jbc.273.42.27587. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y, Shin-ya K, Brosh RM., Jr FANCJ helicase defective in Fanconia anemia and breast cancer unwinds G-quadruplex DNA to defend genomic stability. Mol Cell Biol. 2008;28(12):4116–4128. doi: 10.1128/MCB.02210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribeyre C, et al. The yeast Pif1 helicase prevents genomic instability caused by G-quadruplex-forming CEB1 sequences in vivo. PLoS Genet. 2009;5(5):e1000475. doi: 10.1371/journal.pgen.1000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaughn JP, et al. The DEXH protein product of the DHX36 gene is the major source of tetramolecular quadruplex G4-DNA resolving activity in HeLa cell lysates. J Biol Chem. 2005;280(46):38117–38120. doi: 10.1074/jbc.C500348200. [DOI] [PubMed] [Google Scholar]
- 17.Phan AT, et al. An interlocked dimeric parallel-stranded DNA quadruplex: A potent inhibitor of HIV-1 integrase. Proc Natl Acad Sci USA. 2005;102(3):634–639. doi: 10.1073/pnas.0406278102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuryavyi V, Cahoon LA, Seifert HS, Patel DJ. RecA-binding pilE G4 sequence essential for pilin antigenic variation forms monomeric and 5′ end-stacked dimeric parallel G-quadruplexes. Structure. 2012;20(12):2090–2102. doi: 10.1016/j.str.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallo A, et al. Structure of nucleophosmin DNA-binding domain and analysis of its complex with a G-quadruplex sequence from the c-MYC promoter. J Biol Chem. 2012;287(32):26539–26548. doi: 10.1074/jbc.M112.371013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horvath MP, Schultz SC. DNA G-quartets in a 1.86 Å resolution structure of an Oxytricha nova telomeric protein-DNA complex. J Mol Biol. 2001;310(2):367–377. doi: 10.1006/jmbi.2001.4766. [DOI] [PubMed] [Google Scholar]
- 21.Russo Krauss I, Pica A, Merlino A, Mazzarella L, Sica F. Duplex-quadruplex motifs in a peculiar structural organization cooperatively contribute to thrombin binding of a DNA aptamer. Acta Crystallogr D Biol Crystallogr. 2013;69(Pt 12):2403–2411. doi: 10.1107/S0907444913022269. [DOI] [PubMed] [Google Scholar]
- 22.Phan AT, et al. Structure-function studies of FMRP RGG peptide recognition of an RNA duplex-quadruplex junction. Nat Struct Mol Biol. 2011;18(7):796–804. doi: 10.1038/nsmb.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi T, et al. Binding of an RNA aptamer and a partial peptide of a prion protein: Crucial importance of water entropy in molecular recognition. Nucleic Acids Res. 2014;42(11):6861–6875. doi: 10.1093/nar/gku382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalupníková K, et al. Recruitment of the RNA helicase RHAU to stress granules via a unique RNA-binding domain. J Biol Chem. 2008;283(50):35186–35198. doi: 10.1074/jbc.M804857200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lattmann S, Stadler MB, Vaughn JP, Akman SA, Nagamine Y. The DEAH-box RNA helicase RHAU binds an intramolecular RNA G-quadruplex in TERC and associates with telomerase holoenzyme. Nucleic Acids Res. 2011;39(21):9390–9404. doi: 10.1093/nar/gkr630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lattmann S, Giri B, Vaughn JP, Akman SA, Nagamine Y. Role of the amino terminal RHAU-specific motif in the recognition and resolution of guanine quadruplex-RNA by the DEAH-box RNA helicase RHAU. Nucleic Acids Res. 2010;38(18):6219–6233. doi: 10.1093/nar/gkq372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meier M, et al. Binding of G-quadruplexes to the N-terminal recognition domain of the RNA helicase associated with AU-rich element (RHAU) J Biol Chem. 2013;288(49):35014–35027. doi: 10.1074/jbc.M113.512970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Do NQ, Phan AT. Monomer-dimer equilibrium for the 5′-5′ stacking of propeller-type parallel-stranded G-quadruplexes: NMR structural study. Chemistry. 2012;18(46):14752–14759. doi: 10.1002/chem.201103295. [DOI] [PubMed] [Google Scholar]
- 29.Phan AT, Kuryavyi V, Luu KN, Patel DJ. Structure of two intramolecular G-quadruplexes formed by natural human telomere sequences in K+ solution. Nucleic Acids Res. 2007;35(19):6517–6525. doi: 10.1093/nar/gkm706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heddi B, Phan AT. Structure of human telomeric DNA in crowded solution. J Am Chem Soc. 2011;133(25):9824–9833. doi: 10.1021/ja200786q. [DOI] [PubMed] [Google Scholar]
- 31.Xue Y, et al. Human telomeric DNA forms parallel-stranded intramolecular G-quadruplex in K+ solution under molecular crowding condition. J Am Chem Soc. 2007;129(36):11185–11191. doi: 10.1021/ja0730462. [DOI] [PubMed] [Google Scholar]
- 32.Lech CJ, Heddi B, Phan AT. Guanine base stacking in G-quadruplex nucleic acids. Nucleic Acids Res. 2013;41(3):2034–2046. doi: 10.1093/nar/gks1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung WJ, et al. Structure of a left-handed DNA G-quadruplex. Proc Natl Acad Sci USA. 2015;112(9):2729–2733. doi: 10.1073/pnas.1418718112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balasubramanian S, Hurley LH, Neidle S. Targeting G-quadruplexes in gene promoters: A novel anticancer strategy? Nat Rev Drug Discov. 2011;10(4):261–275. doi: 10.1038/nrd3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martadinata H, Phan AT. Formation of a stacked dimeric G-quadruplex containing bulges by the 5′-terminal region of human telomerase RNA (hTERC) Biochemistry. 2014;53(10):1595–1600. doi: 10.1021/bi4015727. [DOI] [PubMed] [Google Scholar]
- 36.Phan AT, Kuryavyi V, Gaw HY, Patel DJ. Small-molecule interaction with a five-guanine-tract G-quadruplex structure from the human MYC promoter. Nat Chem Biol. 2005;1(3):167–173. doi: 10.1038/nchembio723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adrian M, et al. Structure and conformational dynamics of a stacked dimeric G-quadruplex formed by the human CEB1 minisatellite. J Am Chem Soc. 2014;136(17):6297–6305. doi: 10.1021/ja4125274. [DOI] [PubMed] [Google Scholar]
- 38.Amrane S, et al. Formation of pearl-necklace monomorphic G-quadruplexes in the human CEB25 minisatellite. J Am Chem Soc. 2012;134(13):5807–5816. doi: 10.1021/ja208993r. [DOI] [PubMed] [Google Scholar]
- 39.Luu KN, Phan AT, Kuryavyi V, Lacroix L, Patel DJ. Structure of the human telomere in K+ solution: An intramolecular (3 + 1) G-quadruplex scaffold. J Am Chem Soc. 2006;128(30):9963–9970. doi: 10.1021/ja062791w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phan AT, Luu KN, Patel DJ. Different loop arrangements of intramolecular human telomeric (3+1) G-quadruplexes in K+ solution. Nucleic Acids Res. 2006;34(19):5715–5719. doi: 10.1093/nar/gkl726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim KW, et al. Structure of the human telomere in K+ solution: A stable basket-type G-quadruplex with only two G-tetrad layers. J Am Chem Soc. 2009;131(12):4301–4309. doi: 10.1021/ja807503g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim KW, et al. Sequence variant (CTAGGG)n in the human telomere favors a G-quadruplex structure containing a G⋅C⋅G⋅C tetrad. Nucleic Acids Res. 2009;37(18):6239–6248. doi: 10.1093/nar/gkp630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drew HR, et al. Structure of a B-DNA dodecamer: Conformation and dynamics. Proc Natl Acad Sci USA. 1981;78(4):2179–2183. doi: 10.1073/pnas.78.4.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim KW, Phan AT. Structural basis of DNA quadruplex-duplex junction formation. Angew Chem Int Ed Engl. 2013;52(33):8566–8569. doi: 10.1002/anie.201302995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.