Significance
Flowering plants attract pollinators in part by emitting volatile scents from their petals. This emission of scent is highly regulated, and is often restricted to a specific portion of the day. Although the biochemical pathways of scent production are well characterized, little is known of their transcriptional regulation. Here we describe a direct molecular link between the circadian clock and floral volatile emissions. We find that a clock transcription factor regulates the timing of multiple genes involved in the production of floral volatiles in Petunia. This work provides key insights into the complex yet relatively unexplored transcriptional regulation of scent production, and also sheds light on how the circadian clock can regulate the timing of large metabolic pathways.
Keywords: circadian rhythm, floral volatile, benzenoids, Petunia hybrida, LHY
Abstract
Flowers present a complex display of signals to attract pollinators, including the emission of floral volatiles. Volatile emission is highly regulated, and many species restrict emissions to specific times of the day. This rhythmic emission of scent is regulated by the circadian clock; however, the mechanisms have remained unknown. In Petunia hybrida, volatile emissions are dominated by products of the floral volatile benzenoid/phenylpropanoid (FVBP) metabolic pathway. Here we demonstrate that the circadian clock gene P. hybrida LATE ELONGATED HYPOCOTYL (LHY; PhLHY) regulates the daily expression patterns of the FVBP pathway genes and floral volatile production. PhLHY expression peaks in the morning, antiphasic to the expression of P. hybrida GIGANTEA (PhGI), the master scent regulator ODORANT1 (ODO1), and many other evening-expressed FVBP genes. Overexpression phenotypes of PhLHY in Arabidopsis caused an arrhythmic clock phenotype, which resembles those of LHY overexpressors. In Petunia, constitutive expression of PhLHY depressed the expression levels of PhGI, ODO1, evening-expressed FVBP pathway genes, and FVBP emission in flowers. Additionally, in the Petunia lines in which PhLHY expression was reduced, the timing of peak expression of PhGI, ODO1, and the FVBP pathway genes advanced to the morning. Moreover, PhLHY protein binds to cis-regulatory elements called evening elements that exist in promoters of ODO1 and other FVBP genes. Thus, our results imply that PhLHY directly sets the timing of floral volatile emission by restricting the expression of ODO1 and other FVBP genes to the evening in Petunia.
Plant development and physiology are extensively influenced by the circadian clock (1). The precise timing of a single plant behavioral output often requires a suite of internal mechanisms to occur in coincidence or in quick succession before the behavior taking place. Transcriptome analysis revealed that the circadian clock controls transcription of one third of genes in Arabidopsis (2). In this way, the clock can exert a holistic effect on a complex mechanism at a precise moment in time. The effectiveness of the clock’s ability to coordinate complex behaviors has been used by many aspects of plant physiology, such as photosynthesis, stem and leaf growth, and flowering (3, 4).
The precise timing of sexual reproductive events is critical, as plants are sessile and individuals are often spread over large distances. In addition to regulating the timing of flower formation, when they have opened, many flowers emit floral scents to lure pollinators. Attractive floral volatiles are often emitted in a rhythmic fashion, with peaks of emission coinciding with the primary pollinator’s period of activity (5). Although studies have shown that rhythmic emission of scent requires the influence of a circadian clock (6–8), no study of which we are aware has shown a mechanistic link between clock function and floral volatile production.
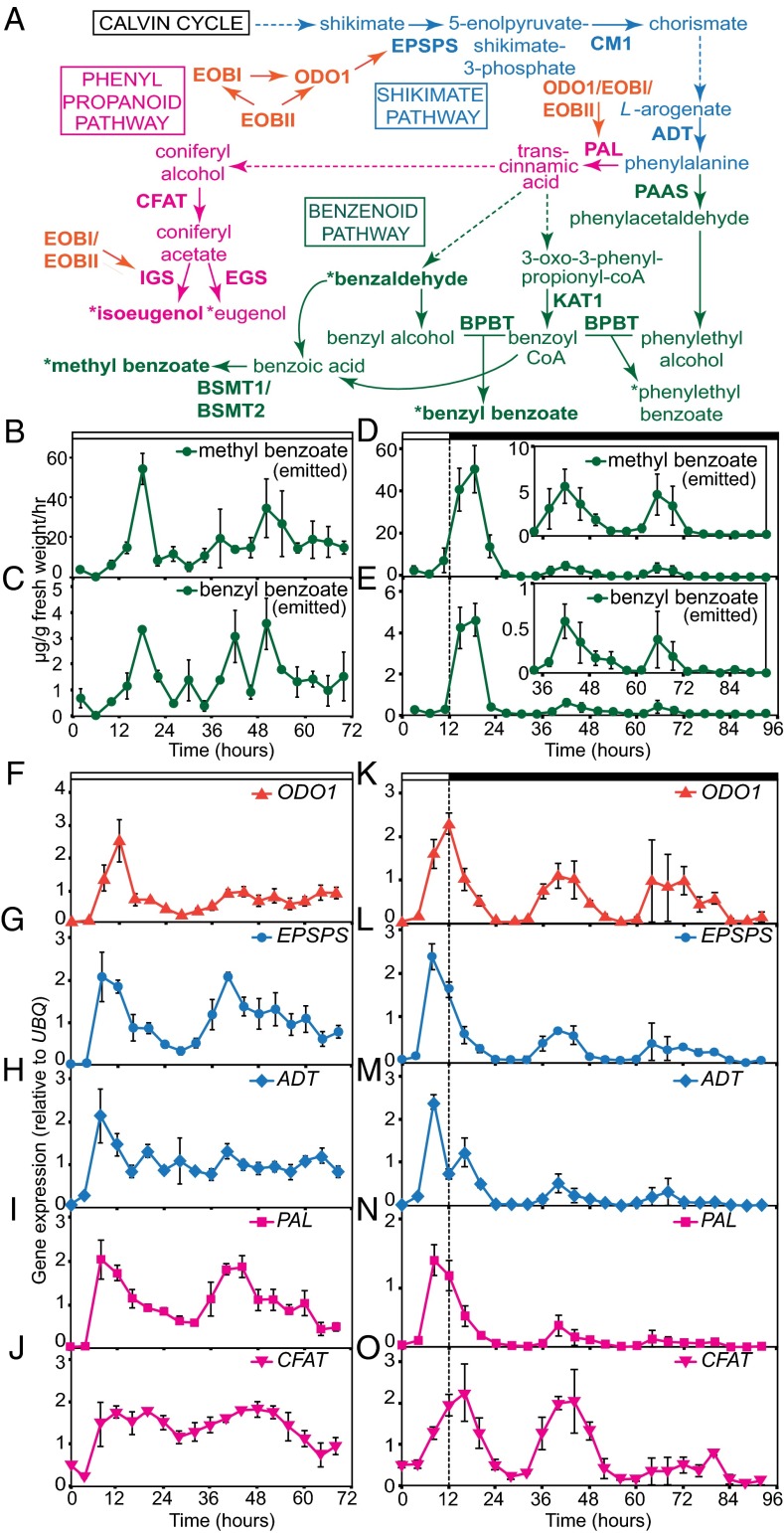
Research on floral volatile synthesis has often used the common garden petunia, Petunia hybrida cv. Mitchell, which exhibits white flowers that peak in scent emission in the middle of night (9). Floral scent in Petunia is dominated by volatile benzenoid/phenylpropanoids (FVBP), a group of organic compounds originally derived from phenylalanine (5). FVBP emission relies on the availability of precursor compounds that flow from enzymatic reactions in the shikimate pathway and later throughout the FVBP metabolic pathways (Fig. 1A). ODORANT1 (ODO1), a transcriptional activator gene of FVBP gene expression, exhibits a daily oscillatory expression with an evening peak (10). By binding to the promoters of several key enzymes, such as 5-enolpyruvylshikimate 3-phosphate synthase (EPSPS), ODO1 facilitates the introduction of precursor molecules to the FVBP synthesis pathway, suggesting that ODO1 is a master regulator of FVBP emissions in Petunia. Two transcription factors, EMISSION OF BENZENOIDS I (EOB I) and EOBII, up-regulate the expression of ODO1 and other FVBP-related genes (11, 12).
Fig. 1.
The floral volatile emission and expression profiles of the genes in the FVBP pathway. (A) An overview of selected parts of the FVBP pathway. Different colors indicate steps and products that are categorized in shikimate, benzenoid, and phenyl propanoid pathways. Arrows with dashed lines in the pathway are representative of multiple steps between products. Volatile products are presented with asterisks. We analyzed expression patterns of enzyme genes and products in bold. Transcription factors are in orange, and enzymes shown are EPSPS, CM1, ADT, phenylacetaldehyde synthase (PAAS), BPBT, KAT1, S-adenosyl-l-methionine:benzoic acid/salicylic acid carboxyl methyltransferase (BSMT) 1, BSMT2, PAL, CFAT, IGS, and eugenol synthase 1 (EGS). (B–E) Volatile emission data of methyl benzoate (B and D) and benzyl benzoate (C and E) in Petunia in continuous light (B and C) and dark (D and E). (Insets, D and E) Graphs with enlarged y-axes showing the same 32–96 time point results. (F–O) Gene expression patterns of transcription factors and enzymes associated with the FVBP pathway in Petunia in continuous light (F–J) and dark (K–O). The line and symbol color of the graphs corresponds to its placement within the FVBP pathway shown in A. Values are relative to UBIQUITIN (UBQ), and normalized by the average expression values of hours 0–12. Results represent means ± SEM from three biological replicates. White and black bars at the top indicate periods of light and dark, respectively.
Analysis of the ODO1 promoter (12), as well as the promoters of several other regulatory genes in the FVBP pathway, revealed the presence of specific cis-elements referred to as evening elements (EEs). The EE is a binding site for two similar circadian-clock transcription factors, CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY), and a related factor, REVEILLE 8, in Arabidopsis (13). CCA1 and LHY, which are highly expressed during the morning, function as repressors for evening expressed genes (14, 15) and activators for morning expressed genes (16). Thus, we hypothesized that the Petunia ortholog of CCA1/LHY, through the repression of ODO1 and several other regulatory genes, might restrict the emission of floral volatiles to the evening. Increasing levels of CCA1/LHY in the morning represses the expression of genes necessary for volatile synthesis, and as the levels of CCA1/LHY decrease in the evening, it facilitates the induction of ODO1 and downstream FVBP enzyme gene expression. Here we report that the circadian clock regulates scent emission timing through the function of LHY ortholog in Petunia.
Results
The Circadian Clock Regulates Volatile Emission and the Expression of Enzyme Genes in the FVBP Pathway in the Dark.
Previous work has shown that daily light/dark transitions are the predominant cues mediating temporal control of the major floral volatile, methyl benzoate, in Petunia (17). However, emission of the terpenoid compound β-ionone and the expression of its synthase enzyme showed circadian oscillations, particularly in the dark (8), indicating that the influence of the circadian clock on scent emission can be conditional. To more comprehensively assess the involvement of the circadian clock in scent emission, we measured the emission of four major floral volatiles (methyl benzoate, benzyl benzoate, benzaldehyde, and isoeugenol) generated through the FVBP pathway (Fig. 1A) under continuous light and dark conditions (Fig. 1 B–E and Fig. S1 A–D). In continuous light, volatile emissions ceased to oscillate, and moderate levels of emission were maintained for all four analyzed volatiles for 3 d (Fig. 1 B and C and Fig. S1 A and B). In continuous dark, volatile peak strength diminished rapidly (Fig. 1 D and E and Fig. S1 C and D); however, smaller peaks (of approximately one tenth of the size of the peaks that occurred in the light/dark conditions) were seen for all volatiles examined in the two following subjective nights, indicating the presence of circadian timing mechanisms in the dark.
Our current understanding of the transcriptional regulation of the FVBP pathway is limited to a few transcription factors: ODO1, EOBI, and EOBII (10–12). ODO1 exhibits an evening peak (Fig. 1K), whereas EOBI and EOBII exhibit morning peaks (Fig. S1 O and P). To investigate the molecular link between the circadian clock and the FVBP pathway, we next analyzed the expression patterns of these three transcriptional regulators and 12 enzyme-encoding genes in the FVBP pathway under continuous light and dark conditions (Fig. 1 and Fig. S1). All FVBP synthesis genes examined showed strong daily oscillations in light/dark cycles (see the first 24-h pattern in Fig. 1 K–O and Fig. S1 O–X). In continuous light, the expression profiles of almost all genes did not sustain daily oscillatory patterns, and often the expression levels became higher than the trough levels observed in 24-h light/dark conditions (Fig. 1 F–J and Fig. S1 E–N). An exception was the benzoyl-CoA:benzyl alcohol/phenylethanol benzoyltransferase (BPBT) gene, which is expressed in a circadian fashion even in continuous light (Fig. S1L). Overall, their expression patterns resembled the emission profiles of the four volatiles examined under the same conditions (Fig. 1 B and C and Fig. S1 A and B). These results suggest that the circadian clock does not have much influence on the timing expression of the FVBP pathway genes as well as scent emission in continuous light in P. hybrida flowers.
Conversely, in continuous dark, ODO1, direct target genes of ODO1 [EPSPS and phenylalanine ammonia-lyase 1 (PAL) genes (10)], and several other enzyme-coding genes in the shikimate, benzenoid, and phenylpropanoid pathways [e.g., arogenate dehydratase 1, coniferyl alcohol acyltransferase (CFAT), and 3-ketoacyl-CoA thiolase 1 (KAT1) genes] displayed dampened but oscillatory mRNA expression patterns for 3 d (Fig. 1 K–O and Fig. S1 O–X), indicating the contribution of the circadian clock to the expression timing of these genes. EOBI and EOBII did not show any rhythmic expression patterns under these conditions, suggesting that daily rhythmic expression of EOBI and EOBII could largely be regulated by light/dark transitions. Also, this indicates that the daily rhythmic expression of EOBI and EOBII does not control the daily ODO1 expression, even though EOBs are direct activators of ODO1 (11, 12). These results indicate that the circadian clock may contribute in the timing regulation of these genes in the dark in Petunia flowers. In addition, the present work and previous findings led us to hypothesize that the clock’s regulation of volatile production may be modulated by light conditions. Another interesting finding is that ODO1, which directly up-regulates EPSPS and PAL expression (18), is expressed at the same time as EPSPS and PAL, especially in the dark (Fig. 1 K, L, and N). This suggests that additional factors are responsible for controlling the timing of the expression of these genes. We found that the EPSPS promoter possesses the EE cis-element (see Fig. 5B). As the ODO1 (12) and the EPSPS promoters contain EEs, we hypothesized that CCA1/LHY homologs in Petunia may be negative factors that set the expression timing of these genes.
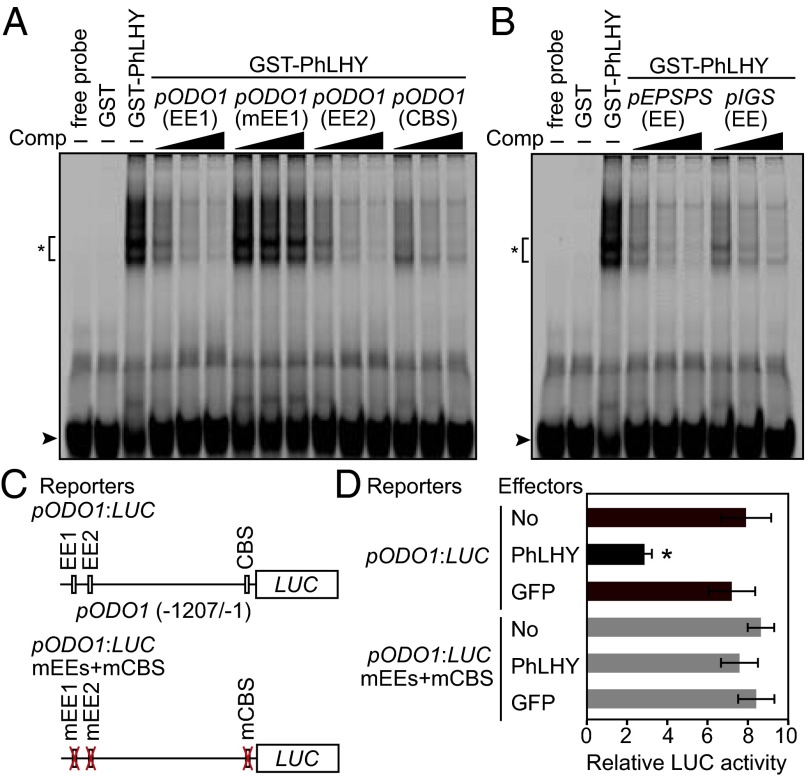
Fig. 5.
PhLHY binds to EEs. (A) An EMSA shows direct interaction of GST-PhLHY with EEs in the ODO1 promoter. The EE1 in the ODO1 promoter (pODO1) was used as a labeled probe. Competition with different concentrations of unlabeled EE1, EE2, and CBS fragments and the mutated EE1 are shown along the top. (B) EMSA of GST-PhLHY with EEs in the EPSPS (pEPSPS) and IGS (pIGS) promoters. For A and B, GST served as a negative control. Asterisks and arrowheads indicate GST-PhLHY/DNA complexes and free probes, respectively. (C) Schematic of reporters used in transient assay. (D) The effect of PhLHY protein on the ODO1 promoter activities. The activities of firefly LUC were normalized by the activities of 35S:Renilla LUC. Results represent means ± SEM of nine independent samples (*P < 0.01 vs. no effector, Student t test).
Identification of LHY Homolog in Petunia.
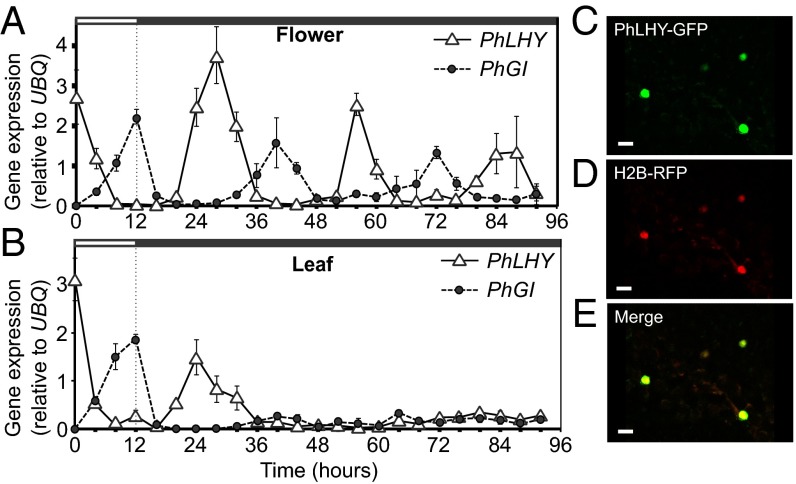
To identify the CCA1/LHY homologous gene in Petunia, we first identified partial sequences that showed homology to Arabidopsis CCA1/LHY from publically available EST databases. After cloning the entire coding region of the cDNA based on the sequences of the EST clones, the entire sequence showed a higher homology to LHY than CCA1; therefore, we named it P. hybrida LHY (PhLHY). By using deduced amino acid sequences, Bayesian posterior probability and maximum-likelihood phylogenetic analyses placed PhLHY nested within the clade of core eudicots—clustered with Nicotiana attenuata LHY (NaLHY) and Solanum lycopersicum (SlLHY) (Fig. S2A). In addition, quantitative PCR (qPCR) analysis of PhLHY expression in Petunia leaves and flowers revealed peak expression at dawn under light/dark conditions (Fig. 2 A and B and Fig. S3 A and B), which is similar to Arabidopsis CCA1/LHY expression patterns (14, 15). To monitor the status of the Petunia circadian clock, the expression patterns of the Petunia homolog of GIGANTEA (PhGI), a clock gene directly regulated by CCA1 (19–21), was also analyzed (Fig. S2C). Similar to the Arabidopsis counterpart, PhGI expression peaks in the evening, demonstrating an antiphasic pattern to PhLHY expression. Interestingly, circadian oscillation of PhLHY and PhGI expression showed distinct patterns in tissue- and light-dependent manners. In Petunia flowers kept in continuous dark, robust oscillation of PhLHY and PhGI was observed for 3 d, whereas the circadian oscillation of PhLHY and PhGI in leaves ceased in the first day in the dark (Fig. 2 A and B). In continuous light, the amplitude of PhLHY expression levels was severely reduced in flowers and leaves, whereas the expression levels of PhGI remained similar to that in the light/dark cycle (Fig. S3 A and B). In addition, PhGI expression levels showed circadian oscillation in leaves, but not in flowers under continuous light conditions. These results indicate the presence of tissue-specific clocks between flowers and leaves in Petunia. Although the circadian oscillation patterns of PhLHY vary depending on tissues and light conditions, the daily expression patterns of PhLHY resemble those of LHY/CCA1.
Fig. 2.
Circadian expression pattern of PhLHY and intracellular localization of PhLHY protein. (A and B) Expression profiles of PhLHY and PhGI in continuous dark over 92 h in Petunia petals (A) and leaves (B). Results represent means ± SEM from three biological replicates. (C–E) PhLHY-GFP is a nuclear localized protein. PhLHY-GFP protein (C) and H2B-RFP protein (reference for nuclei) (D) were expressed in epidermal cells of Petunia petals. (E) Merged image of C and D. (Scale bar: 10 μm.)
Next we examined the intracellular localization of PhLHY protein, as its homologs CCA1 and LHY are nuclear-localized proteins (22, 23). In comparing the intracellular localization patterns of the PhLHY-GFP protein with that of RFP-tagged histone 2B (H2B), PhLHY-GFP localized in the nuclei of Petunia flower and leaf cells (Fig. 2 C–E and Fig. S3 C–E), indicating that PhLHY is a nuclear protein. Based on the sequence similarity, and the temporal and intracellular expression patterns, PhLHY is likely an orthologous gene of Arabidopsis LHY.
PhLHY Maintains Functional Relevance in Arabidopsis.
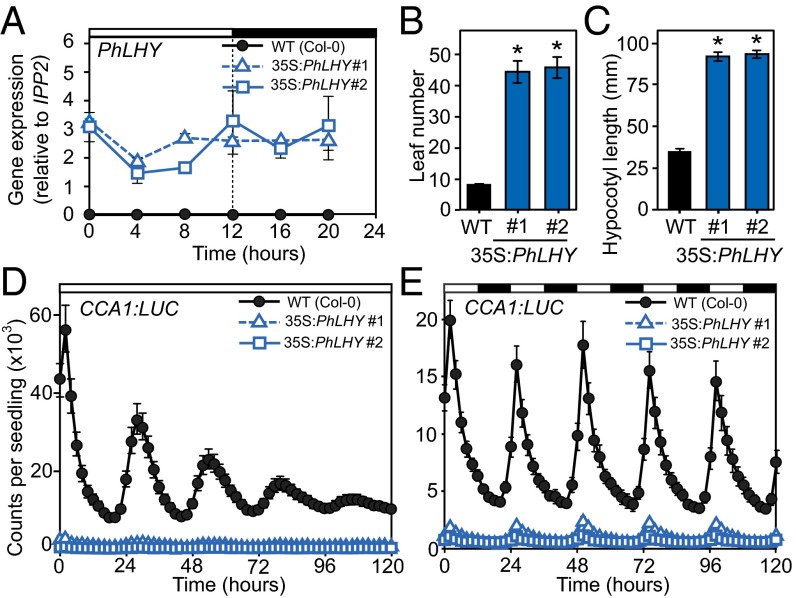
To assess the function of PhLHY, we first tested whether it maintains a similar role with Arabidopsis LHY. PhLHY was overexpressed in Arabidopsis by using the cauliflower mosaic virus (CaMV) 35S promoter (Fig. 3A). PhLHY-overexpressing lines (35S:PhLHY) displayed distinct developmental phenotypes typically observed in Arabidopsis CCA1/LHY overexpressors: a significantly delayed flowering time, and a longer hypocotyl length in comparison with WT plants (Col-0; Fig. 3 B and C) (14, 24). The 35S:PhLHY lines also exhibited a similar clock arrhythmia phenotype to the CCA1/LHY overexpressors under continuous light conditions (14, 24), as measured by a CCA1:luciferase (LUC) reporter (Fig. 3D). In addition, the CCA1:LUC expression in 35S:PhLHY barely responded to the dark-to-light transitions in the morning (Fig. 3E), indicating that the oscillation of circadian clock genes was severely attenuated even under light/dark conditions. Last, we analyzed the expression profiles of the circadian clock genes LHY, CCA1, PSEUDO RESPONSE REGULATOR 9 (PRR9), PRR7, and TIMING OF CAB EXPRESSION 1 (TOC1) in 35S:PhLHY lines. The daily oscillation patterns of these genes became relatively constant in 35S:PhLHY lines throughout the day (Fig. S4). Together, these results imply that PhLHY may possess a similar clock function to CCA1/LHY in Arabidopsis.
Fig. 3.
PhLHY functionally resembles CCA1/LHY in Arabidopsis. (A) Expression of PhLHY in 35S:PhLHY plants under light/dark conditions. (B) Flowering time of 35S:PhLHY lines and WT plants is shown. (*Significant difference vs. WT at P < 0.05, Student t test; n = 16.) (C) Hypocotyl length of 35S:PhLHY lines and WT plants (*P < 0.05; n = 30). (D and E) CCA1:LUC activity as measured by luminescence counts per seedling over 5 d of continuous light (D) and light/dark (E) conditions in a comparison between 35S:PhLHY lines and WT. Results represent means ± SEM (n = 16).
Constitutive Expression of PhLHY Eliminates Floral Volatile Emission in Petunia.
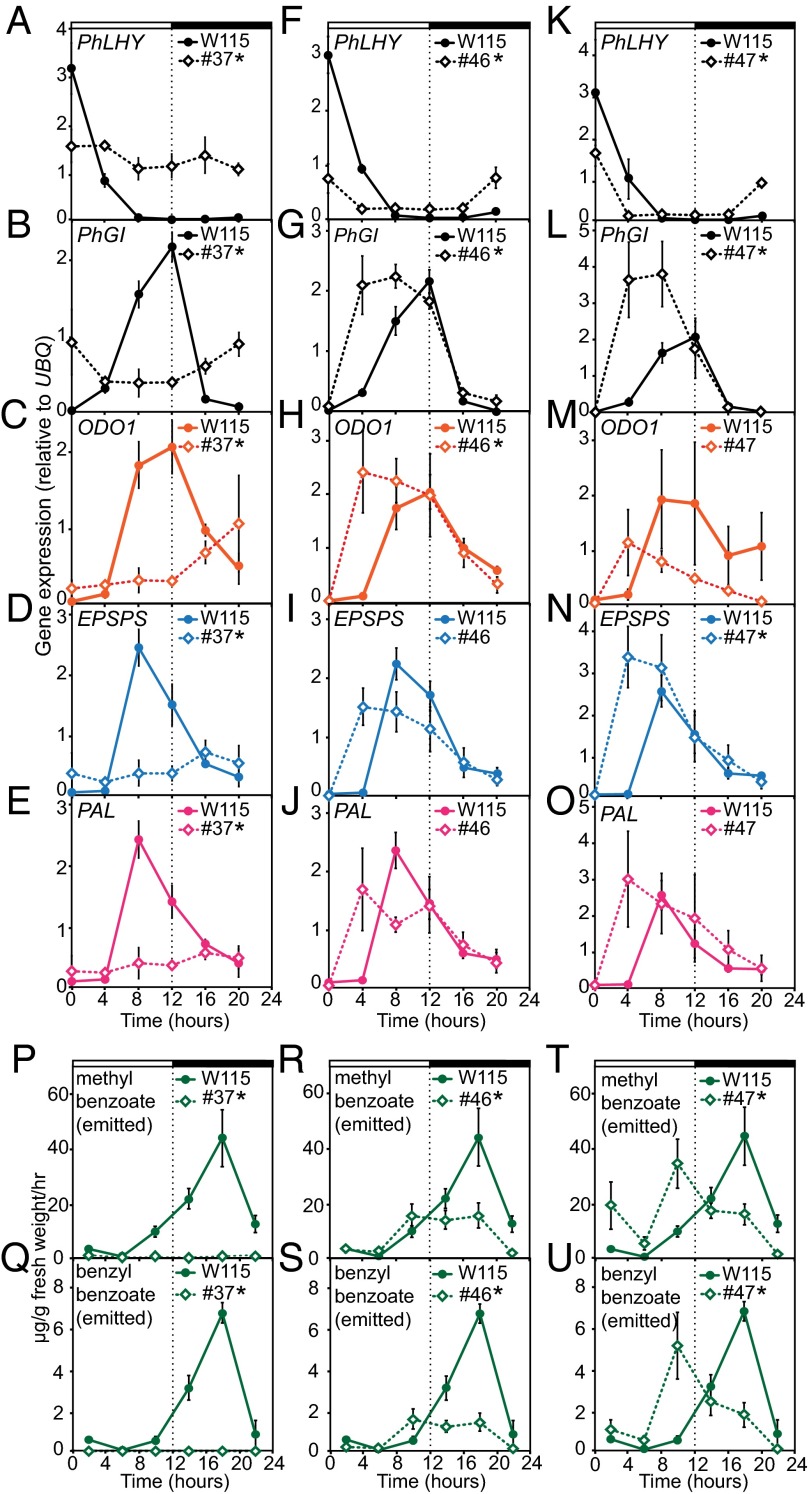
As PhLHY is likely an orthologous gene of Arabidopsis LHY, we next examined PhLHY’s influence on the daily production of floral scent in Petunia. Constitutive expression of PhLHY in Petunia (35S:PhLHY no. 37 line) altered expression patterns of two putative clock gene homologs [PhGI and P. hybrida PSEUDO RESPONSE REGULATOR 5 (PhPRR5; Fig. S2D)] and 15 genes involved in the FVBP pathway in flowers (Fig. 4 A–E and Fig. S5 A–M). The expression levels of the genes [PhGI, PhPRR5, ODO1, EPSPS, chorismate mutase 1 (CM1), arogenate dehydratase 1 (ADT), and PAL], which peak at approximately 8–12 h from the morning in WT plants, became severely reduced and constitutive throughout the day. We further investigated that overexpression of PhLHY severely represses ODO1 promoter activity in vivo. Transient coinfiltration of ODO1 promoter-controlled firefly LUC gene reporter (pODO1:LUC) with 35S:PhLHY (but not with 35S:GFP) suppresses diurnal oscillation patterns of the ODO1 promoter activities in multiple independent Petunia flowers (Fig. S6). Taken together, these expression patterns of evening-expressed genes in 35S:PhLHY no. 37 and in the transient assay were similar to those in CCA1/LHY overexpressors in Arabidopsis (14, 24). Additionally, constitutive expression of PhLHY in Petunia resulted in a near-total decrease in floral volatile production and emission (Fig. 4 P and Q, and Fig. S5 N and O). These results indicate that the PhLHY expression pattern controls the expression patterns of other clock gene homologs and FVBP pathway genes in addition to the scent emission patterns in flowers.
Fig. 4.
PhLHY regulates daily timing of gene expression and volatile emission in the FVBP pathway. (A–O) Daily expression patterns of clock genes and genes encoding proteins in the FVBP pathway in the transgenic line (line 37) with constitutive PhLHY expression (A–E) and in the transgenic lines (lines 46 and 47) with altered PhLHY expression (F–O). Gene expression values were normalized by the average expression values of hours 0–12. (P–U) Daily scent emission patterns of methyl benzoate (P, R, and T) and benzyl benzoate (Q, S, and U) in transgenic line 37 (P and Q) and in transgenic lines 46 and 47 (R–U). Results represent means ± SEM from three biological replicates. The line color of the graphs corresponds to its placement within the FVBP pathway (Fig. 1A; *P < 0.05, daily expression and scent emission patterns of transgenic lines differ significantly from WT Petunia; two-way ANOVA).
Repression of PhLHY Leads to the Early Production of Scent.
Petunia is well known for gene silencing resulting from attempts to express transgenes (25, 26). Our attempt to constitutively express PhLHY also resulted in transgenic plants that exhibited silencing of PhLHY. In the plants with reduced expression levels of PhLHY, the phases of peak expression of PhGI and PhPRR5 as well as most of the FVBP pathway genes responsible for volatile precursor synthesis (EOBI, EOBII, ODO1, EPSPS, CM1, ADT, and PAL), shifted approximately 4–8 h toward the morning (Fig. 4 F–O and Fig. S5 U–AG and AO–BA). This phase-advance phenotype is also observed in cca1 lhy double mutants in Arabidopsis (15, 27). Interestingly, the volatile emission peaks in these plants also occurred at the end of the light period (approximately 8–12 h from the morning) instead of the middle of the night, although the total scent emission levels were slightly lowered in line 46 (Fig. 4 R–U and Fig. S5 AH–HI, BB, and BC). These results imply that PhLHY sets the expression timing of evening-expressed FVBP pathway genes.
PhLHY Binds to the EEs in ODO1, EPSPS, and Isoeugenal Synthase 1 Promoters.
Our results indicated that PhLHY regulates the timing of FVBP pathway genes in Petunia. The ODO1 promoter contains several EEs (12), and we found that the promoter sequences of the evening-expressing EPSPS and morning-expressing isoeugenol synthase 1 (IGS) also contain EEs. Thus, we hypothesized that PhLHY directly binds to these EEs to regulate the temporal expression of these genes. To analyze the direct binding of PhLHY to the EEs, we performed an EMSA of PhLHY (Fig. 5 A and B). The glutathione S-transferase (GST)-fused PhLHY protein specifically bound to the two EEs (EE1 and EE2) and the CCA1-binding site (CBS) (28) derived from the ODO1 promoter, but not to the mutated EE (mEE1; Fig. 5A). In addition, PhLHY also bound to the EEs in EPSPS and IGS promoters (Fig. 5B). We also tested the functional interaction of PhLHY on the EEs and CBS in the ODO1 promoter in vivo by using transient expression assay (Fig. 5C). We found that coinfiltration with 35S:PhLHY specifically reduced luminescence from pODO1:LUC in flowers while having no effect on an activity of the ODO1 promoter with its EE and CBS sites mutated (pODO1:LUC mEEs + mCBS; Fig. 5D), further confirming that PhLHY represses ODO1 by directly binding to its promoter.
Discussion
Identification of the Circadian Clock Gene PhLHY in Petunia.
Our investigation into the clock’s regulation of scent emission implicated a P. hybrida ortholog of CCA1/LHY, which we named PhLHY, as a mechanistic component. Our phylogenetic analysis placed PhLHY as the closest homolog of other Solanaceae LHY homologs (Fig. S2). The structure of this phylogenetic tree also resembled independent tree structures found in separate analyses (29, 30). qPCR analysis revealed rhythmic oscillation in PhLHY expression in Petunia floral tissues in continuous dark (Fig. 2). The daily expression patterns of PhLHY transcripts and the nuclear localization of the PhLHY protein (Fig. 2) provided additional support to the notion that PhLHY acts within Petunia in a manner consistent with its homologs (29, 30).
Arabidopsis PhLHY-overexpressing lines displayed several phenotypes similar to established clock-disrupting CCA1/LHY-overexpressing phenotypes. These lines showed delayed flowering times and elongated hypocotyls compared with WT plants (Fig. 3) (29, 30). In these lines, the disruption of the rhythmic expression of many circadian-clock genes was observed (Fig. 3 and Fig. S4). The disruption of these rhythms by PhLHY overexpression suggests that the function of PhLHY is similar to LHY and CCA1, in which overexpression caused general impairment of clock function (29, 30). As seen in numerous examples in animals and plants, clock components are often a part of a negative autoregulatory feedback loop in which the protein products of clock component genes suppress the expression of their own genes (29, 30). By comparing the CCA1:LUC luminescence and CCA1 and LHY expression patterns of Arabidopsis PhLHY overexpressors and WT plants under continuous light and light/dark conditions, PhLHY overexpression depressed the rhythmic circadian oscillation of LHY and CCA1 (Fig. 3 and Fig. S4). This same pattern of feedback regulation on LHY and CCA1 was observed under conditions in which LHY and CCA1 were overexpressed separately (29, 30), further supporting that the function of PhLHY resembles that of LHY and CCA1, and that PhLHY encodes a core clock component in Petunia.
PhLHY Regulates the Daily Timing of the FVBP Pathway.
ODO1 is a key transcriptional regulator for many steps in the FVBP pathway (10). ODO1 regulates fragrance biosynthesis by administering the flux of precursor molecules through the shikimate pathway (5). Although ODO1 exhibits rhythmic expression that peaks in the evening, its peak of expression closely mirrors that of the known ODO1 target genes (Fig. 1). This coincidence of expression suggests that ODO1 is not wholly responsible for the precise timing of FVBP biosynthesis.
Under light/dark conditions, PhLHY morning expression is antiphasic to the evening expression of many FVBP-related genes including ODO1, EPSPS, and PAL (Fig. 4). Constitutive expression of PhLHY resulted in the abolishment of nearly all FVBP emission (Fig. 4 and Fig. S5) and suppressed most of the known genes responsible for the synthesis of FVBPs (Fig. 4 and Figs. S5 and S6). Under constitutive expression of PhLHY, BPBT and BSMT expression levels were increased (Fig. S5). As ODO1 RNAi knockdown lines also showed increased levels of BPBT and BSMT expression (10), this phenotype might be induced by the low expression level of ODO1, which was caused by constitutive PhLHY expression. In PhLHY-suppressed lines, the peak expression of many FVBP genes examined shifted from late afternoon to earlier in the day (Fig. 4 and Fig. S5). This shift in peaks was seen most clearly in the precursor level of FVBP synthesis, with distinct shifts occurring in EOBI, EOBII, ODO1, EPSPS, CM1, ADT, and PAL. These data clearly suggest that PhLHY regulates the timing of FVBP emission by temporally controlling the expression profiles of enzyme-encoding genes that affect the synthesis of FVBP precursors. Furthermore, PhLHY directly bound to the EE and/or CBS cis-elements in the promoters of ODO1, EPSPS, and IGS (Fig. 5), indicating that PhLHY is likely setting the phase of ODO1 and EPSPS expression by inhibition (and IGS through activation) during the early portion of the day. Based on the similarities of the expression patterns, our expression analyses also suggest that PhLHY may interact with the promoters of other FVBP genes (in perhaps both suppression and activation).
Our analysis of endogenous FVBP compound concentrations (Fig. S5) revealed that the temporal availability of endogenous compounds mirror the external emission levels of those same compounds. These findings support previous evidence that emission of floral volatiles is simply based on diffusion, and regulated primarily at the synthesis level (31, 32).
Petunia Likely Possesses a Tissue-Specific Clock that Regulates the FVBP Pathway.
Another interesting result was the apparent tissue specificity of the clock homolog expression in Petunia. PhLHY and PhGI show circadian rhythmic expression in flower tissue during continuous dark conditions, but not in continuous light. Correspondingly, a small but significant oscillation is observed in many FVBP genes also only during continuous dark. In leaf tissue, no circadian rhythm was present in continuous dark, but the expression profile of PhGI (but not that of PhLHY) shows a strong daily oscillation in continuous light. To regulate the robust oscillation of PhGI in leaves, clock components other than PhLHY may be involved. These results clearly indicate that P. hybrida plants likely possess circadian clocks that have different properties in flowers and leaves. Although tissue specificity of the circadian clock has been shown in Arabidopsis previously (33–35), current examples in other plant species are still limited (36). Of note is the fact that the common study organism for FVBP research, P. hybrida, is a hybrid of Petunia axillaris and Petunia integrifolia. P. axillaris shows robust oscillation of floral scent in continuous light conditions, but P. integrifolia does not (37). This indicates that the light- and tissue-specific clock phenotypes observed in P. hybrida might be caused by mix of features of P. axillaris and P. integrifolia circadian clocks. This also suggests that there may be different characteristics in the molecular clocks even among Petunia species. Although robust circadian rhythms do not seem to persist in P. hybrida’s volatile release under continuous light conditions, the strong effect PhLHY has on FVBP synthesis under light/dark cycle conditions clearly indicates the importance of the clock to the timing of floral emissions in a real world in this species. Together with the light-dependent tissue specificity of rhythmic gene expression, it appears that light and the circadian clock work in concert to regulate floral volatile emissions in Petunia.
In summary, we describe what is, to our knowledge, the first molecular-level evidence that the clock component, PhLHY, plays an important role in controlling the timing of volatile synthesis and emission in Petunia. During an early part of the day, PhLHY represses the expression of many enzyme-encoding genes in the FVBP biosynthesis pathway, primarily in the upstream area of the pathway responsible for precursor availability. It is also possible that PhLHY is up-regulating certain morning-peaked FVBP genes, such as IGS. Further identification and analysis of FVBP regulatory components is required to provide a more precise level of control within the FVBP metabolic pathway. A fuller comprehension of the regulatory network surrounding the FVBP pathway will allow us to not only regulate the abundance of floral volatiles, but the period of emission as well. Such manipulation would allow us to create “designer crops” whose emissions could be adjusted to suit the temporal availability of local pollinators.
Materials and Methods
A full description of the materials and methods is provided in SI Materials and Methods. In brief, we created transgenic Petunia plants through tissue culture transformation by using Agrobacterium tumefaciens. Gene expression was measured through qPCR analysis, and all primer information for qPCR in this research is provided in Table S1. Volatile emissions were collected from live plants in a custom-designed apparatus (Fig. S7) and analyzed by GC/MS. Physical binding of PhLHY to EEs was confirmed by using EMSA and transient LUC assays.
Supplementary Material
Acknowledgments
We thank D. Clark for P. hybrida seeds; N. Dudareva and A. Klempien for transformation protocols; D. Ewing for Petunia care/horticulture advice; B. Medina, M. Clifford, and J. Villanueva for volatile work help; and J. Chau for phylogeny assistance. This work was supported by National Institutes of Health (NIH) Training Grant T32GM007270 (to M.P.F.), National Science Foundation Grant IOS-1354159 (to J.A.R.), and NIH Grant GM079712 (to T.I.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. KP017483).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1422875112/-/DCSupplemental.
References
- 1.Harmer SL. The circadian system in higher plants. Annu Rev Plant Biol. 2009;60:357–377. doi: 10.1146/annurev.arplant.043008.092054. [DOI] [PubMed] [Google Scholar]
- 2.Covington MF, Maloof JN, Straume M, Kay SA, Harmer SL. Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biol. 2008;9(8):R130. doi: 10.1186/gb-2008-9-8-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dodd AN, et al. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309(5734):630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- 4.Kinmonth-Schultz HA, Golembeski GS, Imaizumi T. Circadian clock-regulated physiological outputs: Dynamic responses in nature. Semin Cell Dev Biol. 2013;24(5):407–413. doi: 10.1016/j.semcdb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muhlemann JK, Klempien A, Dudareva N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014;37(8):1936–1949. doi: 10.1111/pce.12314. [DOI] [PubMed] [Google Scholar]
- 6.Loughrin JH, Hamilton Kemp TR, Andersen RA, Hildebrand DF. Circadian rhythm of volatile emission from flowers of Nicotiana sylvestris and N. suaveolens. Physiol Plant. 1991;83(3):492–496. [Google Scholar]
- 7.Kolosova N, Gorenstein N, Kish CM, Dudareva N. Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. Plant Cell. 2001;13(10):2333–2347. doi: 10.1105/tpc.010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simkin AJ, et al. Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of β-ionone, a fragrance volatile of petunia flowers. Plant Physiol. 2004;136(3):3504–3514. doi: 10.1104/pp.104.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerats T, Strommer J. Petunia: Evolutionary, Developmental And Physiological Genetics. Springer; New York: 2008. [Google Scholar]
- 10.Verdonk JC, Haring MA, van Tunen AJ, Schuurink RC. ODORANT1 regulates fragrance biosynthesis in petunia flowers. Plant Cell. 2005;17(5):1612–1624. doi: 10.1105/tpc.104.028837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spitzer-Rimon B, et al. The R2R3-MYB-like regulatory factor EOBI, acting downstream of EOBII, regulates scent production by activating ODO1 and structural scent-related genes in petunia. Plant Cell. 2012;24(12):5089–5105. doi: 10.1105/tpc.112.105247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Moerkercke A, Haring MA, Schuurink RC. The transcription factor EMISSION OF BENZENOIDS II activates the MYB ODORANT1 promoter at a MYB binding site specific for fragrant petunias. Plant J. 2011;67(5):917–928. doi: 10.1111/j.1365-313X.2011.04644.x. [DOI] [PubMed] [Google Scholar]
- 13.Hsu PY, Devisetty UK, Harmer SL. Accurate timekeeping is controlled by a cycling activator in Arabidopsis. eLife. 2013;2:e00473. doi: 10.7554/eLife.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaffer R, et al. The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell. 1998;93(7):1219–1229. doi: 10.1016/s0092-8674(00)81465-8. [DOI] [PubMed] [Google Scholar]
- 15.Mizoguchi T, et al. LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev Cell. 2002;2(5):629–641. doi: 10.1016/s1534-5807(02)00170-3. [DOI] [PubMed] [Google Scholar]
- 16.Farré EM, Harmer SL, Harmon FG, Yanovsky MJ, Kay SA. Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr Biol. 2005;15(1):47–54. doi: 10.1016/j.cub.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 17.Underwood BA, et al. Ethylene-regulated floral volatile synthesis in petunia corollas. Plant Physiol. 2005;138(1):255–266. doi: 10.1104/pp.104.051144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Moerkercke A, Galván-Ampudia CS, Verdonk JC, Haring MA, Schuurink RC. Regulators of floral fragrance production and their target genes in petunia are not exclusively active in the epidermal cells of petals. J Exp Bot. 2012;63(8):3157–3171. doi: 10.1093/jxb/ers034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fowler S, et al. GIGANTEA: A circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J. 1999;18(17):4679–4688. doi: 10.1093/emboj/18.17.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park DH, et al. Control of circadian rhythms and photoperiodic flowering by the Arabidopsis GIGANTEA gene. Science. 1999;285(5433):1579–1582. doi: 10.1126/science.285.5433.1579. [DOI] [PubMed] [Google Scholar]
- 21.Lu SX, et al. CCA1 and ELF3 Interact in the control of hypocotyl length and flowering time in Arabidopsis. Plant Physiol. 2012;158(2):1079–1088. doi: 10.1104/pp.111.189670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yakir E, et al. Posttranslational regulation of CIRCADIAN CLOCK ASSOCIATED1 in the circadian oscillator of Arabidopsis. Plant Physiol. 2009;150(2):844–857. doi: 10.1104/pp.109.137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu SX, Knowles SM, Andronis C, Ong MS, Tobin EM. CIRCADIAN CLOCK ASSOCIATED1 and LATE ELONGATED HYPOCOTYL function synergistically in the circadian clock of Arabidopsis. Plant Physiol. 2009;150(2):834–843. doi: 10.1104/pp.108.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang ZY, Tobin EM. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell. 1998;93(7):1207–1217. doi: 10.1016/s0092-8674(00)81464-6. [DOI] [PubMed] [Google Scholar]
- 25.Napoli C, Lemieux C, Jorgensen R. Introduction of a chimeric chalcone synthase gene into Petunia results in reversible co-suppression of homologous genes in trans. Plant Cell. 1990;2(4):279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Krol AR, Mur LA, Beld M, Mol JN, Stuitje AR. Flavonoid genes in petunia: Addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell. 1990;2(4):291–299. doi: 10.1105/tpc.2.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alabadí D, Yanovsky MJ, Más P, Harmer SL, Kay SA. Critical role for CCA1 and LHY in maintaining circadian rhythmicity in Arabidopsis. Curr Biol. 2002;12(9):757–761. doi: 10.1016/s0960-9822(02)00815-1. [DOI] [PubMed] [Google Scholar]
- 28.Wang ZY, et al. A Myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. Plant Cell. 1997;9(4):491–507. doi: 10.1105/tpc.9.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takata N, et al. Molecular phylogeny and expression of poplar circadian clock genes, LHY1 and LHY2. New Phytol. 2009;181(4):808–819. doi: 10.1111/j.1469-8137.2008.02714.x. [DOI] [PubMed] [Google Scholar]
- 30.Okada R, et al. Functional characterization of CCA1/LHY homolog genes, PpCCA1a and PpCCA1b, in the moss Physcomitrella patens. Plant J. 2009;60(3):551–563. doi: 10.1111/j.1365-313X.2009.03979.x. [DOI] [PubMed] [Google Scholar]
- 31.Oyama-Okubo N, et al. Emission mechanism of floral scent in Petunia axillaris. Biosci Biotechnol Biochem. 2005;69(4):773–777. doi: 10.1271/bbb.69.773. [DOI] [PubMed] [Google Scholar]
- 32.Kondo M, Oyama-Okubo N, Ando T, Marchesi E, Nakayama M. Floral scent diversity is differently expressed in emitted and endogenous components in Petunia axillaris lines. Ann Bot (Lond) 2006;98(6):1253–1259. doi: 10.1093/aob/mcl212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thain SC, Murtas G, Lynn JR, McGrath RB, Millar AJ. The circadian clock that controls gene expression in Arabidopsis is tissue specific. Plant Physiol. 2002;130(1):102–110. doi: 10.1104/pp.005405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.James AB, et al. The circadian clock in Arabidopsis roots is a simplified slave version of the clock in shoots. Science. 2008;322(5909):1832–1835. doi: 10.1126/science.1161403. [DOI] [PubMed] [Google Scholar]
- 35.Endo M, Shimizu H, Nohales MA, Araki T, Kay SA. Tissue-specific clocks in Arabidopsis show asymmetric coupling. Nature. 2014;515(7527):419–422. doi: 10.1038/nature13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SG, Yon F, Gaquerel E, Gulati J, Baldwin IT. Tissue specific diurnal rhythms of metabolites and their regulation during herbivore attack in a native tobacco, Nicotiana attenuata. PLoS ONE. 2011;6(10):e26214. doi: 10.1371/journal.pone.0026214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoballah ME, et al. The composition and timing of flower odour emission by wild Petunia axillaris coincide with the antennal perception and nocturnal activity of the pollinator Manduca sexta. Planta. 2005;222(1):141–150. doi: 10.1007/s00425-005-1506-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.