Significance
Reinfection after treatment is a problem that plagues efforts to control parasites with complex transmission pathways, such as schistosomiasis, which affects at least 220 million people worldwide and requires an obligate snail intermediate host. Our study highlights a potential ecological solution to this global health problem: We show that a species of river prawn indigenous to the west coast of Africa, Macrobrachium vollenhovenii, could offer a low-cost, sustainable form of snail control that, when used in synergy with existing drug distribution campaigns, could reduce or locally eliminate the parasite. Biological conservation does not always benefit human health, but our results show that where it does, it could provide a win-win outcome for humans and nature.
Keywords: disease, ecology, control, elimination, neglected tropical disease
Abstract
Eliminating human parasitic disease often requires interrupting complex transmission pathways. Even when drugs to treat people are available, disease control can be difficult if the parasite can persist in nonhuman hosts. Here, we show that restoration of a natural predator of a parasite’s intermediate hosts may enhance drug-based schistosomiasis control. Our study site was the Senegal River Basin, where villagers suffered a massive outbreak and persistent epidemic after the 1986 completion of the Diama Dam. The dam blocked the annual migration of native river prawns (Macrobrachium vollenhoveni) that are voracious predators of the snail intermediate hosts for schistosomiasis. We tested schistosomiasis control by reintroduced river prawns in a before-after-control-impact field experiment that tracked parasitism in snails and people at two matched villages after prawns were stocked at one village’s river access point. The abundance of infected snails was 80% lower at that village, presumably because prawn predation reduced the abundance and average life span of latently infected snails. As expected from a reduction in infected snails, human schistosomiasis prevalence was 18 ± 5% lower and egg burden was 50 ± 8% lower at the prawn-stocking village compared with the control village. In a mathematical model of the system, stocking prawns, coupled with infrequent mass drug treatment, eliminates schistosomiasis from high-transmission sites. We conclude that restoring river prawns could be a novel contribution to controlling, or eliminating, schistosomiasis.
Multiply just (US) $0.32, the annual cost to treat a child infected with schistosomiasis (1), by the 114 million children requiring treatment (2), and cheap drug-based treatment can become too costly to sustain. Drug-based control programs for human helminth diseases, termed preventive chemotherapy, have led to spectacular improvements in health and reductions of many worm infections (3). The success has led to new challenges in devising strategies to eliminate these parasites over the long-term, sometimes called the “end game” of infectious disease control (4). For schistosomiasis, this new focus on elimination has sparked a debate over the best strategies to complement drug treatment and interrupt the transmission cycle (5–7). In its May 2012 resolution to eliminate schistosomiasis (World Health Assembly Resolution 65.21) (8), the World Health Assembly called for new procedures to interrupt transmission. Here, we offer evidence that the restoration of a natural predator of the obligate snail hosts of schistosomiasis could be an effective strategy to eliminate disease when performed along with drug treatment. We present the results of a recent pilot program to control schistosomiasis after the construction of the Diama Dam on the Senegal River as an example.
The Diama Dam is only 18 m high, but that is enough to keep the tide from pushing brackish water up the Senegal River. The impounded river is now a stable reservoir of freshwater for people in Dakar and Saint-Louis, Republic of Senegal, and for irrigating surrounding farmland. Unfortunately, just after the dam was built in 1986, an unprecedented, massive, and persistent schistosomiasis epidemic swept through the villages along the river and its tributaries (9, 10). As some had predicted (11), the dam created an ideal freshwater habitat for the snail intermediate hosts of schistosomiasis by reducing flows and saltwater intrusion and increasing algal and plant growth (12). Moreover, the dam extirpated a chief snail predator (13), whose role in the control of schistosomiasis is the focus of our current study.
Schistosomiasis infects an estimated 220–240 million people globally, and 790 million are at risk for infection, more than 90% of whom are in Sub-Saharan Africa (14). Infected humans contaminate water sources with urine or feces containing schistosome eggs that release larvae (miracidia) infectious to snails in the genus Biomphalaria (for Schistosoma mansoni) or Bulinus (for Schistosoma hematobium) (Fig. 1). Each infected snail sheds thousands of cercariae, which seek and penetrate human skin. After entering the skin, the parasites migrate to the blood vessels of the intestines (S. mansoni) or urinary bladder (S. hematobium), where female worms lay 350–2,200 eggs per day (15). Eggs have sharp spines that promote passage through the tissues across the intestines or the urinary bladder. Nonetheless, many eggs do not complete their passage, lodging in the liver, bladder, or other organs, where they trigger chronic inflammatory processes (16). Death from liver failure or bladder cancer can be preceded by chronic anemia, cognitive impairment in children, growth stunting, infertility, and a higher risk of contracting HIV in women (17, 18). These effects, combined with the poverty of its victims, make schistosomiasis one of the world’s most important, but most neglected, human diseases (19). Three decades after the Diama Dam’s completion, schistosomiasis is still the chief health concern among the region’s rural poor (20).
Fig. 1.
(A) Adult M. vollenhovenii prawn. (B) Evidence of prawn predation via characteristic damage on snail shells (arrows). (C) Net enclosure for prawns at Lampsar village.
Our current research was inspired by an experimental study showing persistent and cost-effective schistosomiasis control in Kenyan villages following snail predation by the exotic North American crayfish (Procambarus clarkii) (21). Although crayfish are not present in Senegal, a large river prawn, Macrobrachium vollenhovenii (Fig. 1), is native to the Atlantic coast of Africa and was reported in fishery catches before the construction of the Diama Dam in Senegal (22). River prawns, like crayfish, feed on the snails that transmit schistosomiasis (Fig. 1), and, as reported in laboratory experiments, captive prawns can control snail abundance (23).
Ecological theory supports the idea that the river prawn can eliminate the parasite. The prawn acts as an “intraguild predator” of the schistosome because it both preys on the larval worm when eating infected snails and competes with it by eating the parasite’s host. An intraguild predator can extirpate an intraguild prey, such as the schistosome, when the intraguild predator, like the river prawn, is a generalist and its competitor prey, the larval schistosome, is a specialist (24).
The natural history of the region also supports the hypothesis that river prawns can control snails. Before the dam, when river prawns were more common, human schistosomiasis prevalence was low (25). The Diama Dam impeded the annual downstream female migration to the estuary and blocked the upstream return of larvae, after which the river prawn fishery collapsed (13, 23). Prawn extirpation upriver of the dam was concurrent with a dramatic escalation in the prevalence and intensity of human schistosomiasis in the Lower Senegal River Basin (9, 10). It is plausible that the consequent release of snail populations from predation contributed to snail population expansion and to the schistosomiasis epidemic. If so, restoring prawn populations could reduce snail populations and help curb schistosomiasis transmission. Moreover, this hypothesis raises three questions: (i) Can prawns reduce snail abundance at a village water-contact site as they do in aquaria, (ii) can snail population reduction by prawns control schistosomiasis in humans, and (iii) how might the combination of prawn stocking and chemotherapy affect snail populations and parasite elimination?
We addressed these questions with the combination of a field experiment and a mathematical model (Materials and Methods). Logistical constraints limited our field experiment to a single control and experimental village (we discuss the limitations imposed by lack of replication below). Briefly, after recruiting two similar villages upriver of the dam, screening participants for schistosomiasis, and confirming (through trapping) that prawns were scarce in nature (13), we stocked prawns at the downstream village in the pair into a 10-m × 20-m net that enclosed an opening in the reeds along the shoreline that villagers used to access the river (Fig. 1). The other village was a control site without prawns. Then, we tracked snail infection prevalence and abundance at these two sites over 18 mo after adding prawns. Unfortunately, there were no comparable snail data collected before the prawn intervention. After treating the village residents enrolled in the study at both intervention and control sites with the anthelminthic praziquantel in three follow-up visits, we measured schistosomiasis reinfection rates. Finally, we created a mathematical model, parameterized with independent data derived from the literature, as well as published and unpublished laboratory data, to simulate the effect of prawn stocking on schistosome transmission dynamics and to compare model outcomes with those outcomes observed in the field.
Results
Prawn Enclosure Experiment.
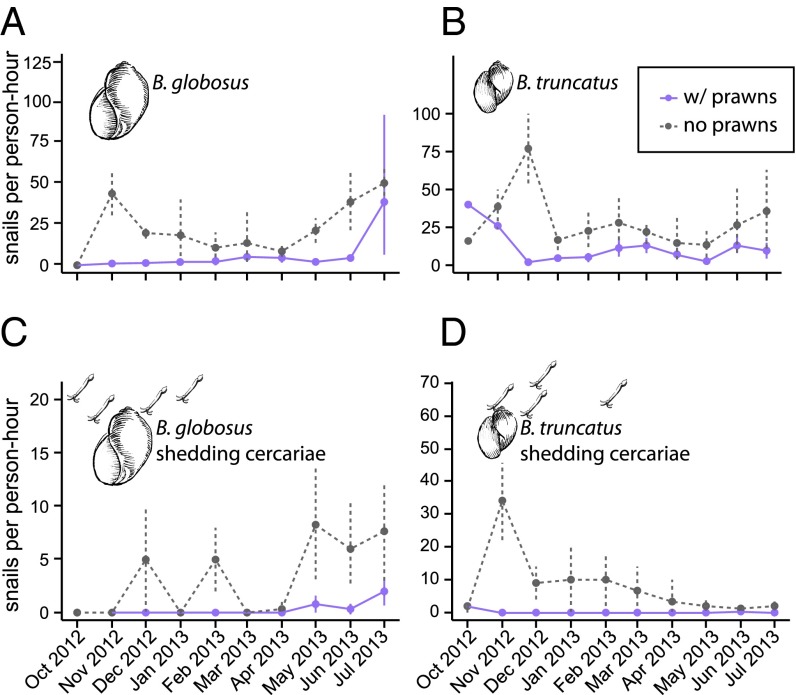
Snails were less abundant in the prawn enclosure after prawns were added (intervention village) than at the control village without prawns (Fig. 2). There were ∼50% fewer Bulinus spp. snails at the village where we added prawns compared with the control village (mixed effects Poisson regression with time as a random effect, P < 0.0001). More importantly from a human health risk perspective, there were ∼80% fewer schistosome-infected (shedding) Bulinus spp. snails (mixed effects Poisson regression with time as a random effect, P = 0.0001). The results were similar for both Bulinus globosus and Bulinus truncatus.
Fig. 2.
Relative density of snails after prawns were installed at the intervention site (w/prawns) and control site (no prawns) from October 2012 to July 2013; (A) total Bulinus globosus, (B) total Bulinus truncatus, (C) B. globosus shedding schistosome cercariae, and (D) B. truncatus shedding schistosome cercariae.
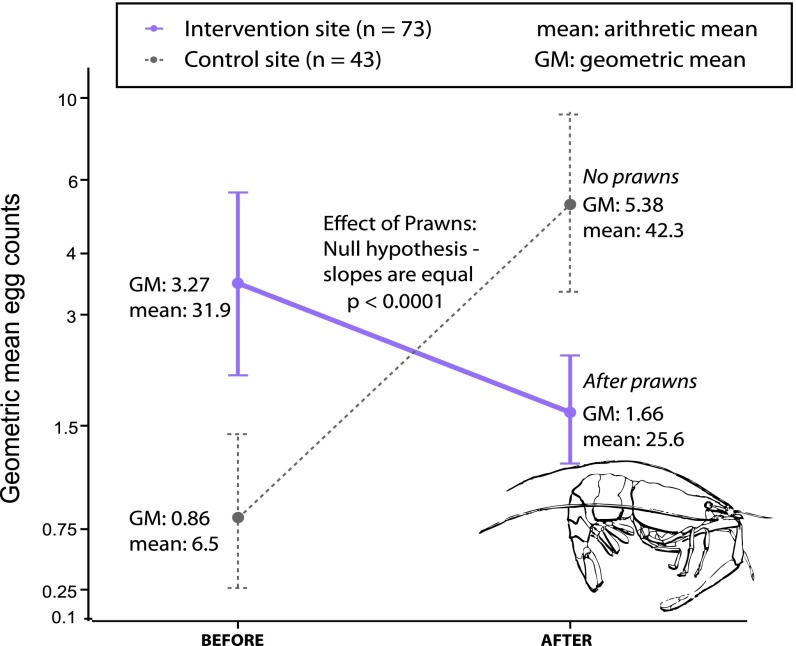
Praziquantel treatment, especially when given in two consecutive doses a few weeks apart, results in high cure rates and egg reduction rates for schistosome parasites (26). Thus, treatment presumably cured many participants, after which some were reinfected. Although the village where we added prawns started out with a significantly higher schistosome prevalence (proportion of individuals infected) in humans [before prawns, odds ratio (OR) intervention/control = 5.2, 95% confidence interval (CI): 2.2–12.2], reinfection prevalence was lower than in the control village after prawns were stocked (OR intervention/control = 0.27, 95% CI: 0.12–0.60; Table 1). Results were similar for heavy infections (Table 1), defined for S. hematobium as >50 eggs per 10 mL of urine, with a lower prevalence of heavy reinfection among people living at the prawn site during all follow-up visits (OR intervention/control = 0.22, 95% CI: 0.08–0.61). The statistical difference in reinfection prevalence between the villages is best shown as a significant interaction between village and time in the before-after-control-impact (BACI) analysis (P < 0.0001). Even more important are the results for our proxy of infection intensity (number of eggs in the urine; Fig. 3 and Table 1), which is the best predictor of human disease (27). That is, despite having an initially higher egg burden before adding prawns, villagers had significantly lower egg burdens after we stocked prawns compared with controls at all follow-up time points (BACI interaction term, P < 0.0001; Fig. 3). These results, although not proof of a prawn effect per se, are consistent with the hypothesis that prawns, by reducing snail abundance, can reduce reinfection rates in humans.
Table 1.
S. hematobium prevalence and infection intensity among study participants
| Site type | Variable | Baseline | 18 mo | Change, % |
| Intervention (prawn) site | Prevalence | 64% | 58% | −9 |
| Heavy infection (>50 eggs per 10 mL) | 11% | 6% | −46 | |
| Eggs/10 mL urine (AM) | 31.9 | 10.2 | −68 | |
| Eggs/10 mL urine (GM) | 3.27 | 2.9 | −11.3 | |
| Control site | Prevalence | 30% | 78% | +160 |
| Heavy infection (>50 eggs per 10 mL) | 4% | 12% | +200 | |
| Eggs/10 mL urine (AM) | 6.5 | 18.3 | +181 | |
| Eggs/10 mL urine (GM) | 0.86 | 6.1 | +616 |
AM, arithmetic mean; GM, geometric mean.
Fig. 3.
BACI of prawns on the intensity of S. hematobium infection (GM egg burdens) among participants at the intervention and control sites.
Mathematical Model.
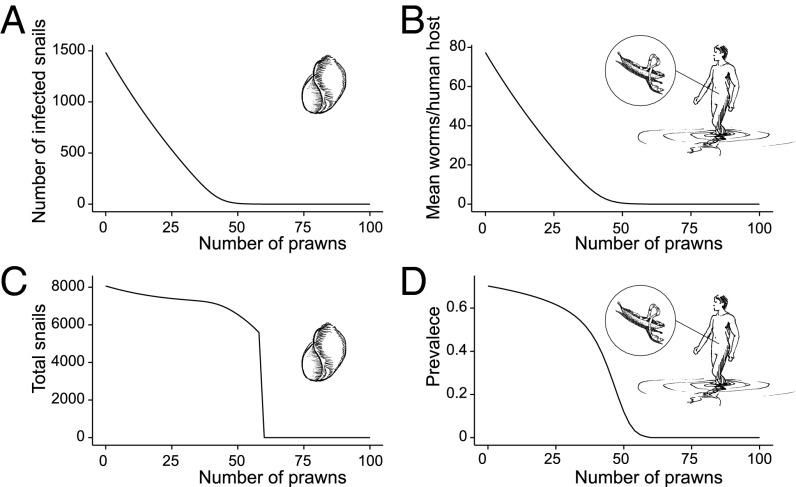
The mathematical model revealed three categorical outcomes resulting from increasing prawn stocking density, consistent with models of generalist intraguild predation (24): (i) reduced disease in humans with increasing prawn density, (ii) parasite extirpation from the snail population, and (iii) snail extirpation. These states switch at specific prawn densities. At low prawn densities (as in the field experiment), each added prawn reduces infected snail density through intraguild predation on schistosomes (Fig. 4). The decline in infected snail density leads to a similar decline in the worm burdens in humans (Fig. 4B) even though the infection prevalence in humans declines little (Fig. 4 A and C), just as we observed in the field. Above 0.3 prawns per square meter (range: 0.16–0.9; Fig. S1), intraguild predation eliminates the parasite from the snail population because prawns eat newly infected snails before they can shed infective cercariae. Ironically, the decline in human egg burdens releases uninfected snails from parasitic castration; therefore, although the abundance of infected snails decreases, the number of susceptible snails may increase, peak, and then decrease at increasing prawn densities. A second critical threshold in prawn density, 0.6 prawns per square meter (range: 0.25–2.0) exists above which prawns locally extirpate the snail population in the model (Fig. S1), much as in laboratory experiments, where prawns cause recruitment failure in the snail population (23).
Fig. 4.
Effect of prawn density on number of shedding snails (A), human worm burden (B), total snail population (C), and human infection prevalence (D) in response to an average density of prawns ranging from 0 to 100 prawns per 200 m2.
Prawns are able to persist in the system after extirpating snails because they can switch to other foods as snails become rare. The gap between the first and second critical densities widens as the prawn-free R0, defined here as the expected number of adult parasites generated per adult parasite (p. 138 in ref. 28), or attack rate of prawns toward snails decreases (Figs. S2 and S3). In every scenario, prawns have their strongest effect on infected snail density, and fewer infected snails translates to a lower parasite burden in villagers.
The parameterized model (Table S1) found a good fit between observed and predicted differences in disease prevalence (−18% vs. −24%, respectively) and egg burden (−50% vs. −57%, respectively) in villagers at the intervention and control villages (Table S2). By including seasonality in snail population growth, the model was further able to reproduce the seasonal reinfection patterns observed in the field (Fig. S4).
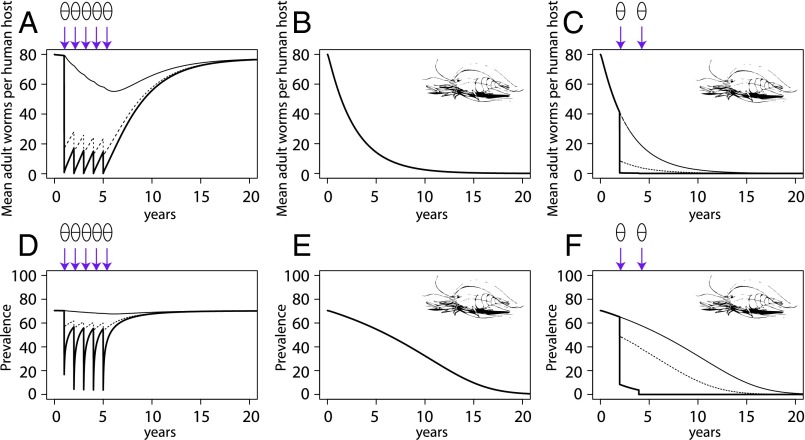
The correspondence between field patterns and model predictions gave us the confidence to use the model to explore hypothetical control scenarios. Specifically, we were interested in comparing the control achieved by mass drug administration (MDA) of praziquantel, by prawn stocking, or by a combination of both. Modeling MDA by itself (Fig. 5 A and D) first reduces human prevalence and worm burden in the treated population (i.e., those individuals who received praziquantel, which was 80% of the total population in this simulation) and then slowly reduces infection in the untreated population. However, in this high-transmission scenario, without continual MDA treatment, prevalence and worm burden return to baseline levels within 5–10 y. In the prawns-only example, prawns maintained at 0.25 per square meter (Fig. 5 B and E) can eradicate schistosomiasis without MDA, but only after stocking prawns for 20 y. In contrast to prawns or MDA alone, the integration of prawn stocking with MDA leads to a rapid decline in disease (<5 y) and local extirpation of the parasite from the snails (Fig. 5 C and F).
Fig. 5.
Trajectory of human schistosomiasis worm burdens and prevalence in a local population of 1,000 individuals under three control scenarios: MDA using praziquantel alone yearly for 5 y with 80% coverage (A and D), prawns maintained at a density of 0.25 per square meter for 20 y (B and E), or prawns maintained at a density of 0.25 per square meter and MDA applied in years 2 and 4 only (C and F). Thick, solid lines indicate the treated population (those participants who received praziquantel); thin, solid lines indicate the untreated population (those participants who did not receive praziquantel); and dashed lines indicate the mean population (including treated and untreated individuals). Arrows show the timing of MDA.
Discussion
Adding prawns to a village water contact area was associated with a subsequent decline in snail densities and reduced schistosomiasis transmission. Although the trajectories of the two villages are consistent with a prawn stocking effect, unseen differences between the villages could have led to changes in snail populations and reinfection, including changes in snail habitat, human behavior, or outside medical care, or it is possible that the prawn enclosure reduced snail abundance for reasons other than prawn stocking. Despite these alternative explanations, the results of the prawn stocking experiment in Senegal were consistent with (i) laboratory experiments showing that river prawns can control snail populations (23), (ii) Kenyan field experiments showing that crayfish reduce snail populations and human reinfection (21), and (iii) the predictions of our simple mechanistic model of schistosomiasis transmission. For these reasons, and assuming that our study villages are characteristic of the region, restoring M. vollenhovenii prawns to the Senegal River system could benefit villagers now subject to chronic schistosomiasis. The model indicates that health benefits would increase with prawn stocking density and that high stocking density has the potential to achieve local disease elimination, especially along with medical intervention, such as MDA.
Although the net pens at the intervention village encompassed the area used by villagers for their daily water needs, participants were in contact with water outside the “prawn-protected” area. For example, most families were in contact with the water at nearby garden plots or rice farming allotments, or while fishing or working in the commercial rice or sugar cane fields (Table S3). This observation helps explain the moderate effect of prawns on human reinfection. Disease control should benefit from a broader distribution of prawns than our net pens allowed.
Wide fluctuations in reinfection rates across seasons were evident, leading to synchronized patterns of reinfection at the intervention and control villages, with some seasons (primarily the rainy season) having high reinfection at both sites (SI Text). This seasonality in reinfection highlights the importance of timing when considering MDA or prawn-stocking interventions.
There were also limits to our manipulation. For instance, transport stress reduced prawn survival, and monitoring of the enclosure over time suggested that the realized prawn density, ∼0.125 prawns per square meter, was one-half to one-quarter of the density required to eradicate infected snails locally in the model. Furthermore, our enclosure was not impervious to immigration by infected snails or cercariae, and snails might have more refuges in the river environment than in aquaria. A more extensive prawn enclosure, with higher stocking densities, would likely have greater public health benefits than seen in our experiment.
An obvious question is how to restore river prawns. One way is to build a prawn passage (ladder) into the Diama Dam. If successful, a prawn passage could replenish natural recruitment and migration, thus restoring prawns to the upper reaches of the river, where they are now excluded. It is important to note that natural prawn densities found in undisturbed ecosystems approach (29, 30)–and the prawn densities in Macrobrachium aquaculture (31) exceed–the critical threshold required for local disease elimination predicted by the model. For this reason, aquaculture, if made cost-effective, could make it easier for native river prawns to reduce schistosomiasis and might also boost the economic benefits of restoring prawn fisheries.
One might worry that harvesting prawns would lead to a trade-off between profits and disease control. Fortunately, because small, fast-growing prawns offer the highest snail-killing efficiency per gram (23), large prawns can be harvested for food or sale without affecting snail control, so long as prawn densities are maintained through natural recruitment (now blocked by the dam) or restocking via aquaculture. In fact, small prawns might do better in the absence of large prawns because of cannibalism (32). Therefore, we hypothesize that scaling up river prawn restoration and aquaculture and optimizing its economic benefits could provide a needed, affordable, and sustainable tool to complement drug distribution campaigns, and perhaps even eliminate schistosomiasis in the Senegal River Basin, while offering nutritional protein and marketable goods for local populations.
Schistosomiasis epidemics often follow dam projects (14). There are many river prawn species around the world (33), and their restoration to dammed areas could also reduce schistosomiasis. In other countries, like Kenya, exotic crayfish have become naturalized and might provide health benefits if fostered near transmission sites. Moreover, other parasitic diseases transmitted by snails may also be controlled by prawn restoration. For example, cattle and sheep trematodes cause economic losses and contribute to food insecurity in Africa (34). Many of these trematodes are transmitted by snails that share similar habitats and morphology with those snails carrying human schistosomes. As generalist feeders and prey for top predators, prawns could have many additional unanticipated effects. Overall, we suspect that these effects would help restore the Senegal River food web to its preexisting state before the construction of the Diama Dam.
Where drugs alone fail to control schistosomiasis due to rapid reinfection, prawns may offer a complementary strategy. In some endemic settings, reinfection is rapid because people lacking alternatives are soon reexposed to snail- and parasite-infested water. Neither the worm nor the treatment triggers a long-lasting immune response; therefore, drug treatment, without addressing environmental exposures, offers a temporary solution at best. Eliminating transmission of schistosomiasis, as called for by World Health Assembly Resolution 65.21 (8), seems unlikely without a more sustainable approach. The synergy between drug treatment and snail predators shown here suggests that with a combined strategy, schistosomiasis might be locally eliminated in endemic areas. The next steps include replicating this study to improve confidence in the results, formally assess economic benefits, and evaluate options for prawn restoration in the Senegal River Basin.
Biological conservation does not always benefit human health, but where it does, it provides a win-win outcome for humans and nature (35). Add the economic benefit of aquaculture to the equation, and river prawn restoration might become a win-win-win-win: for disease control, biodiversity restoration, poverty alleviation, and improved nutrition.
Materials and Methods
Ethical Statement.
The Human Research Ethics Committee of the Senegalese Ministry of Health reviewed and approved the study protocols. After obtaining informed consent, schistosome prevalence and parasite infection intensities were quantified among human study participants using standard protocols: urine filtration for S. hematobium (36) and the Kato–Katz thick smear for S. mansoni (37).
Epidemiological and Ecological Field Data Collection.
We surveyed villages in June 2011 before adding prawns. The two matched villages recruited to this study were situated 0.4 km apart on the Lampsar River in the Lower Senegal River Basin. The water access sites were well defined, and thick reeds (Typha sp.) impeded all other nearby water access. Population size was ca. 1,000 and 300 in the intervention and control villages, respectively. Participant characteristics were comparable between the two matched sites (Table 2). Human schistosomiasis was measured as infection prevalence (proportion of people with eggs present in the urine), mean egg burden (arithmetic mean of the number of eggs per 10-mL urine sample), or geometric mean (GM) egg “intensity” among participants. The following GM formula was used [also known as the “Williams mean” (38)]: GM (egg count + 1) − 1, to allow for inclusion of participants with no eggs, which is consistent with previous literature (39). Before stocking prawns, both villages had high S. hematobium infection rates (Table 1), but few S. mansoni eggs were detected: Four of 161 patients evaluated (2.5%) had low-level S. mansoni infection at baseline, and none passed any S. mansoni eggs at the first follow-up. Thus, only S. hematobium infections were further analyzed for this study.
Table 2.
Human subject (participant) characteristics at the experimental (prawn stocking) and control sites
| Site characteristic | Intervention (prawn) site | Control site |
| Median age (range) | 11 (4–50) | 11 (4–51) |
| Female/male ratio | 1:1.2 | 1:1.8 |
| Sample size (no. recruited at baseline) | 176 | 129 |
In December 2011, a three-sided net enclosure, ca. 20 m wide by 10 m long, was constructed around the naturally defined perimeter at one village’s water contact site; the enclosure’s fourth side was the beach at which villagers access the water (Fig. 1). The net was constructed of durable nylon with a mesh size of 1 cm. It was attached to vertical metal poles and secured at the bottom by sand bags. In January 2012, wild river prawns were collected from the Lobe River in Cameroon (Gulf Aquatics, owned by Cyrille Dening Touokong, Douala, Cameroon), shipped by airfreight, and trucked to the village. Prawn stocking was repeated every 5–7 mo (July and November) in 2012 and bimonthly in 2013.
We monitored snail density and schistosome prevalence in snails at the intervention and control sites after prawns were stocked at the intervention village. Two trained technicians sampled snails biweekly from October 2012 to September 2013, by searching for 1.5 min with standardized snail scoops at 10 equally spaced points along the site edges (40). Those snails susceptible to schistosome infection (Biomphalaria pfeifferi, B. globosus, and B. truncatus) were put in jars filled with river water and returned to the laboratory. By midday, all snails were cleaned in distilled water and placed under bright artificial light for 30 min. Those snails shedding cercariae, with morphologies consistent with Schistosoma spp., were recorded as infected. Those snails not shedding cercariae or those snails shedding cercariae not consistent with Schistosoma spp. morphology were recorded as uninfected.
In February–March 2012, 7 mo after baseline testing, all study participants received praziquantel, regardless of infection status, at a dose of 40 mg/kg, based on a standard dose pole (41). The same praziquantel dose was given again 3 wk later in a double-dose protocol designed to treat both adult and juvenile worm infections for enhanced cure rates (26). Participants were then advised to go about business as usual. Participants were next surveyed in July 2012, February 2013, and September 2013. At each follow-up, participants were tested for Schistosoma eggs in urine or feces, and those participants who were positive were offered treatment as a single 40-mg/kg praziquantel dose.
We compared schistosomiasis prevalence and intensity in study participants living at the intervention and control villages before and after prawns were installed at the intervention village. Statistically, this comparison was achieved by testing for an interaction effect (BACI interaction) between the control/intervention and before/after groups within a generalized linear mixed model, including individual patient identification and testing date as random effects to account for repeated measures at multiple time points (Fig. 3). A binomial error distribution was used for human schistosomiasis prevalence (infected/uninfected), and a negative binomial error distribution was used for egg intensity data. The statistical tests also controlled for several covariates: subject age, gender, and their interaction. As an unreplicated comparison, the statistical tests evaluated whether the villages were significantly different, and not whether there was a significant prawn effect per se (which would have required a substantial replication of villages far beyond the means of this study).
Schistosomiasis Model That Includes Predation on Intermediate Host Snails.
Building on classic models (42–45), we extended the models by including snail population dynamics and logistic population growth, with parasite infections partially to fully castrating snails (46, 47), as well as a managed snail-predator population.
Disease dynamics in snails followed a compartmental susceptible-exposed-infected framework, whereas human infections tracked intensity as mean worm burden (43). The model tracked infection dynamics among snails and humans at a single site (we assumed no immigration), with a spatial scale consistent with a homogeneous mixing assumption (e.g., scale of the 20-m × 10-m prawn enclosure). The basic model had the following four differential equations:
| [1] |
| [2] |
| [3] |
| [4] |
with S, E, and I being susceptible, exposed, and infectious snail classes, respectively, and with N being snail density and M being the mean parasite burden among human hosts. The model was parameterized using data from the literature and published and unpublished laboratory studies performed previously by the authors (SI Text).
The model simulations were run in R, version 3.0.2 (48), using the DeSolve package to calculate numerical solutions to the system of ordinary differential equations. Equilibrium solutions were achieved by running simulations for 30 y in daily time steps. Mass drug treatments, with or without prawns, were simulated by applying a pulse function that reduced the treated population’s mean parasite burden by 99% [a plausible, if not optimistic, level of drug efficacy consistent with other field studies (49, 50)].
We assessed whether the model could qualitatively replicate the observed patterns of reinfection in the human population at the control and experimental sites (SI Text). Because Eqs. 1–4 could not reproduce the seasonal variation in reinfection patterns, we extended the model by allowing snail reproductive rate, f, to vary as a sine function, f(t), with peak reproduction in February through May. The seasonal model was able to reproduce the fluctuating reinfection patterns at the three follow-up visits in the field trial (SI Text).
After assessing equilibrium solutions and the model’s qualitative fit with field data, we used the model to explore a set of hypothetical scenarios for disease control and elimination: (i) yearly MDA at 80% coverage, applied for 5 y; (ii) prawn stocking (indefinitely) at a constant density of 0.25 per square meter; and (iii) prawn density at 0.25 per square meter and MDA applied twice, 2 y apart (with the same 80% coverage as in the first scenario). We then plotted the resultant trajectories of human worm burdens and infection prevalence for 20 y (Fig. 5).
Supplementary Material
Acknowledgments
The authors thank Oumar Talla Diaw, Mouhamadane Mbacke Seye, Chelsea Wood, Ryan Hechinger, and Denis Massenet for helpful assistance. We acknowledge and thank the Senegalese villagers who participated in the study. Greg Galin assisted with figures and graphics. S.H.S. received support from NIH-K08 Grant PHS K08AI082284 from the National Institute of Allergy and Infectious Disease and a grant by the Woods Institute's Environmental Venture Projects at Stanford University. S.H.S. and A.M.K. received support from NSF CNH 1414102. M.H.H. received support from NIH K08DK087895. E.H., N.J., and G.R., as well as field activities, were supported through three seed grants: Grant OPP1024600 from the Bill and Melinda Gates Foundation; a three-year seed grant from The Seaver Institute, Los Angeles, CA; and Grand Challenges Canada's Grant RS-0141-1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502651112/-/DCSupplemental.
References
- 1.Hotez PJ, Fenwick A, Kjetland EF. Africa’s 32 cents solution for HIV/AIDS. PLoS Negl Trop Dis. 2009;3(5):e430. doi: 10.1371/journal.pntd.0000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO (2015) Preventive Chemotherapy and Transmission Control (PCT) databank. Available at www.who.int/neglected_diseases/preventive_chemotherapy/databank/en/. Accessed June 15, 2014.
- 3.WHO . Schistosomiasis: Progress Report 2001-2011 and Strategic Plan 2012-2020. WHO; Geneva: 2011. [Google Scholar]
- 4.Bockarie MJ, Kelly-Hope LA, Rebollo M, Molyneux DH. Preventive chemotherapy as a strategy for elimination of neglected tropical parasitic diseases: Endgame challenges. Philos Trans R Soc Lond B Biol Sci. 2013;368(1623):20120144. doi: 10.1098/rstb.2012.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray DJ, et al. Schistosomiasis elimination: Lessons from the past guide the future. Lancet Infect Dis. 2010;10(10):733–736. doi: 10.1016/S1473-3099(10)70099-2. [DOI] [PubMed] [Google Scholar]
- 6.Fenwick A, Savioli L. Schistosomiasis elimination. Lancet Infect Dis. 2011;11(5):346, author reply 346–347. doi: 10.1016/S1473-3099(11)70110-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Jiang Q. Schistosomiasis elimination. Lancet Infect Dis. 2011;11(5):345, author reply 346–347. doi: 10.1016/S1473-3099(11)70109-8. [DOI] [PubMed] [Google Scholar]
- 8.World Health Assembly . Elimination of Schistosomiasis in WHA65/2012/REC/1 Sixty-Fifth World Health Assembly: Resolutions and Decisions Annexes. WHO; Geneva: 2012. [Google Scholar]
- 9.Sow S, de Vlas SJ, Engels D, Gryseels B. Water-related disease patterns before and after the construction of the Diama dam in northern Senegal. Ann Trop Med Parasitol. 2002;96(6):575–586. doi: 10.1179/000349802125001636. [DOI] [PubMed] [Google Scholar]
- 10.Talla I, et al. Outbreak of intestinal schistosomiasis in the Senegal River Basin. Ann Soc Belg Med Trop. 1990;70(3):173–180. [PubMed] [Google Scholar]
- 11.Diakhate M. Le barrage de Diama: Essai sur l’evaluation de ses impacts potentiels. Rev Geogr Lyon. 1986;61(1):43–61. French. [Google Scholar]
- 12.Southgate VR. Schistosomiasis in the Senegal River Basin: Before and after the construction of the dams at Diama, Senegal and Manantali, Mali and future prospects. J Helminthol. 1997;71(2):125–132. doi: 10.1017/s0022149x00015790. [DOI] [PubMed] [Google Scholar]
- 13.Alkalay AS, et al. The prawn Macrobrachium vollenhovenii in the Senegal River basin: Towards sustainable restocking of all-male populations for biological control of schistosomiasis. PLoS Negl Trop Dis. 2014;8(8):e3060. doi: 10.1371/journal.pntd.0003060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6(7):411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 15.Cheever AW, Macedonia JG, Mosimann JE, Cheever EA. Kinetics of egg production and egg excretion by Schistosoma mansoni and S. japonicum in mice infected with a single pair of worms. Am J Trop Med Hyg. 1994;50(3):281–295. doi: 10.4269/ajtmh.1994.50.281. [DOI] [PubMed] [Google Scholar]
- 16.Mohammed AZ, Edino ST, Samaila AA. Surgical pathology of schistosomiasis. J Natl Med Assoc. 2007;99(5):570–574. [PMC free article] [PubMed] [Google Scholar]
- 17.Linde HJ, Hahn J, Holler E, Reischl U, Lehn N. Septicemia due to Acinetobacter junii. J Clin Microbiol. 2002;40(7):2696–2697. doi: 10.1128/JCM.40.7.2696-2697.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jobin WR, Negrón-Aponte H, Michelson EH. Schistosomiasis in the Gorgol Valley of Mauritania. Am J Trop Med Hyg. 1976;25(4):587–594. doi: 10.4269/ajtmh.1976.25.587. [DOI] [PubMed] [Google Scholar]
- 19.Utzinger J, et al. Neglected tropical diseases: Diagnosis, clinical management, treatment and control. Swiss Med Wkly. 2012;142:w13727. doi: 10.4414/smw.2012.13727. [DOI] [PubMed] [Google Scholar]
- 20.Webster BL, et al. Praziquantel treatment of school children from single and mixed infection foci of intestinal and urogenital schistosomiasis along the Senegal River Basin: Monitoring treatment success and re-infection patterns. Acta Trop. 2013;128(2):292–302. doi: 10.1016/j.actatropica.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Mkoji GM, et al. Impact of the crayfish Procambarus clarkii on Schistosoma haematobium transmission in Kenya. Am J Trop Med Hyg. 1999;61(5):751–759. doi: 10.4269/ajtmh.1999.61.751. [DOI] [PubMed] [Google Scholar]
- 22.Gannett Fleming Corddry and Carpenter I, ORGATEC Societé Africaine d’Études Techniques . Assessment of Environmental Effects of Proposed Developments in the Senegal River Basin. Gannett, Fleming, Corddry, and Carpenter; Harrisburg, PA: 1980. Fisheries appendices. [Google Scholar]
- 23.Sokolow SH, Lafferty KD, Kuris AM. Regulation of laboratory populations of snails (Biomphalaria and Bulinus spp.) by river prawns, Macrobrachium spp. (Decapoda, Palaemonidae): Implications for control of schistosomiasis. Acta Trop. 2014;132:64–74. doi: 10.1016/j.actatropica.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang Y, Wedekin L. Dynamics of a intraguild predation model with generalist or specialist predator. J Math Biol. 2013;67(5):1227–1259. doi: 10.1007/s00285-012-0584-z. [DOI] [PubMed] [Google Scholar]
- 25.Chaine JP, Malek EA. Urinary schistosomiasis in the Sahelian region of the Senegal River Basin. Trop Geogr Med. 1983;35(3):249–256. [PubMed] [Google Scholar]
- 26.King CH, et al. Utility of repeated praziquantel dosing in the treatment of schistosomiasis in high-risk communities in Africa: A systematic review. PLoS Negl Trop Dis. 2011;5(9):e1321. doi: 10.1371/journal.pntd.0001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheever AW. A quantitative post-mortem study of Schistosomiasis mansoni in man. Am J Trop Med Hyg. 1968;17(1):38–64. doi: 10.4269/ajtmh.1968.17.38. [DOI] [PubMed] [Google Scholar]
- 28.Diekman O, Heesterbeek A. Mathematical Epidemiology of Infectious Diseases. Wiley; West Sussex, UK: 2000. [Google Scholar]
- 29.Covich A, Crowl T, Heartsill-Scalley T. Effects of drought and hurricane disturbances on headwater distributions of palaemonid river shrimp (Macrobrachium spp.) in the Luquillo Mountains, Puerto Rico. J North Am Benthol Soc. 2006;25(1):99–107. [Google Scholar]
- 30.Snyder MN, Anderson E, Pringle CM. A migratory shrimp’s perspective on habitat fragmentation in the neotropics: Extending our knowledge from Puerto Rico. In: Asakuro A, editor. New Frontiers in Crustacean Biology: Proceedings of The Crustacean Society's Summer Meeting, Tokyo, 20–24 September 2009. Vol 15. Koninklijke Brill NV; Leiden, The Netherlands: 2009. pp. 169–182. [Google Scholar]
- 31.New MB. Farming Freshwater Prawns: A Manual for the Culture of the Giant River Prawn (Machrobrachium rosenbergii. Food and Agriculture Organization of the United Nations; Rome: 2002. Tech Rep 428. [Google Scholar]
- 32.Tidwell JH, Coyle SD, Bright LA, VanArnum A, Weibel C. The effects of size grading and length of nursery period on growth and population structure of freshwater prawns stocked in temperate zone ponds with added substrates. Aquaculture. 2003;218(1-4):209–218. [Google Scholar]
- 33.De Grave S, Fransen CHJM. Carideorum catalogus: The recent species of the dendrobranchiate, stenopodidean, procarididean and caridean shrimps (Crustacea: Decapoda) Zool Med Leiden. 2011;85:195–589. [Google Scholar]
- 34.Nzalawahe J, Kassuku AA, Stothard JR, Coles GC, Eisler MC. Trematode infections in cattle in Arumeru District, Tanzania are associated with irrigation. Parasit Vectors. 2014;7:107. doi: 10.1186/1756-3305-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood CL, et al. Does biodiversity protect humans against infectious disease? Ecology. 2014;95(4):817–832. doi: 10.1890/13-1041.1. [DOI] [PubMed] [Google Scholar]
- 36.Mott KE, Baltes R, Bambagha J, Baldassini B. Field studies of a reusable polyamide filter for detection of Schistosoma haematobium eggs by urine filtration. Tropenmed Parasitol. 1982;33(4):227–228. [PubMed] [Google Scholar]
- 37.Kato K, Miura M. Comparative examinations. Japanese Journal of Parasitology. 1954;3:35–37. [Google Scholar]
- 38.Williams C. The use of logarithms in the interpretation of certain entomological problems. Ann Appl Biol. 1937;24:404–414. [Google Scholar]
- 39.Alexander N. Review: Analysis of parasite and other skewed counts. Trop Med Int Health. 2012;17(6):684–693. doi: 10.1111/j.1365-3156.2012.02987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massenet D, Jouanard N, Huttinger E. [Collection of mollusk intermediate hosts of Schistosoma at two sites in Lampsar (Senegal River valley): Quantitative reproducibility] Med Sante Trop. 2014;24(1):80–82. French. doi: 10.1684/mst.2014.0299. [DOI] [PubMed] [Google Scholar]
- 41.Montresor A, et al. Development and validation of a ‘tablet pole’ for the administration of praziquantel in sub-Saharan Africa. Trans R Soc Trop Med Hyg. 2001;95(5):542–544. doi: 10.1016/s0035-9203(01)90034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macdonald G. The dynamics of helminth infections, with special reference to schistosomes. Trans R Soc Trop Med Hyg. 1965;59(5):489–506. doi: 10.1016/0035-9203(65)90152-5. [DOI] [PubMed] [Google Scholar]
- 43.Anderson R, May R. Infectious Diseases of Humans. Oxford Univ Press; Oxford: 1991. [Google Scholar]
- 44.Woolhouse MEJ. On the application of mathematical models of schistosome transmission dynamics. II. Control. Acta Trop. 1992;50(3):189–204. doi: 10.1016/0001-706x(92)90076-a. [DOI] [PubMed] [Google Scholar]
- 45.Cohen JE. Mathematical models of schistosomiasis. Annu Rev Ecol Syst. 1977;8:209–233. [Google Scholar]
- 46.Toledo R, Fried B. Biomphalaria Snails and Larval Trematodes. Springer; New York: 2011. [Google Scholar]
- 47.Beideman D, Fried B, Sherma J. Effects of Schistosoma mansoni infection on the survival, fecundity, and triacylglycerol content of Biomphalaria glabrata snails. J Vet Sci Med Diagn. 2013;2(3):1–3. [Google Scholar]
- 48.R Development Core Team . A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- 49.Ferrari ML, Coelho PM, Antunes CM, Tavares CA, da Cunha AS. Efficacy of oxamniquine and praziquantel in the treatment of Schistosoma mansoni infection: A controlled trial. Bull World Health Organ. 2003;81(3):190–196. [PMC free article] [PubMed] [Google Scholar]
- 50.Tchuenté LA, Shaw DJ, Polla L, Cioli D, Vercruysse J. Efficacy of praziquantel against Schistosoma haematobium infection in children. Am J Trop Med Hyg. 2004;71(6):778–782. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.