Significance
γδ T cells producing IL-17 (γδ-17 cells) play an important role in promoting inflammation, but the mechanisms that regulate their development are still being explored. IL-15 and its receptor, IL-15Rα, function largely to promote the development and survival of lymphoid cells. Here we show that IL-15Rα signaling has an opposing effect on the γδ-17 population, such that a deficiency in IL-15Rα increases γδ-17 cells and their precursors. This work establishes a direct role for IL-15Rα signaling in vivo and raises questions about the role of IL-15 itself in this process.
Keywords: IL-15Rα, γδ T cells, IL-17
Abstract
The development and homeostasis of γδ T cells is highly dependent on distinct cytokine networks. Here we examine the role of IL-15 and its unique receptor, IL-15Rα, in the development of IL-17–producing γδ (γδ-17) T cells. Phenotypic analysis has shown that CD44high γδ-17 cells express IL-15Rα and the common gamma chain (CD132), yet lack the IL-2/15Rβ chain (CD122). Surprisingly, we found an enlarged population of γδ-17 cells in the peripheral and mesenteric lymph nodes of adult IL-15Rα KO mice, but not of IL-15 KO mice. The generation of mixed chimeras from neonatal thymocytes indicated that cell-intrinsic IL-15Rα expression was required to limit IL-17 production by γδ T cells. γδ-17 cells also were increased in the peripheral lymph nodes of transgenic knock-in mice, where the IL-15Rα intracellular signaling domain was replaced with the intracellular portion of the IL-2Rα chain (that lacks signaling capacity). Finally, an analysis of neonatal thymi revealed that the CD44lo/int precursors of γδ-17 cells, which also expressed IL-15Rα, were increased in newborn mice deficient in IL-15Rα signaling, but not in IL-15 itself. Thus, these findings demonstrate that signaling through IL-15Rα regulates the development of γδ-17 cells early in ontogeny, with long-term effects on their peripheral homeostasis in the adult.
Both αβ and γδ T cells rely heavily on cytokine signaling for their development and survival. Many of these cytokines belong to the IL-2 cytokine family, whose receptors all share a common γ receptor chain (γc; CD132). Among these, IL-15 has a unique, nonredundant role in both T-cell and natural killer (NK)-cell biology, such that CD8+ memory T cells and NK cells are absent in IL-15–deficient environments (1, 2). The predominant mechanism through which IL-15 functions is termed transpresentation, whereby IL-15 is preassociated with its specific α-chain (IL-15Rα) inside the cell and presented at the cell surface in trans to a responding cell expressing γc and IL-2/15Rβ (CD122) (3–6). IL-15 is unique among its family members owing to its ability to act either in cis or in trans. Whether or not direct signaling (in cis) via IL-15Rα plays a significant biological role in immunobiology has not been resolved (7–11).
Certain populations of γδ T cells are known to be sensitive to the availability of IL-15 for their development and/or survival. A population of specialized γδ T cells called dendritic epidermal T cells are absent from the skin of IL-15 knockout (KO) and IL-15Rα KO mice (12, 13). The CD8αα+ γδ T-cell receptor (TCR) intraepithelial lymphocytes are also decreased in these two KO mouse strains (1, 2). In addition, IL-15 KO mice have a reduced population of IFN-γ+ γδ T cells in the peritoneum (14). All of these populations express CD122, suggesting they can receive IL-15–dependent signals via transpresentation.
Recently, γδ T cells have emerged as important contributors to the generation of immune responses. The innate-like γδ T cells that produce IL-17 (γδ-17 cells) have been implicated in immune responses generated during bacterial and fungal infections, experimental autoimmune encephalomyelytois (EAE), psoriasis, and anticancer immunity (reviewed in ref. 15). The γδ-17 subset of γδ T cells has a restricted TCR use that is largely limited to Vγ2 and Vγ4 expression (Garmin nomenclature; Vγ4 and Vγ6 in Tonegawa nomenclature) and a molecularly distinct gene expression profile (16). Considering the strictly regulated developmental progression of γδ T-cell subsets (17), the vast majority of γδ-17 cells are known to emerge during a limited window early in ontogeny, ∼E16.5 up to shortly after birth (18). The exogenous signals that impact γδ-17–cell development during this window remain unclear. Here we identify the IL-15Rα chain as a critical determinant through which γδ-17–cell development is regulated. Furthermore, in contrast to IL-15Rα’s predominant role in the immune system via IL-15 transpresentation, we found IL-15Rα–dependent changes in both neonatal thymic development and peripheral homeostasis in adulthood, suggesting that γδ-17 cells are dependent on cell-intrinsic signals received through IL-15Rα in cis.
Results
Individual Populations of γδ T Cells Expressed Varying Levels of the IL-2 and IL-15 Receptor Chains.
Several studies have suggested that the presence or absence of IL-15 can impact the homeostasis of γδ T-cell populations; however, most of those studies focused on γδ T-cell populations existing in nonlymphoid tissues rather than secondary lymphoid organs. Because γδ-17 cells are localized in the peripheral lymph nodes (pLNs) of naïve adult mice (19), we examined γδ T cells in the pLNs of IL-15– and IL-15Rα–deficient mice (15KO and RKO, respectively). We first examined cytokine receptor expression on individual populations of γδ T cells as an indicator of their potential dependency on cytokine-based survival signals.
Baccala et al. (20) demonstrated the existence of at least two distinct populations in the pLNs of wild-type (WT) mice that can be identified using CD44 and CD122 (Fig. 1A). The CD44intCD122+ cells correspond to γδ T cells that emerge from T-cell precursors in the adult thymus. This population also expresses CD62L, CD27, and NK1.1. Considering their increased expression of CD122, our finding that CD44intCD122+ cells were absent from the pLNs of 15KO and RKO mice is not surprising (Fig. 1A). Functionally, the CD44intCD122+ population of γδ T cells has been shown to produce IFN-γ (21, 22); thus, we also found an absence of IFN-γ+ γδ T cells in 15KO and RKO mice, as expected (Fig. 1B).
Fig. 1.
CD44highCD122-/low γδ T cells express IL-15Rα. (A and C) pLNs were harvested from WT, 15KO, and RKO mice and stained with antibodies for detection of CD122 (A) or IL-15Rα (C). In A, numbers indicate the average ± SEM percentage of CD3+GL3+TCRβ− cells that are CD44intCD122+ (Lower) or CD44highCD122-/low (Upper). In C, cells were further gated on CD44intCD122+ (open circles) or CD44highCD122-/low (filled circles), and the MFI of IL-15Rα is shown graphically. Baseline IL-15Rα staining in RKO mice is also indicated (filled square). (B) For the detection of IFN-γ, cells were cultured in vitro for 5 h with PMA/ionomycin and brefeldin A. The percentage of IFN-γ–producing γδ T cells is shown graphically. Data are representative of two to four independent experiments with similar results. Statistical analyses were performed using the Student t test (C) and one-way ANOVA (B).
A second population of CD44highCD122-/low cells was significantly increased in both 15KO and RKO mice. Others have shown that the CD44highCD122-/low population is phenotypically CD62LlowCD27−CCR6+ (19, 20, 22) and has the capacity to produce IL-17 (14). We found that the CD44highCD122-/low cells in the pLNs also expressed elevated CD25 (IL-2Rα) compared with the CD44intCD122+ population (Fig. S1A), similar to γδ T cells located in the peritoneal cavity (14). Because CD132 is also required for IL-15 signaling, we examined receptor expression on the two populations of pLN γδ T cells and found that CD132 was expressed at significantly higher levels on the CD44highCD122-/low cells compared with on the CD44intCD122+ cells (Fig. S1B).
Fig. S1.

CD44highCD122-/low γδ T cells express CD25 and CD132. pLNs were harvested from WT mice and stained with antibodies for detection of CD25 (A) or CD132 (B) within the CD3+GL3+TCRβ− population. In B, cells were further gated on CD44intCD122+ (open circles) or CD44highCD122-/low (filled circles), and the MFI of CD132 is shown graphically. The Student t test was used for statistical analysis. Data are representative of three independent experiments with similar results.
Finally, we measured the expression of IL-15Rα itself. IL-15Rα is expressed largely by antigen-presenting cells, but it can be up-regulated by αβ T cells (23). Using an IL-15Rα KO mouse as a negative control, we were able to detect IL-15Rα expression on both CD44intCD122+ and CD44highCD122-/low γδ T cells (Fig. 1C); however, the CD44intCD122+ cells expressed significantly higher levels of IL-15Rα than the CD44highCD122-/low population, as measured by the mean fluorescence intensity (MFI). Thus, our data are consistent with previously published data regarding elevated CD25 and negative or low CD122 expression on CD44high γδ T cells, but also demonstrate the expression of two additional IL-15R chains: IL-15Rα and the γc.
IL-17+ γδ T Cells Were Increased in the Absence of IL-15Rα.
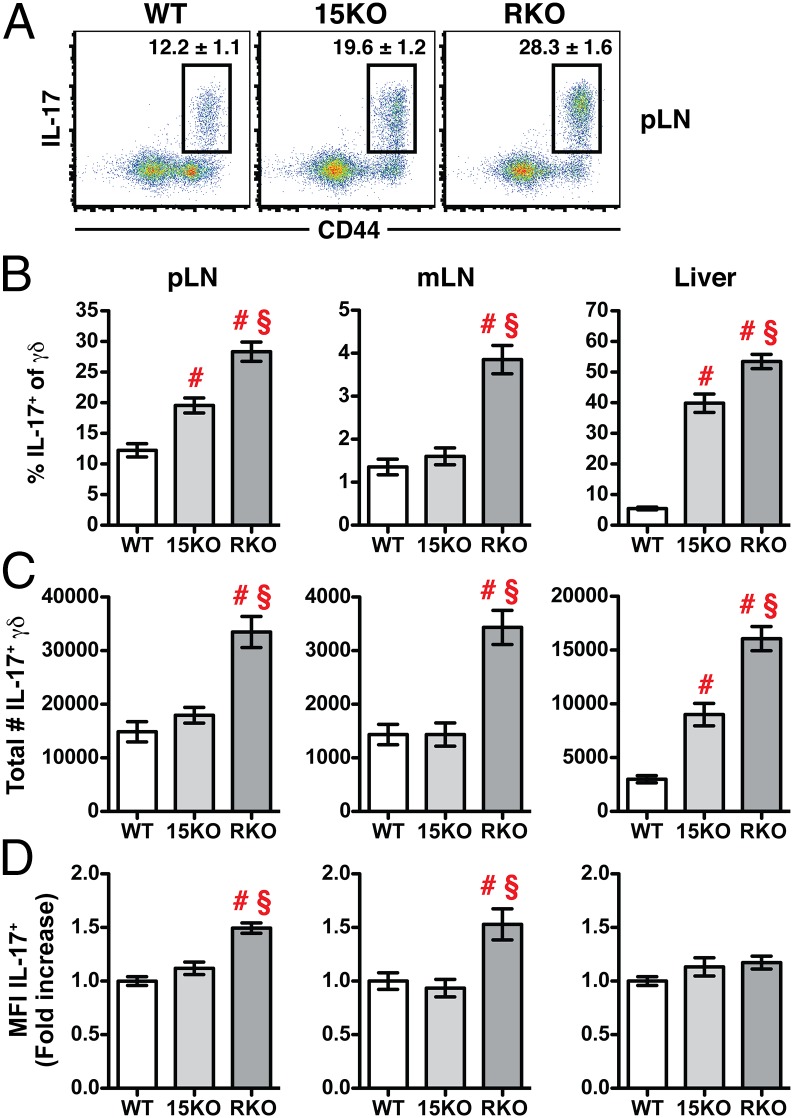
Our data indicate that in IL-15–deficient environments, there is an increased population of γδ T cells with the phenotype typical of γδ-17 cells. Therefore, we next isolated cells from both secondary lymphoid and nonlymphoid tissues and subjected them to phorbol myristate acetate (PMA)/ionomycin stimulation to quantify the frequency and total number of γδ-17 cells present in the absence of IL-15 or IL-15Rα. Similar to our phenotypic analysis, we found an increase in the percentage of IL-17+ cells in the pLNs of both 15KO and RKO mice, all of which had a CD44high phenotype (Fig. 2 A and B, Left); however, we were surprised to find a significant increase in the total number of γδ-17 cells in the pLNs of RKO mice, but not of 15KO mice (Fig. 2C, left column). Normally, γδ-17 cells are largely absent from the mesenteric lymph nodes (mLNs) of WT mice, but we found an increased percentage and number of γδ-17 cells only in the mLNs of RKO mice (Fig. 2 B and C, Middle). In the liver, 15KO mice exhibited an intermediate phenotype marked by a significant increase in the frequency and total number of γδ-17 cells compared with WT controls, but still a greater increase was observed between 15KO and RKO mice (Fig. 2 B and C, Right). Finally, when we measured the MFI of IL-17 within the γδ-17 population, we found that γδ-17 cells in the RKO mice were making significantly more IL-17 on a per cell basis compared with γδ-17 cells in the WT and 15KO mice (Fig. 2D). Thus, our results suggest an essential role for IL-15Rα in modulating the size of the γδ-17 population and its functional potential to produce IL-17. Importantly, we identified a discrepancy between the two known IL-15–deficient environments, 15KO and RKO mice, which to date have phenocopied each other in terms of all other lymphocyte-related developmental or homeostatic defects.
Fig. 2.
IL-17+ γδ T cells are increased in IL-15Rα-deficient mice. Cells from the pLNs, mLNs, and liver of WT, 15KO, and RKO mice were stimulated for 5 h with PMA/ionomycin and brefeldin A and subjected to intracellular cytokine staining for IL-17A. (A) Representative dot plots showing CD44 expression vs. IL-17A production. The numbers indicate the average ± SEM percentage of CD3+GL3+TCRβ− pLN cells in the indicated gate. (B) Graph showing values for the pLNs, mLN, and liver. (C) The total number of IL-17+ γδ T cells calculated for each tissue. (D) Graph showing differences in the MFI of IL-17 based on the fold increase over the average MFI of IL-17 in the WT control group. In B–D, # indicates a significant difference of at least P ≤ 0.01 from WT controls, and § indicates a significant difference of P ≤ 0.001 from 15KO mice, using one-way ANOVA for statistical analysis. Data are inclusive of six independent experiments with three to six mice per group per experiment.
Cell-Intrinsic IL-15Rα Expression Limited IL-17 Production by γδ T Cells.
Our data presented thus far indicate that IL-15Rα is integral in regulating the IL-17+ population of γδ T cells. Because these experiments were conducted in RKO mice in which all cells were deficient in normal IL-15Rα expression, we were unable to examine whether IL-15Rα expression by γδ T cells themselves is required for IL-17 modulation. Owing to the unique developmental biology of γδ-17 cells, we used a methodology described by Gray et al. (24) to generate γδ-17 cells in adult mice following lethal irradiation. In brief, neonatal thymi (neoThy) were harvested from <72-h-old WT (CD45.1+/CD45.2+) and RKO (CD45.1+) pups. The cells were mixed at a 1:1 ratio and transferred to congenic WT recipients (CD45.2+) that had been lethally irradiated. After >7 wk, the pLNs of mixed neoThy chimeras were stimulated with PMA/ionomycin to examine IL-17 production in the CD3+GL3+ cells derived from the WT and RKO neoThy precursors (Fig. 3A). This allowed us to study IL-17 production by γδ T cells that lack IL-15Rα expression side-by-side with γδ T cells expressing IL-15Rα in the lymphoreplete environment of the reconstituted WT mice.
Fig. 3.

Cell-intrinsic IL-15Rα expression modulates γδ T-cell IL-17 production. (A) pLN cells from chimeric mice at 7–10 wk after reconstitution with WT (CD45.1/2+; green gate) and RKO (CD45.1+; blue gate) neonatal thymocytes. (B) Representative dot plots showing CD44 expression and IL-17 production for the cells derived from each neonatal thymocyte donor. (C) Percentage of IL-17+ cells within the CD44high gate. (D) Fold increase in the MFI of IL-17 within the CD44highIL-17+ gate. All values have been normalized to the average MFI of IL-17 from the WT-derived cells. In C and D, a line connects WT-derived cells (green circles) to RKO-derived cells (blue squares) that developed within the same recipient mouse. Data are inclusive of three independent experiments with four to nine chimeras per experiment. The paired Student t test was used to determine significance.
Whereas neoThy preferentially gave rise to γδ-17 cells, we found a significant increase in the percentage of CD44high γδ T cells making IL-17 in those γδ T cells derived from RKO neoThy compared with control WT-derived cells (Fig. 3 B and C). In addition, RKO-derived γδ T cells expressed more IL-17 on a per cell basis compared with the WT-derived γδ T cells in the same mouse (Fig. 3D). Thus, our findings suggest that cell-intrinsic expression of IL-15Rα is required to constrain IL-17 production by γδ T cells.
Ablation of IL-15Rα Signaling Increased the Population of γδ-17 T Cells.
Our data suggest a preferential role for cell-intrinsic IL-15Rα function in modulating IL-17+ γδ T cells, but IL-15Rα has the capacity to act either in cis or in trans. To distinguish between these two potential mechanisms of action, we made use of a transgenic knock-in mouse strain previously generated by Wu et al. (10). These mice [IL-15Rαext/IL-2Rαint, herein termed receptor transgenic (RTg)] express a chimeric IL-15Rα protein in which the external portion of IL-15Rα is unperturbed, but the IL-15Rα intracellular domain is replaced with the intracellular domain of IL-2Rα (which lacks signaling capacity) (Fig. S2). Using RTg mice in this study allowed us to distinguish the effects of IL-15 transpresentation, which occurs normally, from cis IL-15Rα signaling, which is ablated.
Fig. S2.
Diagram of transpresentation vs. cis signaling via IL-15Rα. (A) The most common means by which IL-15 functions in vivo is through transpresentation. Both IL-15 and IL-15Rα are expressed by antigen-presenting cells (APC). IL-15 is then presented in trans to responding cells, including T cells, expressing the common γ (γc; CD132) chain and IL-2/15Rβ (CD122) to initiate downstream signaling cascades. (B) It is believed that most signaling originates from CD122, because the intracellular domain of γc is very short; however, some evidence exists of a trimeric receptor expressed on T cells consisting of γc, CD122, and IL-15Rα, similar to the trimeric receptor for IL-2. Given that the intracellular domain of IL-15Rα has the capacity to initiate downstream signaling, the combined triggering of IL-15Rα and CD122 could modify the overall downstream signaling cascades in some fashion. (C and D) In RTg mice, the intracellular portion of IL-15Rα has been replaced with the intracellular domain of IL-2Rα. In the context of transpresentation (C), this modification has no effect on the ability of an APC to effectively transpresent IL-15 to an IL-15–dependent cell, and the signaling cascades initiated by transpresentation are not disrupted; however, because IL-2Rα has no signaling capacity of its own, this replacement essentially ablates any signaling downstream of IL-15Rα engagement (D). Thus, in RTg mice, transpresentation functions normally, and cis signaling through IL-15Rα does not occur.
As described above, we harvested lymphoid tissues from RTg mice and WT controls and stimulated the cells with PMA/ionomycin to quantify IL-17 production by γδ T cells. When we first examined the γδ T-cell population using CD44 and CD122, we found that the population of CD44intCD122+ γδ T cells was ∼60–70% of the size observed in WT controls (Fig. 4A) but not absent, as was observed in complete RKO mice. This finding confirms our suspicion, based on the data presented in Fig. 1A, that transpresentation largely sustains the CD122+ population of CD44int γδ T cells. When we examined IL-17 production by intracellular staining, however, we found that, just as RKO mice had a significant increase in γδ-17 cells, RTg mice also exhibited significant increases in the percentage and total number of CD44highIL-17+ γδ T cells in pLNs (Fig. 4 B and C). Nevertheless, in contrast to RKO mice, RTg mice did not exhibit an increase in the MFI of IL-17 by γδ-17 cells (Fig. S3A) or a global increase in γδ-17 cells in other lymphoid tissues, such as the mLNs (Fig. 4C). Interestingly, when we examined the blood of RKO and RTg mice, only the RKO mice showed an increased percentage of CD44highCD27− γδ T cells in the blood (Fig. S3B), which may suggest an enhanced ability to migrate in the complete absence of IL-15Rα expression. Taken together, these results provide evidence that a lack of cis IL-15Rα signaling is responsible for the increased population of γδ-17 cells.
Fig. 4.
Increased γδ T-cell IL-17 production occurs in the absence of IL-15Rα signaling. (A and B) Representative dot plots of CD3+GL3+TCRβ− γδ T cells in the pLNs of WT or RTg mice showing CD44 expression vs. CD122 expression (A) or IL-17A production (B). The numbers in A indicate the average percentage of CD44intCD122+ (Upper) or CD44highCD122-/low (Lower) cells ± the SEM. (C) Graph showing the percentage and total number of CD44highIL-17+ γδ T cells in the pLNs and mLNs. The Student t test was used to determine significance. Data are inclusive of two to three independent experiments.
Fig. S3.
No increase in the MFI of IL-17 or migrating γδ-17 cells was observed in the absence of IL-15Rα signaling. (A) CD3+GL3+TCRβ− γδ T cells in the pLNs of WT or RTg mice were gated on CD44highIL-17+ cells. The MFI of IL-17 is shown as the fold increase over the average MFI of IL-17 in the WT control group. (B) Percentage of CD44highCD27− cells within the γδ T cells in the blood for WT, RKO, and RTg mice. One-way ANOVA was used to determine significance. Data are inclusive of two to three independent experiments.
Increased γδ-17 Precursors Were Present in the Thymus of IL-15Rα–Deficient Neonates.
Given that the vast majority of γδ-17 cells emerge from the neoThy during a brief window early in ontogeny (18), we hypothesized that IL-15Rα could play a role in the development of this population. To explore this possibility, we next examined IL-15Rα expression on the developing γδ-17 precursors. Others have proposed that the progenitors of the CD44high γδ-17 cells in the periphery of adult mice can be found within the CD44lo/int population of the neoThy (25). To readily identify this population, we used a fluorescent IL-17A reporter mouse to characterize IL-15Rα expression on CD44lo/int cells that were positive for GFP/IL-17 production (Fig. S4A). We found that CD44lo/intGFP/IL17+ cells expressed low but detectable levels of IL-15Rα (Fig. S4 B and C). The CD44lo/intGFP/IL17− population also expressed low levels of IL-15Rα. Interestingly, the cells that had already acquired a CD44highGFP/IL17+ γδ-17 phenotype in the neoThy expressed significantly higher IL-15Rα levels, similar to the γδ-17 cells identified in the WT adults (Fig. 1C).
Fig. S4.
γδ-17 precursors express IL-15Rα. Neonatal thymi were harvested from C57BL/6-Il17atm1Bcgen/J pups within the first 24 h after birth. (A) Representative dot plot showing CD44 expression vs. GFP/IL17 production in the CD3+GL3+TCRβ− γδ population. (B) Representative histograms depicting IL-15Rα expression on each of the indicated quadrants from A. The gray- filled histograms show IL-15Rα expression in the negative control sample, which lacked the primary IL-15Rα–biotin antibody but included the secondary streptavidin-PE-Texas red detection reagent. (C) The average MFI of IL-15Rα for each population. One-way ANOVA was used to determine significance. Data are representative of two independent experiments with nine neoThy per experiment.
Having shown that γδ-17 precursors express IL-15Rα, we proceeded to test our hypothesis that IL-15Rα could play a role in γδ-17 development in the neoThy. After stimulation with PMA/ionomycin, we examined the γδ T cells of the neoThy for CD44 expression and IL-17 production (Fig. S5). Importantly, we found no significant differences in the total number of thymocytes or γδ T cells among the WT, RKO, and RTg neonates (Fig. 5 A and B). The total size of the 15KO neoThy was significantly reduced only in comparison with WT controls (Fig. 5A), and there was a significant reduction in the total number of γδ T cells in the neoThy of 15KO mice compared with all of the other three genotypes (Fig. 5B); however, there was a significant increase in the percentage of IL-17+ cells within the CD44lo/int subset in the complete absence of IL-15Rα, as well as when cis signaling was ablated in RTg mice (Fig. 5C). This resulted in a significant increase in the total number of CD44lo/intIL-17+ γδ-17 precursors in the neoThy of both RKO and RTg mice compared with WT controls and 15KO mice (Fig. 5D). The percentage of IL-17+ cells within the CD44lo/int precursors was not significantly different between WT and 15KO mice, whereas the total number of cells with this phenotype was significantly reduced in 15KO mice compared with the WT controls (Fig. 5 C and D).
Fig. S5.
Identification of CD44lo/intIL-17+ γδ-17 precursors in the neonatal thymus. Representative dot plots showing CD44 expression and IL-17 production by the CD3+GL3+TCRβ− population in the neonatal thymus of WT, 15KO, RKO, and RTg mice. Numbers in the upper left quadrant indicate the average percentage of the total γδ cells that are CD44lo/intIL-17+ (Q1). The statistical significance between each pair of genotypes is presented in the table below. Note that owing to the unpredictable timing of birth, samples from each litter were collected independently on different days. Differences in the staining intensity of CD44 and IL-17A are not biologically significant and can be attributed to lot-to-lot variations in purchased antibodies, the use of antibodies conjugated to different fluorochromes, and variations in flow cytometer detection on different days and instruments. As a control for each independent experiment, a nonstimulated (brefeldin A only) control was included to promote consistency when drawing the quadrant gates.
Fig. 5.
γδ-17 precursors are increased in the absence of IL-15Rα and IL-15Rα signaling. Neonatal thymi were harvested from pups of the indicated genotypes within the first 24 h after birth. Cells were stimulated with PMA/ionomycin and brefeldin A for 5 h. (A) Total number of cells in the neonatal thymus for WT (circles), 15KO (inverted triangles), RKO (squares), and RTg (triangles) pups. (B) The total number of γδ T cells (CD3+GL3+TCRβ−). (C) Percentage of IL-17+ cells within the CD44lo/int gate. (D) Total number of CD44lo/intIL-17+ γδ T cells. (E) Cells were first gated on the CD44lo/intIL-17+ population to determine the total number of γδ-17 progenitors that were Vγ1.1−Vγ2−. In all graphs, the blue line indicates the mean for each group. Data are inclusive of 46 WT pups, 54 15KO pups, 48 RKO pups, and 38 RTg pups from five to eight individual litters per genotype. One-way ANOVA was used to determine significance. In E, WT, n = 36; 15KO, n = 52; RKO, n = 46; and RTg, n = 38. n.s., not significant.
As mentioned above, γδ-17 cells exhibit limited TCR use, restricted to Vγ2 and Vγ4 expression (16). Thus, to gain further insight into the dysregulation of γδ-17 development in the absence of IL-15 vs. the absence of IL-15Rα, we examined the CD44lo/intIL-17+ γδ-17 precursor population for Vγ expression. Although an antibody detecting Vγ4 has been used in some cases, here we used the Vγ1.1−Vγ2− population as an approximate means of identifying Vγ4+ cells, because Vγ3+ and Vγ5+ cells are largely absent from the thymus by this point in neonatal thymopoiesis (17). We found that the Vγ1.1−Vγ2− (∼Vγ4+) population was present in normal numbers in 15KO mice, but significantly increased in RKO and RTg neonates (Fig. 5E). Taken together, our data further support unique roles for IL-15 and IL-15Rα in the development of γδ-17 cells.
Discussion
IL-15 acts primarily as a survival cytokine for many subsets of immune cells, delivering a positive signal via transpresentation through γc and CD122. For this reason, 15KO and RKO mice have many similar deficiencies in distinct populations of lymphocytes, including NK cells, NK T cells, and CD8+ memory T cells (1, 2). Certain populations of γδ T cells also are unable to develop and/or survive when IL-15 transpresentation via IL-15Rα is absent. Under steady-state conditions, Vγ3+ dendritic epidermal T cells are absent from the skin, Vγ5+ CD8αα+ intraepithelial lymphocytes are reduced in the intestinal mucosa, and IFN-γ+ γδ T cells are decreased in the peritoneal cavity (1, 2, 12–14). Indeed, we also found that CD122+ IFN-γ–producing γδ T cells were absent from the pLNs of both 15KO and RKO mice. Interestingly, we have now shown that a second population of γδ T cells present in the pLNs, the γδ-17 cells, can develop in the absence of IL-15 or IL-15Rα.
Here we describe a novel difference between 15KO and RKO mice. We found that RKO mice had a global increase in γδ-17 cells and their individual levels of IL-17 production, whereas 15KO mice had minimal dysregulation of γδ IL-17 production, as was previously observed in the peritoneal cavity (14). We also identified unique differences between 15KO and RKO mice in the development of Vγ4+ (Vγ1.1−Vγ2−) γδ-17 cells at the site of their developmental origin, the neoThy. Thus, an interesting question raised by this work is how IL-15 itself contributes to γδ-17–cell development and homeostasis. Currently there are no other known binding partners of IL-15Rα; it is possible that an as-yet identified cytokine or receptor chain could interact with IL-15Rα to directly modulate the development of γδ-17 cells. One possibility is that its expression is restricted to the fetal period, which thus far has precluded identification of such a molecule.
Several other studies have identified novel differences between 15KO and RKO mice in terms of nonimmunologic parameters. Wu et al. (26) subjected RKO, 15KO, and γc KO mice to a series of tests designed to measure anxiety behavior, and found that RKO mice, but not 15KO or γc KO mice, displayed less anxiety in the open field test (as determined by a significant increase in the total number of inner grids crossed during a 5- min test interval). Similarly, RKO mice exhibited dramatically less fear and anxiety than 15KO mice on an elevated plus maze test. These findings suggest a distinct role for IL-15Rα in modulating anxiety-related behaviors that is independent of IL-15 and γc signaling. Because skeletal muscle is one of the most abundant sources of IL-15 mRNA (27), the roles of IL-15 and IL-15Rα also have been studied in the context of muscle physiology. Pistilli et al. (28) showed that fast muscles from RKO mice are significantly more resistant to fatigue compared with those from WT mice, whereas fast muscles from 15KO mice fatigue at a rate similar to those from controls. Interestingly, these authors also tested transgenic mice that overexpress IL-15 under the control of a skeletal muscle promoter and found no effects of increased IL-15 on muscle fatigue, further supporting the notion that IL-15 and IL-15Rα have distinct roles in vivo with regard to muscle function.
Whereas the studies discussed above have examined 15KO and RKO mice under steady-state conditions, IL-15 itself is often considered a proinflammatory cytokine, and its up-regulation has been noted in many autoimmune disorders (29). For example, IL-15 expression is increased in psoriatic lesions (30), and blocking the biological activity of IL-15 with a monoclonal antibody can reduce psoriasis severity (31). When modeled in vivo, IL-15 produced by dendritic cells induces the expansion of IL-17+ γδ T cells, and soluble IL-15Rα, proteolytically cleaved and shed from keratinocytes, functions as an IL-15 antagonist to blunt the effects of IL-15–mediated disease exacerbation (32). Thus, an opposing difference in disease severity between 15KO and RKO mice has been identified. Compared with WT controls, 15KO mice exhibit reduced inflammation, whereas RKO mice exhibit exacerbated inflammation. Given that Vγ2+ γδ T cells are largely responsible for IL-17 production in this model (13, 33), it would be informative to determine which Vγ subsets are present in the skin of naïve and inflamed 15KO and RKO mice, especially considering the distinct differences in the total number of γδ-17 precursor cells that we observed in the neoThy.
Contrary to the psoriasis model, three independent studies have shown that disease symptoms are exacerbated in 15KO mice compared with WT controls in the EAE mouse model of multiple sclerosis (34–36). Related to our findings, Pandiyan et al. (35) described a role of IL-15 as a negative regulator of CD4+ Th17 responses and determined, using an in vitro approach, that IL-15Rα expression on CD4+ T cells is required to mediate this effect. Similar to IL-2R signaling, IL-15Rα has the capacity to deliver a limiting signal to T cells through STAT5 (35), which can then oppose the IL-17–promoting effects of STAT3 (37). To our knowledge, no studies have directly compared EAE onset and severity in 15KO and RKO mice. Because γδ T cells also play a role in driving inflammation in EAE (38), it would be interesting to examine the contribution of Th17-derived IL-17 vs. γδ-derived IL-17 in both KO mouse strains, considering that we have now shown that signaling through IL-15Rα also limits the development of γδ-17 cells, leading to an increased population of these cells in RKO mice.
Although cis presentation of IL-15 has been demonstrated in vitro (7–9, 11), evidence of its activity in vivo is limited. Using RTg mice in which only cis signaling is ablated (10), we have shown a clear role for IL-15Rα signaling in cis in modulating the population of γδ-17 cells found in the pLNs of adult mice and in the neoThy. We considered the possibility that in the complete absence of IL-15Rα, available γc has the potential to increase its association with other cytokine receptors in the γc family. This could induce a positive developmental signaling pathway, perhaps through IL-2 or IL-7, because both IL-2Rα and IL-7Rα are expressed by γδ-17 cells (20). In the RTg mice, we would expect a normal distribution of γc among its family members, including the chimeric IL-15Rαext/IL-2Rαint protein. Given the similar increase in γδ-17 cells in the pLNs and neoThy of RTg and RKO mice, we believe that our data are more supportive of a model in which signaling through IL-15Rα limits γδ-17 development.
The study of γδ T-cell development has been complicated by the presence of unique subsets of γδ T cells with distinct developmental requirements and functions in vivo. The identification of γδ T cells producing IL-17 and details pertaining to their development have emerged only recently (14, 18, 25). Indeed, others have described unique developmental differences between the Vγ2+ and Vγ4+ γδ T-cell subsets in the dermis, despite their similar transcriptional profiles and common ability to produce IL-17 (16) and have shown that Vγ2+, but not Vγ4+, γδ T cells can be derived from adult bone marrow (39–41). Here we describe, to our knowledge, the first instance of a cytokine system whose absence enhances γδ-17–cell development. The increased γδ-17 population in RKO and RTg mice suggests that IL-15Rα could be exerting its effects on developing γδ-17 cells or their precursors before their appearance in the adult pLNs independent of IL-15 itself. Indeed, we found a significant increase in the total number of γδ-17 precursors in the neoThy of RKO and RTg mice compared with 15KO mice and WT controls. These disparate observations in 15KO and RKO mice are intriguing, especially because they are the first to be described in the context of lymphoid hematopoiesis. Unfortunately, at present, we lack the many of the fundamental details specifically regarding γδ-17 development that would allow us to further pinpoint the mechanism by which IL-15Rα limits the emergence of this population; for example, the upstream progenitor of the CD44lo/intIL-17+ γδ-17 precursors has yet to be identified. Exactly how γδ-17 cells (or their progenitors) exit the neoThy, settle in the pLNs, and restrict their circulation through individual lymph nodes is currently unknown. When considering the origins of individual lymph nodes during embryogenesis, it has been shown that the mLNs develop at least 1 wk before the window of γδ-17 development, whereas the brachial, axillary, and inguinal lymph nodes develop only days before or simultaneously with the γδ-17 population (42); thus, it is possible that this developmental order itself or the stromal elements unique to the environment of the pLNs vs. the mLNs could impact the ability of γδ-17 cells to seed or be maintained in individual secondary lymphoid tissues. In addition, although γδ-17 cells do not normally develop from progenitors in the adult bone marrow (18, 24), it is possible that in the absence of IL-15Rα signaling, the window of γδ-17–cell development is extended, allowing for the observed increases in the total population. Thus, further studies are needed to determine (i) how IL-15Rα signaling restricts γδ-17 development and homeostasis, (ii) whether alternative signaling networks can promote γδ-17 development in the absence of IL-15Rα, and (iii) if IL-5Rα has the capacity to interact with a novel ligand to drive downstream effects independent of IL-15. Similar to Th17 cells, it is likely that a balance exists between opposing extrinsic signals that either inhibit or promote γδ-17 development (37), and here we show that the effects of IL-15Rα signaling should be considered in mediating that balance.
Materials and Methods
Mice.
WT C57BL/6 mice (both CD45.2+ and CD45.1+) were either purchased directly from the National Cancer Institute/Charles River Laboratories or maintained in our facility from breeders purchased from the same vendor. IL-15 and IL-15Rα KO mice, originally obtained from Dr. Jacques Peschon (Immunex) and Averil Ma (University of California San Francisco), were maintained in our facility (1, 2). Cryopreserved sperm from RTg mice (10) were obtained from the Mutant Mouse Regional Resource Center at the University of North Carolina, and mice were rederived by the Gene Targeting and Transgenic Facility at UConn Health. C57BL/6-Il17atm1Bcgen/J mice were purchased from The Jackson Laboratory and bred in our facility. All adult mice were analyzed at age 9–14 wk. All experiments were performed in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee of UConn Health.
Cell Isolation and Stimulation.
Tissues were harvested, minced, subjected to collagenase digestion for 30 min at 37°, and crushed through a 70-μm filter to obtain single-cell suspensions. pLN cells were pooled from inguinal, brachial, and axillary nodes. Liver cells were further separated using a Percoll gradient. Cells were counted with a Vi-Cell XR cell viability analyzer (Beckman Coulter). To detect intracellular IL-17 production, 5 × 106 cells were stimulated in 96-well round-bottom plates for 5 h in harvest medium (RPMI supplemented with 5% bovine serum and Hepes, GlutaMAX, penicillin/streptomycin, and gentamyacin) with Leukocyte Activation Cocktail with GolgiPlug (PMA, ionomycin, and brefeldin A) or GolgiPlug alone (BD Pharmingen) as a negative control. For the detection of IFN-γ, the harvest medium used for the 5-h stimulation was produced with Iscove's Modified Dulbecco's Medium supplemented as above.
Flow Cytometry.
The following antibodies directly conjugated to various fluorochromes were used to stain 5–10 × 106 cells: CD3, CD25, CD27, CD44, CD45.1, CD45.2, CD122, CD132, γδ TCR (clone GL3), TCRβ, Vγ1.1, and Vγ2. Antibodies were purchased from BD Biosciences, BioLegend, and eBioscience. All samples were treated with Fc block (anti-CD16/32; clone 2.4G2; Bio X Cell). To detect IL-15Rα expression, cells were stained with polyclonal goat IgG anti-murine IL-15Rα-biotin (R&D Systems) alone for 30 min at 4° C, followed by streptavidin-PE-Texas red (BD Biosciences). Cells were fixed with 2% PFA. For intracellular cytokine staining, cells were fixed and permeabilized with the Cytofix/Cytoperm Kit (BD Biosciences) and stained with antibodies specific for IL-17A or IFN-γ. All data were collected using an LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star). For all analyses, cells were first gated based on forward/side scatter parameters and exclusion of a live/dead dye.
Generation of Mixed Neonatal Thymocyte Chimeras and neoThy Analysis.
WT (CD45.1+ x CD45.2+) and RKO (CD45.1+ x CD45.1+) mice were mated overnight (<18 h) in our facility, and once visibly pregnant, monitored every 12 h for delivery. As described by Gray et al. (24), thymi were isolated from pups within 72 h after birth. Neonatal thymocytes from WT (CD45.1/2+) and RKO (CD45.1+) mice were mixed at a 1:1 ratio, and a total of 5–10 × 106 cells were transferred retro-orbitally to lethally irradiated (1200 rads) WT recipients (CD45.2+). The next day, recipient mice received 2 × 106 WT CD45.2+ bone marrow cells. Recipient mice were allowed to reconstitute for at least 7 wk. For direct analysis of WT neonatal thymocytes, some litters were derived from timed pregnant C57BL/6 mice (National Cancer Institute/Charles River Laboratories) that were delivered to our facility at E13.5 and then monitored every 24 h. The 15KO, RKO, RTg, and C57BL/6-Il17atm1Bcgen/J mice were bred in our facility and monitored every 24 h.
Statistical Significance.
All statistical analyses were performed using GraphPad Prism software. Either the Student t test or one-way ANOVA followed by a Tukey posttest was used, as indicated in the figure legends. For all figures, *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 unless noted otherwise.
Acknowledgments
We thank Quynh-Mai Pham for assistance with these experiments, along with Dr. Evan Jellison (UConn Health Flow Cytometry Facility). This work was supported by National Institutes of Health Grants AI051583 and AI056172 and a postdoctoral fellowship from the American Cancer Society (Grant PF-11-152-01-LIB, to S.L.C.).
Footnotes
Conflict of interest statement: L.L. once had and L.P. currently has financial interests in BioLegend and eBioscience. The authors have no additional financial interests.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1420741112/-/DCSupplemental.
References
- 1.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lodolce JP, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9(5):669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 3.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 in trans to neighboring cells. Immunity. 2002;17(5):537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 4.Burkett PR, et al. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200(7):825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandau MM, Schluns KS, Lefrancois L, Jameson SC. Cutting edge: Transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15R alpha by the same cells. J Immunol. 2004;173(11):6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- 6.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205(5):1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen SK, et al. Crystal structure of the interleukin-15⋅interleukin-15 receptor alpha complex: Insights into trans and cis presentation. J Biol Chem. 2007;282(51):37191–37204. doi: 10.1074/jbc.M706150200. [DOI] [PubMed] [Google Scholar]
- 8.Ota N, Takase M, Uchiyama H, Olsen SK, Kanagawa O. No requirement of trans presentations of IL-15 for human CD8 T cell proliferation. J Immunol. 2010;185(10):6041–6048. doi: 10.4049/jimmunol.0901834. [DOI] [PubMed] [Google Scholar]
- 9.Rowley J, Monie A, Hung CF, Wu TC. Expression of IL-15RA or an IL-15/IL-15RA fusion on CD8+ T cells modifies adoptively transferred T-cell function in cis. Eur J Immunol. 2009;39(2):491–506. doi: 10.1002/eji.200838594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z, et al. The IL-15 receptor alpha chain cytoplasmic domain is critical for normal IL-15Ralpha function but is not required for trans presentation. Blood. 2008;112(12):4411–4419. doi: 10.1182/blood-2007-03-080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro I, Yu A, Dee MJ, Malek TR. The basis of distinctive IL-2– and IL-15–dependent signaling: Weak CD122-dependent signaling favors CD8+ T central-memory cell survival but not T effector-memory cell development. J Immunol. 2011;187(10):5170–5182. doi: 10.4049/jimmunol.1003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Creus A, et al. Developmental and functional defects of thymic and epidermal V gamma 3 cells in IL-15–deficient and IFN regulatory factor-1–deficient mice. J Immunol. 2002;168(12):6486–6493. doi: 10.4049/jimmunol.168.12.6486. [DOI] [PubMed] [Google Scholar]
- 13.Pantelyushin S, et al. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J Clin Invest. 2012;122(6):2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibata K, et al. Identification of CD25+ gamma delta T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol. 2008;181(9):5940–5947. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 15.Kisielow J, Kopf M. The origin and fate of γδT cell subsets. Curr Opin Immunol. 2013;25(2):181–188. doi: 10.1016/j.coi.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Narayan K, et al. Immunological Genome Project Consortium Intrathymic programming of effector fates in three molecularly distinct γδ T cell subtypes. Nat Immunol. 2012;13(5):511–518. doi: 10.1038/ni.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335(6189):443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 18.Haas JD, et al. Development of interleukin-17–producing γδ T cells is restricted to a functional embryonic wave. Immunity. 2012;37(1):48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Haas JD, et al. CCR6 and NK1.1 distinguish between IL-17A and IFN-gamma–producing gammadelta effector T cells. Eur J Immunol. 2009;39(12):3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 20.Baccala R, et al. Gamma delta T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J Immunol. 2005;174(8):4606–4612. doi: 10.4049/jimmunol.174.8.4606. [DOI] [PubMed] [Google Scholar]
- 21.Jensen KD, et al. Thymic selection determines gammadelta T cell effector fate: Antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29(1):90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribot JC, et al. CD27 is a thymic determinant of the balance between interferon gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10(4):427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schluns KS, Williams K, Ma A, Zheng XX, Lefrançois L. Cutting edge: Requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168(10):4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 24.Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17–producing gammadelta T cell population in the dermis. J Immunol. 2011;186(11):6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Do JS, et al. Cutting edge: Spontaneous development of IL-17–producing gamma delta T cells in the thymus occurs via a TGF-beta 1–dependent mechanism. J Immunol. 2010;184(4):1675–1679. doi: 10.4049/jimmunol.0903539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, et al. Essential role of interleukin-15 receptor in normal anxiety behavior. Brain Behav Immun. 2010;24(8):1340–1346. doi: 10.1016/j.bbi.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabstein KH, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264(5161):965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 28.Pistilli EE, et al. Loss of IL-15 receptor α alters the endurance, fatigability, and metabolic characteristics of mouse fast skeletal muscles. J Clin Invest. 2011;121(8):3120–3132. doi: 10.1172/JCI44945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Sabatino A, Calarota SA, Vidali F, Macdonald TT, Corazza GR. Role of IL-15 in immune-mediated and infectious diseases. Cytokine Growth Factor Rev. 2011;22(1):19–33. doi: 10.1016/j.cytogfr.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Ong PY, et al. Decreased IL-15 may contribute to elevated IgE and acute inflammation in atopic dermatitis. J Immunol. 2002;168(1):505–510. doi: 10.4049/jimmunol.168.1.505. [DOI] [PubMed] [Google Scholar]
- 31.Villadsen LS, et al. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J Clin Invest. 2003;112(10):1571–1580. doi: 10.1172/JCI18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouchaud G, et al. Epidermal IL-15Rα acts as an endogenous antagonist of psoriasiform inflammation in mouse and man. J Exp Med. 2013;210(10):2105–2117. doi: 10.1084/jem.20130291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai Y, et al. Pivotal role of dermal IL-17–producing γδ T cells in skin inflammation. Immunity. 2011;35(4):596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez-Nicola D, Spagnolo A, Guaza C, Nieto-Sampedro M. Aggravated experimental autoimmune encephalomyelitis in IL-15 knockout mice. Exp Neurol. 2010;222(2):235–242. doi: 10.1016/j.expneurol.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 35.Pandiyan P, et al. The role of IL-15 in activating STAT5 and fine-tuning IL-17A production in CD4 T lymphocytes. J Immunol. 2012;189(9):4237–4246. doi: 10.4049/jimmunol.1201476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu P, Bamford RN, Waldmann TA. IL-15–dependent CD8+ CD122+ T cells ameliorate experimental autoimmune encephalomyelitis by modulating IL-17 production by CD4+ T cells. Eur J Immunol. 2014;44(11):3330–3341. doi: 10.1002/eji.201444675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang XP, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12(3):247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petermann F, et al. γδ T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23–dependent mechanism. Immunity. 2010;33(3):351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray EE, et al. Deficiency in IL-17–committed Vγ4(+) γδ T cells in a spontaneous Sox13-mutant CD45.1(+) congenic mouse substrain provides protection from dermatitis. Nat Immunol. 2013;14(6):584–592. doi: 10.1038/ni.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malhotra N, et al. Immunological Genome Project Consortium A network of high-mobility group box transcription factors programs innate interleukin-17 production. Immunity. 2013;38(4):681–693. doi: 10.1016/j.immuni.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai Y, et al. Differential developmental requirement and peripheral regulation for dermal Vγ4 and Vγ6T17 cells in health and inflammation. Nat Commun. 2014;5:3986. doi: 10.1038/ncomms4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184(5):1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]