Significance
G protein-coupled receptors constitute an important class of receptors and represent a family of choice for therapeutic interventions for multiple diseases and disorders. One of these metabotropic glutamate receptors, mGluR5, is highly relevant because of its biological importance for various medical conditions, including major depressive disorder (MDD). In a search of mGluR5 modulators we identified Norbin as a key regulator. Here we demonstrate in vivo that Norbin significantly influences hippocampal neurogenesis and that both the proliferation and survival of newborn neurons are impaired in the absence of Norbin. Furthermore, the use of three well-established behavioral paradigms indicated that Norbin-KO mice display depressive-like characteristics. Thus interventions that could boost Norbin function might be beneficial to treat MDD symptoms.
Keywords: Norbin, mGluR5, major depressive disorder, neurogenesis, glutamate
Abstract
Adult neurogenesis in the hippocampus subgranular zone is associated with the etiology and treatment efficiency of depression. Factors that affect adult hippocampal neurogenesis have been shown to contribute to the neuropathology of depression. Glutamate, the major excitatory neurotransmitter, plays a critical role in different aspects of neurogenesis. Of the eight metabotropic glutamate receptors (mGluRs), mGluR5 is the most highly expressed in neural stem cells. We previously identified Norbin as a positive regulator of mGluR5 and showed that its expression promotes neurite outgrowth. In this study, we investigated the role of Norbin in adult neurogenesis and depressive-like behaviors using Norbin-deficient mice. We found that Norbin deletion significantly reduced hippocampal neurogenesis; specifically, the loss of Norbin impaired the proliferation and maturation of newborn neurons without affecting cell-fate specification of neural stem cells/neural progenitor cells (NSCs/NPCs). Norbin is highly expressed in the granular neurons in the dentate gyrus of the hippocampus, but it is undetectable in NSCs/NPCs or immature neurons, suggesting that the effect of Norbin on neurogenesis is likely caused by a nonautonomous niche effect. In support of this hypothesis, we found that the expression of a cell–cell contact gene, Desmoplakin, is greatly reduced in Norbin-deletion mice. Moreover, Norbin-KO mice show an increased immobility in the forced-swim test and the tail-suspension test and reduced sucrose preference compared with wild-type controls. Taken together, these results show that Norbin is a regulator of adult hippocampal neurogenesis and that its deletion causes depressive-like behaviors.
Major depressive disorder (MDD) is one of the most common and debilitating psychiatric illnesses with a lifetime prevalence of ∼17% (1). Clinical symptoms of MDD include anhedonia, depressed mood, helplessness, and cognitive disruption. Because of the clinical and etiological heterogeneity of MDD, the pathophysiology of MDD remains elusive (2), and the biological underpinnings remain unclear. Decades of research have clearly established that various neurotransmitters, especially monoamine neurotransmitters, as well as neurotrophic factors, contribute to MDD (3). More recently glutamatergic signaling also has been linked to MDD. With the confirmation of continuous neurogenesis in adult brains of most mammals, including humans, a neurogenic hypothesis of MDD has been put forward and continues to drive research (4).
The neurogenic hypothesis of MDD is based on several correlative studies. Brain imaging and postmortem studies of MDD patients suggest that reduction in hippocampal volume could be reflective of reduced neurogenesis in addition to mature neuronal cell loss (5, 6). Antidepressant treatment in animals increases hippocampal neurogenesis with a lag time that is reminiscent of the delayed onset of antidepressant efficacy typically observed in humans (7). In addition, stress, a common risk factor for depression, inhibits neurogenesis in nonhuman primates, and this phenomenon can be corrected by antidepressant treatment (8). In rodents, ablation of hippocampal neurogenesis causes a depressive phenotype and abolishes the effect of certain antidepressants (9). Therefore, it is hypothesized that reduced adult hippocampal neurogenesis may underlie the pathological mechanism of MDD and that an up-regulation of neurogenesis would oppose the action of stress and/or depression, having a potential therapeutic benefit for MDD.
In the hippocampus, adult neurogenesis involves the five following steps: (i) proliferation of neural stem/progenitor cells (NSCs/NPCs) in the subgranular zone (SGZ) of the dentate gyrus (DG); (ii) fate specification (neurons versus astrocytes); (iii) massive loss of progenitor cells; (iv) neuronal differentiation and maturation; and (v) ultimate integration to the existing neuronal circuitry of the DG (10). Each step is modulated by both physiological stimuli and pathophysiological conditions. Two important determinants, cell-autonomous factors and neurogenic niche, play a key role in determining the ultimate outcome of adult neurogenesis. Cell-autonomous factors are intrinsic NSC/NPC genetic traits that dictate their fate, and the neurogenic niche corresponds to their local microenvironment defined by various parameters (e.g., neuronal inputs, vasculature, and glia composition) that interact with NSCs/NPCs and also influence their fate (11).
Previous studies indicated that neurotransmitter glutamate regulates DG neurogenesis (12, 13). Of the eight metabotropic glutamate receptors (mGluRs), mGluR5 is most highly expressed in NSCs (14, 15). In vivo activation of mGluR5 receptors benefits the proliferation and/or survival of NSCs in the hippocampus. In vitro, pharmacological blockade of mGluR5 reduced NSC proliferation and survival, whereas activation of mGluR5 receptors substantially enhanced cell proliferation, suggesting that mGluR5 has a cell-autonomous role of in neurogenesis (16, 17).
We previously identified Norbin as a positive regulator of mGluR5 (18). Forebrain-specific Norbin-KO mice display phenotypic characteristics that resemble phenotypes observed in cases of reduced mGluR5 activity, including impaired synaptic plasticity and sensitivity to psychostimulants. In the CNS, Norbin is a neuronal gene whose expression is up-regulated concomitantly with long-term potentiation, the electrophysiological mechanism underlying learning and memory. Norbin expression correlates with neurite outgrowth and also was found to have an impact on both the quantity and the length of neurites in cultured neuroblastoma N2a cells. In summary, Norbin (i) regulates mGluR5, whose activity affects DG neurogenesis; (ii) promotes neurite outgrowth; (iii) is highly expressed in the DG of the hippocampus, the niche for adult DG neurogenesis. Norbin-KO mice also display impaired cognitive functions. For all these reasons, we investigated the role of Norbin in adult hippocampal neurogenesis and whether the loss of Norbin could contribute to depression-like behaviors.
We found here that neuron-specific Norbin ablation in mice, using two distinct Cre systems, results in a significant reduction in hippocampal neurogenesis. Both the proliferation and survival of newborn neurons are impaired. This impairment is likely the result of a nonautonomous niche effect because Norbin expression is limited to mature granular neurons in the DG. In addition, gene-expression analysis of Norbin-KO mice compared with wild-type controls indicated that Norbin deletion results in a significant reduction in desmoplakin (DSP) expression. In light of the observed reduction in adult hippocampal neurogenesis in Norbin-KO mice, we used three well-established behavioral paradigms to determine if there also was a depressive-like consequence of Norbin KO. Results from the forced-swim test (FST), the tail-suspension test (TST), and the sucrose preference test indicated that Norbin-KO mice display depressive-like characteristics.
Results
Adult Hippocampal Neurogenesis Is Significantly Impaired in Norbin-KO Mice.
To test whether Norbin plays a role in adult neurogenesis, we analyzed the impact of genetic ablation of Norbin on SGZ neurogenesis in the hippocampus. For this purpose, we crossed a genetically modified mouse line harboring lox-P sites flanking exon 3 and 4 of the Norbin gene with a mouse line expressing the Cre recombinase under the calcium/calmodulin-dependent protein kinase II α (CAMK2α) promoter. This CAMK2α-Cre line causes specific Norbin deletion in neurons of the forebrain, mostly postnatally (18).
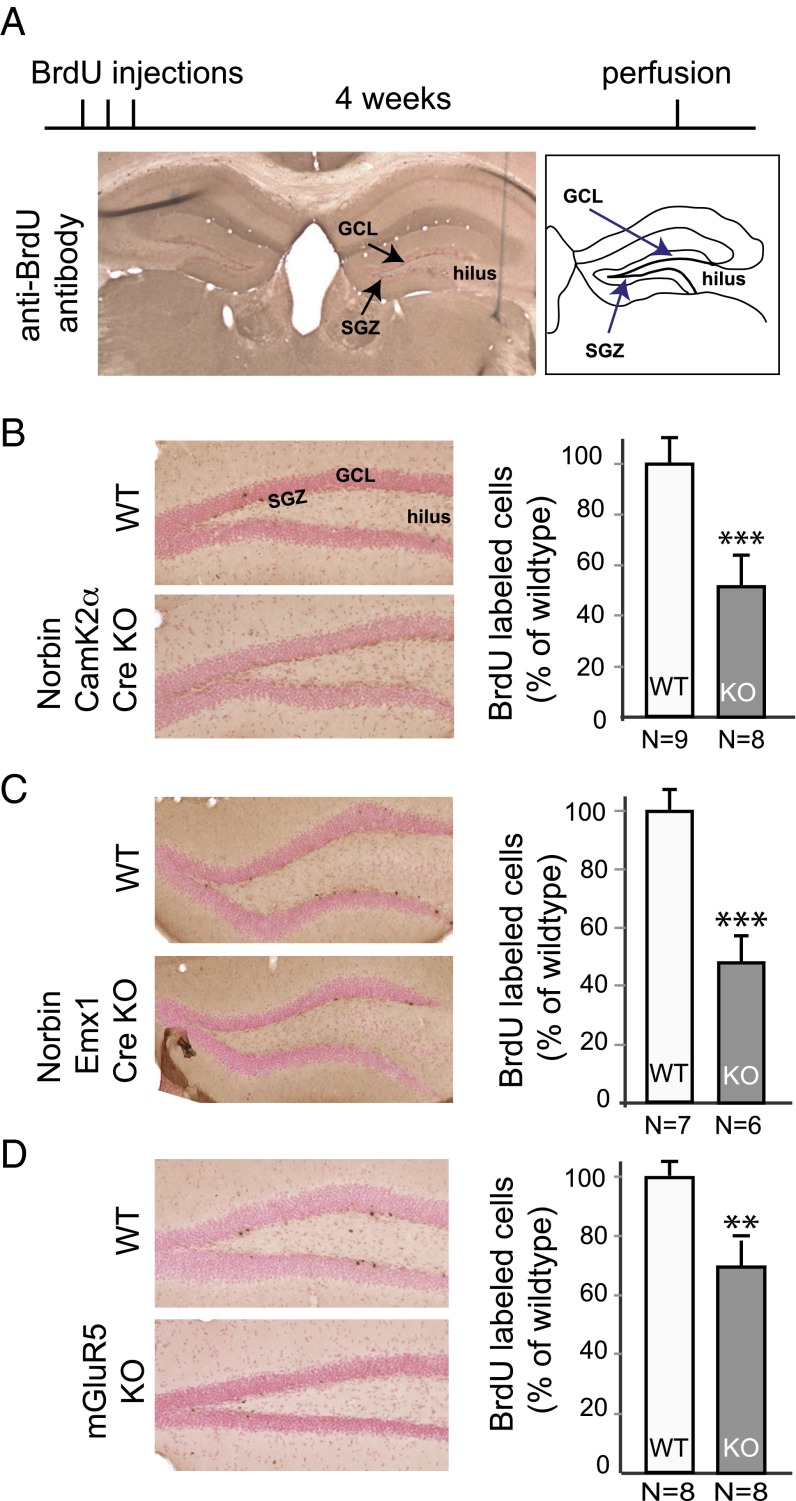
Two-month-old mice were injected with BrdU to label adult-born neurons. Four weeks later, mouse brains were fixed and analyzed by immunocytochemistry using a BrdU-specific antibody (Fig. 1A). The number of labeled neurons in the SGZ was evaluated by stereological cell counting. BrdU+ neurons were dramatically reduced in Norbin-KO mice compared with wild-type controls (51.4 ± 11.1% of wild type, P < 0.001) (Fig. 1B). To confirm this result, we generated a second Norbin-KO line using the empty spiracles homeobox 1 (Emx1)-Cre line, which caused specific Norbin deletion in the cortical and hippocampal pyramidal neurons (19). Norbin flox/flox-Emx1-Cre mice also showed significant reduction in adult hippocampal neurogenesis compared with wild-type controls (47.8 ± 8.2% of wild type, P < 0.001) (Fig. 1C).
Fig. 1.
Genetic deletion of Norbin or mGluR5 impairs adult hippocampal neurogenesis. (A) Experimental diagram of the adult neurogenesis study. Two-month-old mice received i.p. BrdU injections (100 mg/kg) twice a day for three consecutive days. Four weeks later, mice were perfused with a PFA solution, and brain slices were analyzed by immunocytochemistry using an anti-BrdU antibody. BrdU+ cells in the DG region were counted using stereologic methods. GCL, granule cell layer; SGZ, subgranular zone. (B) Norbin-KO mice (CamK2α-Cre) show impaired neurogenesis in the hippocampus DG. (Left) Representative images of the BrdU+ cells in the DG of wild-type (n = 9) and Norbin-KO mice (n = 8). (Right) Quantification of total numbers of BrdU+ cells. Values are shown as means ± SEM, ***P < 0.001. (C) Norbin-KO mice (EMX1-Cre) show impaired neurogenesis in the hippocampus. (Left) Representative images of BrdU+ cells in the DG of wild-type (n = 7) and Norbin-KO (n = 6) mice. (Right) Quantification of total numbers of BrdU+ cells. Values are shown as means ± SEM, ***P < 0.001. (D) mGluR5-KO mice show impaired neurogenesis in the hippocampus. (Left) Representative images of the BrdU+ cells in the DG of wild-type (n = 8) and mGluR5-KO mice (n = 8). (Right) Quantification of total numbers of BrdU+ cells. Values are shown as means ± SEM, *P < 0.01.
Because Norbin is a positive regulator of mGluR5, and considering the role of mGluR5 in NSC proliferation (15), we next tested how mGluR5 affects the survival of adult-born neurons using mGluR5-KO mice. As in Norbin-KO mice, at 4 wk after BrdU injection the number of neurons was significantly reduced in mGluR5-KO mice compared with wild-type controls (70.0 ± 5.5% of wild type, P < 0.01) (Fig. 1D).
Surface and volume measurements of the DG, the granule cell layer, and the subventricular zone/hilus did not reveal significant differences in size when comparing the Norbin flox/flox-CAMK2α-Cre line or the Norbin flox/flox-Emx1-Cre mice with control mice.
Hippocampal NSC Proliferation Is Decreased in Norbin-KO Mice.
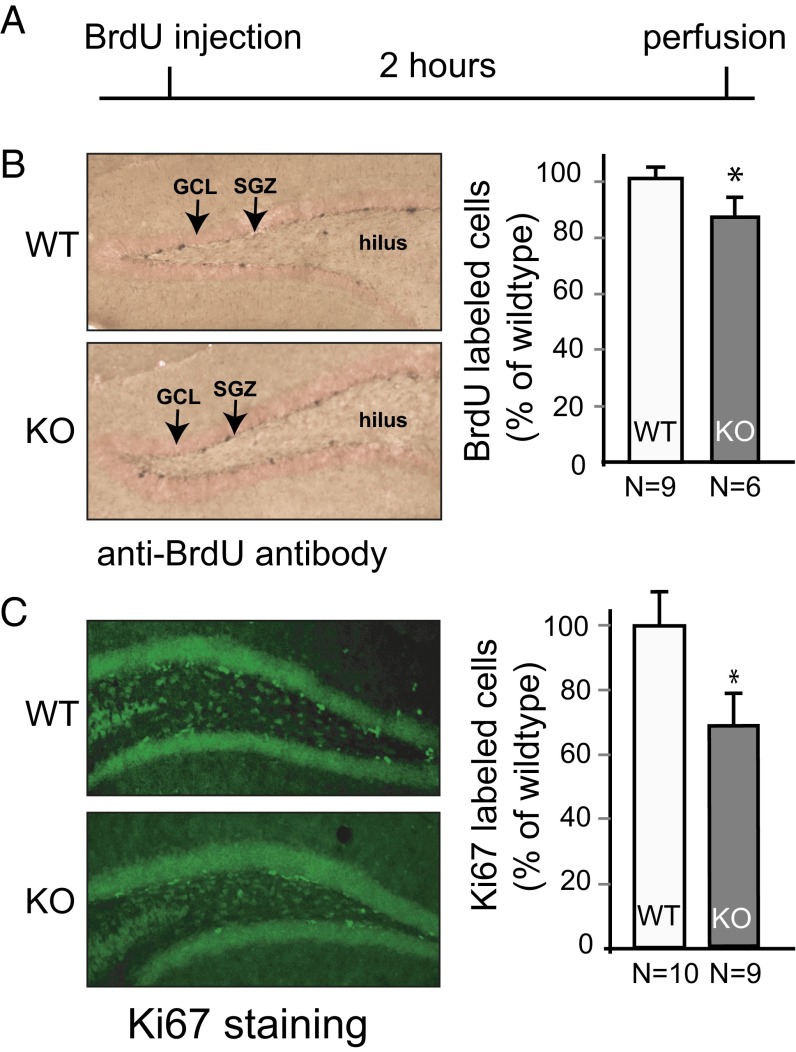
At least three steps are involved in the overall 4-wk survival of adult-born neurons, and the disruption of any of these steps could lead to the results observed in Norbin-KO mice. These steps include (i) NSC proliferation; (ii) NSC/NPC cell-fate specification (either toward hippocampal neurons or astrocytes); and (iii) survival, maturation, and migration of young neurons. To determine at which step(s) Norbin KO contributes to the observed reduction of neurogenesis, we first quantified the proliferation of NSCs/NPCs in vivo in wild-type and Norbin-KO mice 2 h after BrdU injection. Mice were perfused, and BrdU+ cells were counted (Fig. 2A). We observed fewer proliferating SGZ cells in Norbin-KO mice than in wild-type controls (85.9 ± 4.9% of wild type, P < 0.05) (Fig. 2B). To validate this observation, and to avoid potential injection-induced stress which might affect hippocampal cell proliferation, a different cohort of mice was used to evaluate cell proliferation using the endogenous mitotic marker Ki-67 by immunolabeling. Similar results were obtained with this alternative technique (78.4 ± 8.2% of wild type) (Fig. 2C).
Fig. 2.
Hippocampal proliferation of NPCs/NSCs is impaired in Norbin-KO mice. (A) Experimental diagram of the proliferation assay of adult neurogenesis. Two-month-old mice were injected with BrdU (i.p. 100 mg/kg). Mice were perfused 2 h later, and brain slices were analyzed by immunocytochemistry using an anti-BrdU antibody. (B) Norbin-KO mice show reduced proliferation of NPCs/NSCs. (Left) Representative images of the BrdU+ cells in the DG of wild-type (n = 9) or Norbin-KO mice (n = 6). GCL, granule cell layer; SGZ, subgranular zone. (Right) Quantification of total numbers of BrdU+ cells. (C) A different cohort of 2-mo-old wild-type (n = 10) and Norbin-KO mice (n = 9) were perfused without injections, and Ki-67 immunofluorescence staining was performed. (Left) Representative images of Ki-67+ cells in the DG are shown. (Right) Quantification of total numbers of Ki67+ cells. Values are shown as means ± SEM, *P < 0.05.
Norbin Deletion Does Not Affect Cell-Fate Specification of NSCs/NPCs.
In the hippocampus, SGZ NSCs/NPCs can differentiate into neurons or astrocytes. We next evaluated the role of Norbin in the fate specification of SGZ NSCs/NPCs, asking if the reduced neurogenesis could be caused by abnormal astrogenesis. Four weeks after BrdU labeling, hippocampal slices of wild-type mice and Norbin-KO mice were labeled with neuronal marker NeuN and the astrocyte marker GFAP (Fig. 3A, Upper). About 81.3% of BrdU+ cells are NeuN+ in Norbin-KO mice, similar to the percentage in wild-type mice (83.6%), suggesting that Norbin ablation did not affect the fate determination of NSCs or NPCs (Fig. 3A, Lower).
Fig. 3.

Norbin deletion does not affect fate specification of NSCs/NPCs. (A) Four weeks after BrdU injection, brain slices were immunostained with anti-BrdU, NeuN, and GFAP antibodies. (Left) Representative confocal images of the BrdU and NeuN double-positive cells in the DG. (Right) Quantification of BrdU and NeuN double-positive mature neurons in the DG of wild-type (n = 3) and Norbin-KO (n = 3) mice 4 wk after BrdU injection. (B) Norbin-KO mice show significantly fewer immature neurons (Dcx+), presenting a lower dendritic density. Two-month-old wild-type (n = 6) and Norbin-KO (n = 6) mice were perfused, and brain slices were analyzed by immunostaining with anti-Dcx antibody. (Left) Representative confocal images of Dcx+ immature neurons in the adult brain of wild-type or Norbin-KO mice. (Right) Fluorescence intensity was quantified using Image J software in the SGZ and in the DG region. Values are shown as means ± SEM. **P < 0.01, ***P < 0.001.
Norbin-KO Mice Show Significantly Reduced Immature Neurons.
To test whether Norbin affects the maturation of adult-born neurons, hippocampal slices of wild-type and Norbin-KO mice were immunolabeled with doublecortin (Dcx), which is expressed specifically in immature neurons (Fig. 3B, Upper). We quantified the Dcx signal by measuring the fluorescence intensity in the SGZ and DG region. Significantly fewer Dcx+ cells and lower dendritic density were found in Norbin-KO mice than in wild-type controls, suggesting that neuronal maturation is impaired in the absence of Norbin (Fig. 3B, Lower).
Mature Granular Neurons of the Hippocampal DG Express Norbin.
The results described so far highlight the importance of Norbin in adult hippocampal neurogenesis. We next investigated whether the effects of Norbin on neurogenesis are a cell-autonomous (NSC/NPC) or a non–cell-autonomous neurogenic niche effect.
We first tested whether Norbin is expressed in the NSCs/NPCs. Coimmunostaining with antibodies directed toward Norbin and cell-type–specific markers was performed. Norbin protein was detected throughout the mature granular neurons that express NeuN but not in immature neurons that express Dcx or in proliferating cells or astrocytes that express GFAP (Fig. 4A). To examine the possibility that the absence of Norbin in the neurogenic zone results from the low sensitivity of the Norbin antibody for immunostaining, we analyzed Norbin expression by Western blotting and RT-PCR. Norbin is highly expressed by hippocampal extracts but not by cultured neurospheres that contain NSCs/NPCs as indicated by sex-determining region Y-box 2 (Sox2) expression. In contrast, mGluR5 is expressed by cultured neurospheres, as previously reported (Fig. 4B). In line with our Western blot results, Norbin expression also is undetectable in neurospheres by RT-PCR analysis (Fig. 4C). These results indicate that Norbin is scarcely expressed, if at all, by NSCs/NPCs and therefore suggest that Norbin has a non–cell-autonomous effect on adult neurogenesis.
Fig. 4.
Norbin is enriched in mature DG granular neurons. (A) Confocal images of the DG of an adult mouse brain, coimmunostained for Norbin (green) and NeuN (red), Dcx (red), or GFAP (red). Norbin localizes with mature (NeuN+) neurons but not with immature neurons (Dcx+) or glia (GFAP+). (B) Western blotting detection of Norbin and mGluR5 in adult mouse hippocampal tissues and cultured neurospheres (NS). Protein extracts (20 µg) isolated from wild-type and Norbin-KO mice and mouse embryonic day 14 (E14) neurospheres were analyzed by Western blotting using antibodies against Norbin, mGluR5, β-actin, or Sox2. (C) RT-PCR detection of Norbin and Sox2 transcripts in adult mouse hippocampal tissues and cultured neurospheres. mRNAs were isolated from wild-type and Norbin-KO mice and from mouse E14 neurospheres. RT-PCR was performed using TaqMan gene-expression assays. ***P < 0.001.
The Expression of DSP, a Cell–Cell Contact Gene, Is Reduced in Norbin-KO Mice.
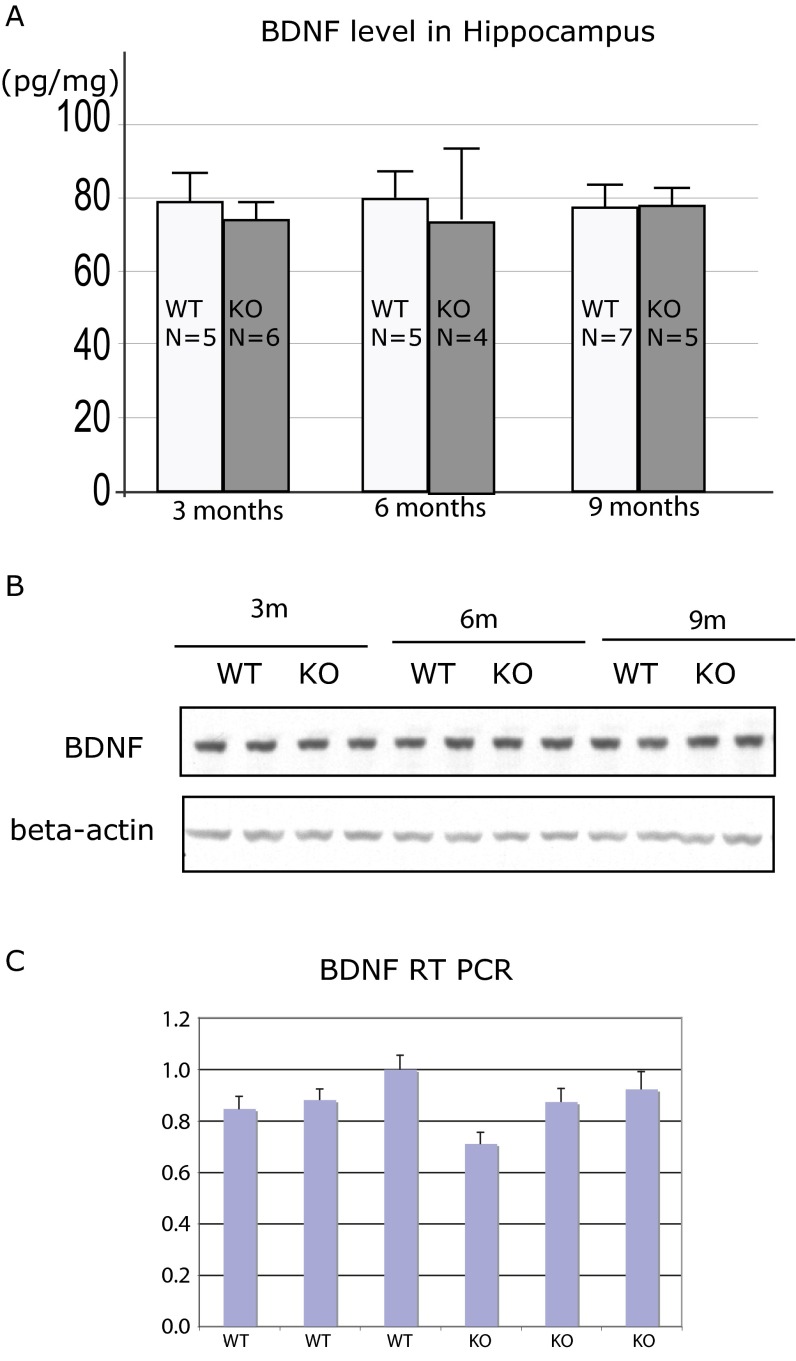
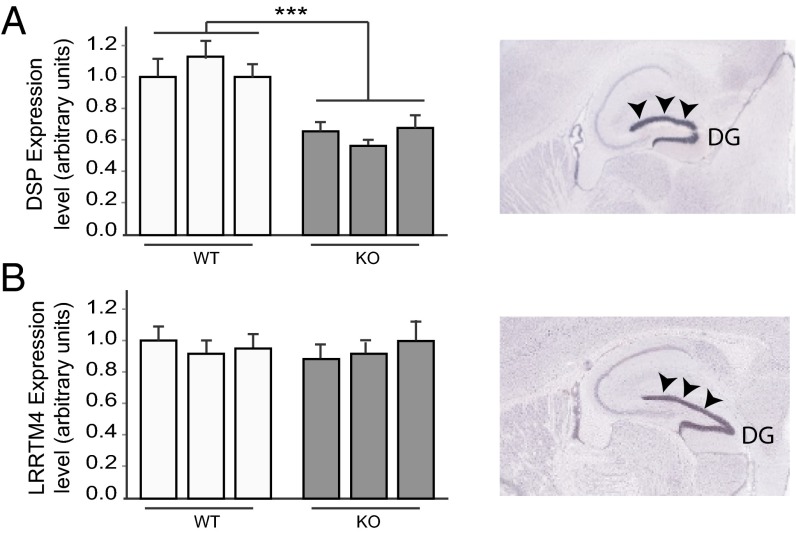
One well-established factor affecting adult neurogenesis is BDNF. We therefore tested whether the level of BDNF expression is changed in Norbin-KO mice. We isolated BDNF from 3-, 6-, and 9-mo-old animals and tested the BDNF level by ELISA, Western blotting, and RT-PCR. No changes were detected (Fig. S1). To pinpoint how Norbin KO affects the neurogenic niche, we performed DG gene-expression profiling of wild-type and Norbin-KO mice. Fewer than 30 genes were found to be differentially expressed. Out of this pool of genes tested by quantitative PCR, only a few were significantly affected. Among the significantly differentially expressed genes, DSP was found to be the most affected, with a 42% reduction observed in Norbin-KO mice. The other genes involved in the housekeeping function were not relevant for this study and were not studied further. DSP is an obligatory component of functional desmosomes, the highly organized adhesive intercellular junction that couples intermediate filaments to the cell surface at a site of cell–cell adhesion. Mutation in DSP leads to defective cell–cell junctions and causes cardiomyopathies and keratodermas as well as the autoimmune disease paraneoplastic pemphigus in humans. Remarkably, in the brain, DSP is expressed specifically in the DG (Fig. 5A) (20) although its function in the CNS has not been explored. The reduced DSP expression in Norbin-KO mice was confirmed by RT-PCR using three wild-type and three KO animals. An approximately 40% reduction in DSP expression was observed (Fig. 5A, Left). The expression of LRRTM4, another DG-specific gene, was not affected (Fig. 5B), indicating that the DSP reduction in Norbin KO is not caused by general abnormal gene expression in DG.
Fig. S1.
The BDNF level is normal in Norbin-KO mice. Hippocampi of 3-, 6-, or 9-mo-old wild-type and Norbin-KO mice were dissected, and protein or RNA was isolated. (A) BDNF ELISA was performed using the Amersham kit according to the manufacturer’s instructions. (B) Proteins were analyzed by Western blotting using an anti-BDNF antibody. (C) RNAs isolated from 6-mo-old wild-type (n = 3) and Norbin-KO (n = 3) mice were analyzed by RT-PCR using a TaqMan gene-expression assay.
Fig. 5.
DSP expression is reduced in Norbin-KO mice. RNAs isolated from the DG region of 2-mo-old wild-type or Norbin-KO mice were analyzed by RT-PCR using a TaqMan gene-expression assay for DSP (A) and LRRTM4 (B). In situ hybridizations (Right) showing the high levels of expression of DSP or LRRTM in the DG (arrowheads). ***P < 0.001. Reprinted with permission from the Allen Brain Atlas.
Norbin-KO Mice Display Depressive-Like Behavior.
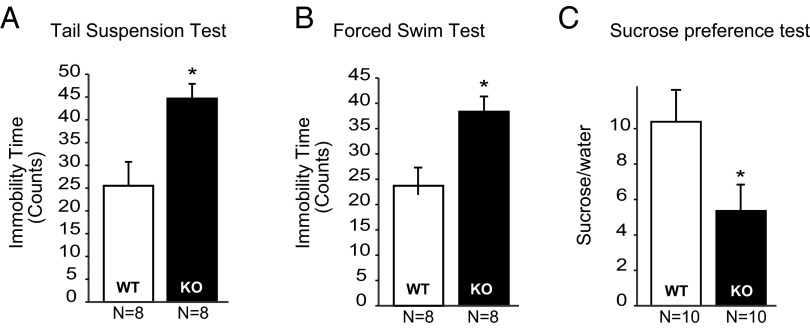
Reduced adult neurogenesis has been associated with depressive behaviors (21). We behaviorally characterized Norbin-KO mice using well-established mouse behavioral paradigms, including the TST, the FST, and the sucrose preference test. Increased immobility in both the TST and FST has been defined as a behavioral measurement for despair. The immobility times during the TST and FST are reduced by several antidepressant treatments, whereas factors that increase depression in humans, such as stress, postpartum state, or genetic predisposition, all increase TST and/or FST immobility times (22, 23). Compared with wild-type mice, Norbin-KO mice displayed longer immobility time in the TST (in 5-s bins, 42.5 ± 3.1% vs. 24.3 ± 5.0%, P < 0.05) (Fig. 6A) and the FST (in 5-s bins, 38.8 ± 2.5% vs. 22.9 ± 4.1%, P < 0.05) (Fig. 6B). To ensure that locomotor activity was not a confounding factor for the outcome of these tests, wild-type and Norbin-KO mice were tested in the open-field paradigm; no difference in locomotor activity was observed (Fig. S2A). Similarly no difference in the body weights of the two cohorts was found (Fig. S2B). Finally, in the sucrose preference test, which measures anhedonia (a core feature in depression), Norbin-KO mice display reduced preference for a palatable sucrose solution (5.3 ± 2.1-fold over water) compared with wild-type mice (10.2 ± 3.1-over water).
Fig. 6.
Norbin-KO mice showed a depressive-like behavioral phenotype. (A) Four-month-old wild-type (n = 8) and Norbin-KO mice (n = 8) were subjected to the TST, and immobility time was scored using a behavioral sampling method (Materials and Methods). (B) Four-month-old wild-type (n = 8) and Norbin-KO mice (n = 8) were subjected to the FST, and immobility time was scored using a behavioral sampling method (Materials and Methods). (C) Four-month-old wild-type (n = 10) and Norbin-KO mice (n = 10) were subjected to a sucrose preference test. The amount of sucrose versus water consumed was calculated as a fold of preference. Data are presented as means ± SEM, *P < 0.05.
Fig. S2.
Norbin-KO mice showed normal locomotor activity measured by the open-field test (A) and normal body weights (B). Data are presented as means ± SEM.
Discussion
We have identified Norbin as a player in adult hippocampal neurogenesis. A 4-wk neuron-survival assay revealed that ablation of Norbin expression in mice caused nearly a 50% reduction in adult hippocampal neurogenesis. This reduction was caused by reduced NSC/NPC proliferation and survival, not by cell-fate specification. Norbin deletion also resulted in a depression-like phenotype based on all three behavioral tests used.
Because Norbin expression is hardly detectable in NSCs and NPCs, we favor the hypothesis that Norbin has a non–cell-autonomous role in adult hippocampal neurogenesis. It is well established that NSCs/NPCs are regulated by the surrounding microenvironment, the neurogenic niche, such as granule cells in the DG, and innervations from different brain regions such as the amygdala. Norbin is highly expressed in mature DG neurons and in the amygdala. It is possible that Norbin deletion in these neurons may indirectly affect NSCs/NPCs in the SGZ.
To explore how Norbin deletion affects the function of DG granule neurons, we performed a gene-expression profiling study and identified DSP as the most dysregulated gene in Norbin-KO mice. DSP has been well studied in the peripheral tissue for its function in desmosomes. Desmosomes are molecular complexes of cell-adhesion proteins and linking proteins. Desmoglein and desmocollin, members of the cadherin family, are cell transmembrane adhesion proteins that mediate cell–cell interaction. DSP and plakoglobin are linking proteins that attach the cell-surface adhesion proteins to intracellular cytoskeletal filaments. Although DSP mRNA is expressed specifically in the DG region of the hippocampus, the expression of other desmosome components is not detectable in the DG (20), making the existence of functional desmosomes in the DG unlikely. However, it is possible that DSP interacts with other cadherin family members to ensure cell–cell interactions.
The role of adult hippocampal neurogenesis in the pathophysiology of major depression is still under extensive inquiry. Ablation of neurogenesis does not systematically induce depressive-like phenotypes, and not all antidepressants are associated with increased neurogenesis (for a review, see ref. 24). Although we report here that Norbin-KO mice display depressive-like behaviors that correlate with reduced hippocampal neurogenesis, it will be important to investigate further whether reduced neurogenesis has a causative role in the behavioral differences observed. We cannot exclude the possibility that some of the cognitive deficiencies observed in the Norbin-KO mice might interfere partially with some of the depression-like paradigms used. However, to minimize this possibility, we used three different behavioral tests that are relatively simple and that do not require higher cognitive abilities (e.g., the sucrose preference test). It also will be interesting to test whether the depressive-like phenotype of Norbin-KO mice can be rescued by antidepressant treatments, including treatments that are known to exert their antidepressant effect independently of neurogenesis (e.g., ketamine). For future studies, measuring the influence of the mouse strain used for depression-like behaviors also might shed some light on the underlying mechanisms.
This study identifies Norbin as a player in adult hippocampal neurogenesis and shows that its deletion in key brain areas causes a depressive-like phenotype in mice. We hypothesize that Norbin deletion affects the neurogenic niche of NSCs/NPCs, possibly through a pathway involving the cell–cell contact gene DSP. We believe that understanding Norbin function might help uncover the molecular and cellular mechanisms underlying neurogenesis and that these studies also could contribute to the development of novel antidepressants.
Materials and Methods
Animal Studies.
Experiments requiring animals were approved by the Institutional Animal Care and Use Committee of The Rockefeller University. Mice were maintained on a 12-h light/dark cycle with food and water ad libitum. Eight- to twelve-week-old animals were used for studies. GRM5-KO mice were kindly provided by A. Nishi, Kurume University School of Medicine, Kurume, Fukuoka, Japan (25). Norbin flox/flox mice were generated in house as described earlier (17). The CamK2α-Cre and Emx1-Cre lines were obtained from the Jackson Laboratory.
BrdU Labeling and Immunocytochemistry.
To evaluate cell proliferation and differentiation, BrdU (100 mg/kg; Sigma-Aldrich) was prepared using a saline solution and administered to mice i.p. (10 mL/kg). For analysis of cell proliferation, 10-wk-old mice received a single i.p. injection of BrdU, and 2 h later, animals were perfused transcardially with 4% (wt/vol) paraformaldehyde (PFA). For analysis of cell survival, 10-wk-old mice received six injections of BrdU (twice daily on three consecutive days) and were perfused with PFA 4 wk later (at age 14 wk), as previously described (26). BrdU immunostaining was performed as previously described (26). See also SI Materials and Methods.
Stereological Cell Counting.
BrdU+ cells from every eighth section covering the entire rostrocaudal axis of the DG were counted using a high-power (40×) microscope. Cells were counted in a blind manner. At least eight sections from both sides of the DG were counted per animal. The number for each group of animals is indicated in figure legends.
Immunofluorescence.
For confocal observations of BrdU and NeuN or GFAP colabeling, the sections first were pretreated with 2N HCl and incubated at 40 °C for 20 min, neutralized with 0.1 M borate buffer (pH 8.5), and then blocked with 10% (vol/vol) goat serum (Thermo Fisher Scientific). Sections then were incubated with anti-BrdU (1:500; Roche Diagnostics), anti-NeuN (1:500; EMD Millipore Corporation), and anti-GFAP (1:1,000; Sigma-Aldrich) antibodies overnight at 4 °C. Fluorophore-conjugated secondary antibody was used to visualize the staining. For Ki-67 staining, a slightly different protocol was used (see SI Materials and Methods for details).
Behavioral Analysis.
All behavioral studies were performed using adult (3- to 6-mo-old) male mice. Wild-type and KO mice were housed together. The TST and FST were performed as described (26). Tests were scored using behavioral sampling methods: The last 4 min of a 6-min testing session were scored. Every 5 s, a blinded observer noted whether a test subject was immobile. The immobility “counts” represent the number of times the mouse was scored as immobile out of a possible 48 total counts.
Sucrose Preference.
The sucrose preference test was conducted using a two-bottle choice procedure. Mice were housed individually for 3 d. Four days before the experiment (day −4), mice were given 24 h of continuous two-bottle exposure to tap water. On day −3 one bottle of tap water was replaced with 1.5% (wt/vol) sucrose for 24 h. On day −2, the bottle positions were switched. On day −1, mice were denied access to fluid but had free access to food. On the test day, mice were given access to the two preweighed bottles, one containing tap water and one containing 1.5% (wt/vol) sucrose, and were left undisturbed. Fluid consumption over 24 h was measured the next day. The preference for sucrose over water (sucrose/water) was used as the measure of the preference for sucrose (27).
Microarray Analysis and RT-PCR.
Hippocampi from wild-type and Norbin-KO mice were dissected, total RNAs were isolated and quantified, and 1 µg of total RNA was used for microarray analysis according to standard procedures (see SI Materials and Methods for details).
Statistical Analysis.
All data for quantitative studies are expressed as means ± SEM and are described in the figure legends. Parametric analyses were performed with the unpaired Student's t test for two groups or one- or two-way ANOVA for comparisons of more than two groups. A value of P < 0.05 was considered to be significant.
SI Materials and Methods
Immunocytochemistry for BrdU.
Sections were pretreated with 50% (vol/vol) formamide/2× SSC for 1 h at 65 °C, denatured with 2N HCl for 30 min at 37 °C, neutralized with 0.1 M borate buffer (pH 8.5), and washed three times with PBS. The sections were incubated with the primary antibody (anti-BrdU; Roche Diagnostics) overnight at 4 °C, followed by incubation with the secondary antibody (Vector Laboratories, Inc.) for 1 h at room temperature, and were developed using the ABC staining system (Vector Laboratories, Inc.). Sections then were rinsed five times, dehydrated, and mounted with DPX mounting medium (Sigma-Aldrich).
PFA Perfusion.
Fourteen-week-old mice were deeply anesthetized and transcardially perfused with 50 mL of cold 4% (vol/vol) PFA in 0.1 M PBS (pH 7.4) as described previously (24). Brains were removed, postfixed with 4% (vol/vol) PFA for 1 h at 4 °C, cryoprotected with 30% (wt/vol) sucrose, embedded in optimum cutting temperature (OCT) compound (Tissue-Tek; Sakura Finetek USA, Inc.), and frozen and stored at −80 °C. Coronal brain sections (40 µm thick) covering the entire hippocampus were obtained using a cryostat and were collected free-floating at 320-µm intervals and stored at −20 °C until use.
Immunofluorescence.
For Ki-67 staining, brain sections were incubated for 30 min in 10 mM sodium citrate buffer (pH 6.0) at 80 °C before blocking. Anti-Ki67 antibody was purchased from DakoCytomation and was used at a 1:100 dilution. Dcx antibody was purchased from Santa Cruz Biotechnology and was used at a 1:200 dilution.
Microarray and RT-PCR.
Hippocampi from wild-type and Norbin-KO mice were dissected, immediately frozen in liquid nitrogen, and stored at −80 °C. Total RNAs were isolated using the Qiagen RNeasy Mini kit (Qiagen). RNA quality and quantity were assessed by microfluidics chip (Agilent 2100 Expert Bioanalyzer Nano Chip; Agilent Technologies, Inc.) and NanoDrop ND1000 (Thermo Fisher Scientific), respectively. One microgram of total RNA was used for microarray analysis (The GeneChip Mouse Genome 430 2.0 Array; Affymetrix, Inc.). Differential gene expression was considered statistically significant when a combination of pairwise absolute value exhibited a fold-change of at least 1.4. Gene expression levels were confirmed by RT-PCR. Probes and the cDNA synthesis kit were obtained from Life Technology (Thermo Fisher Scientific).
Acknowledgments
We thank Tatyana Michurina and Julia Fram for technical support. This work was supported, in part, by NIH/National Institute of Mental Health Grant MH090963, by grants from the Fisher Center for Alzheimer’s Disease Research Foundation (to M.F. and P.G.) and by Army Medical Research and Materiel Command Grant W81XWH-10-1-0691 (to M.F.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510291112/-/DCSupplemental.
References
- 1.Kessler RC, et al. National Comorbidity Survey Replication The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Tanti A, Belzung C. Open questions in current models of antidepressant action. Br J Pharmacol. 2010;159(6):1187–1200. doi: 10.1111/j.1476-5381.2009.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: Novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35(1):47–56. doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: A road to remission? Science. 2012;338(6103):72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8(4):189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]
- 6.Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12(10):585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czéh B, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001;98(22):12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 10.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Ming GL, Song H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54(4):559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laplagne DA, et al. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4(12):e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Giorgi Gerevini VD, et al. The mGlu5 metabotropic glutamate receptor is expressed in zones of active neurogenesis of the embryonic and postnatal brain. Brain Res Dev Brain Res. 2004;150(1):17–22. doi: 10.1016/j.devbrainres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Melchiorri D, et al. Metabotropic glutamate receptors in stem/progenitor cells. Neuropharmacology. 2007;53(4):473–480. doi: 10.1016/j.neuropharm.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Cappuccio I, et al. Endogenous activation of mGlu5 metabotropic glutamate receptors supports self-renewal of cultured mouse embryonic stem cells. Neuropharmacology. 2005;49(Suppl 1):196–205. doi: 10.1016/j.neuropharm.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Di Giorgi-Gerevini V, et al. Endogenous activation of metabotropic glutamate receptors supports the proliferation and survival of neural progenitor cells. Cell Death Differ. 2005;12(8):1124–1133. doi: 10.1038/sj.cdd.4401639. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, et al. Norbin is an endogenous regulator of metabotropic glutamate receptor 5 signaling. Science. 2009;326(5959):1554–1557. doi: 10.1126/science.1178496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo H, et al. Specificity and efficiency of Cre-mediated recombination in Emx1-Cre knock-in mice. Biochem Biophys Res Commun. 2000;273(2):661–665. doi: 10.1006/bbrc.2000.2870. [DOI] [PubMed] [Google Scholar]
- 20. Allen brain atlas. Available at www.brain-map.org. Accessed July 7, 2015.
- 21.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29(4-5):571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology (Berl) 2005;177(3):245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- 24.Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Curr Opin Neurobiol. 2015;30:51–58. doi: 10.1016/j.conb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahide S, Nishi A. Effect of acute and chronic administration of methamphetamine on locomotor activity in mGluR5 knockout mice. J Pharmacol Sci. 2007;103(Suppl 2):229–239. [Google Scholar]
- 26.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci USA. 2007;104(11):4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen CK, Arnt J, Sánchez C. Intracranial self-stimulation and sucrose intake differ as hedonic measures following chronic mild stress: Interstrain and interindividual differences. Behav Brain Res. 2000;107(1-2):21–33. doi: 10.1016/s0166-4328(99)00110-2. [DOI] [PubMed] [Google Scholar]