An intriguing report by Dettmer et al. (1) in PNAS describes a link between the in vivo multimerization state of the neuronal protein α-synuclein (αSyn) and neurotoxicity. In this paper, it is demonstrated that αSyn mutations that abolish the formation of soluble αSyn tetramers in live neurons decrease αSyn solubility, induce αSyn-rich cytoplasmic inclusions, and cause neurotoxicity similar to that observed due to a known proapoptotic agent. The reporting authors form a convincing link between the presence of stable αSyn tetramers and neuron viability. With this discovery, it would seem that the controversy over the relevance (and indeed, the existence) of the physiological αSyn tetramer might be laid to rest (2–5).
It has been over 20 years since the protein now known as αSyn was identified as a component of Alzheimer’s plaques from human brain tissue (6). The discovery that αSyn was also the primary protein component of Lewy bodies, the insoluble aggregates that are the hallmark of Parkinson’s disease (PD) (7), sent research on this otherwise-nondescript 140-residue polypeptide into high gear: A current key word search in Web of Science yields over 11,000 hits on αSyn. However, despite the person-years expended trying to understand the role of αSyn in both normal brain function and PD pathology, we still know remarkably little about this enigmatic protein. We know that αSyn is abundant in presynaptic termini of dopaminergic neurons, especially in the substantia nigra, and that it appears to play a role in synaptic vesicle trafficking (8). Knockout of all three (α, β, and γ) synuclein genes in mice results in some age-related neurodegeneration (9). Nevertheless, young synuclein knockout mice remain stubbornly healthy, so whatever role the synucleins play in normal neurons, it is either nonessential or can be at least partially compensated by other actors.
What is undoubtedly true is that a number of point mutations in the SNCA gene that codes for αSyn have been definitively linked to familial PD in humans, as have SNCA gene duplications and up-regulation of αSyn expression (10, 11). Based on this, it is safe to assume that, whatever αSyn gets up to in normal neurons, too much αSyn or particular mutations of αSyn are bad things. Furthermore, the fact that both Lewy bodies and Alzheimer’s plaques are made up of insoluble aggregates of normally soluble proteins seems to indicate that PD and other synucleinopathies are the result of αSyn misfolding. As long as αSyn remains soluble, Lewy bodies do not form, and PD is avoided. So what keeps αSyn soluble in vivo, and how can we sustain that solubility? To answer these questions, we first need to know what form(s) native soluble αSyn can take.
For most of the last 20 years, αSyn has been presumed to be a disordered monomeric protein in vivo, assuming a helical hairpin structure only when associated with membranes or lipid vesicles in vitro (12). One reason for this presumption is the fact that the primary source of αSyn used for biophysical studies is heterologous expression in Escherichia coli, often with heating as the first step in purification. Several years ago, however, the Selkoe group at Harvard Medical School (also the source of the current report) (4) found evidence that, under nondenaturing conditions, αSyn could occur as tetramers with relatively high helical content in human cell lines, including neuroblastoma and red blood cells. At the same time, our laboratories found evidence for a tetramer of heterologously expressed αSyn, again with high helical content, purified under nondenaturing conditions, that resisted aggregation and precipitation longer and under conditions of higher concentration than αSyn purified from E. coli by other means (5). Interestingly, mutations in our construct corresponding to known PD-causing mutations resulted in much more rapid insoluble aggregate formation than in the wild-type construct. To our groups, at least, the idea of a helical tetramer as a stable form of storage for αSyn at high concentrations seems a clean answer to the problem of maintaining αSyn solubility in vivo. It also addresses the unresolved problem of the stability of αSyn: For a nominally disordered protein, αSyn exhibits an unusually long half-life in vivo. This is despite the fact that monomeric αSyn is readily degraded by the 20S proteasome, indicating that the monomeric protein is not inherently resistant to proteasomal degradation (13). Furthermore, our model for the synuclein tetramer rationalized the localization of mutations known to bring on early-onset PD (10).
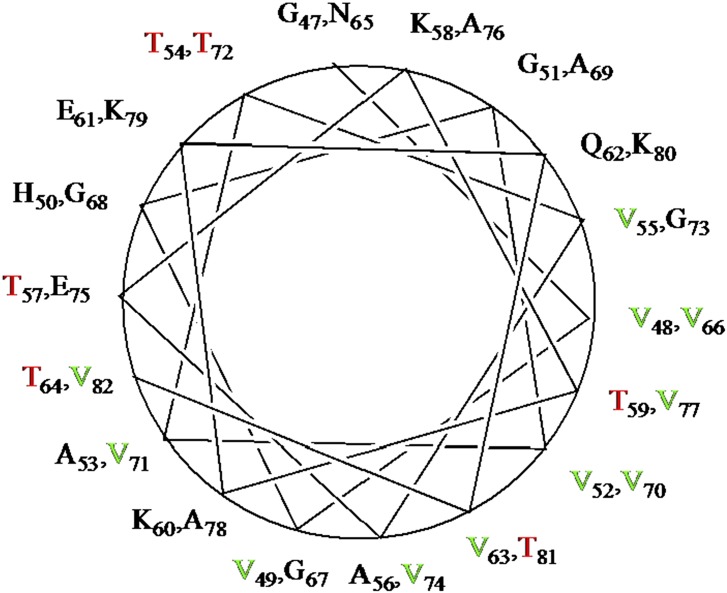
Still, the tetramer has proven to be elusive. Although several groups have seen evidence of multimeric forms of αSyn from in vivo chemical cross-linking experiments, these results are often ascribed to random association of synuclein monomers (or even toxic oligomers). Analysis of the αSyn sequence (Fig. 1) in terms of secondary structural propensity is somewhat contradictory. On the one hand, the presence of seven (or nine, depending upon how one counts) imperfect KTKEGV repeats in the first 100 residues of αSyn suggests a propensity for amphipathic α-helix formation (Fig. 2). On the other hand, the presence of multiple sequential pairings of the β-branched amino acids threonine (T) and valine (V) between residues 47 and 82 (highlighted in red in Fig. 1 and red/green in Fig. 2) hints at a tendency toward β-sheet formation: Steric interactions between adjacent β-branched side chains tend to favor the alternating extended polypeptide conformations found in β-strands. Indeed, solid-state NMR confirms that the region containing adjacent VV, VT, or TV pairs assumes extended conformations in insoluble αSyn fibrils (presumed to be the form αSyn takes in Lewy bodies) (14). As a result, the αSyn polypeptide appears to be poised on a knife edge: If one can stabilize the helical tendency in some way, the balance is tipped away from fibril formation. Destabilize the helix and fibrils will form. The proposed role of the recently discovered tetramer is to bury the hydrophobic edges of the amphipathic helices away from the cytosol, resulting in their mutual stabilization. It has been shown that the helical αSyn tetramer is much more efficient at burying hydrophobic surfaces than any other monomeric or oligomeric form that is likely to be populated in solution (15).
Fig. 1.

Amino acid sequence of human αSyn. The nine imperfect six-residue repeats are underlined, and adjacent VT, TV, and VV pairs are shown in red (see text).
Fig. 2.
Helical wheel showing amphiphilic helix arrangement of residues 47–82 of αSyn. β-Branched valine is shown in green, and β-branched threonine is shown in red.
In the current work, Dettmer et al. (1) demonstrate that the imperfect KTKEGV repeats are in fact redundant promoters of tetramer formation. The researchers serially deleted 10 residues at a time from αSyn, removing ∼2.8 potential turns of α-helix per deletion. The mutant αSyn constructs were transfected into human M17D neural cells, and the expressed protein cross-linked in the live cells using cell-permeable cross-linking agents, with the results examined by gel electrophoresis. Although some variability in the tetramer/monomer ratios are observed (particularly for deletions in the range of residues 41–60), it was clear that the tetramer is formed by all of the deletion mutants. (Note that only small amounts of dimer or trimer are observed in any case, indicating that the multimers are not artifacts due to statistical cross-linking of monomers.) Of course, the worry is that negative evidence is insufficient, especially for a controversial result. So the next step was to introduce changes into the imperfect repeats, by replacing β-branched threonine (T) with the γ-branched leucine (KLK), an acidic residue with a basic residue (KGV), or a small residue with a large one (EIV and EGW). The replacement mutations were made in each repeat where it was appropriate. The results are remarkable: The KLK, KGV, EIV, and EGW replacements all essentially abolished tetramer formation, whereas other substitutions in the consensus ([K→G]TKEGV, KT[K→E]EGV, and KTKEG[V→R]) did not. Furthermore, those mutants that abolish tetramer formation proved to be “frankly neurotoxic,” in the words of the authors. One assay for cytotoxicity [cleaved poly-(ADP-ribose) polymerase as a marker for activated apoptosis] was clearly binary: Only in the presence of the tetramer-abolishing mutants is the marker observed. Yellow fluorescent protein-tagged αSyn variants that abolish tetramer formation also gave rise to (presumably insoluble) protein inclusions, as measured by fluorescence microscopy, in M17D cells, as did the respective untagged αSyn variants expressed in primary rat neurons, whereas only background fluorescence was observed for the tetramer-neutral variants.
Further strengthening the link between tetramer formation and neuronal health, this same group (16) has recently published a communication describing experiments demonstrating that known familial PD-causing mutations in αSyn also shift the tetramer/monomer ratio in favor of monomer in both mouse models and human cell lines. These two publications place the ball firmly in the biophysicists’ court. With the importance of the tetramer in maintaining αSyn homeostasis now established, it will be necessary to get clear structural and dynamic data on the tetramer. Once this is done, it will be possible to start thinking about how one goes about stabilizing the physiological tetramer as a means of preventing or delaying the onset of PD.
Footnotes
The author declares no conflict of interest.
See companion article on page 9596.
References
- 1.Dettmer U, Newman AJ, von Saucken VE, Bartels T, Selkoe D. KTKEGV repeat motifs are key mediators of normal α-synuclein tetramerization: Their mutation causes excess monomers and neurotoxicity. Proc Natl Acad Sci USA. 2015;112:9596–9601. doi: 10.1073/pnas.1505953112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burré J, et al. Properties of native brain α-synuclein. Nature. 2013;498(7453):E4–E6, discussion E6–E7. doi: 10.1038/nature12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fauvet B, et al. α-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287(19):15345–15364. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartels T, Choi JG, Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477(7362):107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W, et al. A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 2011;108(43):17797–17802. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwai A, et al. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14(2):467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 7.Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, et al. α-Synuclein multimers cluster synaptic vesicles and attenuate recycling. Curr Biol. 2014;24(19):2319–2326. doi: 10.1016/j.cub.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greten-Harrison B, et al. αβγ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc Natl Acad Sci USA. 2010;107(45):19573–19578. doi: 10.1073/pnas.1005005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kara E, et al. α-Synuclein mutations cluster around a putative protein loop. Neurosci Lett. 2013;546:67–70. doi: 10.1016/j.neulet.2013.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singleton AB, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 12.Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human alpha-synuclein. J Biol Chem. 2005;280(10):9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Castelao B, Castaño JG. Synphilin-1 inhibits alpha-synuclein degradation by the proteasome. Cell Mol Life Sci. 2011;68(15):2643–2654. doi: 10.1007/s00018-010-0592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heise H, et al. Molecular-level secondary structure, polymorphism, and dynamics of full-length alpha-synuclein fibrils studied by solid-state NMR. Proc Natl Acad Sci USA. 2005;102(44):15871–15876. doi: 10.1073/pnas.0506109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurry T, et al. The dynamic structure of α-synuclein multimers. J Am Chem Soc. 2013;135(10):3865–3872. doi: 10.1021/ja310518p. [DOI] [PubMed] [Google Scholar]
- 16.Dettmer U, et al. Parkinson-causing α-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nat Commun. 2015;6:7314. doi: 10.1038/ncomms8314. [DOI] [PMC free article] [PubMed] [Google Scholar]