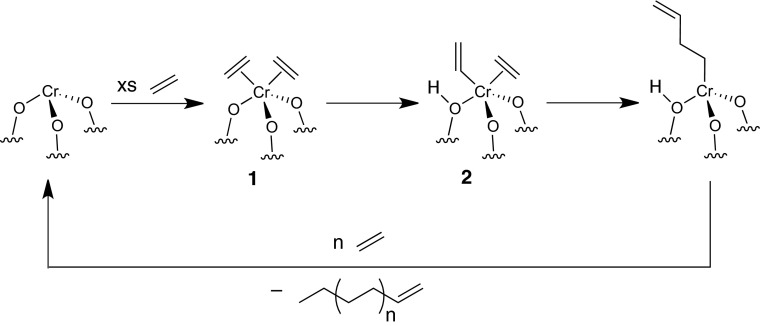
In a recent PNAS report, we propose that heterolytic activation of a C–H bond in ethylene on a Cr–O bond in silica-supported Cr(III) sites forms a Cr–vinyl species; this surface species coordinates and inserts ethylene to propagate polymer growth (Fig. 1) (1). In their letter, Peters et al. show that the bands at 3,640 cm−1 and 3,605 cm−1 in the infrared spectrum can result from combination bands from the polymer, and not from Si(OH)Cr–R (2). We had already corrected this assignment in a recent publication (3). Using computational data on cluster models presented in ref. 1, Peters et al. describe a kinetic model for the proposed mechanism (2). Their analysis concludes that 1 acts as an “off-cycle trap” (resting state) for most Cr sites, and the C–H bond activation barrier obtained from density functional theory (DFT) studies to form 2 would lead to small amounts of active Si(OH)Cr–R, suggesting alternative initiation pathways. Peters et al. (2) also suggest different chain transfer mechanisms.
Fig. 1.
Proposed ethylene polymerization mechanism for Cr(III) silicates.
Models, by definition, are a simplified view of complex systems. Cluster models, by necessity, omit elements that can be important in real systems. For example, van der Waals interactions of the growing polymer chain with the surface or the presence of strained Si–O–M bonds are not included in a cluster model. Both will play critical roles in determining actual reaction- and transition-state energies. This is why this cluster model was used to distinguish possible and probable from impossible. Predicting reaction rates and comparing absolute rates that govern selectivity and molecular weight of the polymer from a DFT calculated potential energy surface with this simple cluster model are not possible. Peters et al. agree with us on this point and mention that DFT is not accurate enough to predict rates (2, 4).
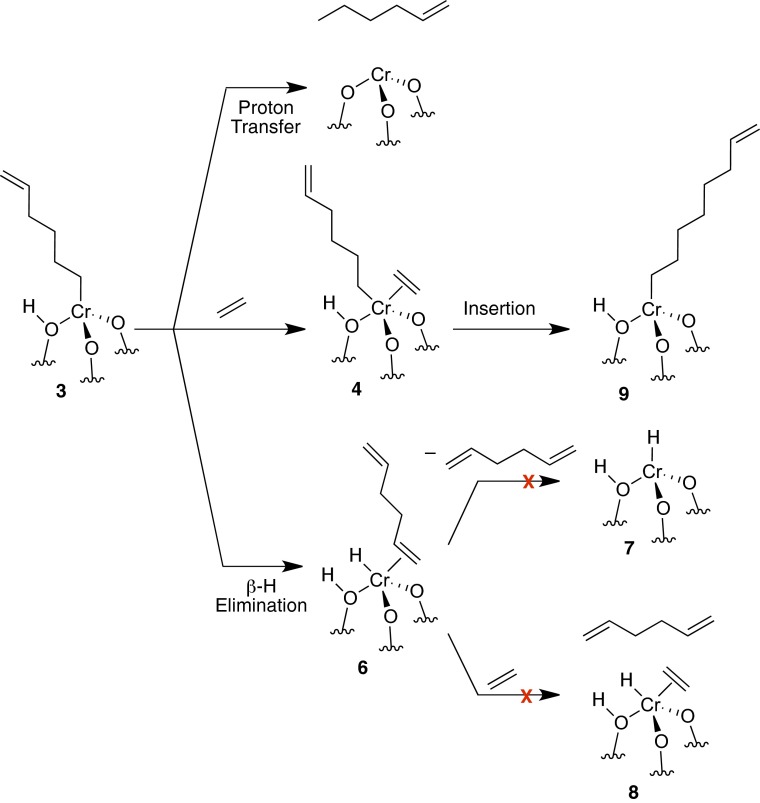
Peters et al. (2) suggest that other chain transfer mechanisms may be more plausible than proton transfer. In the Supporting Information of ref. 1, we compare the chain transfer reaction pathways from the hexenyl complex using B3LYP-D3, summarized in Fig. 2. β-Hydride elimination has a low energy barrier, but forms unstable Cr–H intermediates after olefin decoordination. We were unable to locate an ethylene-assisted transition state from 6 to 8 using B3LYP-D3, suggesting a high-energy intermediate (4). Proton transfer is the lowest energy-chain transfer mechanism, and even lower if ethylene is coordinated, as recently proposed by Peters et al. using a different functional (4).
Fig. 2.
Proposed termination steps for ethylene polymerization on Cr(III) sites.
Peters et al. (2) suggest that our mechanism is not plausible because Si(OH)Cr–R is unobservable as a result of the high barrier associated with its formation from DFT results. Although the coverage of Si(OH)Cr–R may be low on the surface, this does not preclude Si(OH)Cr–R from being a reaction intermediate. Further experimental investigations will be needed to confirm or deny this mechanism (5). Our computational and experimental results show that these proposals are reasonable.
Footnotes
The authors declare no conflict of interest.
References
- 1.Delley MF, et al. Proton transfers are key elementary steps in ethylene polymerization on isolated chromium(III) silicates. Proc Natl Acad Sci USA. 2014;111(32):11624–11629. doi: 10.1073/pnas.1405314111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters B, Scott SL, Fong A, Wang Y, Stiegman AE. Reexamining the evidence for proton transfers in ethylene polymerization. Proc Natl Acad Sci USA. 2015;112:E4160–E4161. doi: 10.1073/pnas.1422589112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conley MP, Delley MF, Nuñez-Zarur F, Comas-Vives A, Copéret C. Heterolytic activation of C–H bonds on CrIII–O surface sites is a key step in catalytic polymerization of ethylene and dehydrogenation of propane. Inorg Chem. 2015;54(11):5065–5078. doi: 10.1021/ic502696n. [DOI] [PubMed] [Google Scholar]
- 4.Fong A, Yuan Y, Ivry SL, Scott SL, Peters B. Computational kinetic discrimination of ethylene polymerization mechanisms for the Phillips (Cr/SiO2) catalyst. ACS Catalysis. 2015;5(6):3360–3374. [Google Scholar]
- 5.Espenson JH. Chemical Kinetics and Reaction Mechanisms. McGraw Hill; New York: 1981. [Google Scholar]