Significance
Benign prostatic hyperplasia (BPH) is characterized by an enlargement of the prostate gland, a common disease in elderly men. Excessive testosterone is considered to cause BPH. However, its etiologic mechanisms are elusive. We found that ANO1, a Ca2+-activated Cl− channel, is essential for the testosterone-induced BPH. ANO1 was highly expressed in dihydrotestosterone (DHT)-treated prostate epithelial cells. The selective knockdown of ANO1 suppressed DHT-induced cell proliferation. Surprisingly, we found that there were three androgen-response elements in the ANO1 promoter region, which were relevant for the DHT-dependent induction of ANO1. Intraprostate treatment of Ano1 siRNA inhibited the prostate enlargement in vivo. Thus, ANO1 appears essential for the development of prostate hyperplasia and becomes a useful target for treating BPH.
Keywords: prostate, hyperplasia, anoctamin 1, testosterone, proliferation
Abstract
Benign prostatic hyperplasia (BPH) is characterized by an enlargement of the prostate, causing lower urinary tract symptoms in elderly men worldwide. However, the molecular mechanism underlying the pathogenesis of BPH is unclear. Anoctamin1 (ANO1) encodes a Ca2+-activated chloride channel (CaCC) that mediates various physiological functions. Here, we demonstrate that it is essential for testosterone-induced BPH. ANO1 was highly amplified in dihydrotestosterone (DHT)-treated prostate epithelial cells, whereas the selective knockdown of ANO1 inhibited DHT-induced cell proliferation. Three androgen-response elements were found in the ANO1 promoter region, which is relevant for the DHT-dependent induction of ANO1. Administration of the ANO1 blocker or Ano1 small interfering RNA, inhibited prostate enlargement and reduced histological abnormalities in vivo. We therefore concluded that ANO1 is essential for the development of prostate hyperplasia and is a potential target for the treatment of BPH.
Benign prostatic hyperplasia (BPH) is characterized by the anatomical enlargement of the prostate gland and is one of the most common diseases among elderly men (1). More than 50% of men aged more than 60 suffer from lower urinary tract symptoms, including urinary hesitancy, weak stream, and nocturia, which are commonly caused by bladder obstruction (2). The availability of testosterone or dihydrotestosterone (DHT) is known to cause the development of histologically characterized BPH (3). Clinical reports on BPH have suggested a positive association between BPH and prostate cancer, with increased risk of and mortality from prostate cancer among BPH patients (4). However, some epidemiologic studies have reported that BPH is not a cause of prostate cancer (5). Despite the controversy on the association between prostate cancer and BPH, common risk factors for the two diseases include chronic inflammation, metabolic disturbance, and genetic variation (6, 7). Regardless of its association with prostate cancer, BPH is still a social issue for the elderly, but the etiologic mechanisms of its pathology remain unknown.
Anoctamin1 (ANO1, also known as TMEM16A) encodes a Ca2+-activated chloride channel (CaCC) (8–10), and is widely expressed in secretory epithelia, including the salivary gland, trachea (11), and intestine, smooth muscle (12), and sensory neurons (13). ANO1 is known to mediate various physiological functions, such as fluid and electrolyte secretion, gut motility, vascular smooth muscle contraction, and thermal nociception (14).
ANO1 has been suggested to be a regulator of cell proliferation and tumorigenesis, even before it was discovered as a CaCC, and is highly expressed in several carcinomas, including gastrointestinal stromal tumors (15), esophageal squamous cell carcinoma (16), head and neck squamous cell carcinoma (17), oral cancer (18), breast cancer (19), and prostate cancer (20). The disruption of Ano1 or the administration of a pharmacological ANO1 inhibitor impairs the proliferation of interstitial cells of Cajal (21) and numerous cancer cells (19, 20, 22). ANO1 promotes tumorigenesis and cancer progression by inducing epidermal growth factor receptor-activated mitogen-activated protein kinase (MAPK)/AKT signaling (19) and regulates tumor cell motility and metastasis via the ezrin/radixin/moesin protein family (23). Thus, ANO1 is considered to be a potential target for anticancer therapy (24).
BPH and prostate cancer share common characteristics, such as testosterone-dependent growth and response to hormone therapy, which indicates a causal link between the two diseases (25). Notably, ANO1 is highly overexpressed in prostate cancer cells (20). Knockdown of Ano1 results in reduced cell proliferation and the suppression of tumor progression in the breast cancer model (19). Thus, it is conceivable that ANO1 may be involved in BPH, which may progress to prostate cancer. Therefore, this study was performed to determine whether ANO1 plays a key role in testosterone-dependent prostate hyperplasia.
Results
Dihydrotestosterone Up-Regulates ANO1 in Prostate Epithelial Cells.
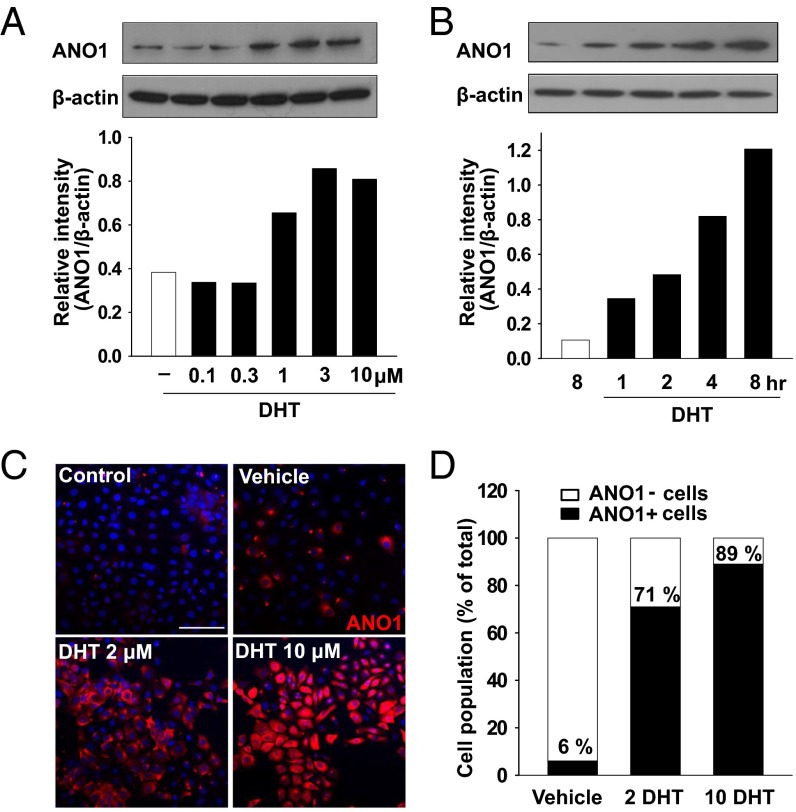
The main phenotype of BPH is an increase in cell numbers (3). We therefore determined whether the expression of ANO1 was related to prostate hyperplasia. To induce prostate hyperplasia, we treated normal human prostate epithelial RWPE-1 cells with DHT, an immediate metabolite of testosterone that is metabolized in stromal cells by 5α-reductase and a critical mediator of prostatic growth (3), and studied the changes in the ANO1 expression. Western blot analysis revealed a dose-dependent increase in ANO1 expression 24 h after DHT treatment (Fig. 1A). The threshold concentration was 1 μM. ANO1 expression was detected as early as 1 h after incubation with 2 μM DHT (Fig. 1B). ANO1 immunoreactivity in RWPE-1 cells increased in a dose-dependent manner after the treatment with DHT, whereas only weak ANO1 immunoreactivity was observed in vehicle-treated RWPE-1 cells (Fig. 1 C and D). Following the treatment with 10 µM DHT, 89% of cells were ANO1-positive. These results indicate that ANO1 is up-regulated by DHT in prostate epithelial cells.
Fig. 1.
Dihydrotestosterone (DHT) increases ANO1 expression of human prostate epithelial cells. (A and B) Western blot analysis of ANO1 in the RWPE-1 cells cultured with DHT in a dose- (0.1, 0.3, 1, 3, 10 μM for 24 h) (A) and time- (1, 2, 4, 8 h at 2 μM) (B) dependent manner. The ANO1 band intensity was normalized to β-actin (lower blot). (C) Representative microscopy images stained with ANO1 (red) and Hoechst 33342 (blue) in vehicle- or DHT-treated RWPE-1 cells for 48 h. (Scale bar: 50 μm.) (D) Immunofluorescence quantification of ANO1-positive and -negative cells.
Androgen-Response Elements in the Ano1 Promoter Region.
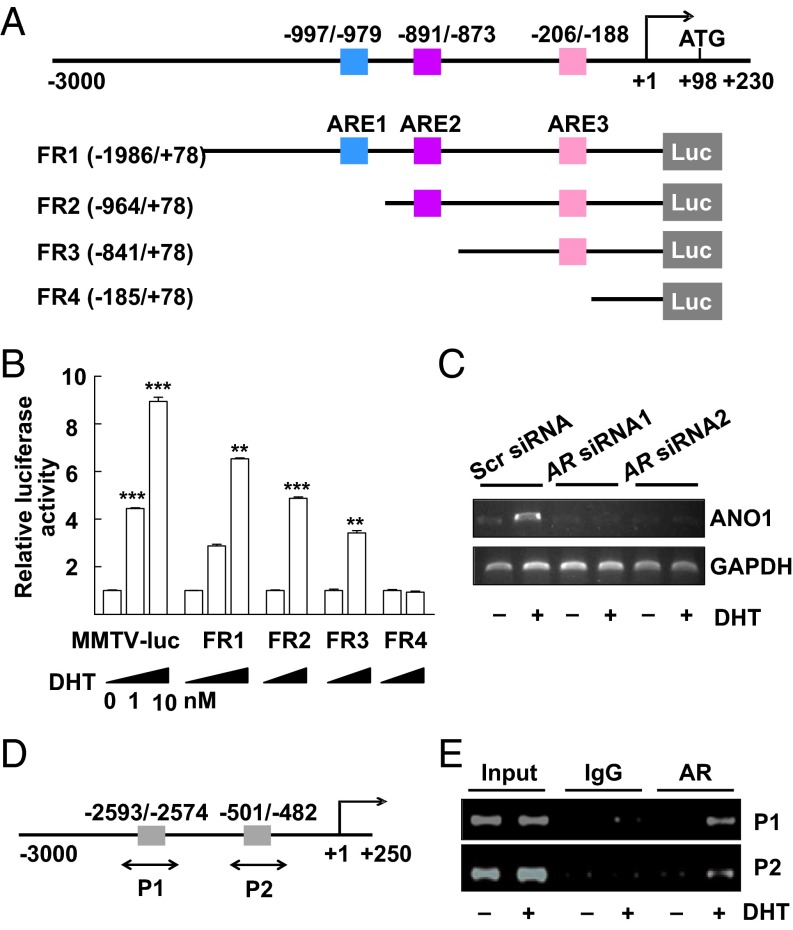
Because of the increase in ANO1 expression after DHT treatment, it was conceivable that DHT controls the transcription of Ano1. Using the MatInspector search engine program (www.genomatix.de), the mouse Ano1 promoter region was searched for the androgen-response element (ARE) that is known to regulate the transcription of androgen-responsive genes (26). After ligand binding, androgen receptors (ARs) are known to recognize and bind the ARE region leading to subsequent transcription (26). When the −3,000-bp promoter region upstream of the transcriptional initiation site of Ano1 was analyzed, three ARE consensus sites were found. As shown in Fig. 2A, the three putative AREs (ARE1, ARE2, and ARE3) were located at −997 ∼ −979, −891 ∼ −873, and −206 ∼ −188 bp upstream of the start codon, respectively. We then performed a luciferase reporter assay to determine whether testosterone acted on the AREs to stimulate the transcription of Ano1. The promoter–luciferase constructs FR1, FR2, FR3, and FR4 were created, containing ARE1 + ARE2 + ARE3, ARE2 + ARE3, ARE3 alone, and none of the three, respectively. The mouse mammary tumor virus promoter (MMTV)–luciferase reporter plasmid (MMTV-luc) containing four inverted repeats of the 5′-TGTTCT-3′ sequence was used as a positive control (27). The promoter activities of these constructs were examined in RWPE-1 cells, which after transfection of the reporter plasmid were treated with DHT for 24 h and lysed for luciferase activity. A 5∼6-fold increase in luciferase activity was observed in cells transfected with the FR1 and FR2 constructs in a dose-dependent manner (Fig. 2B). A smaller increase was found in FR3-transfected cells, but no increase occurred in cells transfected with the blank (FR4) constructs (Fig. 2B). Simultaneously, the level of the Ano1 transcript was increased in RWPE-1 cells after treatment with DHT, but was blocked after transfection with small interfering RNAs (siRNAs) of the AR (Fig. 2C).
Fig. 2.
Activation of the ANO1 promoter-driven luciferase reporter gene and ChIP analysis. (A) Schema of ANO1 promoter-luciferase (Luc) constructs used for reporter gene assays. (B) The luciferase reporter assay. The RWPE-1 cells were transiently transfected with mouse ANO1 promoter FR1, FR2, FR3, and FR4-Luc or MMTV-Luc and with CMV-β-gal as an internal control. After 4 h of transfection, the cells were treated with 1 or 10 nM DHT or vehicle for 24 h. The luciferase activity was normalized to β-gal activity. **P < 0.01 and ***P < 0.001 (one-way ANOVA). (C) Ano1 transcripts in RWPE-1 cells transfected with scrambled siRNA or AR siRNAs for 48 h and treated with 10 nM DHT or vehicle for 24 h. (D) Schematic representation of hAno1 promoter fragments. Putative ARE sites are illustrated (gray box). (E) A ChIP assay for the AR binding to the ANO1 promoter. The chromatin was cross-linked to proteins, digested with micrococcal nuclease, and immunoprecipitated with control IgG (IgG) or anti-AR (AR) antibodies. DNA was purified and the regions (P1 and P2) containing the each ARE site was amplified with two probes.
We next performed a chromatin immunoprecipitation (ChIP) assay to verify in vivo interaction of the AR with the ANO1 promoter region. To do this assay, the human promoter region of Ano1 was searched for the putative ARE regions. There were two putative ARE regions found in the human promoter region (Fig. 2D). The chromatin fragments from RWPE-1 cells cultured in presence of vehicle or 10 nM DHT were immunoprecipitated with anti-AR antibody or control rabbit IgG. Then, approximately 200-bp fragments of the ANO1 promoter (designated as P1 and P2) were amplified by PCR using two sets of primers directed to cover the two putative ARE sites. In agreement with the results from the reporter gene assay (Fig. 2B), the binding of AR to the two putative AR-binding sites was clearly increased in RWPE-1 cells treated with DHT for 24 h (Fig. 2E). These results further confirm that testosterone can activate the Ano1 promoter through direct interaction of AR and ARE in the ANO1 promoter.
Ano1 Knockdown Abolishes DHT-Induced Cell Proliferation.
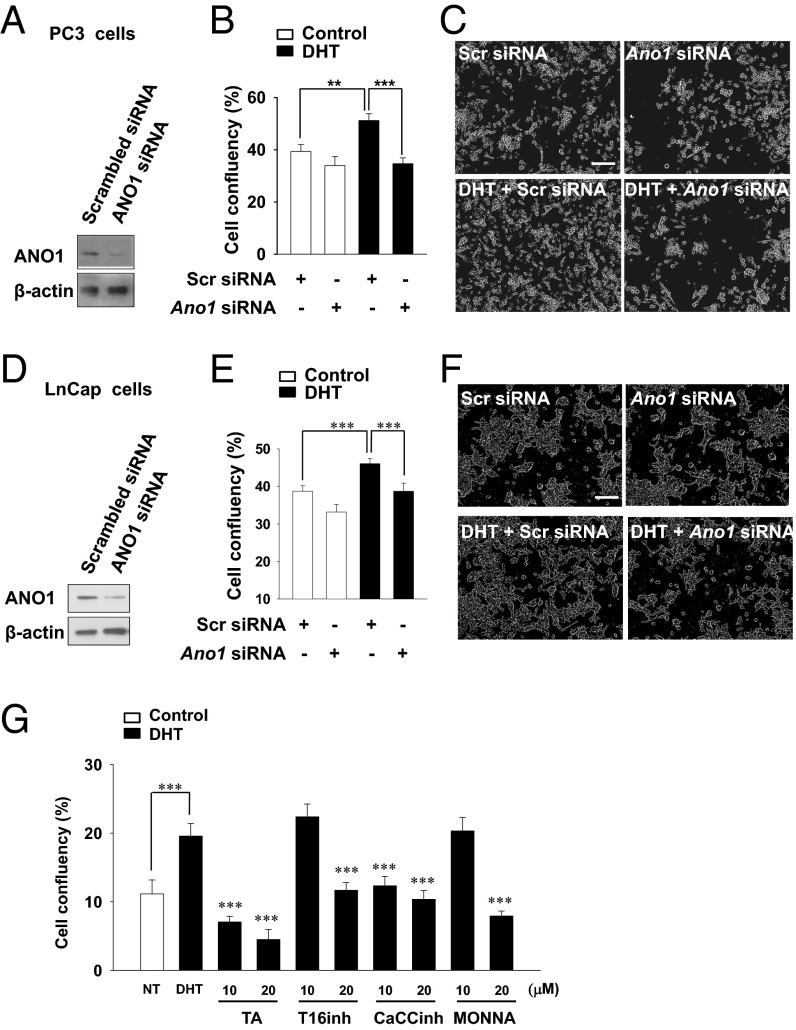
Because DHT up-regulates ANO1, it is conceivable that the ANO1 expression contributes to the hyperplasia. To address this issue, we examined whether the knockdown of Ano1 affects cell proliferation. Because RWPE-1 cells have a low level of endogenous ANO1, human prostate cancer PC3 and LnCap cells that have a high basal level of endogenous ANO1 were used to determine the effect of Ano1 knockdown (20). Western blot analysis showed that PC3 and LnCap cells expressed ANO1 at the basal level, and the transfection of Ano1 siRNA effectively silenced the endogenous expression of ANO1 in both cells (Fig. 3 A and D). DHT treatment induced the proliferation of PC3 and LnCap cells transfected with scrambled siRNA, whereas it failed to induce the proliferation of PC3 and LnCap cells transfected with Ano1 siRNA (Fig. 3 B, C, E, and F). Similarly, ANO1 blockers, such as tannic acid, T16inh-A01, CaCCinh-A01, and MONNA (28–30), also effectively inhibited the DHT-induced proliferation of LnCap cells (Fig. 3G). These results indicate that ANO1 is essential for the DHT-induced proliferation of prostate cells.
Fig. 3.
Ano1 siRNA or antagonist treatment inhibits DHT-induced proliferation. (A) Western blot analysis of ANO1 in PC3 cells transfected with scrambled (Scr) siRNA or Ano1 siRNA. (B) After transfected PC3 cells were incubated with 100 nM DHT for 3 d, the cells were photographed every hour for 120 h with an IncuCyte ZOOM automated microscope system. Confluency was calculated by measuring the fraction area occupied by cells of each well. (C) Representative live-cell images of PC3 cells. (D) Western blot analysis of LnCap cells transfected with scrambled (Scr) siRNA or Ano1 siRNA. (E) Effect of Ano1 knock-down on LnCap cell proliferation. (F) Representative live cell images of LnCap cells. (G) Effects of ANO1 blockers, 10 μM or 20 μM tannic acid (TA), T16Ainh-A01, CACCinh-A01, and MONNA on LnCap cell proliferation.
DHT Induces ANO1 Currents in RWPE-1 Cells.
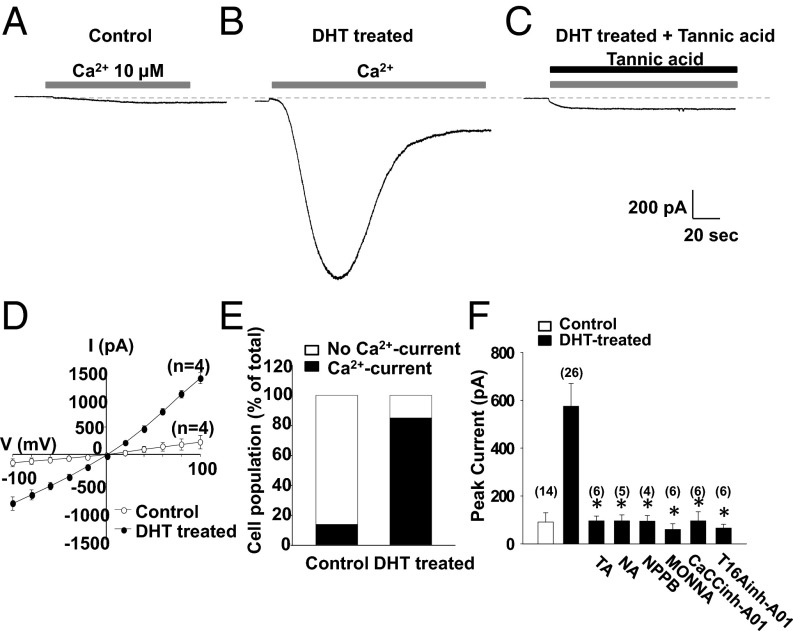
We next determined whether DHT up-regulates functional ANO1 in RWPE-1 cells by measuring their Ca2+-activated Cl− currents after treatment with DHT. Cells were voltage clamped to record the whole-cell currents. To obtain Ca2+-activated Cl− currents, 10 μM Ca2+ was added to the pipette solution. Both the pipette and bath solutions contained 140 mM N-methyl-D-glucamine-Cl to ensure that Cl− was the only charge carrier. When a whole cell was formed at a holding potential of −60 mV, small Cl− currents with an average amplitude of 91 ± 39 pA (n = 14) were activated in the control cells (Fig. 4A). In contrast, RWPE-1 cells treated with 2 μM DHT for 18–24 h elicited large and robust currents with an amplitude of 575 ± 95 pA (n = 26) (Fig. 4 B and F). In addition, the DHT treatment induced an increase in the number of cells responding to the intracellular Ca2+ from 14.3% (2/14 cells) to 84.6% (22/26 cells) (Fig. 4E). These Ca2+-activated currents were inhibited by ANO1 inhibitors, such as tannic acid, niflumic acid, and 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB), MONNA, CaCCihn-A01, and T16Ainh-A01 (28–30) (Fig. 4 C and F). These results clearly suggest that DHT treatment induces the expression of functional ANO1 in prostate epithelial cells.
Fig. 4.
DHT induces Ca2+-activated chloride currents in RWPE-1 cells. (A–C) Whole-cell currents of RWPE-1 cells at a holding potential of −60 mV. Ca2+-induced inward current in control (A), DHT-treated (B), and DHT-treated RWPE-1 cells with 10 μM tannic acid (C). Pipette solution contained 10 μM Ca2+. Pipette and bath solutions contained 140 mM NMDG-Cl. (D) Mean current-voltage (I-V) relationship for Ca2+-induced current in control (open circles) or DHT-treated RWPE-1 cells (closed circles). (E) The cell population with Ca2+-induced current of total cells is shown. (F) Effects of ANO1 blockers on DHT-induced, Ca2+-activated Cl− currents in RWPE-1 cells. Blockers; 10 μM tannic acid (TA), 10 μM niflumic acid (NA), 10 μM 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB), 5 μM T16Ainh-A01, 20 μM CACCinh-A01, and 10 μM MONNA.
Tannic Acid Suppresses Prostate Enlargement.
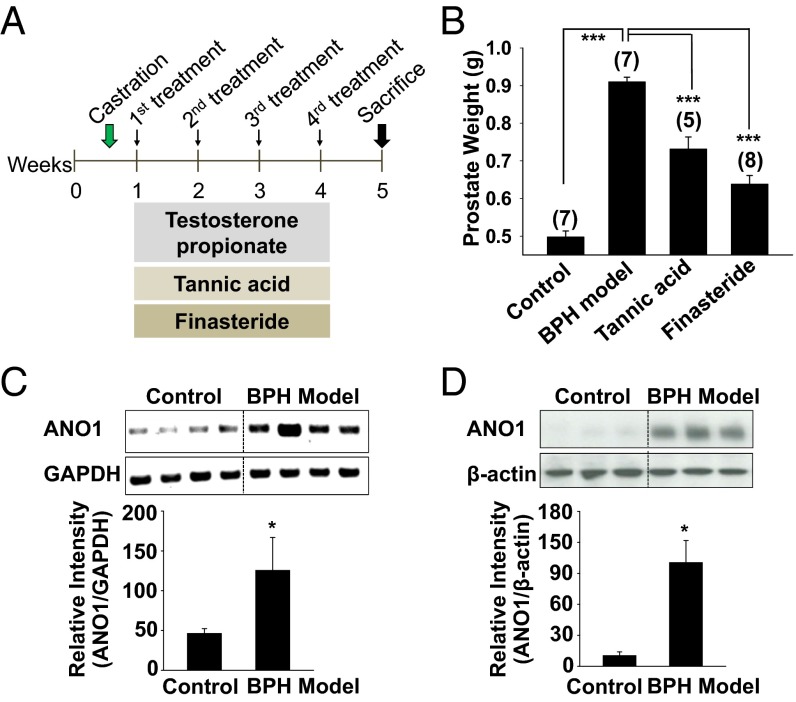
Next we examined whether the functional inhibition of ANO1 can block testosterone-induced prostate enlargement in vivo. To induce prostate enlargement (BPH model), 6-wk-old male rats were castrated and then treated with testosterone propionate (3 mg/kg, s.c. injection) for 4 wk (Fig. 5A). The testosterone-treated group showed a considerable increase in prostate weight compared with vehicle-treated, castrated controls (Fig. 5B). As a positive control, a 5α-reductase inhibitor, finasteride, was administered to castrated male rats. Chronic administration of finasteride (10 mg/kg, orally) reduced prostate weight significantly (P < 0.001, n = 8), suggesting that the development of prostate hyperplasia requires the conversion of testosterone to DHT (3) (Fig. 5B). Similarly, when tannic acid (150 mg/kg, orally for 4 wk) was administered, prostate weight was also significantly reduced (P < 0.001, n = 5) (Fig. 5B). A postmortem analysis of ANO1 expression in the BPH-model rats revealed that the levels of both ANO1 messenger RNA (mRNA) and protein were markedly increased (Fig. 5 C and D).
Fig. 5.
ANO1 is amplified in the prostate of BPH rat model. (A) Experimental schema. Castration control: After castration, rats were treated with s.c. injection of corn oil and oral saline; BPH model: s.c. injection of 3 mg/kg testosterone propionate and oral saline; Tannic acid and Finasteride: s.c. injection of 3 mg/kg testosterone propionate and oral tannic acid (150 mg/kg) or finasteride (10 mg/kg). (B) Effect of tannic acid on prostate weight. (C and D) RT-PCR (C) and Western blot (D) analysis of ANO1 in rat prostates. ANO1 band intensity was normalized to GAPDH (C) or β-actin (D). *P < 0.01.
In Vivo Knockdown of Ano1 Reduces Prostate Hyperplasia.
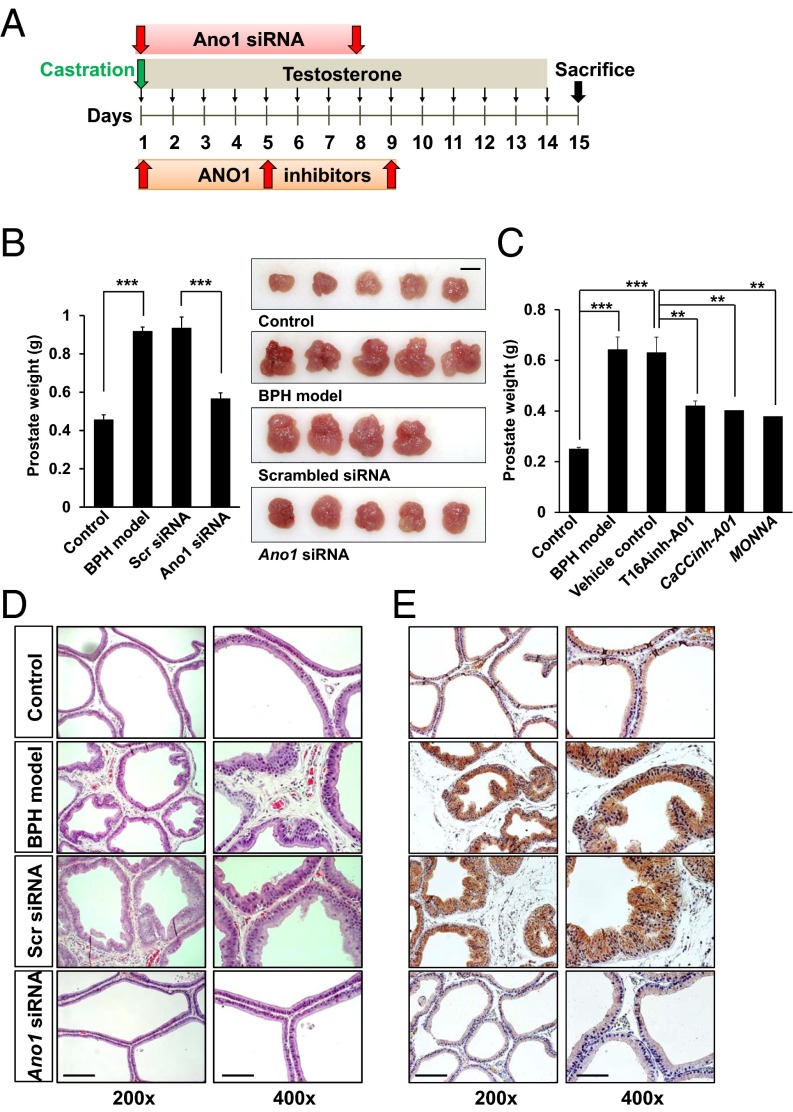
We then induced the in vivo depletion of Ano1 in the rat prostate by treatment with Ano1 siRNA to investigate whether it can effectively block BPH in vivo. Ano1 siRNA (32-40 μg per rat) was injected directly into the prostate twice (day 1 and 8) after castration of BPH-model rats (Fig. 6A). The prostates from testosterone-treated rats showed marked increase in size and weight relative to castrated controls (Fig. 6B). In contrast, treatment with Ano1 siRNA reduced the prostate weight significantly (60.5%, P < 0.001, n = 4–5), whereas prostates injected with scrambled siRNA showed a similar increase in weight to those of BPH-model rats (Fig. 6B). Similarly, intraprostatic injections (days 1, 5, and 9) of ANO1 inhibitors with some nonspecific activity as well, T16Ainh-A01, CaCCinh-A01, and MONNA (300 μM in 50 μL per rat) (28–30), significantly reduced the prostate weights of BPH model rats (Fig. 6C).
Fig. 6.
Intraprostatic injection of Ano1 siRNA or inhibitors reduces the prostate weight in the BPH rat model. (A) Experimental schema. After castration (day 1), corn oil or 3 mg/kg testosterone propionate was injected s.c. for 14 consecutive days. At day 1 and day 8, scrambled siRNA or ANO1 siRNA was injected slowly into the lateral prostate lobes. For the ANO1 inhibitor test, ANO1 inhibitors, T16Ainh-A01, CaCCinh-ANO1, and MONNA (300 μM, 50 μL per rat) were injected to prostates on days 1, 5, and 9. (B Left) Summary of the effect of Ano1 siRNA or scrambled siRNA injection on prostate weight. (Right) Comparison of the prostate size. (Scale bar: 1 cm.) (C) Summary of the effects of orthotopic injections of ANO1 antagonists on prostate weight. (D) Representative H&E histology of the prostate sections from the rat BPH model. (Magnifications: Left, 200×; Right, 400×.). (Scale bars: 20 μm.) (E) Representative immunostainings using ANO1 antibody.
We examined whether abnormal histologic changes in the prostates of BPH rats were affected by Ano1 depletion. Hematoxylin/eosin (H&E) staining showed highly overgrown epithelial cells in a multilayer array in the prostates from the BPH-model group compared with those in the control group that had well-arranged epithelial cells in a single layer (Fig. 6D). However, the histologic abnormalities were considerably reduced following Ano1 siRNA treatment (Fig. 6D). ANO1-immunoreactive cells that were markedly increased along the epithelia in the prostates of BPH-model rats were reduced by treatment with Ano1 siRNA, but not with scrambled siRNA (Fig. 6E). These results further suggest that ANO1 plays a key role in testosterone-induced prostate hyperplasia.
Ano1 Knockdown Suppresses Cell Proliferation.
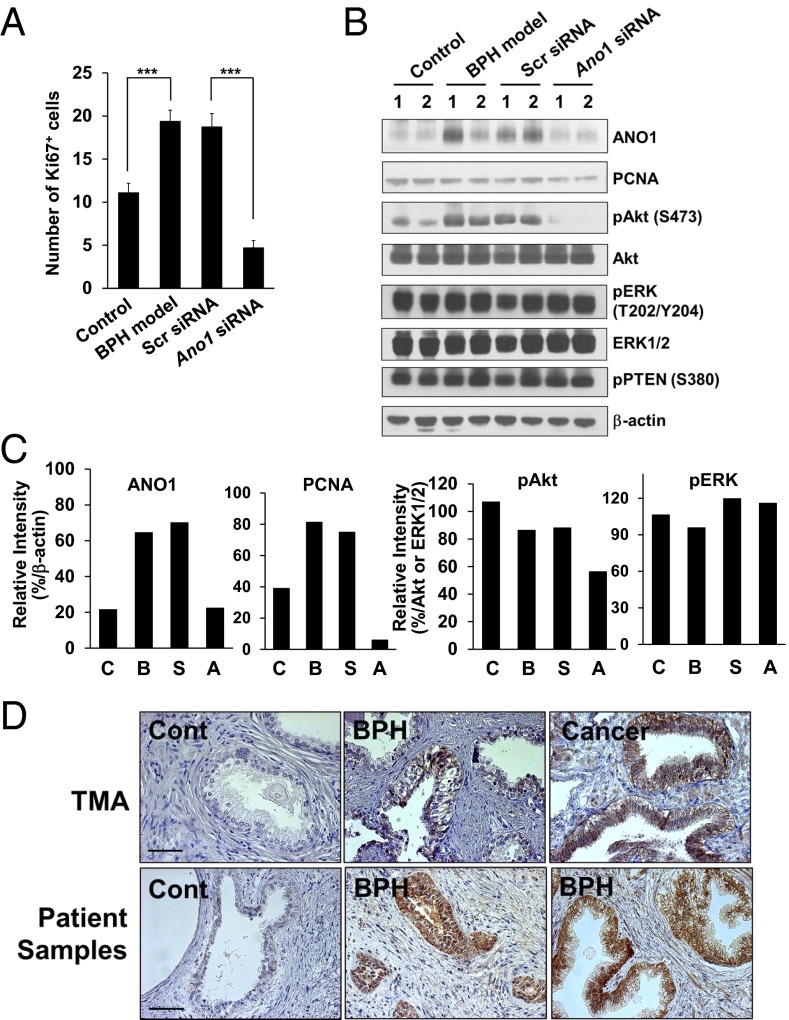
To determine whether Ano1 knockdown suppressed cell proliferation in vivo, we examined the expression of specific markers, such as Ki-67 and proliferating cell nuclear antigen (PCNA) (31), in the prostates of BPH-model rats. Treatment with Ano1 siRNA markedly reduced the number of Ki-67–positive cells compared with that in the scrambled siRNA-treated BPH-model rats (Fig. 7A) and the level of PCNA (Fig. 7C).
Fig. 7.
Intraprostatic injection of Ano1 siRNA suppresses cell proliferation. (A) The number of Ki-67–positive cells in prostates of control, rat BPH model and BPH model with scrambled or Ano1 siRNA-treated group. (B) Immunoblots of ANO1, PCNA, AKT, ERK, and phosphorylated PTEN in rat prostate lysates of control, rat BPH model, and BPH model with scrambled or Ano1 siRNA-treated group. Phosphorylated AKT (pAKT) and ERK (pERK) were also immunoblotted. The experiments were repeated twice. Numbers above the blots represent the number of experiments. (C) Quantification of immunoblot intensities of ANO1, PCNA, pAKT, and pERK shown in B. (D) ANO1 expression in human hyperplastic prostates. Representative images of ANO1 expression in human hyperplastic prostate samples obtained from tissue microarray (TMA) (Upper) and BPH patients (Lower). BPH, prostate from BPH patient; Cancer, prostate adenocarcinoma; Cont, ANO1 negative control. (Scale bar: 20 μm.)
The extracellular signal-regulated kinase (ERK) and protein kinase B (AKT) pathways are the main pathways regulating cell proliferation, survival, and differentiation (32). In addition, ERK and AKT are known to vary with ANO1 expression in prostate and other cancer tissues (19, 20, 33). Thus, to define the downstream mechanisms underlying the effects of ANO1 on prostate hyperplasia, we performed Western blots in the prostates of BPH-model rats with antibodies of phosphorylated AKT (S473) and ERK (T202/Y204). In addition, because gene ablation of a tumor suppressor phosphatase, PTEN, leads to prostate cancer (34), its expression along with BPH was also determined. The phosphorylation of AKT was markedly increased in BPH prostates compared with those in castration control prostates and completely inhibited by ANO1 knockdown in BPH prostate (Fig. 7 B and C). The level of phosphorylated ERK was not affected by the change in prostate size. Taken together, these results suggest that Ano1 knockdown inhibits cell proliferation in prostates by blocking of the AKT phosphorylation.
Finally, we investigated whether ANO1 expression is increased in BPH patient samples. Tissue microarray (PR804, Biomax) was prepared from 70 cases of BPH and 10 cases of prostate adenocarcinoma. Notably, ANO1 immunoreactivity was observed in 36 of 70 prostate samples (51%) from BPH patients. In addition, among the 10 samples from adenocarcinoma prostates, 9 samples (90%) were immunoreactive to ANO1-specific antibody (Fig. 7D). To further confirm the ANO1 expression in human hyperplastic prostates, we also obtained pathological tissue specimens from BPH patients. As shown in Fig. 7D, six of eight BPH specimens (75%) were positive to ANO1 immunoreactivity. The ANO1 immunoreactivity was found in multilayer-thickening epithelia (Fig. 7D).
Discussion
BPH is characterized by an enlargement of the prostate, which compresses the urethral canal and results in urinary tract obstruction. The major symptoms of BPH are urinary hesitancy, frequent urination, incomplete voiding, and urinary retention leading to renal failure (2). Androgen signaling through the AR is known to play a role in the development of BPH by promoting the proliferation of epithelial or stromal cells (35). The blockage of this signaling can reduce the volume of BPH and relieve the lower urinary tract symptoms (35). Despite the androgen dependence of hyperplasic cell growth, its downstream signals are still unclear. In this study, we showed that the treatment of prostate epithelial cells with DHT increased endogenous ANO1 expression and enhanced cell proliferation. Because the level of ANO1 was correlated with the concentration of DHT applied to the RWPE-1 cells, ANO1 transcription was suspected to be directly controlled by androgen. Indeed, ARE domains were identified in the Ano1 promoter region, by which the expression of ANO1 was controlled transcriptionally through in vivo interaction between the AR and AREs of ANO1 promoter in the presence of DHT. The application of an ANO1 blocker or ANO1 knock-down suppressed DHT-induced cell proliferation in vitro and testosterone-induced prostate hyperplasia in vivo.
AR downstream signaling is mediated by transcriptional activation when the AR complex binds to the ARE in the promoter regions of target genes. The ARE consensus sequence comprises two 5′-TGTTCT-3′ inverted repeats separated by three nucleotides (36). Nucleotide sequencing of the promoter region of Ano1 revealed that the Ano1 promoter contains three putative AREs that had high transcriptional activities in the luciferase reporter assay. ANO1 encodes a CaCC (8–10), and the main function of ANO1 is transepithelial Cl− secretion (8). When Cl− is fluxed out of epithelial cells, water also moves out, resulting in fluid secretion into the lumen. Because ANO1 does not appear to control the cell cycle or mitogenic activity directly, the increased expression of ANO1 in proliferating cells, including tumor cells, is enigmatic. ANO1 is highly expressed in many types of cancer tissues including prostates (see for review; ref. 24). The ANO1 amplification is linked to the Ras-ERK pathway in breast cancer or head and neck carcinoma (19, 33). However, the AKT pathway also appears to mediate the ANO1 downstream signals for the cell proliferation in breast cancer cells (19). Consistent with the latter observation, the level of AKT covaries with cell proliferation in prostates in the present study. The change in the ERK or AKT level in the ANO1-dependent cell proliferation largely depends on the activity of ANO1 as a channel (19, 33). However, how the channel activity of ANO1 is linked to the ERK or AKT pathway is not known. One theory is that the ANO1 activity leads to the increase in intracellular Cl− concentration, which is often found in cancer cells (24). However, when ANO1 is active as a channel, the intracellular concentration of Cl− would decrease, if any, because the Cl− flows out of the cell due to electrochemical gradient in epithelial cells. Depolarization is known to stimulate PI3K/AKT pathway in lung epithelial cells (37), which provides a clue to a missing link between the channel activity of ANO1 and cell proliferation in hyperplastic prostates as well as in cancer cells. Thus, it is likely that the membrane depolarization due to the activity of ANO1 would stimulate the PI3K/AKT pathway, resulting in the cell proliferation. However, this idea needs to be clarified. Because ANO1 mediates the secretion in epithelia as a CaCC (8), the secretion of fluid into the interstitial space via ANO1 could conceivably be advantageous for the proliferative microenvironment. Another hypothesis would be the regulation of cell volume, which is under the control of Cl− channel functions. A volume-regulated anion channel (VRAC) regulates the decrease in volume when a cell is exposed to hypoosmotic shock, and is also known to control the apoptosis-regulated volume (38). Thus, the regulation of cell volume is closely associated with apoptosis. Although ANO1 is not sensitive to hypoosmotic shock, the activity of ANO1 is probably involved in the control of cell volume, which eventually leads to changes in apoptotic activity.
Several clinical studies have supported the association between BPH and prostate cancer. Prospective and retrospective epidemiologic studies and clinical diagnostic examinations have suggested that BPH is a risk factor for prostate cancer (39). A 27-y follow-up study suggested a connection between BPH and the incidence of and mortality from prostate cancer (4). In contrast, other studies found a partial association, depending on surgical interventions in BPH patients (40), or no association between BPH and prostate cancer (41). Although no clear evidence of a close relationship between BPH and prostate cancer has been found, they share some common features such as hormone-dependent growth, responses to antiandrogen therapy, and common risk factors such as chronic inflammation and metabolic and genetic factors (6, 7).
Interestingly, ANO1 is also highly expressed in prostate cancer cells and in prostate adenocarcinoma in patients in vivo (20). Thus, regardless of the association between BPH and prostate cancer, ANO1 is expressed both in prostate hyperplasia and cancer cells (20). Because they are susceptible to androgens that appear to regulate ANO1 expression, unsurprisingly the level of ANO1 has been found to be up-regulated in both diseases. Furthermore, the suppression of ANO1 expression reduces both tumor growth and invasiveness in human prostate carcinoma and the size of the prostate in the BPH model (20). Thus, ANO1 probably plays a key role in the progression of BPH and prostate cancer.
Materials and Methods
Cell Culture and Transfection.
RWPE-1, PC3, and LnCap cells were purchased from ATCC and cultured in Keratinocyte-SFM (Gibco) and RPMI medium 1640 (Invitrogen), supplemented with 10% (vol/vol) FBS, respectively.
Reporter Gene Assay.
For the luciferase reporter encoding FR1, FR2, FR3, and FR4, the mouse Ano1 promoter region from −3000 to +78 relative to the transcription start site was amplified by PCR and cloned into the pGL3 basic vector. The MMTV-luciferase reporter plasmid (MMTV-luc) containing four inverted repeats of the 5′-TGTTCT-3′ sequence was used as a positive control.
Chromatin Immunoprecipitation Assay.
The chromatin, cross-linked to proteins, was digested with micrococcal nuclease. The digested chromatin was immunoprecipitated with control IgG or anti-AR antibodies (Millipore). The DNA-protein cross-links of immunoprecipitants were reversed, and then the DNA was purified. The association between AR and the putative AR binding regions of the ANO1 promoter was analyzed by PCR.
Rat BPH Model.
Animal care was carried out according to the guidelines of the Institutional Animal Care of the Seoul National University. Six-week-old male Wistar rats weighing 150–200 g were castrated to exclude the influence of intrinsic testosterone. BPH was generated in rats by s.c. injections of 3 mg/kg testosterone propionate for 4 wk after castration. The drugs were administered orally once daily for 4 wk. The rats were weighed weekly during the experiments. Under heavy anesthesia on day 29, prostates were removed, weighed, fixed in paraformaldehyde, and snap frozen.
Acknowledgments
This study was supported by National Research Foundation of Korea Grants NRF-2013R1A1A2063015 (to U.O.) and NRF-2014R1A2A1A10052265 (to M.O.L.) funded by the Ministry of Science, ICT and Future Planning and a grant from BK21+ program of Ministry of Education of Korea.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 9506.
This article is a PNAS Direct Submission.
References
- 1.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132(3):474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 2.Sarma AV, Wei JT. Clinical practice. Benign prostatic hyperplasia and lower urinary tract symptoms. N Engl J Med. 2012;367(3):248–257. doi: 10.1056/NEJMcp1106637. [DOI] [PubMed] [Google Scholar]
- 3.Bartsch G, Rittmaster RS, Klocker H. Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia. Eur Urol. 2000;37(4):367–380. doi: 10.1159/000020181. [DOI] [PubMed] [Google Scholar]
- 4.Ørsted DD, Bojesen SE, Nielsen SF, Nordestgaard BG. Association of clinical benign prostate hyperplasia with prostate cancer incidence and mortality revisited: A nationwide cohort study of 3,009,258 men. Eur Urol. 2011;60(4):691–698. doi: 10.1016/j.eururo.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Schenk JM, et al. Association of symptomatic benign prostatic hyperplasia and prostate cancer: Results from the prostate cancer prevention trial. Am J Epidemiol. 2011;173(12):1419–1428. doi: 10.1093/aje/kwq493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Nunzio C, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: The role of inflammation. Eur Urol. 2011;60(1):106–117. doi: 10.1016/j.eururo.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 7.De Nunzio C, Aronson W, Freedland SJ, Giovannucci E, Parsons JK. The correlation between metabolic syndrome and prostatic diseases. Eur Urol. 2012;61(3):560–570. doi: 10.1016/j.eururo.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Yang YD, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455(7217):1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134(6):1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caputo A, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322(5901):590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 11.Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol. 2008;321(1):141–149. doi: 10.1016/j.ydbio.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Hwang SJ, et al. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587(Pt 20):4887–4904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho H, et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci. 2012;15(7):1015–1021. doi: 10.1038/nn.3111. [DOI] [PubMed] [Google Scholar]
- 14.Jang Y, Oh U. Anoctamin 1 in secretory epithelia. Cell Calcium. 2014;55(6):355–361. doi: 10.1016/j.ceca.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 15.West RB, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165(1):107–113. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashyap MK, et al. Genomewide mRNA profiling of esophageal squamous cell carcinoma for identification of cancer biomarkers. Cancer Biol Ther. 2009;8(1):36–46. doi: 10.4161/cbt.8.1.7090. [DOI] [PubMed] [Google Scholar]
- 17.Carles A, et al. Head and neck squamous cell carcinoma transcriptome analysis by comprehensive validated differential display. Oncogene. 2006;25(12):1821–1831. doi: 10.1038/sj.onc.1209203. [DOI] [PubMed] [Google Scholar]
- 18.Huang X, Gollin SM, Raja S, Godfrey TE. High-resolution mapping of the 11q13 amplicon and identification of a gene, TAOS1, that is amplified and overexpressed in oral cancer cells. Proc Natl Acad Sci USA. 2002;99(17):11369–11374. doi: 10.1073/pnas.172285799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Britschgi A, et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc Natl Acad Sci USA. 2013;110(11):E1026–E1034. doi: 10.1073/pnas.1217072110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W, Lu M, Liu B, Huang Y, Wang K. Inhibition of Ca(2+)-activated Cl(-) channel ANO1/TMEM16A expression suppresses tumor growth and invasiveness in human prostate carcinoma. Cancer Lett. 2012;326(1):41–51. doi: 10.1016/j.canlet.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Stanich JE, et al. Ano1 as a regulator of proliferation. Am J Physiol Gastrointest Liver Physiol. 2011;301(6):G1044–G1051. doi: 10.1152/ajpgi.00196.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bill A, et al. Small molecule facilitated degradation of ANO1: A new targeting approach for anticancer therapeutics. J Biol Chem. 2014;289(16):11029–11041. doi: 10.1074/jbc.M114.549188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Cornejo P, et al. Anoctamin 1 (Tmem16A) Ca2+-activated chloride channel stoichiometrically interacts with an ezrin-radixin-moesin network. Proc Natl Acad Sci USA. 2012;109(26):10376–10381. doi: 10.1073/pnas.1200174109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu Z, et al. The Ca2+-activated Cl− channel, ANO1 (TMEM16A), is a double-edged sword in cell proliferation and tumorigenesis. Cancer Med. 2014;3(3):453–461. doi: 10.1002/cam4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ørsted DD, Bojesen SE. The link between benign prostatic hyperplasia and prostate cancer. Nat Rev Urol. 2013;10(1):49–54. doi: 10.1038/nrurol.2012.192. [DOI] [PubMed] [Google Scholar]
- 26.Nelson PS, et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci USA. 2002;99(18):11890–11895. doi: 10.1073/pnas.182376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonneveld E, Jansen HJ, Riteco JA, Brouwer A, van der Burg B. Development of androgen- and estrogen-responsive bioassays, members of a panel of human cell line-based highly selective steroid-responsive bioassays. Toxicol Sci. 2005;83(1):136–148. doi: 10.1093/toxsci/kfi005. [DOI] [PubMed] [Google Scholar]
- 28.Namkung W, Thiagarajah JR, Phuan PW, Verkman AS. Inhibition of Ca2+-activated Cl- channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. FASEB J. 2010;24(11):4178–4186. doi: 10.1096/fj.10-160648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Namkung W, Phuan PW, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem. 2011;286(3):2365–2374. doi: 10.1074/jbc.M110.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh SJ, et al. MONNA, a potent and selective blocker for transmembrane protein with unknown function 16/anoctamin-1. Mol Pharmacol. 2013;84(5):726–735. doi: 10.1124/mol.113.087502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong W, et al. Ki-67 and PCNA expression in prostate cancer and benign prostatic hyperplasia. Clin Invest Med. 2008;31(1):E8–E15. doi: 10.25011/cim.v31i1.3136. [DOI] [PubMed] [Google Scholar]
- 32.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem Sci. 2011;36(6):320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duvvuri U, et al. TMEM16A induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res. 2012;72(13):3270–3281. doi: 10.1158/0008-5472.CAN-12-0475-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4(3):209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 35.Izumi K, Mizokami A, Lin WJ, Lai KP, Chang C. Androgen receptor roles in the development of benign prostate hyperplasia. Am J Pathol. 2013;182(6):1942–1949. doi: 10.1016/j.ajpath.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Claessens F, et al. Selective DNA binding by the androgen receptor as a mechanism for hormone-specific gene regulation. J Steroid Biochem Mol Biol. 2001;76(1-5):23–30. doi: 10.1016/s0960-0760(00)00154-0. [DOI] [PubMed] [Google Scholar]
- 37.Chatterjee S, et al. Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS. Am J Physiol Heart Circ Physiol. 2012;302(1):H105–H114. doi: 10.1152/ajpheart.00298.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okada Y, et al. Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J Membr Biol. 2006;209(1):21–29. doi: 10.1007/s00232-005-0836-6. [DOI] [PubMed] [Google Scholar]
- 39.Hammarsten J, Högstedt B. Calculated fast-growing benign prostatic hyperplasia—a risk factor for developing clinical prostate cancer. Scand J Urol Nephrol. 2002;36(5):330–338. doi: 10.1080/003655902320783827. [DOI] [PubMed] [Google Scholar]
- 40.Chokkalingam AP, et al. Prostate carcinoma risk subsequent to diagnosis of benign prostatic hyperplasia: A population-based cohort study in Sweden. Cancer. 2003;98(8):1727–1734. doi: 10.1002/cncr.11710. [DOI] [PubMed] [Google Scholar]
- 41.Greenwald P, Kirmss V, Polan AK, Dick VS. Cancer of the prostate among men with benign prostatic hyperplasia. J Natl Cancer Inst. 1974;53(2):335–340. doi: 10.1093/jnci/53.2.335. [DOI] [PubMed] [Google Scholar]