Abstract
STUDY OBJECTIVE
To determine the effect of tacrolimus trough concentrations on clinical outcomes in kidney transplantation, while assessing if African-American (AA) race modifies these associations.
DESIGN
Retrospective longitudinal cohort study of solitary adult kidney transplants.
SETTING
Large tertiary care transplant center.
PATIENTS
Adult solitary kidney transplant recipients (n=1078) who were AA (n=567) or non-AA (n =511).
EXPOSURE
Mean and regressed slope of tacrolimus trough concentrations. Subtherapeutic concentrations were lower than 8 ng/ml.
MEASUREMENTS AND MAIN RESULTS
AA patients were 1.7 times less likely than non-AA patients to achieve therapeutic tacrolimus concentrations (8 ng/ml or higher) during the first year after kidney transplant (35% vs 21%, respectively, p<0.001). AAs not achieving therapeutic concentrations were 2.4 times more likely to have acute cellular rejection (ACR) as compared with AAs achieving therapeutic concentrations (20.8% vs 8.5%, respectively, p<0.01) and 2.5 times more likely to have antibody-mediated rejection (AMR; 8.9% vs 3.6%, respectively, p<0.01). Rates of ACR (8.3% vs 6.7%) and AMR (2.0% vs 0.9% p=0.131) were similar in non-AAs compared across tacrolimus concentration groups. Multivariate modeling confirmed these findings and demonstrated that AAs with low tacrolimus exposure experienced a mild protective effect for the development of interstitial fibrosis/ tubular atrophy (IF/TA; hazard ratio [HR] 0.78, 95% confidence interval [CI] 0.47–1.32) with the opposite demonstrated in non-AAs (HR 2.2, 95% CI 0.90–5.1).
CONCLUSION
In contradistinction to non-AAs, AAs who achieve therapeutic tacrolimus concentrations have substantially lower acute rejection rates but are at risk of developing IF/TA. These findings may reflect modifiable time-dependent racial differences in the concentration-effect relationship of tacrolimus. Achievement of therapeutic tacrolimus trough concentrations, potentially through genotyping and more aggressive dosing and monitoring, is essential to minimize the risk of acute rejection in AA kidney transplant recipients.
Keywords: kidney transplantation, African-American, tacrolimus, therapeutic drug monitoring, acute rejection
Tacrolimus (TAC) is the most commonly used immunosuppressant in transplantation and considered the cornerstone of contemporary maintenance immunosuppressive therapy. Therapeutic drug monitoring (TDM) of TAC in kidney transplantation, using 12-hour trough concentrations to approximate total exposure, is advocated in national guidelines and utilized in most transplant centers.1, 2 Despite this commonly accepted practice,3 evidence to support TAC TDM in improving clinical outcomes, either through reduction in acute rejection rates or toxicities, is conflicting. Recent data, pooled from three randomized controlled trials and conducted in a predominantly low-risk cohort of kidney transplant recipients, suggests that achieving therapeutic TAC trough concentrations early posttransplant is not associated with reduced rejection rates.4 However, other studies have demonstrated that TAC trough concentrations are associated with improved efficacy in higher risk patients.5–7
It is well established that African-Americans (AAs) are at substantially higher risk of acute rejection and graft loss following transplant.8–10 In addition, AAs require significantly higher doses of TAC to achieve therapeutic trough concentrations,11, 12 which is likely a reflection of differences in gene variants associated with drug absorption and metabolism.13 Outside of pharmacokinetic issues, AA patients are also at higher immunologic risk, which may be related to differences in the pharmacodynamics of immunosuppressants including TAC.8, 14, 15 Despite these data, a paucity of studies have assessed if AA race modifies the impact of TAC trough concentrations on clinical outcomes in kidney transplantation. This is likely because of an underrepresentation of AA patients in previous studies analyzing associations between TAC trough concentrations and clinical outcomes in transplantation.4–6, 16–19 Thus the objectives of this study were to determine the overall impact of TAC trough concentrations on clinical outcomes in kidney transplantation while assessing if AA race modifies these associations.
Methods
Study Design
This was a single-center longitudinal cohort study of a large sample of racially diverse adult solitary kidney recipients transplanted between 2005 and 2012 at the Medical University of South Carolina in Charleston, South Carolina. Data were retrospectively collected, starting at the time of transplant (baseline data) and longitudinally following patients through graft loss, death, or end of follow-up (July 2013). For the initial univariate comparisons, cohorts were delineated based on TAC 12-hour trough concentrations during the first year posttransplant (lower than 8 ng/ml vs 8 ng/ml or higher). This cutoff was chosen to be consistent with previous U.S. studies. Once it was established that AA race significantly modifies the impact of TAC exposure on acute rejection, cohorts were then stratified across recipient race, and final multivariate modeling is displayed in this context. Local institutional review board approval was obtained before conducting this study.
Patients
Patients were considered eligible for inclusion in this study if they received a kidney transplant from the study institution between 2005 and 2012 and were adults at the time of transplant (18 years or older). Patients were excluded if they received a nonrenal organ transplant before, with, or after the kidney transplant, received a maintenance immunosuppression regimen that did not consist of TAC, mycophenolate, and corticosteroids, had graft loss within 3 months of transplant, or were lost to follow-up.
Outcomes
Outcome measurements were time to development of acute rejection and interstitial fibrosis/ tubular atrophy (IF/TA) based on the 12-hour trough concentrations of TAC. Histology was obtained from both for cause and protocol biopsies that were scored using Banff criteria by a single pathologist. Time to these events was computed as the number of years starting at the time of transplant to the development of acute rejection and IF/TA or to graft loss, death, or end of follow-up (July 2013). Data from patients who did not develop any of these events were censored.
Data Variables and Study Definitions
Data were collected in a retrospective longitudinal manner using both paper and electronic medical records. Baseline information was collected at the time of transplant and included recipient sociodemographics and past medical history as well as donor and transplant characteristics. Following transplant, clinical data including laboratory values, medication regimens, and vital signs were collected at prespecified time points in relation to transplant date: day 3, 5, 7, and 14, month 1, 3, and 6, and yearly thereafter. All documented posttransplant events were captured including acute rejections, hospitalizations, graft failures, and deaths.
Graft failure was defined as a documented return to chronic dialysis or death. Acute rejection was defined as biopsy proven and at least Banff grade of 1A per the 1997 staging criteria. IF/ TA was considered to be a biopsy-proven development of at least mild IF/TA that occurred at least 1 month after transplant. IF/TA present on biopsies taken at the time of transplant or within 1 month of transplant was considered donor disease. Delayed graft function was defined as the need for dialysis within 7 days following transplant. Cytomegalovirus (CMV) infection was defined as the presence of CMV viremia of any detectable level (200 copies/ml or more) or any CMV viremia with signs and symptoms consistent with infection. BK infection was defined as BK viremia of any detectable level (200 copies/ml or more) and/or biopsy-proven BK nephropathy.
Intrapatient TAC concentrations were assessed using two methods, means and trajectories. For the mean comparisons, trough concentrations were averaged for each patient during the first year posttransplant (or up until having acute rejection or IF/TA), and cohorts were assigned based on a cutoff of 8 ng/ml. This cutoff trough concentration was chosen because our center considers this protocol to be therapeutic, as shown by previous studies conducted in the United States.4 For the trajectory analysis, each patient had a slope determined using linear regression. Both the mean and trajectory data were used as variables within multivariable modeling. These two methods of assessing TAC trough concentrations were chosen because they provide different information, with the mean analysis serving as a proxy for the average exposure prior to the event and the trajectory analysis informing assessment of the direction the concentrations were headed prior to the event.
Statistical Analysis
For the initial analysis, cohorts were divided into an exposure groups with TAC mean trough concentrations lower than 8 ng/ml and an exposure group with concentrations of 8 ng/ml or higher (defined as concentrations within the first year and prior to acute rejection or IF/TA events). Univariate comparisons between cohorts for baseline and follow-up data were made using the Pearson χ2 test for categorical data and the Student t test for continuous variables; nonparametric analyses were made using the Mann-Whitney U test when applicable.
Multivariable modeling, using Cox regression, was used to study the association between time to development of acute rejection and IF/TA and 12-hour trough concentrations of TAC in AAs and non-AAs. In this time-to-event analysis, variables were included in the model based on their univariate association or if they were known to influence the clinical outcomes of acute rejection or IF/TA. AA race effect modification was determined in a multiplicative fashion by including an interaction term (AA race × TAC concentration [both mean and trajectory]) within the models. Statistical significance was defined as a p value <0.05. Statistical analyses were performed using SPSS v. 22 (IBM Corp., Armonk, NY) and SAS v.9.4 (SAS Institute Inc., Cary, NC).
Results
Patients and Baseline Characteristics
Between January 1, 2005, and December 31, 2012, a total of 1501 kidney transplants were performed in our institution. Of these transplants, 423 patients were excluded because of nonkidney transplant (n=185), age younger than 18 years at time of transplant (n=79), administration of a non-TAC/mycophenolate mofetil maintenance immunosuppression regimen at baseline (n=63), graft loss within 3 months of transplant (n=38), and lost to follow-up (n=58), leaving 1078 patients who were included in the final analyses. The mean time of follow-up was 3.8 ± 2.1 years, which was similar between TAC exposure cohorts (3.8 ± 2.2 years in the TAC 8 ng/ml group or higher vs 3.7 ± 2.1 years in the TAC lower than 8 ng/ml group; p=0.524).
Baseline recipient demographics, past medical history, donor demographics, and transplant characteristics were reported across exposure cohorts (mean TAC 8 ng/ml or higher [n=767] vs < 8 ng/ml [n=311]) (Table 1). The groups were fairly similar for baseline information, except that the low TAC exposure cohort was more likely to be AA (65% vs 48%, p<0.001), to have received an expanded criteria donor kidney (18% vs 12%, p=0.011), and to develop delayed graft function (19% vs 11%, p=0.001). Because these factors influence the outcomes of acute rejection and IF/TA, they were controlled for in the multivariable modeling. The study population was racially diverse and contained a balanced number of AA (n=567) and non-AA (n =511) patients.
Table 1.
Baseline Characteristics Compared Across Tacrolimus Exposure Cohorts
| Variables | Mean TAC ≥ 8 ng/ml (n=767) | Mean TAC < 8 ng/ml (n=311) | p value |
|---|---|---|---|
| Recipient demographics | |||
| Median age (IQR) | 53.4 (42.5–62.4) | 50.9 (40.1–61.7) | 0.154 |
| Female, % | 41.0 | 42.1 | 0.634 |
| African-American, % | 47.6 | 65.0 | <0.001 |
| Medicare insurance, % | 70.8 | 74.0 | 0.297 |
| Recipient medical history | |||
| Primary cause of end-stage renal disease | |||
| Diabetes, % | 25.4 | 28.3 | 0.332 |
| Hypertension, % | 28.9 | 31.2 | 0.464 |
| Heart disease, % | 19.2 | 18.3 | 0.750 |
| Dialysis, % | 77.6 | 81.4 | 0.170 |
| Median years on dialysis (IQR) | 2 (0.5–4) | 2.5 (1–4) | 0.023 |
| Previous kidney transplant, % | 9.6 | 9.0 | 0.743 |
| Donor characteristics | |||
| Deceased donor, % | 82.5 | 84.6 | 0.419 |
| African-American, % | 23.1 | 28.9 | 0.043 |
| Expanded criteria donor, % | 11.7 | 18.2 | 0.011 |
| Median age (IQR) | 38 (24–47) | 39 (24–49) | 0.213 |
| Transplant characteristics | |||
| Cytolytic induction, % | 40.0 | 48.6 | 0.097 |
| Median panel reactive antibody (IQR) | 0 (0–27) | 0 (0–21) | 0.334 |
| Panel reactive antibody > 20%, % | 30.7 | 28.2 | 0.539 |
| Median HLA mismatch (IQR) | 4 (3–5) | 5 (4–5) | 0.053 |
| Median cold time, hrs (IQR) | 16.6 (9.1–23.1) | 17.3 (11.7–23.5) | 0.256 |
| Median warm time, min (IQR) | 36 (30–41) | 36 (30–40) | 0.723 |
| Delayed graft function, % | 11.2 | 18.6 | 0.001 |
IQR = interquartile range; HLA = human leukocyte antigen; TAC = tacrolimus.
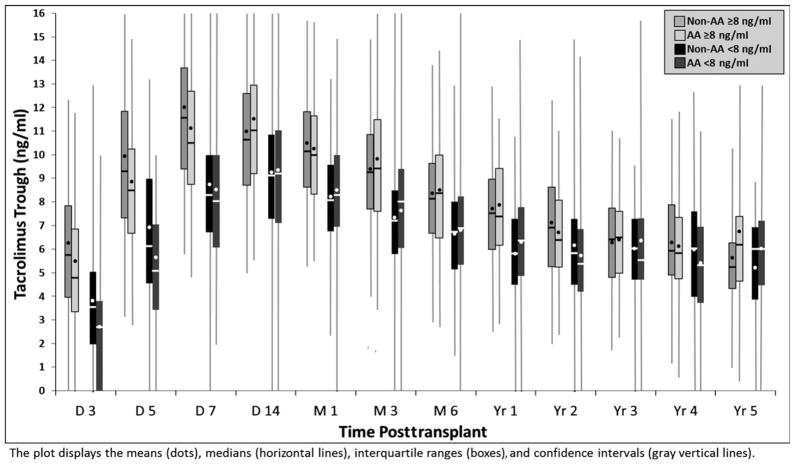
Good delineation was noted between exposure cohorts for TAC trough concentrations during the first year posttransplant, with those in the mean of lower than 8 ng/ml group having roughly a 2 ng/ml lower TAC concentration across all time points during the first year post-transplant (Figure 1). AA recipients in both TAC exposure cohorts had a delay in achieving therapeutic trough concentrations of ~2 days (median days to therapeutic: 7 days in AA vs 5 days in non-AA, p<0.001) and had 1.7 times the risk of not achieving a mean TAC trough concentration of at least 8 ng/ml (p<0.001) during the first year posttransplant.
Figure 1.
Tacrolimus trough concentrations compared over time across exposure cohorts, stratified by race.
Univariate Analysis for Outcomes Based on Mean TAC Concentrations
Table 2 displays the clinical outcomes compared across TAC exposure cohorts. Patients with a mean TAC concentration lower than 8 ng/ml had 2.2 (95% confidence interval [CI] 1.5–3.1, p<0.001) times the risk of developing acute cellular rejection and antibody-mediated rejection (AMR) (95% CI 1.2–4.1, p=0.010). The risk of developing de novo IF/TA was also significantly higher in the low TAC exposure cohort (relative risk 1.6, 95% CI 1.1–2.2, p=0.010). In multivariable modeling, the interaction term between recipient race and TAC exposure was statistically significant for acute rejection (p<0.05), which was consistent for models that included the intrapatient mean TAC trough concentration as well as those including the intra-patient TAC trajectory. The interaction term for the outcome of IF/TA was not statistically significant (p=0.192, 0.197) for either model.
Table 2.
Clinical Outcomes Compared across Tacrolimus Exposure Cohorts
| Clinical outcomes | Mean TAC = 8 ng/ml (n=767), % | Mean TAC < 8 ng/ml (n=311), % | p value |
|---|---|---|---|
| Acute rejection | 7.6 | 16.4 | < 0.001 |
| Borderline rejection | 11.1 | 14.1 | 0.16 |
| Antibody-mediated rejection | 2.7 | 6.1 | 0.008 |
| Interstitial fibrosis/ tubular atrophy | 9.6 | 15.1 | 0.01 |
| Any BK infection | 13.3 | 14.1 | 0.712 |
| BK nephropathy | 5.1 | 5.1 | 0.968 |
| Any cytomegalovirus infection | 16.8 | 15.1 | 0.492 |
| Graft loss | 10.0 | 12.2 | 0.294 |
| Death | 6.4 | 6.8 | 0.826 |
TAC = tacrolimus.
Because of the significant interaction between recipient race and TAC exposure for the outcome of acute rejection, the data were stratified across race (Table 3). Among AAs, acute rejection (p<0.001) and AMR (p=0.007) rates were significantly higher in those with mean TAC concentrations lower than 8 ng/ml. This was not demonstrated for either acute rejection (p=0.577) or AMR (p=0.450) among non-AAs. The rates of IF/TA were similar in those with low TAC exposure among both AAs (p=0.138) and non-AAs (p=0.112). Graft loss rates were similar across TAC exposure cohorts, regardless of race as well.
Table 3.
Clinical Outcomes Compared across Tacrolimus Exposure Cohorts, Stratified by Race
| African-Americans
|
Non–African-Americans
|
|||
|---|---|---|---|---|
| Clinical outcomes | Mean TAC ≥ 8 ng/ml (n=365), % | Mean TAC < 8 ng/ml (n=202), % | Mean TAC ≥ 8 ng/ml (n=402), % | Mean TAC < 8 ng/ml (n=109) |
| Acute rejection | 8.5 | 20.8a | 6.7 | 8.3b |
| Borderline rejection | 12.3 | 14.4b | 10.0 | 13.8b |
| Antibody-mediated rejection | 3.6 | 8.9a | 2.0 | 0.9b |
| Interstitial fibrosis/tubular atrophy | 12.3 | 16.8b | 7.2 | 11.9b |
| Graft loss | 10.7 | 12.4b | 9.5 | 11.9b |
| Death | 6.6 | 5.9b | 6.2 | 8.3b |
TAC = tacrolimus.
p<0.05.
p<0.05.
Multivariate Analyses
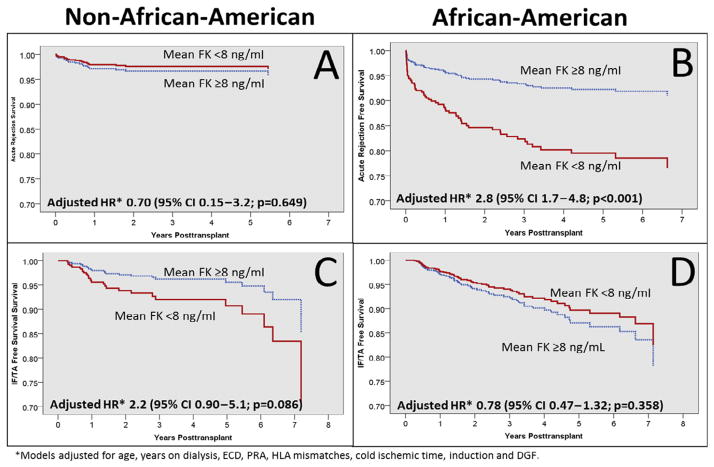
Figure 2 displays the Cox regression models adjusted event-free survival plot estimates, stratified by race for the outcomes of acute rejection (Figure 2A, 2B) and IF/TA (Figure 2C, 2D) for the mean TAC exposure analysis. The figure demonstrates that AA patients with FK lower than 8 ng/ml experienced a significantly higher risk of acute rejection (adjusted hazard ratio [HR] 2.8, 95% CI 1.7–4.8; Figure 2B), which was not demonstrated in non-AAs (adjusted HR 0.7, 95% CI 0.15–3.2; Figure 2A). The multivariate modeling also revealed that AA patients with FK lower than 8 ng/ml experienced a mild protective effect for developing IF/TA (HR 0.78, 95% CI 0.47–1.32; Figure 2D), whereas non-AAs with FK lower than 8 ng/ml were at higher risk of IF/TA (HR 2.2, 95% CI 0.90–5.1; Figure 2C).
Figure 2.
Cox regression event-free survival plots for acute rejection and interstitial fibrosis/tubular atrophy (IF/TA), compared across FK exposure cohorts and stratified by race. CI = confidence interval; DGF = delayed graft function; ECD = expanded criteria donor; HLA = human leukocyte antigen; HR = hazard ratio; PRA = panel reactive antibody.
For the outcome of IF/TA, the multivariate modeling revealed differences as compared with the univariate analysis. AA patients with low TAC exposure experienced a mild protective effect (HR 0.78, 95% CI 0.47–1.32; Figure 2D), whereas non-AAs with low TAC exposure were at higher risk of IF/TA (HR 2.2, 95% CI 0.90–5.1; Figure 2C); however, neither of these adjusted HR estimates reached statistical significance, although the trends were in the opposite direction. Acute rejection (adjusted HR 24–87) was the predominant risk factor for developing IF/TA in both AAs and non-AAs.
Multivariate modeling using Cox regression was performed using intrapatient TAC trajectories as a continuous covariate for the outcomes of acute rejection and graft loss (Table 4). These results demonstrated a similar pattern for effect modification of race on the association of TAC exposure for acute rejection (interaction term p<0.001) but not for IF/TA (p=0.182). The intrapatient TAC trajectory was significantly and positively associated with acute rejection, with a substantially larger effect seen in AAs (HR 16.6) as compared with non-AAs (HR 4.6). Conversely, the intrapatient TAC trajectory was not statistically significantly associated with IF/TA in either AAs (HR 7.9) or non-AAs (0.3), although, similar to the mean TAC lower than 8 ng/ml model, the effect trend was in opposite directions: AAs with increasing intrapatient TAC trajectories were at higher risk of IF/TA, whereas non-AAs with increasing intrapatient TAC trajectories were at a lower risk of developing IF/TA.
Table 4.
Multivariate Modeling Output for Intrapatient Tacrolimus Trajectory for Acute Rejection and Interstitial Fibrosis/ Tubular Atrophy, Stratified by Race
| Outcomea | Cohort | Parameter estimate | Standard error | Adjusted HR | 95% CI | p value |
|---|---|---|---|---|---|---|
| Acute rejectionb | African-American | 2.81 | 0.42 | 16.60 | 7.3–37.9 | < 0.0001 |
| Non–African-American | 1.54 | 0.30 | 4.65 | 2.58–8.36 | < 0.0001 | |
| IF/TAc | African-American | 2.07 | 1.81 | 7.91 | 0.23–275.3 | 0.2534 |
| Non–African-American | −1.21 | 3.89 | 0.30 | 0.00–609.3 | 0.7560 |
CI = confidence interval; HLA = human leukocyte antigen; HR = hazard ratio; IF/TA = interstitial fibrosis/tubular atrophy.
Model adjusted for gender, age, diabetes, insurance, donor type, HLA mismatch, panel reactive antibody, retransplant, and induction.
Interaction term p=0.0003.
Interaction term p=0.1823.
Discussion
The results of this analysis provide novel insights into the markedly different impact of TAC exposure on outcomes across recipient race. AA kidney transplant recipients have a 70% higher risk of not achieving mean therapeutic TAC trough concentrations during the first year posttransplant. Importantly, low TAC exposure substantially increases the risk of acute rejection in AAs, which was not demonstrated in non-AAs. Conversely, AA recipients with therapeutic TAC concentrations may be at a somewhat higher risk of developing IF/TA, which, after confounder adjustment, appears to be a mildly protective factor in non-AAs.
Intrapatient TAC trajectory analysis, which was used as a surrogate measure for the direction that TAC concentrations are trending prior to the event, also demonstrate that AA patients have significantly higher trajectories (slopes) prior to acute rejection as compared with non-AAs. AAs with increasing trajectories are also at mildly higher risk of developing IF/TA, with the opposite effect seen in non-AAs. These analyses, taken in entirety, provide evidence that enhanced monitoring and manipulation of TAC trough concentrations may provide one mechanism to mitigate racial disparities in kidney transplantation.
Evidence supporting the use of TAC TDM to improve outcomes or reduce toxicities is conflicting.3 The most recent study, published in 2013, was a secondary analysis of pooled data from three randomized controlled clinical trials. The analysis failed to find a relationship between TAC troughs at five time points (day 3, 10, 14 and month 1 and 6 posttransplant) and biopsy-proven acute rejection. Although this was a large analysis (n=1304), none of the three trials were originally designed to study this association, and the population of kidney transplant recipients included was low risk, with 12% black, 40% living donor, and 92% with a panel reactive antibody less than 15%.4 In contradistinction, two recent analyses demonstrated that early post-transplant tacrolimus trough concentrations were significantly lower in patients who subsequently developed acute rejection. Both of these studies contained smaller sample sizes (n=57 and 216), but patients appeared to be at higher immunologic risk compared with the Bouamer analysis.5, 7 However, to date, very limited studies have assessed the association between TAC trough concentrations and acute rejection that contain a significant number of AA patients. Most recent studies analyzing the association between TAC concentrations and outcomes are from the United Kingdom, Australia, or Europe. Studies from the United States are either dated (use much higher target TAC trough concentrations than conventional strategies) or have a small sample size.6, 16–20 There are data clearly demonstrating that AA recipients require higher TAC doses to achieve therapeutic concentrations, but these analyses have not correlated this to acute rejection.11, 12 Thus the data presented within this analysis are the first to provide statistically and clinically significant associations between TAC concentrations and clinical outcomes within a large AA population (n=567).
There is a robust biologic explanation why AA patients have a significantly stronger association between TAC concentrations and clinical outcomes when compared with non-AAs. First, AAs are less likely to achieve therapeutic TAC concentrations, which is likely a reflection of pharmacogenomics.14, 15 AA patients are substantially more likely to be CYP 3A5 expressors, a known factor that strongly influences TAC dosing requirements.21, 22 Second, AAs are at higher immunologic risk due to a number of potential etiologies including more HLA mismatching,23, 24 more marginal donors,25 and immune functionality differences.26–28 Thus achieving therapeutic TAC concentrations early posttransplant in AAs is less common yet may be more crucial to preventing acute rejection when compared with non-AAs. Finally, AAs are more likely to receive deceased donor organs,25 which due to their increased marginal status as compared with living donors, may be more prone to TAC-related nephrotoxicity, leading to higher rates of IF/TA in patients achieving therapeutic TAC concentrations.29–31
Moving forward, a number of potential interventions have the strong potential to improve TAC medication therapy management and monitoring in AA recipients. First, prospective genotyping of potential recipients for CYP3A5 and ABCB1 would potentially allow clinicians the ability to develop individualized initial dosing strategies for TAC at the time of transplantation. Because AAs are known to predominantly express the CYP3A5 gene variant, this alone could lead to dramatic improvements in early TAC dosing accuracy. The literature demonstrating a strong association between ABCB1 gene variants and tacrolimus pharmacokinetics and dynamics is less compelling and somewhat contradictory. A number of studies have found that ABCB1 gene variants are associated with tacrolimus dosing requirements and risk of toxicity (calcineurin inhibitor [CNI] nephrotoxicity through accumulation in the allograft); others have failed to find such associations.3, 13, 15, 32 Thus further research is needed in this area. In addition, the interactions and convergence of CYP3A5 and ABCB1 gene variants on TAC pharmacokinetics and dynamics is not well studied, particularly as it relates to racial disparities in transplantation.3, 13, 15, 32 The data presented in this analysis suggest that improved understanding of CYP3A5 and ABCB1 genotyping may allow for more accurate dosing and monitoring of TAC therapy and provide clinicians better information to conduct risk-benefit assessment of this highly efficacious, yet significantly toxic immunosuppressant therapy. This is particularly the case in AA patients.32
In terms of the association between therapeutic TAC concentrations and the development of IF/TA, the path forward is not as well defined. Two studies have demonstrated good success with CNI minimization or withdrawal regimens, using therapy based on mammalian target of rapamycin in AA recipients.33, 34 Perhaps developing a method to identify which AA recipients are at high likelihood to develop significant post-transplant IF/TA may be the first step toward individualizing immunosuppression regimens. ABCB1 gene variant analysis, in conjunction with other known donor and recipient risk factors, may provide the ability to predict which patients are at greater risk of CNI-associated nephrotoxicity.32 It is clear that the predominant risk of developing IF/TA is acute rejection, regardless of race. Thus improving early TAC dosing accuracy in AAs, which will likely lead to lower rejection rates, may, in fact, be the most successful intervention to improve all posttransplant outcomes.
A number of limitations to this study should be discussed. First, this was a retrospective study, and as such, it is prone to bias, misclassification, and confounders. We attempted to limit bias by specifying minimum predefined exclusions. Indeed, very few patients were excluded due to missing data or lost to follow-up. Mis-classification is also a possibility because a number of methods were used to assess TAC exposure. To minimize misclassification, we utilized two methods (intrapatient mean and trajectory) to conduct a robust assessment of exposure. We chose not to use single-time point measurements as previous studies have done, which are limited by a high degree of both intra-and interpatient variability. Because this was a longitudinal cohort study, we were able to classify outcomes in a time-to-event manner (rejection and IF/TA), which allowed us to fully establish temporality, because only the TAC concentrations that were drawn prior to the event were included in the exposure calculations. Confounding was minimized by collecting detailed baseline and follow-up data and including known risk factors in multivariate models. Because we had access to a large sample size with significant event rates, we were able to include all important confounders in models, which was a limitation of previous studies. A number of limitations that we cannot fully address due to the retrospective nature of this study include assessment of gene variants, assessment of medication adherence to TAC therapy, and discerning if all concentrations were true 12-hour predose troughs. The 7-year time period of the study, which may have captured significant evolutions in donor and recipient characteristics and/or clinical practice standards was also a limitation. However, using this time period did allow for a large sample of patients, which increased the power and external validity of this study.
In conclusion, these results provide novel evidence to support that AA kidney transplant recipients are more likely to have early subthera-peutic TAC trough concentrations, which significantly increases their risk of acute rejection. We did not observe this association in non-AAs. Conversely, AAs achieving therapeutic TAC concentrations may be at higher risk of developing IF/TA.
Acknowledgments
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Numbers K23DK099440 and T35 DK007431.
Footnotes
This research was presented at the 2014 ASN Kidney Week, as an oral presentation, in Philadelphia, PA.
References
- 1.Disease K. Improving Global Outcomes (KDIGO) Transplant Work Group: KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 2.Wallemacq P, Armstrong VW, Brunet M, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit. 2009;31:139–52. doi: 10.1097/FTD.0b013e318198d092. [DOI] [PubMed] [Google Scholar]
- 3.Kuypers DR. Immunosuppressive drug monitoring—what to use in clinical practice today to improve renal graft outcome. Transplant Int. 2005;18:140–50. doi: 10.1111/j.1432-2277.2004.00041.x. [DOI] [PubMed] [Google Scholar]
- 4.Bouamar R, Shuker N, Hesselink DA, et al. Tacrolimus pre-dose concentrations do not predict the risk of acute rejection after renal transplantation: a pooled analysis from three randomized-controlled clinical trials. Am J Transplant. 2013;13:1253–61. doi: 10.1111/ajt.12191. [DOI] [PubMed] [Google Scholar]
- 5.Borobia AM, Romero I, Jimenez C, et al. Trough tacrolimus concentrations in the first week after kidney transplantation are related to acute rejection. Ther Drug Monit. 2009;31:436–42. doi: 10.1097/FTD.0b013e3181a8f02a. [DOI] [PubMed] [Google Scholar]
- 6.Undre N, Van Hooff J, Christiaans M, et al. Low systemic exposure to tacrolimus correlates with acute rejection. Transplantation. 1999;31:296–8. doi: 10.1016/s0041-1345(98)01633-9. [DOI] [PubMed] [Google Scholar]
- 7.Richards KR, Hager D, Muth B, Astor BC, Kaufman D, Djamali A. Tacrolimus trough level at discharge predicts acute rejection in moderately sensitized renal transplant recipients. Transplantation. 2014;97:986–91. doi: 10.1097/TP.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 8.Malat GE, Culkin C, Palya A, Ranganna K, Kumar MS. African American kidney transplantation survival: the ability of immunosuppression to balance the inherent pre- and post-transplant risk factors. Drugs. 2009;69:2045–62. doi: 10.2165/11318570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Neylan JF. Racial differences in renal transplantation after immunosuppression with tacrolimus versus cyclosporine. FK506 kidney transplant study group. Transplantation. 1998;65:515–23. doi: 10.1097/00007890-199802270-00011. [DOI] [PubMed] [Google Scholar]
- 10.Padiyar A, Augustine JJ, Bodziak KA, Aeder M, Schulak JA, Hricik DF. Influence of African-American ethnicity on acute rejection after early steroid withdrawal in primary kidney transplant recipients. Transplant Proc. 2010;42:1643–7. doi: 10.1016/j.transproceed.2010.02.081. [DOI] [PubMed] [Google Scholar]
- 11.Mancinelli LM, Frassetto L, Floren LC, et al. The pharmacokinetics and metabolic disposition of tacrolimus: a comparison across ethnic groups. Clin Pharmacol Ther. 2001;69:24–31. doi: 10.1067/mcp.2001.113183. [DOI] [PubMed] [Google Scholar]
- 12.Vadivel N, Garg A, Holt DW, Chang RW, MacPhee IA. Tacrolimus dose in black renal transplant recipients. Transplantation. 2007;83:997–9. doi: 10.1097/01.tp.0000259248.60448.8a. [DOI] [PubMed] [Google Scholar]
- 13.MacPhee IA, Holt DW. A pharmacogenetic strategy for immunosuppression based on the CYP3A5 genotype. Transplantation. 2008;85:163–5. doi: 10.1097/TP.0b013e3181609054. [DOI] [PubMed] [Google Scholar]
- 14.Higgins R, Fishman J. Disparities in solid organ transplantation for ethnic minorities: facts and solutions. Am J Transplant. 2006;6:2556–62. doi: 10.1111/j.1600-6143.2006.01514.x. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda S, Zhang L, Huang S. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84:417–23. doi: 10.1038/clpt.2008.141. [DOI] [PubMed] [Google Scholar]
- 16.Staatz C, Taylor P, Tett S. Low tacrolimus concentrations and increased risk of early acute rejection in adult renal transplantation. Nephrol Dial Transplant. 2001;16:1905–9. doi: 10.1093/ndt/16.9.1905. [DOI] [PubMed] [Google Scholar]
- 17.Bottiger Y, Brattstrom C, Tyden G, Sawe J, Groth CG. Tacrolimus whole blood concentrations correlate closely to side-effects in renal transplant recipients. Br J Clin Pharmacol. 1999;48:445–8. doi: 10.1046/j.1365-2125.1999.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kershner RP, Fitzsimmons WE. Relationship of FK506 whole blood concentrations and efficacy and toxicity after liver and kidney transplantation. Transplantation. 1996;62:920–6. doi: 10.1097/00007890-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 19.Laskow DA, Vincenti F, Neylan JF, Mendez R, Matas AJ. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States multicenter FK506 kidney transplant group. Transplantation. 1996;62:900–5. doi: 10.1097/00007890-199610150-00005. [DOI] [PubMed] [Google Scholar]
- 20.Naesens M, Lerut E, Damme B, Vanrenterghem Y, Kuypers DR. Tacrolimus exposure and evolution of renal allograft histology in the first year after transplantation. Am J Transplant. 2007;7:2114–23. doi: 10.1111/j.1600-6143.2007.01892.x. [DOI] [PubMed] [Google Scholar]
- 21.Haufroid V, Wallemacq P, Van Kerckhove V, et al. CYP3A5 and ABCB1 polymorphisms and tacrolimus pharmacokinetics in renal transplant candidates: guidelines from an experimental study. Am J Transplant. 2006;6:2706–13. doi: 10.1111/j.1600-6143.2006.01518.x. [DOI] [PubMed] [Google Scholar]
- 22.Hesselink DA, van Schaik RH, van der Heiden IP, et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74:245–54. doi: 10.1016/S0009-9236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 23.Leffell MS, Steinberg AG, Bias WB, Machan CH, Zachary AA. The distribution of HLA antigens and phenotypes among donors and patients in the UNOS registry. Transplantation. 1994;58:1119. [PubMed] [Google Scholar]
- 24.Rebellato LM, Arnold AN, Bozik KM, Haisch CE. HLA matching and the United Network for Organ Sharing Allocation System: impact of HLA matching on African-American recipients of cadaveric kidney transplants. Transplantation. 2002;74:1634. doi: 10.1097/00007890-200212150-00024. [DOI] [PubMed] [Google Scholar]
- 25.Matas A, Smith J, Skeans M, et al. OPTN/SRTR 2011 annual data report: kidney. Am J Transplant. 2012;13:11–46. doi: 10.1111/ajt.12019. [DOI] [PubMed] [Google Scholar]
- 26.McDaniel D, Barber W, Nguyan C, et al. Combined analysis of cytokine genotype polymorphism and the level of expression with allograft function in African-American renal transplant patients. Transpl Immunol. 2003;11:107–19. doi: 10.1016/S0966-3274(02)00171-5. [DOI] [PubMed] [Google Scholar]
- 27.Hutchings A, Purcell WM, Benfield MR. Increased costimulatory responses in African-American kidney allograft recipients. Transplantation. 2001;71:692–5. doi: 10.1097/00007890-200103150-00021. [DOI] [PubMed] [Google Scholar]
- 28.Kerman RH, Kimball P, Van Buren CT, Lewis RM, Kahan BD. Possible contribution of pretransplant immune responder status to renal allograft survival differences of black versus white recipients. Transplantation. 1991;51:338. doi: 10.1097/00007890-199102000-00013. [DOI] [PubMed] [Google Scholar]
- 29.de Mattos AM, Olyaei AJ, Bennett WM. Nephrotoxicity of immunosuppressive drugs: long-term consequences and challenges for the future. Am J Kidney Dis. 2000;35:333–46. doi: 10.1016/s0272-6386(00)70348-9. [DOI] [PubMed] [Google Scholar]
- 30.Lo A, Egidi MF, Gaber LW, et al. Observations regarding the use of sirolimus and tacrolimus in high-risk cadaveric renal transplantation. Clin Transplant. 2004;18:53–61. doi: 10.1111/j.1399-0012.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro R, Vivas C, Scantlebury VP, et al. “Suboptimal” kidney donors: the experience with tacrolimus-based immunosuppression. Transplantation. 1996;62:1242–6. doi: 10.1097/00007890-199611150-00010. [DOI] [PubMed] [Google Scholar]
- 32.Hesselink DA, Bouamar R, Elens L, van Schaik RHN, van Gelder T. The role of pharmacogenetics in the disposition of and response to tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2014;53:123–39. doi: 10.1007/s40262-013-0120-3. [DOI] [PubMed] [Google Scholar]
- 33.Hricik DE, Knauss TC, Bodziak KA, et al. Withdrawal of steroid therapy in African American kidney transplant recipients receiving sirolimus and tacrolimus. Transplantation. 2003;76:938–42. doi: 10.1097/01.TP.0000089440.47239.3F. [DOI] [PubMed] [Google Scholar]
- 34.Patel N, Taber DJ, Weimert NA, et al. Potential differences in kidney allograft outcomes between ethnicities when converting to sirolimus base immunosuppression. Transplant Proc. 2009;41:4131–7. doi: 10.1016/j.transproceed.2009.09.088. [DOI] [PubMed] [Google Scholar]