B cell depletion is an effective remission induction and maintenance therapy in patients with antineutrophil cytoplasmic auto-antibody (ANCA)-associated vasculitis (AAV).1–6 Rituximab targets both pathogenic effector B cells and protective regulatory B cells. To avoid infections and adverse events from therapy, clinicians require improved markers of disease activity and impending relapse to guide immunosuppression strategies following B cell depletion. We reported that CD5+ B cells, as a surrogate marker of B regulatory cells, are decreased in patients with active AAV and normalise during disease remission.7 After B cell depletion, patients who repopulated with a low or decreasing percentage of CD5+ B cells and were on low maintenance immunosuppression had a shorter time to relapse than patients on similar levels of immunosuppression with normalised CD5+ B cells or patients with similarly low CD5+ B cells but higher immunosuppression. The CD5+CD24hiCD38hi B cell subpopulation correlates inversely with active disease but parallels both interleukin (IL)-10 production and suppression of ANCA.8 CD5 may identify B cells enriched in IL-10 production, the defining cytokine of B regulatory cells.8,9 Whether CD5+ B cells can serve as an indicator of time to relapse without considering remission maintenance immunosuppression dose is not known. We sought to address this question and confirm our previous findings in a larger cohort by separating patients solely based on their CD5+ B cells at repopulation.
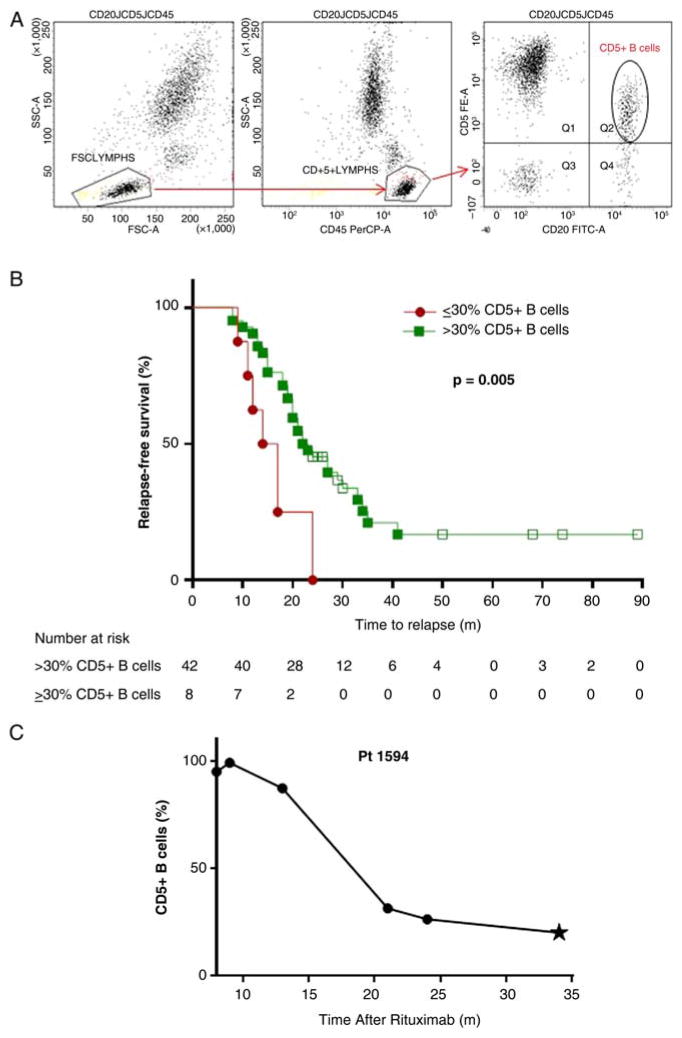
We examined B cell phenotype in 50 patients with AAV following rituximab therapy by flow cytometry (table 1). Patients with ANCA-negative vasculitis or history of other autoimmune disease were excluded. Data available from the University of North Carolina (UNC) Hospitals McLendon Clinical Flow Cytometry Laboratories were reanalysed with FACSDiva software to determine the percentage of CD5+ B cells instead of CD5+ lymphocytes typically reported in this clinical test (figure 1A). Patients were divided into two groups at first B cell repopulation (≥1% CD19+/CD20+ lymphocytes): those who repopulated with >30% (high) CD5+ B cells and those who repopulated with ≤30% (low) CD5+ B cells. Maintenance immunosuppression with other agents did not factor into patient grouping. Patients who repopulated with low CD5+ B cells relapsed sooner (median=16 months (IQR=12–19)) than patients who repopulated with high CD5+ B cells (23 months (18–30); p=0.005) after rituximab (figure 1B). If time to relapse from B cell repopulation was considered, patients who repopulated with low CD5+ B cells relapsed much sooner (3 months (1–9)) than patients who repopulated with high CD5+ (12 months (6–21), p=0.001; table 1). Although patients repopulating with low CD5+ B cells had less upper respiratory involvement, time to relapse remained significantly shorter for these patients after adjusting for upper respiratory involvement by time-to-event proportional hazards modelling (table 1). Controlling for upper respiratory involvement and PR3 serotype, those with low CD5 remained at higher risk for relapse with a HR of 3.7 (95% CI 1.5 to 9.0, p=0.005). HRs and CIs remained constant when controlling for PR3 serotype and lung involvement or with CD5 as a continuous variable. Of 25 patients who relapsed and had additional samples available, 20 (80%) demonstrated a decrease in CD5+ prior to relapse. Longitudinal data following repopulation with high CD5+ B cells depicts decreasing CD5+ B cells prior to relapse (figure 1C).
Table 1.
Clinical characteristics and CD5+ B cell repopulation of patients with AAV after B cell depletion therapy
| Characteristic | Repopulation with >30% CD5+ B cells n=42 | Repopulation with ≤30% CD5+ B cells n=8 | p Value* |
|---|---|---|---|
| Age at time of rituximab infusion, median (IQR) | 54 (41–62) | 56 (52–60) | 0.572 |
| Female gender, n (%) | 23 (55) | 4 (50) | 0.999 |
| Caucasian (non-Hispanic), n (%) | 31 (74) | 7 (88) | 0.661 |
| MPO-ANCA serotype, n (%) | 18 (43) | 3 (38) | 0.999 |
| Disease, n (%) | 0.999 | ||
| GPA | 23 (55) | 4 (50) | |
| MPA | 15 (36) | 3 (38) | |
| ANCA GN (renal-limited) | 4 (10) | 1 (12) | |
| Organ involvement (past and/or present), n (%) | |||
| Upper Respiratory | 31 (74) | 2 (25) | 0.013 |
| Pulmonary | 28 (67) | 7 (88) | 0.407 |
| Renal | 38 (90) | 8 (100) | 0.999 |
| Time to B cell repopulation (months), median (IQR) | 9 (8–13) | 12 (10–13) | 0.549 |
| Percentage of total B cells at time of B cell repopulation, median (IQR) | 5 (2–8) | 2 (1–6) | 0.036 |
| Percentage of CD5+ B cells at time of B cell repopulation, median (IQR) | 74 (57–86) | 24 (21–28) | <0.001 |
| ANCA titre† (U/mL) at time of B cell repopulation, median (IQR) | 34 (21–61) | 31 (16–79) | 0.833 |
| ANCA titre† (U/mL) at time of relapse, median (IQR) | 64 (35–84) | 74 (56–86) | 0.901 |
| Previous rituximab, n (patients) (%) | 16 (38) | 3 (38) | 0.999 |
| Medications following rituximab therapy, n (patients) (%) | |||
| Mycophenolate mofetil | 18 (43) | 1 (13) | 0.112 |
| Azathioprine | 3 (7) | 0 (0) | 0.999 |
| Cyclophosphamide | 1 (2) | 0 (0) | 0.999 |
| Ciclosporin | 1 (2) | 1 (13) | 0.302 |
| Prednisone | 14 (33) | 4 (50) | 0.427 |
| Methylprednisolone | 1 (2) | 0 (0) | 0.999 |
| None | 11 (26) | 2 (25) | 1.000 |
| Time to relapse from rituximab (months), median (IQR) | 23 (18–30) | 16 (12–19) | 0.005‡ |
| Time to relapse from B cell repopulation (months), median (IQR) | 12 (6–21) | 3 (1–9) | 0.001§ |
p Values were calculated by Fisher’s exact test for categorical variables, Wilcoxon rank test for continuous variables and log-rank for time-to-event measures. A Bonferroni correction was applied and p values <0.025 were considered significant.
ANCA titres were determined by the McLendon Clinical Laboratories at the University of North Carolina using ELISA kits specific for either MPO or proteinase 3 (PR3, Inova Diagnostics, San Diego, California, USA). Negative titres are ≤20 U/mL.
After adjusting for differences in upper respiratory involvement by time-to-event proportional hazards modelling, p=0.002.
After adjusting for differences in upper respiratory involvement by time-to-event proportional hazards modelling, p=0.001.
ANCA GN, antineutrophil cytoplasmic autoantibody glomerulonephritis; AAV, antineutrophil cytoplasmic autoantibody-associated vasculitis; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; MPO, myeloperoxidase; n, number.
Figure 1.
Repopulation with <30% CD5+ B cells portends a shorter time to relapse than repopulation with normal levels of CD5+ B cells. (A) Gating scheme for re-analysis of clinical flow cytometry data. Whole blood was stained for a CD20 workup with the following fluorescently labelled antihuman antibodies: CD20-FITC (clone L27), CD45-PerCP (clone 2D1) and CD5-PE (clone L17F12, all from BD Biosciences, San Jose, California, USA). Cells were analysed using a FACSCanto II flow cytometer. Lymphocytes were first selected based on forward versus side scatter; CD45 was then used as a pan lymphocyte marker in combination with side scatter to identify lymphocytes in a strategy known as heterogeneous gating that is required in clinical flow cytometry core facilities.10 CD5+ B cells were defined as cells positive for both CD20 and CD5 as denoted by the oval gate. In samples used for this study, CD20 correlated well with CD19 expression (r2=0.98). (B) Relapse-free survival from the first dose of rituximab is depicted. Patients who repopulated with ≤30%CD5+ B cells (
 ) relapsed sooner than patients who repopulated with >30% CD5+ B cells (
) relapsed sooner than patients who repopulated with >30% CD5+ B cells (
 ; p=0.005). Open squares denote the months of follow-up for patients who did not relapse during the time of our study (n=11), with a minimum 24 months of relapse-free follow-up required. Adjusting for differences in upper respiratory involvement, the low CD5 group at B cell repopulation remained significantly associated with a shorter time to relapse from time of rituximab (p=0.002) and from time of B cell repopulation (p=0.001). (C) CD20+CD5+ B cells decrease prior to relapse. Shown is an example of a patient who repopulates with high CD5+ B cells after rituximab therapy but exhibits decreasing and then low CD5+ B cells prior to disease relapse (denoted by star).
; p=0.005). Open squares denote the months of follow-up for patients who did not relapse during the time of our study (n=11), with a minimum 24 months of relapse-free follow-up required. Adjusting for differences in upper respiratory involvement, the low CD5 group at B cell repopulation remained significantly associated with a shorter time to relapse from time of rituximab (p=0.002) and from time of B cell repopulation (p=0.001). (C) CD20+CD5+ B cells decrease prior to relapse. Shown is an example of a patient who repopulates with high CD5+ B cells after rituximab therapy but exhibits decreasing and then low CD5+ B cells prior to disease relapse (denoted by star).
Our data indicate that a low percentage of CD5+ B cells at B cell repopulation portends a shorter time to relapse following rituximab therapy irrespective of additional immunosuppressive therapy. Monitoring CD5+ B cell repopulation and decrease may serve as a novel immunological biomarker to detect risk of subsequent relapse. We posit that immunosuppression guided by CD5+ B cells to avoid unnecessary treatment when protective CD5+ B cells are present and avoid relapse by proactive treatment when CD5+ B cells are low could offer immeasurable benefit to patients.
Acknowledgments
The authors wish to thank the patients and the other healthcare providers involved in their care. We appreciate Grazy Radulian and Holly Brown’s help in data retrieval and precision analysis and for cheerfully accommodating our presence in the McLendon Clinical Flow Laboratory. The authors thank Jean Brown and Elizabeth McInnis for their assistance with the figure.
Funding This work was supported by a Program Project Grant number 5P01DK058335-14 from NIH/NIDDK and the Vasculitis Foundation.
Footnotes
Contributors RJF, PHN, JAGM and WFP provided clinical care for the patients. JGM and ESK reviewed patients’ clinical information. DOB, LTA and JGM conceived and designed the research. Clinical flow cytometry data were provided by JLS. YH and SLH provided expert statistical analysis and interpretation. CJP obtained institutional review board approval for this study. CEM, ESK, KAC, LTA and CJP collected data. CEM, JGM, SLH, WFP and DOB interpreted the data and wrote the manuscript. All authors participated in reviewing the manuscript and approved the final version.
Competing interests None declared.
Patient consent Obtained.
Ethics approval University of North Carolina Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement This research includes flow cytometry data derived from clinical tests and clinical information regarding disease diagnosis and disease status from 50 subjects. The final dataset includes self-reported demographic data and other clinical laboratory data. Even though the final dataset will be stripped of identifiers prior to release for sharing, we believe that there remains the possibility of deductive disclosure of subjects. Thus, we will make the data and associated documentation available to users only under a data-sharing agreement that provides for (1) a commitment to using the data only for research purposes and not to identify any individual participant; (2) a commitment to securing the data using appropriate computer technology; and (3) a commitment to destroying or returning the data after analyses are completed.
References
- 1.Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363:211–20. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 2.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013;369:417–27. doi: 10.1056/NEJMoa1213277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pendergraft WF, III, Cortazar FB, Wenger J, et al. Long-term maintenance therapy using rituximab-induced continuous B-cell depletion in patients with ANCA vasculitis. Clin J Am Soc Nephrol. 2014;9:736–44. doi: 10.2215/CJN.07340713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calich AL, Puéchal X, Pugnet G, et al. French Vasculitis Study Group. Rituximab for induction and maintenance therapy in granulomatosis with polyangiitis (Wegener’s). Results of a single-center cohort study on 66 patients. J Autoimmun. 2014;50:135–41. doi: 10.1016/j.jaut.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Miloslavsky EM, Specks U, Merkel PA, et al. Rituximab for the treatment of relapses in ANCA-associated vasculitis. Arthritis Rheumatol. 2014;66:3151–9. doi: 10.1002/art.38788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunch DO, McGregor JG, Khandoobhai NB, et al. Decreased CD5+ B cells in active Anti-neutrophil Cytoplasmic Autoantibody (ANCA) vasculitis and relapse after rituximab. Clin J Am Soc Nephrol. 2013;8:382–91. doi: 10.2215/CJN.03950412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aybar LT, McGregor JG, Hogan SL, et al. Reduced CD5+CD24hiCD38hi and interleukin-10+ regulatory B cells in active anti-neutrophil cytoplasmic autoantibody associated vasculitis permit increased circulating autoantibodies. Clin Exp Immunol. 2015;180:178–88. doi: 10.1111/cei.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gary-Gouy H, Harriague J, Bismuth G, et al. Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production. Blood. 2002;100:4537–43. doi: 10.1182/blood-2002-05-1525. [DOI] [PubMed] [Google Scholar]
- 10.Schnizlein-Bick CT, Mandy FF, O’Gorman MR, et al. Use of CD45 gating in three and four-color flow cytometric immunophenotyping: guideline from the National Institute of Allergy and Infectious Diseases, Division of AIDS. Cytometry. 2002;50:46–52. doi: 10.1002/cyto.10073. [DOI] [PubMed] [Google Scholar]