Abstract
Shigella spp. are among the enteric pathogens with the highest attributable incidence of moderate-to-severe diarrhea in children under 5 years of age living in endemic areas. There are no vaccines available to prevent this disease. In this work, we investigated a new Shigella vaccine concept consisting of non-living, self-adjuvanted, Lactococcus lactis bacterium-like particles (BLP) displaying Shigella invasion plasmid antigen (Ipa) B and IpaD and examined its immunogenicity and protective efficacy in adult and newborn/infant mice immunized via the nasal route. Unique advantages of this approach include the potential for broad protection due to the highly conserved structure of the Ipas and the safety and practicality of a probiotic-based mucosal/adjuvant delivery platform. Immunization of adult mice with BLP-IpaB and BLP-IpaD (BLP-IpaB/D) induced high levels of Ipa-specific serum IgG and stool IgA in a dose-dependent manner. Immune responses and protection were enhanced by BLP delivery. Vaccine-induced serum antibodies exhibited opsonophagocytic and cytotoxic neutralizing activity, and IpaB/D IgG titers correlated with increased survival post-challenge. Ipa-specific antibody secreting cells were detected in nasal tissue and lungs, as well as IgG in bronchoalveolar lavage. Bone marrow cells produced IpaB/D-specific antibodies and contributed to protection after adoptive transfer. The BLP-IpaB/D vaccine conferred 90% and 80% protection against S. flexneri and S. sonnei, respectively. Mice immunized with BLP-IpaB/D as newborns also developed IpaB and IpaD serum antibodies; 90% were protected against S. flexneri and 44% against S. sonnei. The BLP-IpaB/D vaccine is a promising candidate for safe, practical and potentially effective immunization of children against shigellosis.
Keywords: Shigella vaccines, L. lactis bacterium-like particles, mucosal immunization, infant diarrheal disease
INTRODUCTION
A variety of new vaccine approaches are being explored to improve the safety and effectiveness of pediatric immunization. Methods that allow administration of vaccines through mucosal routes are highly desirable, as they are more practical, less invasive and easier to implement than parenteral injection, the route typically used for routine immunization. Effective mucosal vaccines that can prevent the devastating burden of childhood diarrhea in less developed areas of the world would make a substantial contribution to public health. The recent Global Enteric Multicenter Study (GEMS), led by the Center for Vaccine Development at the University of Maryland, identified Shigella as one of the organisms associated with the largest incidence of diarrheal disease in children under 5 years of age.1 When incidence of disease was stratified by age, toddlers 11–23 months of age were found to be the most affected group.1 In addition to unacceptably high mortality rates, repeated bouts of diarrheal disease throughout childhood can result in impaired development and long-term disability.2,3 Aiming to identify an effective pediatric prophylactic tool to substantially reduce this burden, we focused on Shigella invasion plasmid antigens (Ipas), which are components of the Type III secretion system, as potential candidates for the development of a broadly protective subunit-based Shigella vaccine. We have recently shown that adjuvanted Shigella IpaB and IpaD were able to induce robust cross-protective immunity in mice immunized via mucosal4,5 or parenteral6,7 routes. The purpose of this study was to investigate the use of non-living Lactococcus lactis bacterium-like particles (BLP) as an adjuvant and vaccine display system for mucosal delivery of Shigella IpaB and IpaD that could potentially be used to immunize susceptible children. The L. lactis BLP consist of peptidoglycan (PGN) shell particles devoid of intracellular content that are produced by heat-acid treatment of L. lactis. They have the same shape and size as the living L. lactis.8 Antigens are displayed on the particle surface as a fusion protein containing a PGN binding domain that attaches non-covalently and with high avidity to the PGN wall.8,9 Because L. lactis is a generally regarded as a safe (GRAS) food additive, the BLP are likely to be safe for immunization of children through a mucosal route. The BLP PGN is a Toll-like receptor 2 (TLR-2) agonist10 and serves as a mucosal adjuvant.11 Because of their larger size (+/− 1–2 μm), the particles interact more efficiently with mucosal antigen-presenting cells (APC) and facilitate vaccine uptake. Conceptually, this approach would be highly advantageous because it combines safety, strong immunogenic capacity and ease of delivery for effective and practical immunization early in life. A precedent exists for efficient mucosal immunization of newborn mice with Yersinia pestis LcrV displayed on L. lactis BLP, which induced mucosal and systemic immunity and afforded complete protection against systemic plague infection.10 Likewise, the BLP technology has been successfully tested in adult mice as a vaccine delivery system for protection against respiratory syncytial virus12, malaria13 and Streptococcus pneumoniae.14 Serving as adjuvants, the BLP have been shown to enhance the immunogenicity15 and protective efficacy16,17 of intranasally (i.n.) delivered influenza virus vaccines.
Herein, we examined the in vivo distribution of i.n. delivered L. lactis BLP and the immunogenicity and protective capacity of combined BLP displaying IpaB or IpaD (BLP-IpaB/D). Increasing doses of BLP-IpaB/D and IpaB/D alone were tested in adult and newborn mice, and a thorough characterization of the mucosal and systemic immune responses was performed, including a detailed analysis of serum antibodies along with their functional capacity and association with protection.
RESULTS
Shigella IpaB and IpaD displayed on L. lactis BLP
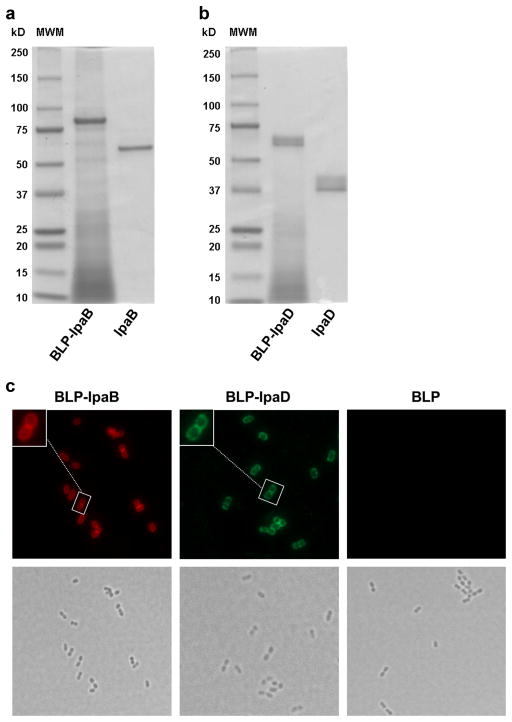
IpaB and IpaD PGN anchor fusion proteins (PA) were successfully produced and attached to the L. lactis BLP. The SDS-PAGE analysis of the vaccine preparations revealed bands near the 87 KDa and 64 KDa expected for the IpaB-PA fusion protein loaded onto the BLP and IpaB alone, respectively (Figure 1a). Bands in the proximity of the theoretical sizes of 61 KDa and 38 KDa were seen for IpaD-PA loaded on the BLP and IpaD alone, respectively (Figure 1b). The IpaB and IpaD displayed on the BLP were recognized by specific monoclonal antibodies as shown by immunofluorescence images (Figure 1c); no signal was detected for the BLP alone.
Figure 1. Analysis of BLP-IpaB/D by SDS-PAGE and fluorescence microscopy.
(a) SDS PAGE analysis of BLP displaying IpaB (BLP-IpaB) or (b) IpaD (BLP-IpaD). The size of molecular weight markers (in kDa) is shown on the left (MWM). Proteins were stained with Coomassie blue. (c) Immunofluorescence (top) and bright field microscopy images (bottom) of BLP-IpaB, BLP-IpaD and BLP alone. BLP display of IpaB and IpaD was revealed by incubation with specific monoclonal antibodies: biotin labeled anti-IpaB followed by Streptavidin-Alexa Fluor® 594 (red) and unlabeled anti-IpaD followed by anti-mouse Alexa Fluor® 488 (green).
In vivo distribution of L. lactis BLP
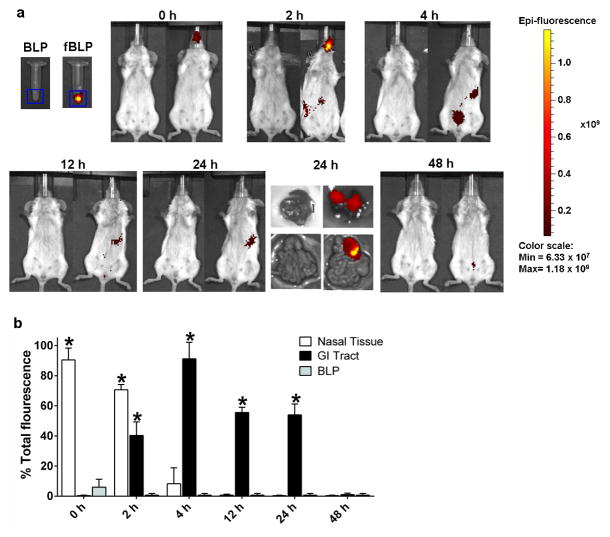
To assess the in vivo distribution of i.n. delivered BLP and identify mucosal tissues potentially involved in immunologic priming, fluorescently labeled BLP (fBLP) were administered to adult mice at a concentration equivalent to the highest dose used in the vaccine studies and following the same immunization procedures (described below). Mice that received unlabeled BLP served as negative controls. Whole body images were obtained every 2–4 h for 2 days. The fBLP were seen in the nasal cavity immediately after vaccination and most of the inoculum remained there for the next 2 h (Figure 2a and b); a small fraction (10%) was still detected in this compartment 4 h after inoculation but had completely disappeared at the 12 h time point. The majority of the particles reached the gastrointestinal tract (most likely due to swallowing reflex) 4 h after inoculation, with a fraction remaining for up to 24 h (Figure 2a and b). Although not evident from the whole body images, the fBLP also localized in the lung, as demonstrated by images of dissected tissue 24 h after inoculation (Figure 2a). Almost all of the fBLP had been cleared from the body 2 days after vaccination (Figure 2a and b).
Figure 2. In vivo imaging of L. lactis BLP distribution.
(a) Unlabeled and fluorescent BLP (fBLP) were administered i.n. to adult mice in a 30 μl volume, under anesthesia, and whole body images were obtained at the indicated time points using a Xenogen IVIS-200 imagining system. (b) Fluorescence distribution among different organs. Each bar represents mean % of total fluorescence+ SEM from 3 mice per time point; total fluorescence was the radiant efficiency of fBLP in the 30-μl inoculum. Significant overall differences were determined by multivariate analysis of variance (MANOVA), with % fluorescence at each time point as the variable. Comparisons between fBLP and BLP were then made at each individual time point using one-way ANOVA with Dunnett’s comparison to a common control (BLP alone); * denotes statistical significance P<0.05 compared to unlabeled BLP.
Protective immunity induced by intranasal immunization with BLP-IpaB/D in adult mice
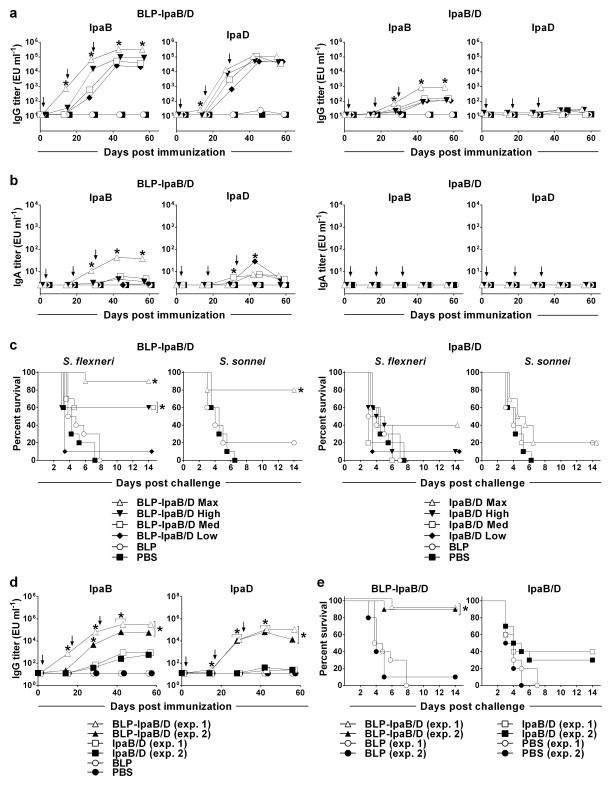
The immunogenicity and protective efficacy of the BLP-IpaB/D in comparison with IpaB/D (the proteins alone) was first examined in a dose-escalation experiment. Adult mice were immunized i.n. with increasing dosage levels of each of the vaccines on days 0, 14 and 28, as described in Materials and Methods and in the legend to Figure 3. Immunization with BLP-IpaB/D resulted in robust serum IgG responses to both IpaB and IpaD, which increased in a dose-dependent manner (Figure 3a). Mice that received the maximum (Max) dose of BLP-IpaB/D (the largest amount that could be given in the 30-μl inoculum) were the first to respond; this group exhibited significant levels of IpaB and IpaD-specific IgG, above all other groups, after the first immunization. Mice that received the high, medium and low doses of BLP-IpaB/D responded only after the second vaccination. The influence of the vaccine dose in the magnitude of serum IgG responses was particularly noticeable at this time point (Figure 3a). Although titers increased and reached a plateau after the 3rd vaccination in all groups, the recipients of the BLP-IpaB/D Max dose remained the best responders throughout. The BLP-IpaB/D Max was also the only group that developed significant and sustained IpaB-specific IgA responses in stool (Figure 3b). Stool IgA responses against IpaD were also detected but did not follow a dose-response pattern. No antibodies were detected in serum or stool from BLP and PBS controls. Importantly, the IgG responses to IpaB and IpaD markedly improved when displayed on the BLP as compared to the proteins given alone. BLP-IpaB/D recipients achieved ~3 and 5 log10 higher IpaB and IpaD serum IgG titers, respectively, than those that received IpaB and IpaD alone. In fact only the group that received the max dose of IpaB and IpaD alone developed a response above the negative controls. Fecal IgA responses were absent following immunization with IpaB/D alone.
Figure 3. Serum IgG, stool IgA and protective efficacy of BLP-IpaB/D and IpaB/D in adult mice.
Adult mice were immunized i.n. with increasing dosage levels (maximum, high, medium and low) of BLP-IpaB/D or IpaB/D alone: BLP carrying 20 μg IpaB and 53 μg IpaD (BLP-IpaB/D-Max), 10 μg IpaB and 40 μg IpaD (BLP-IpaB/D-High), 5 μg IpaB and 20 μg IpaD (BLP-IpaB/D-Med), and 2.5 μg of IpaB and 10 μg of IpaD (BLP-IpaB/D-Low). The same amounts of IpaB and IpaD were given alone. (a) Kinetics of IpaB and IpaD serum IgG (b) and stool IgA titers measured by ELISA. Each data point represents the geometric mean titer in log10 of at least 10 mice per group ± SEM. (c) Mice were challenged with wild-type S. flexneri 2a 2457T or S. sonnei 53G 28 days after the third immunization; the curves represent % survival post-challenge for 10 mice per group. (d) Serum IgG titers and (e) protection following S. flexneri challenge in mice immunized with BLP-IpaB/D-Max or IpaB/D alone in 2 separate experiments. Significant overall differences in log10 antibody titers were determined by MANOVA. Significant differences in titers between all vaccinated groups were analyzed by ANOVA with Tukey-Kramer multiple comparison test to compare all groups. * P<0.05 for mean antibody titers significantly higher compared to all other groups (panels a and b) and compared to Ipas alone and controls (panel d). Significant differences in survival curves were determined by log-rank test; * P<0.05 compared to PBS and BLP.
One month after vaccination all mice were subjected to a lethal pulmonary challenge with fully virulent S. flexneri strain 2a 2457T. Mice immunized with BLP-IpaB/D had the highest survival rates: 90% for those that received the maximum dose and 60% in the high and medium dose groups (Figure 3c). Protection was dramatically reduced when IpaB and IpaD were given alone: less than half (40%) of the mice that received the maximum dose survived and only 10% of those that received the high dose (statistically indistinguishable from the unvaccinated controls) survived. When challenged with wild-type S. sonnei, which together with S. flexneri 2a cause the majority of endemic pediatric shigellosis in the developing world, the BLP-IpaB/D Max dose afforded 80% protection; none of the other vaccine groups exhibited significant protection against this strain (Figure 3c). Negligent protection against S. sonnei and S. flexneri (≤20%) was seen occasionally in mice that received BLP alone, whereas all unvaccinated (PBS) controls succumbed to infection 6 to 8 days after challenge (Figures 3c and d). Results were consistent when different batches of vaccine were tested in separate experiments both in terms of kinetics of antibody production and BLP-immune enhancement (Figure 3d), as well as protective efficacy (Figure 3e).
IpaB- and IpaD antibodies and prediction of protection by titer and functional capacity
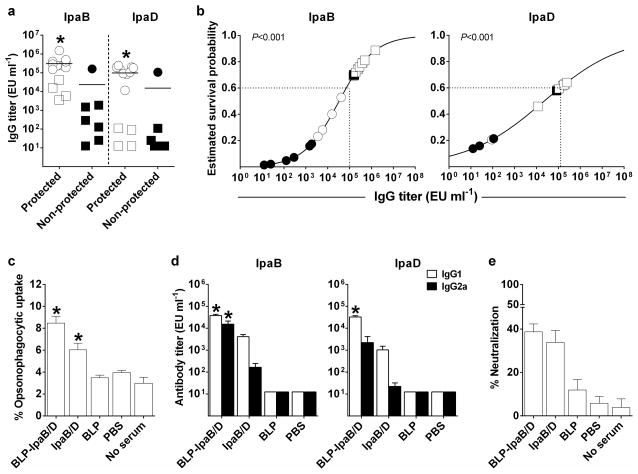
A detailed statistical analysis was performed to establish potential associations between vaccine-induced antibody responses (IgG, IgG1 and IgG2a in serum and IgA in stool) and protection against disease. First, survival rates post challenge were compared among mice immunized with BLP-IpaB/D or IpaB/D classified as responders vs. non-responders. Serum IgG titers ≥ 50 EU ml−1 and stool IgA titers ≥ 10 EU ml−1 were considered the threshold for a positive response (corresponding to 4 times the lower limits of detection, which was also 4 times the titer for all unvaccinated controls) (Table 1). The proportion of survivors was significantly higher among mice that had developed positive responses for IgG and IgG2a against both IpaB and IpaD in serum and IgA against IpaB in stool, compared to non-responders (P<0.05, two-sided Fisher exact test). Furthermore, there was a significant association between survival and the magnitude of serum ELISA titers for each type of antibody measured (P<0.05, likelihood ratio test in logistic regression) (Table 1). Vaccinated mice that were protected from S. flexneri infection (i.e. healthy survivors that maintained at least 80% of body weight) had significantly higher serum IgG titers anti-IpaB and IpaD than those that succumbed to infection at the time of challenge (Figure 4a). A further analysis of vaccine-induced antibodies and probability of survival using logistic regression confirmed the association between IpaB- and IpaD-specific IgG and protection (P<0.001). The models indicated that an IpaB titer ≥1×106 EU ml−1 predicted at least an 80% chance of survival (60% if the titer dropped to 1×105 EU ml−1) and an IpaD titer ≥1×105 EU ml−1 predicted at least 60% probability of survival (Figure 4b).
Table 1.
Association between vaccine-induced antibodies and survival rates post-challenge
| Survival vs. antibody responsesa | Survival and ELISA titersa | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No. mice | Survival rates among responders | Survival rates among non-responders | pb | Odds ratioc | 95% CId | pe | |
| IpaB total IgG | 80 | 27/65 | 1/15 | 0.016 | 3.46 | 1.89–6.33 | <0.0001 |
| IpaD total IgG | 80 | 24/44 | 4/36 | <0.0001 | 1.76 | 1.30–2.38 | <0.0001 |
| IpaB IgG1 | 20 | 12/19 | 0/1 | 0.80 | 2.74 | 0.93–8.08 | 0.038 |
| IpaB IgG2a | 20 | 10/12 | 2/8 | 0.031 | 3.00 | 1.17–7.73 | 0.007 |
| IpaD IgG1 | 20 | 10/13 | 2/7 | 0.10 | 2.31 | 1.12–4.78 | 0.011 |
| IpaD IgG2a | 20 | 6/6 | 6/14 | 0.048 | --f | --f | 0.0002 |
| IpaB stool IgA | 80 | 10/11 | 18/69 | 0.0002 | 19.76 | 2.54–153.87 | <0.0001 |
| IpaD stool IgA | 80 | 6/9 | 22/71 | 0.087 | 4.65 | 1.08–20.07 | 0.024 |
Mice were classified as responders or non-responders according to the total serum IgG, IgG1, IgG2a or stool IgA ELISA titers at day 56 after immunization. A titer of at least 50 ELISA units ml−1, corresponding to 4 times the lower limit of detection (12.5 EU ml−1) was considered a positive response in serum and a titer of at least 10 ELISA units ml−1, corresponding to 4 times the lower limit of detection (2.5 EU ml−1) was considered a positive response in stool. Survivors were mice that remained alive 14 days after pulmonary challenge with S. flexneri 2a. Total IgG and IgA titers were measured in mice immunized with max, high, med and low BLP-IpaB/D and IpaB/D dosage levels; subclasses were measured in mice that received the max dose.
p-value from two-sided Fisher’s exact test.
Odds ratio for survival from logistic regression for an increase of 1 in log10(titer).
Confidence interval for odds ratio, based on Wald test.
p-value from likelihood ratio test.
Calculation did not converge; all mice that died were non-responders (i.e., had titers <50 ELISA units ml−1).
Figure 4. Antibodies and protection analysis, functional activity (opsonophagocytosis and neutralization of cytotoxicity) and IgG subclasses.
Mice were immunized with BLP-IpaB/D or IpaB/D Max (20 μg IpaB and 53 μg IpaD), as described in Figure 3. (a) Antibody titers measured before challenge and ranked according to survival outcome; circles denote mice immunized with BLP-IpaB/D, and squares mice immunized with IpaB/D. Higher levels of IpaB or IpaD-specific serum IgG were associated with protection (survival) by logistic regression on log10-transformed IpaB or IpaD titers; * denotes P < 0.001 by likelihood ratio test in both analyses. (b) Estimated survival probability by logistic regression on log-transformed IpaB or IpaD IgG titers (same analysis as for panel a); mice immunized with BLP-IpaB/D-Max and IpaB/D-Max were included and depicted as circles and square, respectively. Filled and solid circles represent surviving and non-surviving mice, respectively. Dashed lines indicate level of antibodies predicting >60% survival. (c) Opsonophagocytic activity of serum antibodies from vaccine and control groups. Results are shown as mean % opsonophagocytic uptake of S. flexneri 2a 2457T in individual serum samples. (d) IpaB- and IpaD-specific IgG1 and IgG2a subclasses determined by ELISA; data represent mean titers from 20 mice per group. (e) Neutralization of macrophage cytotoxicity by serum from different treatment groups. Results are shown as mean % neutralization from replicate wells by pooled serum samples. In panels c and d, individual measurements from vaccine and control groups were compared by one-way ANOVA with Dunnett’s multiple comparisons test against a common control; the tests on IpaB- and Ipa-D-specific subclasses were done using log10-transformed antibody titers. * denotes P < 0.05 compared to non-serum or PBS for OPA and to PBS for subclasses.
Given the observed correlation between protection against disease and vaccine-induced humoral responses, we investigated the functional capacity of IpaB and IpaD antibodies in facilitating bacterial uptake by phagocytic cells and preventing Shigella-induced cytotoxicity. Antibodies induced by BLP-IpaB/D and IpaB/D exhibited opsonophagocytic activity (OPA) seen by the increased uptake of serum-opsonized S. flexneri 2a 2457T by mouse macrophages, compared to a non-opsonized organism or organisms opsonized with non-immune serum (Figure 4c). The highest OPA responses were seen in the group immunized with BLP-IpaB/D (Figure 4c). These results prompted us to examine the IgG subclass profile induced by the two vaccine treatments to test the hypothesis that the higher OPA could be associated with an increased proportion of antibodies with higher binding capacity for phagocytic cells. Indeed, higher IgG2a levels were produced after immunization with BLP-IpaB/D as compared to IpaB/D alone (Figure 4d). Additionally, we measured the capacity of vaccine-induced antibodies to neutralize macrophage cytotoxicity in the presence of wild-type Shigella. Sera from mice immunized with BLP-IpaB/D and IpaB/D prevented Shigella-induced cell death compared to non-immune sera from mice that received PBS (Figure 4e).
Mucosal ASC, antibodies and innate immune mediators in bronchoalveolar fluid (BALf)
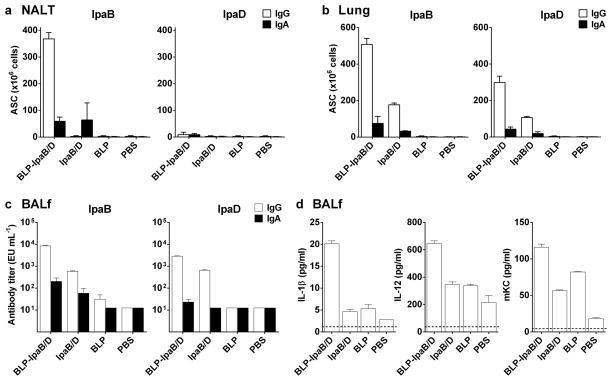
To further assess the mucosal responses induced by BLP-IpaB/D immunization, we measured the frequency of IpaB- and IpaD-specific ASC in the nasal-associated lymphoid tissue (NALT) and lung, as well as the levels of IpaB/D-specific IgG and IgA in BALf, one week after the 3rd and final immunization (Figures 5a and b). High numbers of IpaB-specific IgG ASC were detected in the NALT of BLP-IpaB/D vaccinated mice (Figure 5a). An even higher IpaB-specific ASC response was found in the lungs (Figure 5b). Likewise, IpaD-specific IgG ASC were detected in the lungs, albeit at much lower numbers. IpaB and IpaD-specific IgA ASC were induced in response to BLP-IpaB/D, but their magnitude was marginal compared to the IgG ASC. In all cases, the ASC responses in the BLP-IpaB/D group surpassed those of mice that received IpaB/D alone. Elevated IpaB- and IpaD-specific IgG titers were found in BALf, and, similar to the ASC, there was a trend of higher responses in the BLP-IpaB/D recipients compared to those immunized with IpaB/D alone (Figure 5c). No specific antibodies or ASC were detected in the BLP and PBS controls. A summary of serum and mucosal antibody responses in all groups and the corresponding protection levels is given in Table 2. From a large panel of cytokines measured by microarrays (IFN-γ, IL-10, IL-12, IL-2, IL-4, IL-5, KC, and TNF-α) IL-1β, IL-12 and the neutrophil-attracting chemokine, keratinocyte chemoattractant (KC), were found to be elevated in the BALf of vaccinated mice (Figure 5d). The highest levels were found in the group immunized with BLP-IpaB/D, but positive responses (above the unvaccinated PBS controls) were also found in the IpaB/D and BLP groups (Figure 5d).
Figure 5. Mucosal IgA and IgG secreting cells, antibodies and cytokines following BLP-IpaB/D and IpaB/D vaccination.
Mice were immunized with BLP-IpaB/D or IpaB/D Max dose as described in Figure 3. IgA and IgG ASC were measured by ELISpot in the (a) NALT and (b) lung tissue 35 days after the first immunization. Data correspond to mean ASC counts per 106 cells measured in pooled cell suspensions from 5 mice in each group + SEM from triplicate wells. (c) IpaB- and IpaD-specific IgG and IgA were measured by ELISA in bronchoalveolar lavage fluid (BALf) 35 days after immunization; data represent mean of individual titers from 5 mice per group (d). Cytokines measured in BALf by MSD multiarray technology; data represent mean concentrations as (pg ml−1) + SEM from triplicate wells.
Table 2.
Antibody titers measured in serum and mucosal secretions and protective efficacy
| Serum IgGa | Stool IgAa | BALf IgGb | BALf IgAb | Survivalc | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IpaB | IpaD | IpaB | IpaD | IpaB | IpaD | IpaB | IpaD | S. flexneri | S. sonnei | |
| BLP-Ipa Max | 310,021 | 109,915 | 37.9 | 6.6 | 2,616 | 1,095 | 145.9 | 19.2 | 90 | 80 |
| IpaB/D Max | 918.1 | 25.2 | 2.5 | 2.5 | 315.5 | 57.2 | 29.2 | 12.5 | 40 | 20 |
| BLP | 12.5 | 13.4 | 2.5 | 2.5 | 18.4 | 12.5 | 12.5 | 12.5 | 0 | 20 |
| PBS | 12.5 | 12.5 | 2.5 | 2.5 | 12.5 | 12.5 | 12.5 | 12.5 | 0 | 0 |
Serum and stool values are provided as geometric mean titers (EU ml−1) from 10 mice per group measured on Day 55 (time of challenge).
BALf was collected from 5 mice per group on day 35 following the initial vaccination. Geometric mean titers are provided in EU ml−1.
Survival is listed as the percentage of 10 mice per group surviving lethal pulmonary challenge with the indicated Shigella organism.
Systemic ASC responses and bone marrow adoptive transfer protection
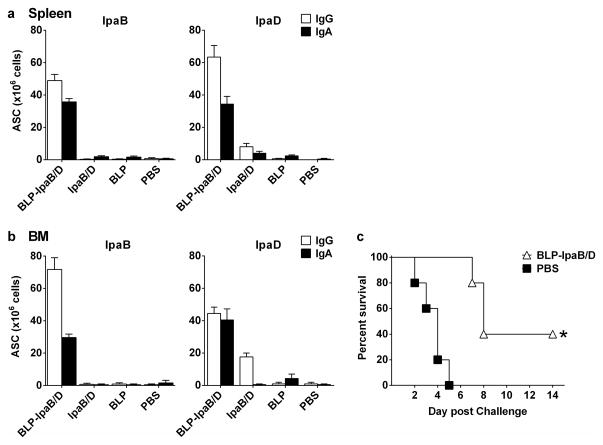
We also examined the presence of antigen-specific ASC in the spleen and in the bone marrow (BM) as potential sources of circulating antibodies and long-lived plasma cells, respectively. IpaB- and IpaD-specific IgG and IgA ASC were found in both tissues mainly in response to BLP-IpaB/D vaccination, whereas no ASCs were detected after vaccination with IpaB/D alone (Figures 6a and b). In most instances, the levels of IgG ASC surpassed those of IgA ASC. BM cells from BLP-IpaB/D recipients adoptively transferred to naïve mice conferred moderate, yet significant protection against lethal Shigella infection; 40% of the BM recipients survived the pulmonary challenge (Figure 6c).
Figure 6. Systemic IgG and IgA ASC and induced by the BLP-IpaB/D and IpaB/D and protection by bone marrow adoptive transfer.
Mice were immunized with BLP-IpaB/D or IpaB/D-Max as described in Figure 3. (a) IgA and IgG ASC measured by ELISpot in spleen and (b) bone marrow (BM) on day 69 after primary immunization. Data correspond to mean ASC counts per 106 cells from pooled cell suspensions from 5 mice in each group + SEM from triplicate wells. (c) Bone marrow cells from BLP-IpaB/D or PBS recipients (obtained 69 days after primary vaccination) were adoptively transferred to naïve mice that were challenged 48 h later with wild type S. flexneri 2a 2457T. Survival curves were compared by log-rank test; * P<0.05 compared to PBS.
Antibody responses and protective efficacy of BLP-IpaB/D in newborn mice
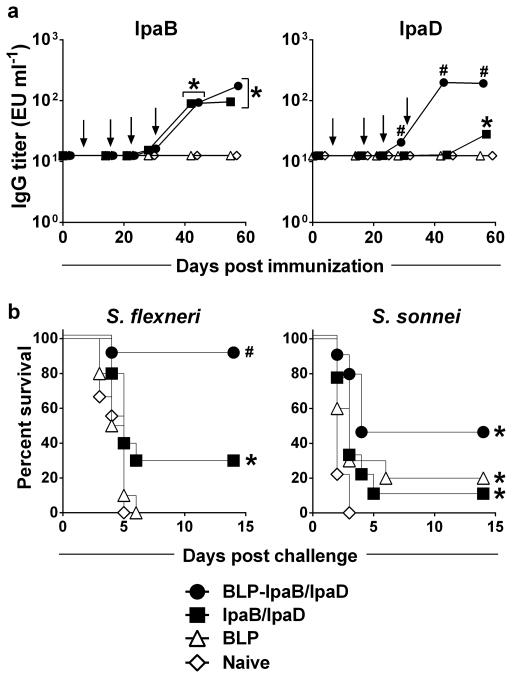
Considering that infants and toddlers would be the main targets of a Shigella vaccine and that immune responses may be influenced by age and immune development, we examined the protective capacity of BLP-IpaB/IpaD in mice immunized as newborns and infants. Seven-day-old mice were administered BLP-IpaB/D or IpaB/D via the nasal route and identical booster doses were given on days 14, 21 and 28 after birth. A series of preliminary studies was performed to determine optimal doses and number of booster immunizations needed to maximize protection. Because the serum IgG anti-IpaD levels were consistently low, the amount of IpaD was increased to the highest that could be included in the vaccine inoculum (24 μg in 10 μl). A three-dose regimen with BLP-IpaB/D, which was highly successful in adults, provided only ~40% protection in mice immunized as newborns (data not shown) and hence a fourth dose was added after weaning (day 28). Immunization with BLP-IpaB/D during the neonatal/infant period resulted in significant levels of IpaB- and IpaD-specific serum IgG. As observed in adult mice, antibody responses markedly increased when the Ipas were displayed on the BLP. Immunization with BLP-IpaB/D elicited serum IgG responses against both IpaB and IpaD, whereas lower IpaB- and barely significant (and delayed) IpaD-specific IgG was produced in response to IpaB/D alone (Figure 7a). Newborn/infant immunization with BLP-IpaB/D afforded 90% protection against S. flexneri 2a and 44% against S. sonnei, which in both cases was significant above the unvaccinated controls (Figure 7b). Protection was greatly reduced upon immunization with IpaB/D alone; only 30% of these mice survived S. flexneri challenge and only 11% survived infection with S. sonnei.
Figure 7. Serum IgG levels and protective efficacy of BLP-IpaB/D and IpaB/D in newborn mice.
Mice were immunized as newborns and infants with BLP carrying 2 μg IpaB and 24 μg IpaD or with the same amounts of IpaB and IpaD given alone. Control groups received BLP alone or remained naïve. Vaccines were given on days 7, 14, 21 and 28 after birth (arrows). (a) IpaB- and IpaD-specific IgG measured by ELISA; data represent geometric mean titers from 12 mice per group. Significant overall differences in log10 antibody titers were determined by MANOVA. Significant differences in titers between all vaccinated groups were analyzed by ANOVA with Tukey-Kramer multiple comparison test to compare all groups. * P<0.05 for mean antibody titers significantly higher compared to BLP and PBS control groups. # P<0.05 for mean antibody titers significantly higher compared to all groups. (b) Protection against lethal pulmonary infection with wild type S. flexneri 2a 2457T or S. sonnei 53G (n=12). Survival curves were compared by log-rank test. * P<0.05 compared to naïve mice. # P<0.05 compared to all groups.
DISCUSSION
The recent estimates of moderate-to-severe diarrhea attributable to Shigella spp. in children living in Asia and sub-Saharan Africa1 re-emphasize the urgent need for effective preventive strategies to reduce mortality rates and alleviate an overall disease burden that results in long-lasting health impairment. Herein, we provide the first report of successful immunization of adult and newborn/infant mice with L. lactis BLP displaying Shigella IpaB and IpaD. The vaccine was well tolerated when administered via the nasal route as early as one week after birth and induced robust immune responses. Most importantly, it was highly protective against lethal challenge with S. flexneri 2a and S. sonnei 53G, the two most prevalent endemic serotypes in the developing world as reported by the GEMS18 and previous large-scale studies.19,20 Immunogenicity and protection were dose-dependent and consistent in multiple experiments using different vaccine lots. The safety and suitability of the BLP-IpaB/D vaccine for mucosal delivery and its capacity to induce strong cross-protective immunity in young hosts make it unique and appealing for the immunization of toddlers and young children, who would benefit the most from a practical and effective prophylactic approach.
The BLP exhibited distinct adjuvant properties and contributed to the protective immunity achieved. Display of IpaB and IpaD on the L. lactis particles significantly increased the magnitude of systemic and mucosal antibody and ASC responses, innate immune mediators in mucosal secretions and importantly, the survival rate post challenge. It is noteworthy that robust serum IgG responses to IpaD, typically the less immunogenic of the two proteins, were only elicited when it was displayed on the BLP. Similarly, stool IgA was detected only in mice that received BLP-IpaB/D, not in the IpaB/D recipients. This immune enhancement could be explained by a more efficient B cell activation and antigen delivery to APC, and/or by the inherent adjuvant properties of the BLP.21,22 Displayed on the BLP surface, the proteins are less likely to suffer degradation and, due to the microbial size and surface charge of the particles, they are more easily captured by activated APC than soluble proteins alone.10,23 We have shown that the L. lactis BLP bind TLR2 in vitro and activate adult and neonatal dendritic cells (from both mice and humans), enhance their maturation and capacity for antigen presentation and stimulate the production of pro-inflammatory cytokines including TNF-α and IL-6.10 Similarly, the interaction of BLP with TLR2 in vivo has been associated with the induction of mucosal IgA responses, as well as splenic IgG ASC and IFN-γ-secreting T cells following co-administration of a split influenza virus vaccine.11
The immunological effectors and the precise mechanisms by which IpaB and IpaD mediate protection against Shigella lethal pulmonary infection in the mouse model are not fully understood. Presumably, antibodies produced locally and/or transudated from circulation impede bacterial-host cell contact (and inhibit translocation of virulence factors) at the mucosal epithelial interface, thereby preventing tissue damage and bacterial spread.4,6 Antibodies may also facilitate uptake of organisms by phagocytic cells. A detailed statistical analysis of BLP-IpaB/D and IpaB/D-induced antibodies and rates of survival from multiple experiments revealed a categorical association between a serum antibody response to each of the proteins and protection against lethal experimental challenge. Survival was also associated with the magnitude of serum IgG (and IgG2a) ELISA titers; the stronger the antibody response, the higher the likelihood of vaccinated mice withstanding the lethal infection, with threshold serum IpaB- and IpaD-specific IgG titers of 1×105 EU ml−1 predicting at least 60% survival.
A new and critical observation was the capacity of IpaB and IpaD antibodies generated through vaccination to reduce Shigella-induced macrophage cytotoxicity and facilitate their uptake of bacteria through opsonophagocytosis. These functional properties are important to prevent tissue damage and further microbial spread during Shigella infection, and might represent mechanisms of host defense in vivo. Antibodies generated by the BLP-IpaB/D vaccine elicited higher OPA activity (P<0.01), which could be explained by the larger proportion of IgG2a induced, compared to the Ipas alone. Unlike IgG1, IgG2a binds the FcγRI receptor on mouse macrophages and phagocytic cells24 with high affinity and is therefore more efficient at activating these cells and promoting microbial clearance. In line with these results, we and others had shown that the BLP promote more balanced Th1/Th2 responses to the carried antigens compared with the same antigens given alone or admixed with alum, which generate a distinct Th2-biased IgG1 response10,17 that is unsuited for the elimination of intracellular pathogens, particularly in the Th2-prevailing environment early in life.25
Among the systemic effectors induced by BLP-IpaB/D immunization, it is worth mentioning the IpaB and IpaD-specific BM ASC (representing long-lived plasma cells or their plasmablasts precursors) that partly protected naïve hosts, as this suggests the potential for this vaccine to elicit effectors that can mediate long-lasting protective immunity. In addition, the BLP-IpaB/D generated potent mucosal immune responses largely represented by IpaB and IpaD-specific IgG ASC in the NALT and lungs, and high levels of IpaB and IpaD-specific IgG in BALf. Some of the antibodies detected in the BALf were absent in serum (i.e., IgA and IpaD-specific IgG in IpaB/D recipients), which suggests that vaccine-induced local ASC produce some of the mucosal antibodies detected. An interesting observation was the predominance of IgG responses in mucosal tissues (NALT and lung ASC, and BALf) as opposed to IgA, which was still sufficient to confer high levels of protection. In humans, serum IgG specific for Shigella LPS has been associated with serotype-specific protection 26–30 and it has been proposed that these antibodies transudate the mucosal epithelial barrier and neutralize and/or kill the organisms in the gut lumen.31 The relative contribution of serum and mucosal antibodies in protection against shigellosis and the mechanisms involved remain to be elucidated.
Another relevant observation was the presence of innate immune mediators (IL-12, IL-1β and mKC) in lung secretions from BLP-IpaB/D recipients. IL-12 plays a major role in host defense against intracellular pathogens by activating macrophages and inducing IFN-γ production. Likewise, KC (CXCL1), a chemokine produced by macrophages and neutrophils (via TLR2 activation pathway), recruits and activates phagocytic cells. Shigella IpaB has been attributed with initiating the inflammatory response through caspase-1, by promoting secretion of IL-1β.32 In a concerted manner, these molecules can facilitate bacterial clearance synergizing with host adaptive immune effectors. This is, to the best of our knowledge, the first demonstration of these cytokines/chemokines being present in mucosal secretions following immunization with a Shigella or BLP vaccine.
In an attempt to identify the tissues involved in vaccine uptake, we tracked the in vivo distribution of i.n. delivered fBLP. The particles localized in the NALT the first few hours after inoculation and subsequently in the gut; they were also found in the lungs for up to at least 24 hours. Antigen-specific ASC were detected in NALT and lung, and it is therefore reasonable to assume their involvement in immunological priming. The 30 μl inoculum volume administered is consistent with particles spreading from the nasal cavity into the lungs, as we have observed in mice given live bacterial vaccines33. Others have similarly reported >10 μl intranasal instillations dispersing into the bronchial region as well as the gastrointestinal tract.34 However, in humans, delivery of a BLP vaccine would be directed to the nasal cavity targeting the NALT as the primary inductive site. The fBLP presumably reached the gut through the swallowing reflex and this likely represents the route excretion. However, some immune activation might have occurred there as well, as suggested by the presence of IgA in stool, while completely absent in circulation. Since Shigella is an enteric pathogen, immunological priming in the gut and induction of local (intestinal) immunity would be an advantageous feature of any new vaccine. We had previously reported that oral delivery of IpaB and IpaD resulted in lower protective efficacy than intranasal vaccination4 and therefore this route was not pursued in the BLP-IpaB/D studies. Since experimental infection of mice with Shigella involves nasal/pulmonary exposure (as opposed to enteric in humans), oral vaccination adds a confounding factor to the proper evaluation of vaccine candidates in this model.
An additional important benefit of the BLP technology is that it allows for successful mucosal (even intranasal) immunization, without the need for potentially reactogenic adjuvants. In previous work from our group, IpaB/D delivered i.n., admixed with a double mutant of the Escherichia coli heat labile toxin (dmLT), afforded similar protection to that described here for the BLP-IpaB/D, using a smaller dose. However, due to safety concerns, an enterotoxin-derived adjuvant would not be suitable for use in humans.35 Parenteral immunization with the IpaB/D plus dmLT was likewise effective in mice6 and potentially suitable for use in children; a drawback is that it would still require special tools (i.e. microneedles) and might not be as practical as mucosal delivery. The BLP were well tolerated when given to 15 human adults via the nasal route (1.25 mg of BLP in 250 μl) mixed with a commercial influenza vaccine.21 If equally safe in children, this route might provide an alternative to oral vaccination, which is known to yield less than desired immune responses to routine vaccines in developing countries.36 Supportive of this concept are the results of Mallet et al37 and Orr et al38, whereby intranasal immunization with a proteosome-based S. sonnei and S. flexneri vaccine induced homologous protection in mice and in the guinea pig keratoconjunctivitis model, respectively. While none of these models accurately represent the course of Shigella infection in humans, these results confirm the potential of a mucosal/ intranasal subunit vaccine approach.
Pre-clinical studies of Shigella vaccine candidates have traditionally involved immunization of adult hosts. Two recent reports investigated protection of young animals through passive protection from maternally derived antibodies.39,40 Gnobiotic piglets have been used to evaluate live vaccine strains.41 Ours is, to the best of our knowledge, the first demonstration of substantial cross protective immunity in mice immunized as newborns with a protein-based vaccine. Although the protection against S. sonnei was lower than anticipated, it could be improved through optimization of vaccine dose and regimen; it may also reflect a more stringent challenge procedure.
In conclusion, the BLP-IpaB/D vaccine is a promising candidate for immunization of young children against shigellosis. Advantages of this vaccine over other approaches include its safety, suitability for mucosal delivery (which makes it a practical for large scale use), strong immunogenicity and broad protective capacity. Our results warrant further assessment of this concept in humans.
METHODS
Preparation of vaccines and antigens
Recombinant IpaB and IpaD were purified by affinity and size exclusion chromatography as previously described.5,42 To be loaded onto the L. lactis BLP particles (formerly called Gram Enhancement Matrix particles), each protein was expressed as a fusion containing the peptidoglycan anchoring (PA) domain (IpaB-PA and IpaD-PA). The plasmid construction, expression and purification of IpaD-PA has been described elsewhere.43 IpaB-PA was produced by inserting the DNA sequence for PA 5′ to the ipaB sequence in pACYC-Duet as previously described.44 The purification of IpaB-PA was the same as for IpaB. L. lactis BLP were produced by boiling L. lactis whole cells in 10% trichloroacetic acid for 30 min, followed by extensive washing in PBS, as previously described.8 One mg of BLP contains approximately 8×109 particles. To produce BLP-IpaD, L. lactis cell-free culture supernatants containing IpaD-PA and BLP were mixed under gentle rotation for 30 min at room temperature as described elsewhere.8 To produce BLP-IpaB, BLP were incubated with purified IpaB-PA for 30 min with gentle rocking at room temperature. Removal of excess IpaB or IpaD-PA was achieved by three sequential centrifugation of the particles, removal of the supernatants, and resuspension. The resulting vaccine preparations BLP-IpaB and BLP-IpaD (along with IpaB and IpaD alone) were analyzed by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS PAGE). Samples were diluted 1:2 in Laemmli sample buffer® (BioRad, Hercules, CA) containing 5% 2β-Mercaptoethanol, boiled at 95°C for 10 min and loaded (1 μg/well) in a 4–12% pre-casted Mini-PROTEAN TGX Gel® (BioRad, Hercules, CA). The separated proteins were detected by Coomassie staining (EZ blue™) (Sigma, St. Louis, MO). The amount of IpaB-PA and IpaD-PA bound to the BLP was determined by SDS PAGE using IpaB-PA or bovine serum albumin as standard, respectively. The BLP-IpaB/D and IpaB/D vaccine components were stored at −80°C until use.
The BLP-IpaB and BLP-IpaD were further analyzed by dual color immunofluorescence. Briefly, suspensions containing 100 μl of BLP-IpaB and BLP-IpaD were washed with ultrapure water (Milli-Q, Millipore, Billerica, MA), resuspended in PBS containing 2% Bovine serum albumin (BSA, Sigma, St. Louis, MO) and incubated for one hour at room temperature with biotinylated mouse anti-IpaB or anti-IpaD monoclonal antibodies (a gift from Dr. Ed Oaks). After washing, BLP-IpaD samples were incubated with anti-mouse Alexa Fluor® 488 (Molecular Probes, Grand Island, NY) and BLP-IpaB samples with Streptavidin Alexa Fluor® 598 (Molecular Probes, Grand Island, NY); 1:50 and 1:200, dilutions, respectively, in PBS containing 2% BSA for one hour at room temperature. After washing, samples were resuspended in 100 μl of ultrapure water, and a 10-μl sample was air dried onto a microscope slide and applied ProLong® Gold antifade reagent (Life Technologies, Eugene, OR) along with a coverslip. Images were acquired at 100× magnification on a Zeiss Axio Imager Z.1 fluorescence microscope using ZEN Imaging Software (Carl Zeiss Microscopy, Oberkochen, Germany).
In vivo tracking of L. lactis BLP
The BLP particles were labeled with Alexa Fluor® 790 Succinimidyl Esters (NHS esters) purchased from Molecular Probes, Grand Island, NY, through chemical binding to non-protonated aliphatic amine groups on the particle surface. Briefly, the BLP (15 mg) were washed and resuspended in 0.1M NaHC03 pH 8.3, and incubated with 1 μg of Alexa Fluor® 790 for 1 h at 4°C in the dark and with agitation. Adequate labeling (>80%) was confirmed by flow cytometry. The fluorescent BLP (fBLP) were washed three times in PBS and resuspended to a final concentration of 10 mg ml−1 in PBS containing 0.01% thimerosal (Sigma-Aldrich, St. Louis, MO). Adult female Balb/c mice (Charles River Laboratories, Wilmington, MA) were placed in an anesthesia induction chamber (Piramal Healthcare, Mumbai, Maharashtra, India) dispensing Isoflurane (Fluriso™, VETone®, Boise, ID) 2.5% and, once fully anesthetized, 30 μl of fBLP (0.3mg) were administered i.n. The mice were then moved to the imaging chamber of a Xenogen IVIS-200 system (Perkin Elmer, Waltham, MA) and placed lying on their backs on a heated shelf surface to maintain body temperature. Isoflurane (0.5%) was administered through nose cones during imaging. Pictures were taken and analyzed using Living Image® Software Version 4.3.1 (Perkin Elmer), creating gates or Regions of Interest (ROIs). The fluorescent intensity (Epi-fluorescence) of the fBLP was measured as radiant efficiency using the flowing equation: . The background was defined as the ROI (nasal tissue, lungs or intestine) of mice that received unlabeled BLP.
Immunization, cell adoptive transfer and experimental infection
Female Balb/c adult mice (7–8 weeks old) were immunized i.n. on days 0, 14 and 28 with increasing doses of BLP-IpaB and BLP-IpaD or IpaB and IpaD (alone): 2.5 μg IpaB and 10 μg IpaD, referred to as BLP-IpaB/D-Low; 5 μg IpaB and 20 μg IpaD (BLP-IpaB/D-Med); 10 μg IpaB and 40 μg IpaD (BLP-IpaB/D-High), and 20 μg of IpaB and 53 μg of IpaD (BLP-IpaB/D-Max). The amount of BLP given with each vaccine was consistently 300 μg. The Max dose was the largest amount of BLP-IpaB/D that could be given in the 30 μl inoculum volume. Other groups received equivalent doses of IpaB and IpaD (without BLP), also referred to as IpaB/D-Low, IpaB/D-Med, IpaB/D-High and IpaB/D-Max, respectively. Newborn (7-days old) mice received 2 μg IpaB and 24 μg IpaD alone or displayed onto the BLP (in a 10 μl volume), on days 7, 14, 21 and 28 after birth. In both experiments, unvaccinated controls received BLP alone or PBS. The vaccine inoculum was dispensed half into each nare, with a pipette and under Isoflurane anesthesia, as previously described.4 Serum and fecal samples were obtained from individual mice before and every two weeks after vaccination. Bronchoalveolar lavage fluid, NALT, spleen and BM cells were collected from subgroups of mice at days 35 or 69 as described before.4,5 For adoptive transfer experiments, BM cells obtained on day 69 post-vaccination were injected i.v. to adult mice; 6×107 cells in 100 μl of PBS were administered to each recipient via the tail vein. One month after the last immunization (day 56) or 48 h after cell adoptive transfer, adult mice were challenged with 6×107 CFU virulent S. flexneri 2a 2457T (corresponding to ~11 MLD50) or with 1.3×108 CFU of S. sonnei 53G (corresponding to ~6 MLD50), as previously described.4,5 Newborn mice were challenged with 3×107 CFU of S. flexneri 2a 2457T (corresponding to ~3 MLD50) or with 9.35×107 CFU of S. sonnei 53G. All animal studies and procedures were approved by the University of Maryland Institutional Animal Care and Use Committee.
Antibody measurements
(i) ELISA IpaB- and IpaD- specific antibodies were measured by ELISA as previously described. 4,5 (ii) Opsonophagocytosis. A Shigella opsonophagocytic assay was developed following previously described format45, with modifications. Briefly, J774A.1 mouse macrophages were seeded into 24-well plates (2×105 cells per well) and grown in Dulbecco’s modified Eagle Medium (DMEM) (Gibco, Grand Island, NY) supplemented with 10% Defined Fetal Bovine Serum (GE Healthcare HyClone, Logan, UT) at 37°C, 5% CO2, until they reached 90% confluence. S. flexneri 2a wild type strain 2457T was grown overnight at 37°C in Tryptic Soy Agar (TSA) (Difco, Becton Dickinson Diagnostics, Sparks, MD) supplemented with 0.02% Congo Red (Sigma-Aldrich), and isolated colonies were sub-cultured in Animal-Product-Free Luria Bertani broth (Lennox) (Athena Enzyme Systems, Baltimore, MD) and grown at 37°C until reaching an optical density measured at a 600 nm wavelength (OD600) of 0.2. Ninety μl bacterial suspension containing ~ 2–3×105 CFU were incubated with 10 μl of individual serum samples (previously inactivated for 20 min at 56°C) for 20 min at room temperature, in constant rotation. A 10 μl aliquot of opsonized organisms (2–3×104 CFU) was added to the cell monolayers (in duplicate wells), centrifuged (10 min, 1,000 × g) and incubated for 45 min. After washing with PBS, 500 μl of fresh medium containing 100 μg ml−1 of gentamicin (Gibco) was added for 1 h to remove external organisms. Cells were washed again, lysed with 500μl of PBS containing 0.5% Triton X-100 (Sigma-Aldrich) and supernatants (25 μl aliquots, in triplicate) were cultured overnight at 37°C in TSA-Congo Red to determine viable counts. The percent of phagocytosis was calculated as the ratio of CFU recovered/CFU added to the wells × 100. (iii) Neutralization of macrophage cytotoxicity. Shigella is known to induce apoptosis of macrophages.46 To determine whether serum from immunized animals could prevent macrophage cytotoxicity upon exposure to wild-type organisms, we established a cytotoxicity neutralization assay based on methods previously described.47–49 A 30 μl suspension of S. flexneri 2a strain 2457T, prepared as described above and containing 2×105 CFU, was incubated with 10 μl of mouse serum in 96-wells round bottom plates (in duplicate) for 20 min at room temperature. The content of each well was then transferred to a 96-well plate seeded with J774A.1 macrophages (3×103 per well, moi: 60) and incubated for 18 h at 37°C, 5% CO2. The plate was centrifuged and lactate dehydrogenase (LDH) release (an indicator of cytotoxicity) was measured in 50 μl of culture supernatants using a colorimetric assay (CytoTox 96, Promega, Madison, WI). Absorbances values at 490 nm were measured using a Multiskan ELISA reader (Thermo Scientific). Cytotoxicity (cell death) was determined as: (experimental LDH release–spontaneous LDH release)/(maximum LDH release–spontaneous LDH release), where spontaneous LDH release was the LDH activity in supernatants from cells incubated in medium alone and total LDH release the activity in lysed macrophages. Percent neutralization was calculated as 1-cell death ×100.
ASC and cytokines
IgA and IgG ASC were measured by ELISpot as previously described.4,5 Cytokines were measured in alveolar lavages or in culture supernatants from cells stimulated with IpaB or IpaD, using a MSD® Mouse Th1/Th2 Multi-array® tissue culture kit (Meso Scale Discovery, Rockville, MD), as previously described.4,6
Statistical analysis
Overall differences over time in % fluorescence and antibody responses (in serum and stool) were assessed by multivariate analysis of variance (MANOVA), using the different each time points measured as variables. Comparisons between vaccinated groups at specific time points were made using one-way analysis of variance (ANOVA) with the Tukey-Kramer multiple comparisons test. Survival for serum IgG responders (titers ≥ 50 ELISA Units ml−1) and non-responders (<50 ELISA Units ml−1) and stool IgA responders (titers ≥ 10 ELISA Units ml−1) and non-responders (<10 ELISA Units ml−1) were analyzed by a Fisher exact test, with the two-sided p-value calculated as twice the one-sided p-value in the direction of the observed difference. Associations between probability of survival and serum IgG titers were evaluated using logistic regression models. Survival after challenge was assessed by Kaplan-Meier curves and log-rank tests. Two-sided P≤0.05 was considered statistically significant. GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla, CA), SAS version 9.3 (SAS Institute, Cary, NC) and NCSS 8 (Number Cruncher Statistical Systems, Kaysville, UT) were used for statistical analyses.
Acknowledgments
This work was funded by NIH R01 AI089519 (to MFP and WLP).
Footnotes
CONFLICT OF INTEREST
KL and MR are employees and shareholders of Mucosis, B.V. The remaining authors declare no conflicts of interest.
References
- 1.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–22. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.Van de Verg LL, Venkatesan MM. A Shigella vaccine against prevalent serotypes. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu471. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 4.Heine SJ, Diaz-McNair J, Martinez-Becerra FJ, Choudhari SP, Clements JD, Picking WL, et al. Evaluation of immunogenicity and protective efficacy of orally delivered Shigella type III secretion system proteins IpaB and IpaD. Vaccine. 2013;31:2919–29. doi: 10.1016/j.vaccine.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Becerra FJ, Kissmann JM, Diaz-McNair J, Choudhari SP, Quick AM, Mellado-Sanchez G, et al. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infect Immun. 2012;80:1222–31. doi: 10.1128/IAI.06174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heine SJ, Diaz-McNair J, Andar AU, Drachenberg CB, Van DV, Walker R, et al. Intradermal delivery of Shigella IpaB and IpaD type III secretion proteins: kinetics of cell recruitment and antigen uptake, mucosal and systemic immunity, and protection across serotypes. J Immunol. 2014;192:1630–40. doi: 10.4049/jimmunol.1302743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Becerra FJ, Scobey M, Harrison K, Choudhari SP, Quick AM, Joshi SB, et al. Parenteral immunization with IpaB/IpaD protects mice against lethal pulmonary infection by Shigella. Vaccine. 2013;31:2667–72. doi: 10.1016/j.vaccine.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 8.van Roosmalen ML, Kanninga R, El Khattabi M, Neef J, Audouy S, Bosma T, et al. Mucosal vaccine delivery of antigens tightly bound to an adjuvant particle made from food-grade bacteria. Methods. 2006;38:144–9. doi: 10.1016/j.ymeth.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Bosma T, Kanninga R, Neef J, Audouy SA, van Roosmalen ML, Steen A, et al. Novel surface display system for proteins on non-genetically modified Gram-positive bacteria. Appl Environ Microbiol. 2006;72:880–9. doi: 10.1128/AEM.72.1.880-889.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez K, Ditamo Y, Rodriguez L, Picking WL, van Roosmalen ML, Leenhouts K, et al. Neonatal mucosal immunization with a non-living, non-genetically modified Lactococcus lactis vaccine carrier induces systemic and local Th1-type immunity and protects against lethal bacterial infection. Mucosal Immunol. 2010;3:159–71. doi: 10.1038/mi.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keijzer C, Haijema BJ, Meijerhof T, Voorn P, de HA, Leenhouts K, et al. Inactivated influenza vaccine adjuvanted with bacterium-like particles induce systemic and mucosal influenza A virus specific T-cell and B-cell responses after nasal administration in a TLR2 dependent fashion. Vaccine. 2014 doi: 10.1016/j.vaccine.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Rigter A, Widjaja I, Versantvoort H, Coenjaerts FE, van RM, Leenhouts K, et al. A protective and safe intranasal RSV vaccine based on a recombinant prefusion-like form of the F protein bound to bacterium-like particles. PLoS ONE. 2013;8:e71072. doi: 10.1371/journal.pone.0071072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nganou-Makamdop K, van Roosmalen ML, Audouy SA, van Gemert GJ, Leenhouts K, Hermsen CC, et al. Bacterium-like particles as multi-epitope delivery platform for Plasmodium berghei circumsporozoite protein induce complete protection against malaria in mice. Malar J. 2012;11:50. doi: 10.1186/1475-2875-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Audouy SA, van Selm S, van Roosmalen ML, Post E, Kanninga R, Neef J, et al. Development of lactococcal GEM-based pneumococcal vaccines. Vaccine. 2007;25:2497–506. doi: 10.1016/j.vaccine.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Saluja V, Amorij JP, van Roosmalen ML, Leenhouts K, Huckriede A, Hinrichs WL, et al. Intranasal delivery of influenza subunit vaccine formulated with GEM particles as an adjuvant. AAPS J. 2010;12:109–16. doi: 10.1208/s12248-009-9168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de HA, Haijema BJ, Voorn P, Meijerhof T, van Roosmalen ML, Leenhouts K. Bacterium-like particles supplemented with inactivated influenza antigen induce cross-protective influenza-specific antibody responses through intranasal administration. Vaccine. 2012;30:4884–91. doi: 10.1016/j.vaccine.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 17.Saluja V, Visser MR, Ter VW, van Roosmalen ML, Leenhouts K, Hinrichs WL, et al. Influenza antigen-sparing by immune stimulation with Gram-positive enhancer matrix (GEM) particles. Vaccine. 2010;28:7963–9. doi: 10.1016/j.vaccine.2010.09.066. [DOI] [PubMed] [Google Scholar]
- 18.Livio S, Strockbine NA, Panchalingam S, Tennant SM, Barry EM, Marohn ME, et al. Shigella Isolates From the Global Enteric Multicenter Study Inform Vaccine Development. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von SL, Kim DR, Ali M, Lee H, Wang X, Thiem VD, et al. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med. 2006;3:e353. doi: 10.1371/journal.pmed.0030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toro CS, Farfan M, Contreras I, Flores O, Navarro N, Mora GC, et al. Genetic analysis of antibiotic-resistance determinants in multidrug-resistant Shigella strains isolated from Chilean children. Epidemiol Infect. 2005;133:81–6. doi: 10.1017/s0950268804003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Braeckel-Budimir N, Haijema BJ, Leenhouts K. Bacterium-like particles for efficient immune stimulation of existing vaccines and new subunit vaccines in mucosal applications. Front Immunol. 2013;4:282. doi: 10.3389/fimmu.2013.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leenhouts K. Mimopath™-Based Vaccine Delivery. In: Singh M, editor. Novel Immune Potentiators and Delivery Technologies for Next Generation Vaccines. New York: Springer Science Business Media; 2013. pp. 245–265. [Google Scholar]
- 23.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–96. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 24.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014;14:94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- 25.Debarry J, Garn H, Hanuszkiewicz A, Dickgreber N, Blumer N, von ME, et al. Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J Allergy Clin Immunol. 2007;119:1514–21. doi: 10.1016/j.jaci.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Cohen D, Green MS, Block C, Rouach T, Ofek I. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J Infect Dis. 1988;157:1068–71. doi: 10.1093/infdis/157.5.1068. [DOI] [PubMed] [Google Scholar]
- 27.Cohen D, Green MS, Block C, Slepon R, Ofek I. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J Clin Microbiol. 1991;29:386–9. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen D, Ashkenazi S, Green MS, Gdalevich M, Robin G, Slepon R, et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–9. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 29.Passwell JH, Ashkenzi S, Banet-Levi Y, Ramon-Saraf R, Farzam N, Lerner-Geva L, et al. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1–4-year-old Israeli children. Vaccine. 2010;28:2231–5. doi: 10.1016/j.vaccine.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black RE, Levine MM, Clements ML, Losonsky G, Herrington D, Berman S, et al. Prevention of shigellosis by a Salmonella Typhi-Shigella sonnei bivalent vaccine. J Infect Dis. 1987;155:1260–5. doi: 10.1093/infdis/155.6.1260. [DOI] [PubMed] [Google Scholar]
- 31.Robbins JB, Schneerson R, Szu SC. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–98. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 32.Sansonetti PJ, Phalipon A, Arondel J, Thirumalai K, Banerjee S, Akira S, et al. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12:581–90. doi: 10.1016/s1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]
- 33.Pickett TE, Pasetti MF, Galen JE, Sztein MB, Levine MM. In vivo characterization of the murine intranasal model for assessing the immunogenicity of attenuated Salmonella enterica serovar Typhi strains as live mucosal vaccines and as live vectors. Infect Immun. 2000;68:205–13. doi: 10.1128/iai.68.1.205-213.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Southam DS, Dolovich M, O’Byrne PM, Inman MD. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L833–L839. doi: 10.1152/ajplung.00173.2001. [DOI] [PubMed] [Google Scholar]
- 35.Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, et al. Transient facial nerve paralysis (Bell’s palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS ONE. 2009;4:e6999. doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qadri F, Bhuiyan TR, Sack DA, Svennerholm AM. Immune responses and protection in children in developing countries induced by oral vaccines. Vaccine. 2013;31:452–60. doi: 10.1016/j.vaccine.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Mallett CP, Hale TL, Kaminski RW, Larsen T, Orr N, Cohen D, et al. Intransal or intragastric immunization with proteosome-Shigella lipopolysaccharide vaccines protects against lethal pneumonia in a murine model of Shigella infection. Infect Immun. 1995;63:2382–6. doi: 10.1128/iai.63.6.2382-2386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orr N, Robin G, Cohen D, Arnon R, Lowell GH. Immunogenicity and efficacy of oral or intranasal Shigella flexneri 2a and Shigella sonnei proteosome-lipopolysaccharide vaccines in animal models. Infect Immun. 1993;61:2390–5. doi: 10.1128/iai.61.6.2390-2395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitra S, Barman S, Nag D, Sinha R, Saha DR, Koley H. Outer membrane vesicles of Shigella boydii type 4 induce passive immunity in neonatal mice. FEMS Immunol Med Microbiol. 2012;66:240–50. doi: 10.1111/j.1574-695X.2012.01004.x. [DOI] [PubMed] [Google Scholar]
- 40.Mitra S, Chakrabarti MK, Koley H. Multi-serotype outer membrane vesicles of Shigellae confer passive protection to the neonatal mice against shigellosis. Vaccine. 2013;31:3163–73. doi: 10.1016/j.vaccine.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Jeong KI, Venkatesan MM, Barnoy S, Tzipori S. Evaluation of virulent and live Shigella sonnei vaccine candidates in a gnotobiotic piglet model. Vaccine. 2013;31:4039–46. doi: 10.1016/j.vaccine.2013.04.076. [DOI] [PubMed] [Google Scholar]
- 42.Choudhari SP, Kramer R, Barta ML, Greenwood JC, Geisbrecht BV, Joshi SB, et al. Studies of the conformational stability of invasion plasmid antigen B from Shigella. Protein Sci. 2013;22:666–70. doi: 10.1002/pro.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choudhari SP, Chen X, Kim JH, van Roosmalen ML, Greenwood JC, Joshi SB, et al. Biophysical Characterization of the Type III Secretion Tip Proteins and the Tip Proteins Attached to Bacterium-Like Particles. J Pharm Sci. 2014 doi: 10.1002/jps.24047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birket SE, Harrington AT, Espina M, Smith ND, Terry CM, Darboe N, et al. Preparation and characterization of translocator/chaperone complexes and their component proteins from Shigella flexneri. Biochemistry. 2007;46:8128–37. doi: 10.1021/bi700099c. [DOI] [PubMed] [Google Scholar]
- 45.Simon R, Tennant SM, Wang JY, Schmidlein PJ, Lees A, Ernst RK, et al. Salmonella enterica serovar enteritidis core O polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. enteritidis. Infect Immun. 2011;79:4240–9. doi: 10.1128/IAI.05484-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–9. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 47.Zauberman A, Cohen S, Levy Y, Halperin G, Lazar S, Velan B, et al. Neutralization of Yersinia pestis-mediated macrophage cytotoxicity by anti-LcrV antibodies and its correlation with protective immunity in a mouse model of bubonic plague. Vaccine. 2008;26:1616–25. doi: 10.1016/j.vaccine.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 48.Hilbi H, Puro RJ, Zychlinsky A. Tripeptidyl peptidase II promotes maturation of caspase-1 in Shigella flexneri-induced macrophage apoptosis. Infect Immun. 2000;68:5502–8. doi: 10.1128/iai.68.10.5502-5508.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zychlinsky A, Kenny B, Menard R, Prevost MC, Holland IB, Sansonetti PJ. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994;11:619–27. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]