Abstract
Positron emission tomography (PET) and magnetic resonance imaging, until recently, have been performed on separate PET and MR systems with varying temporal delay between the two acquisitions. The interpretation of these two separately acquired studies requires cognitive fusion by radiologists/nuclear medicine physicians or dedicated and challenging post-processing. Recent advances in hardware and software with introduction of hybrid PET/MR systems have made it possible to acquire the PET and MR images simultaneously or near simultaneously. This review article serves as a road-map for clinical implementation of hybrid PET/MR systems and briefly discusses hardware systems, the personnel needs, safety and quality issues, and reimbursement topics based on experience at NYU Langone Medical Center and Cleveland Clinic.
Keywords: PET/MR, Hybrid PET/MR, Clinical implementation
Positron emission tomography (PET) and magnetic resonance (MR) imaging until recently have largely been performed by separate PET and MR hardware with varying temporal delay between the two acquisitions. These acquisitions are either cognitively fused (interpreted together by a single radiologist/nuclear medicine specialist) or post-processed and fused using proprietary software. Recently, multiple vendors have been working on various implementations of PET and MR acquisitions either sequentially or simultaneously to create a hybrid modality. The Food and Drug Administration (“FDA”) has recently approved several commercial hybrid PET/MR scanners including the Siemen's Biograph mMR [1] and the Philips Ingenuity TF PET/MRI, with the Signa from General Electric (“GE”) still under FDA review [2].
A number of groups are validating, testing, and investigating the role and clinical utility of PET/MR imaging for a variety of clinical applications in oncology, neurology, and cardiovascular medicine. However, the clinical translation of this modality requires not only identifying the appropriate clinical indications but also understanding various components involved in establishing a PET/MR service which include physical installation of the system, equipment safety, clinical workflow, technician and physician training, and monetary reimbursement. The purpose of this review is to provide a playbook for setting up a clinical/translational PET/MR service. Specifically, we will share our experience at NYU Medical Center and at Cleveland Clinic with the Siemens Biograph mMR simultaneous acquisition scanner.
Hardware
System configuration
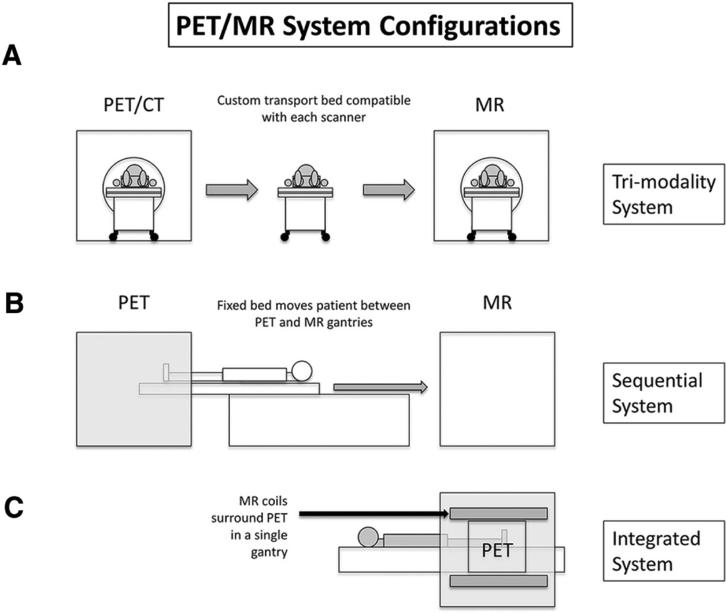
In general, three types of ‘hybrid’ PET/MR imaging constructs are available.
Trimodality system
This technology employs separate PET/CT and MR systems with a shared bed and hence is termed as “tri-modality” system (Fig. 1A). For accurate image registration, the patient stays positioned on the bed for both PET/CT and MR examinations while being transported between the systems. On some systems [3], registration tabs preserve patient positioning between studies. This technology requires advanced software that registers the source information from different acquisitions and produces a single dataset of fused images. Advantages of this technology include low cost due to the fact that most imaging institutions already own PET/CT and MR systems; only the software package and the specialized, dedicated bed are required for purchase. Additional advantages include built in attenuation correction due to CT acquisition. The major disadvantage of the system is the high potential for mis-registration. At our institutions, we do not have direct experience with this system.
Fig. 1.
Various PET/MR system configurations. A “Tri-modality” system employs a single bed with two completely separate scanners, one of which is a conventional PET/CT. B “Sequential” system employs a single bed; MR images are acquired immediately after PET images. C “Integrated” system employs a single bed and images are acquired simultaneously.
Sequential system
This technology employs an MR scanner and PET scanner with a single bed that facilitates moving between the two systems such that the patient is scanned through the PET system and then sequentially scanned through the MR system (Fig. 1B). The table literally rotates 180° after the MR images are acquired and subsequent PET imaging is performed while the patient remains in position. Similar to the trimodality system, this technology requires advanced software for post-processing. Advantages of this system is a reduced risk of mis-registration given the temporal nature of the system (one scan immediately after the other). Furthermore, there are specific, complex shielding requirements given that the PET system is immediately adjacent to the MR system. At our institutions, we do not have experience with this system.
Integrated simultaneous system
This system simultaneously acquires PET and MR data without acquiring CT information (Fig. 1C). MR images are utilized for attenuation correction in addition to anatomic information, thereby eliminating the need for CT acquisition, and thus significantly reducing radiation exposure, a major advantage. Attenuation correction on integrated systems is performed by acquiring Dixon sequences and improving attenuation correction algorithms with MR-based mu maps remains an area of active research. The major advantage is that mis-registration is significantly reduced and that the PET and MR data are acquired without temporal delay. The major disadvantages include technical complexity and cost associated with the purchase of a dedicated system. We will share our experience with this system in this article.
Equipment cost
The less expensive options employ separate MRI and PET/CT units (trimodality systems) in separate rooms. While having two scanners does represent a larger foot print, it should be noted that this allows independent use of the separate MR system and PET/CT system. On the other hand, the sequential and simultaneous construct offers a flexible fusion architecture allowing for wider latitude of scanning parameters utilizing simultaneous or near simultaneous acquisition of MR and PET. Thus, they have smaller footprints but are more expensive options. Sequential and simultaneous hybrid PET/MR systems can also be utilized as a dedicated traditional MR scanner, therefore allowing flexible utilization of the system. Solutions will most likely vary on the institutional need, physical space, and monetary constraints.
Space requirements
Although essentially self-evident depending on the type of system purchased, space requirements will vary. The largest system requirements are associated with the sequential scanners due to the “long” nature of the system (Fig. 1). Integrated system space requirements are similar to those for typical MRI scanners. Control requirements of hybrid PET/MR system are not different than those of traditional MRI systems. Other details of the hybrid PET/MR systems include bore size of 60 cm, system length of 199 cm, system weight 9 tons, and minimum room size of 33 m2. The suggested interior dimensions of the scanner room for the Biograph are approximately 23 × 13 square feet (minimum length and width), while the minimum total space required is approximately 355 square feet.
Dedicated shielding requirements for simultaneous hybrid system
With respect to the hardware elements of the PET machine, there is no real shielding requirement for the detector elements; solid state detector elements are not severely impacted by the MR gradients and radiofrequency signals. There is a normalization process that is performed periodically, which improves the homogeneity of the response, but there is no other specific “shielding” requirement for the PET detector elements.
Room shielding requirements are similar to that of a standalone MR system and a standalone PET system. As is typical for MR systems, we employed a copper “cage” without specific changes given that the PET system was co-located; in other words, the PET system does not induce any separate changes for the MR system shielding. Additionally, similar to standalone PET or PET/CT systems, the walls and doors were outfitted with lead and the observation window contains lead equivalent glass, but no specific changes were necessary due to the presence of the MR system.
Radiation safety & Hotlab considerations
With respect to radiation safety, the operation of a PET/MR scanning department closely mirrors that of a PET/CT center. Hotlabs are configured with the standard array of equipment (dose calibrators, well counters, lead shielding, shielded receptacles for storage of discarded radioactive materials, etc.) following local regulations. Isotopes are delivered from commercial or on-site radio-pharmacies using standard procedures. For short-lived isotopes, a workflow must exist whereby the cyclotron and PET systems are in close physical proximity to one another, so that the decay of the short-half-life radiotracers is not significant. For relatively longer half-life agents, other possibilities such as quick transport between the radiopharmacy and scanner may be a feasible option. Special care is taken when handling lead-shielded syringes in the scanner room due to the risk of the lead-containing shield being pulled toward the magnet. MRI-safe syringe shields made out of tungsten have recently come to market (Lynax, Czech Republic) and should be strongly considered for use in any PET/MR imaging suite. An automated blood-sampling system (Swisstrace “twilite two”, Menzingen, Switzerland) with MR-compatibility has been recently developed, allowing for precise measurement of arterial blood activity concentrations to support quantitative dynamic PET studies using PET/MR scanners. MR-compatible contrast injectors with the capability to automatically inject both radiopharmaceuticals, and MR contrast agents are in a prototype phase with FDA-approved devices expected in the near future. Non-attenuating head cushions and MR safe survey meters are also used at our institution. MR-compatible goggles and video screens are also in development to support neurological and psychiatric PET/MR applications.
Personnel
Technologists and certification
Currently, there is no certification process in place for PET/MR technologists. Various issues exist that require PET/MR technologists to have an expansive skillset. For example, hotlab work, dealing with a patient after radiotracer injection, and monitoring the patient while on the scanner due to the regulatory requirements for supervision of nuclear exams represent separate requirements above and beyond those of the traditional MRI technologists. It should be noted that these nuclear medicine requirements are typically mandated at the state level, and therefore, for example, New York State requires that the nuclear medicine certified technologist must be present at the scanner console to monitor the patient during image acquisition.
Given the above requirements, at NYU Medical Center, we have a certified nuclear medicine/PET technologist and certified MR technologist working together to operate the PET/MR scanner. As outlined above, certifications of each type of technologist typically vary according to state regulations, and at our institution, there are no additional or special certifications held by the two technologists overseeing the scanner.
At Cleveland Clinic, at the present time, three technologists are dual trained in both MRI and PET. One of the dual trained technologists operates the PET/MR scanner with single coverage of the scanner. Although each technologist is dual trained, such that a single technologist can operate the scanner individually, it is important to recognize that redundancy in training for both PET scanning and MR scanning is paramount. The troubleshooting skills required for operating the hybrid machine require good understanding of the physics that underlie image generation via both modalities, as well as the post-processing necessary to produce clinically relevant and interpretable images. These skills sometimes require active management at the scanner by the radiologist along with the technologist.
QA/QC requirements
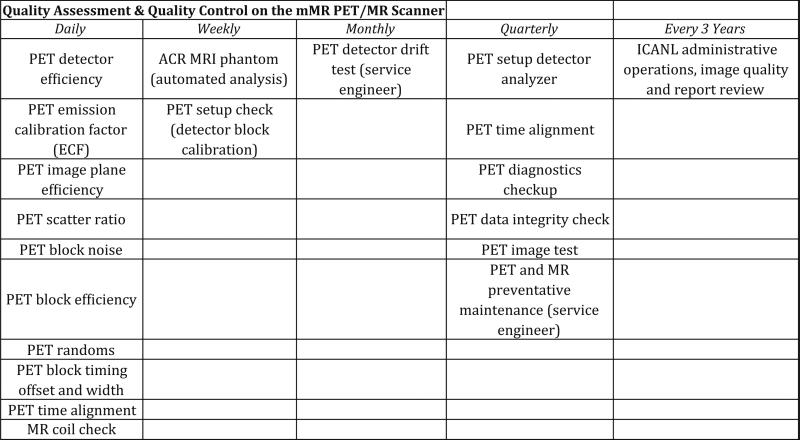
For all regulatory and QA work that needs to be performed for both systems, our dedicated PET and MR physicists work together with the PET and MR technologists to ensure optimal scanner parameters and function. No additional QA is required by the ACR or any local or federal agency for the combined system. In other words, the same routines are followed as if our facility were operating a standalone PET and MR systems, although the frequency of certain tests is different. For the most part, MR QA is weekly and PET/MR (Fig. 2) is daily with weekly “setup” and quarterly “full setup.”
Fig. 2.
Summary of our PET/MR quality assessment and quality control.
The weekly MR QA is standard which includes the weekly American College of Radiology (ACR) phantom scan. Additionally, the technologist has the option at any time to perform an MR coil check with a water bottle phantom on the head and neck coil, which currently takes approximately 10 min.
The PET QA/QC is identical to those for the conventional PET/CT systems, although the frequency of these is increased on the combined PET/MR system for various reasons. It should be noted that any checks that traditionally utilized metal/lead holders or components must be outfitted with plastic so as to comply with the MRI safety guidelines. Similar to PET/CT, a Germanium source is checked daily with a plastic phantom holder. Additional checks which are standard for PET/CT systems that are also checked on our PET/MR system include block noise, block efficiency, scanner efficiency, plain efficiency, and scatter ratio. On our PET/MR, we check time alignment daily, and a gantry setup is performed to monitor gain due to temperature variations and voltage changes that affect the detectors, whereas on the PET/CT, this is checked weekly. Finally, a calibration check of the detector blocks is performed weekly on our PET/MR, whereas it is performed monthly on our PET/CT.
Although there are no additional QA requirements for the integrated system as compared to the standalone PET or MRI scanners, there are certain preventative parameters that require attention. Preventative maintenance checkups for the integrated system are performed by the engineer on a monthly or quarterly basis. Specifically, an extra functionality of the PET/MR that requires QA attention is the “detector drift test” that is supposed to be performed by the engineer monthly. This test checks to see if any detectors have moved or drifted, and also if a given detector should be replaced.
We predict that technologist and physicist experiences will vary widely depending on the comfort level of the radiologists and nuclear medicine physicians overseeing these examinations, as well as on the type of scanning system employed. Additionally, as PET/MR examinations become more common, we predict that certification requirements may change, such that a single technologist will be routinely employed to operate the system regardless of the PET/MR system. We also do not currently have or foresee the need in the future for a separate physicist dedicated to the PET/MR scanner; our current head of physics oversees all nuclear and MR-based QC requirements, and our head nuclear and MR technologists work in concert to oversee technologist training and examination troubleshooting.
Exam workflow
Indications
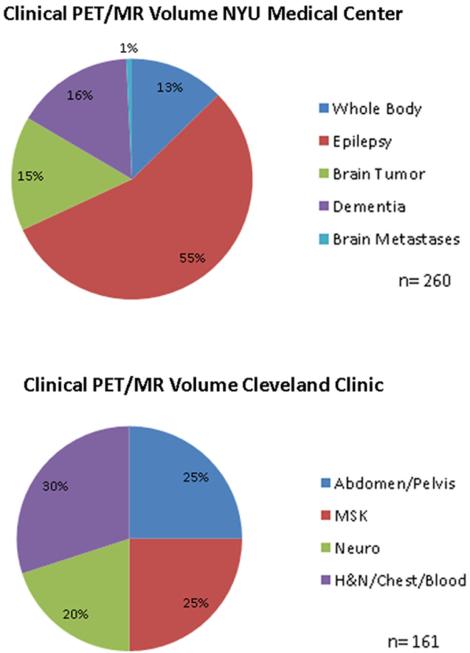
The most common current clinical applications at our institution include neurologic and oncologic indications (Fig. 3). Since July 2012, we at NYU Medical Center have performed over 500 research examinations, over 130 clinical brain FDG PET/MR, and nearly 50 onco-logic clinical PET/MR examinations [4, 5].
Fig. 3.
Summary of clinical PET/MR exams performed at our institution.
Following an IRB phase between September 2013 and February 2014 during which 68 FDG PET/MRI scans were completed at the Cleveland Clinic Center for PET and Molecular Imaging, over 150 clinical FDG PET/MRI scans have been subsequently completed (through November 2014). Of these scans, there have been equal numbers of FDG PET/MR of the brain (e.g., brain tumor and epilepsy) and FDG whole-body (WB) PET/MR for solid tumor oncology (e.g., rectal and gynecologic malignancies) and lymphoma. The remainder of the FDG WB PET/MR scans have been for musculoskeletal pathology (e.g., multiple myeloma and bone and soft tissue sarcoma). About 25 WB PET/MR scans have been performed on pediatric patients (less than 18 years) primarily for epileptogenic focus localization and solid tumor oncology indications.
We believe that in addition to oncologic applications, neurologic and cardiovascular imaging will continue to gain prominence as our field gains more experience with PET/MR scanners.
Exam Referral/Ordering: Currently, in our experience, no category 3 CPT code exists for PET/MR examination. Our referring physicians order either clinically indicated PET examination or clinically indicated PET examination along with clinically indicated MR examination.
Protocols and scanning
Detailed discussion surrounding dedicated PET/MR protocols is beyond the scope of this article. However, briefly, at NYU Medical Center, we employ two types of Oncology protocols: (1) a whole-body (“WB”) imaging protocol alone, similar to PET/CT examinations and (2) WB imaging with the addition of morphologic dedicated MR sequences according to the organ of interest as described below. Typically, [FDG-18] PET radiotracer is utilized, and an approximately 45 min–1 h delay is employed after injection. The WB exam consists of multiple stations from the skull base through the thighs, lasting 6–8 min per station. Each bed acquisition time is dictated generally by the number of MR sequences that are acquired per bed. Total exam acquisition time for protocol “1” is 30–45 min currently and includes the acquisition of following MR sequences per each bed station:
– Dixon for attenuation correction;
– Diffusion weighted imaging (DWI) with three b values acquired axially in free-breathing;
– axial free-breathing radial T1 GRE (Gradient Echo) VIBE (Volumetric Interpolated Breath-hold Examination); and
– breath-hold axial HASTE (Half-Fourier Acquisition Single-shot Turbo Spin Echo) or SSFSE (Single-Shot Fast Spin Echo).
– Depending on the clinical indication as well as the patient's ability to tolerate the examination, we add free-breathing T2 turbo spin echo acquisition (TSE) for the chest, abdomen, and pelvis stations.
Advantages of this protocol include short acquisition time. However, this protocol does not take advantage of strengths of MRI due to abbreviated numbers of MR sequences. Of note, the longer bed times are offset by a relatively longer axial field of view of the PET/MR scanner compared to many traditional PET/CT cameras.
We also perform additional dedicated MR examinations depending on the clinical indication (protocol “2” above). These dedicated sequences are obtained depending on the underlying indication and may be augmented by medication (e.g., furosemide for hematuria/bladder cancer studies), gadolinium contrast (e.g., liver lesions for metastatic breast cancer), or dedicated changes in organ-specific protocols (e.g., small FOV for gynecologic or rectal malignancies). Regardless of the type of protocol employed, at NYU Medical Center, we schedule all patients receiving a PET/MR examination for a 90-min time slot.
At the Cleveland Clinic, three types of exams are performed for clinical FDG PET/MR scans which are a variation of the protocols described above. “Basic (or routine”) WB PET/MR exam can be requested as skull vertex to toes, typically for indications such as multiple myeloma and melanoma, or skull vertex to proximal thighs, analogous to PET/CT exams, and the whole-body protocol ‘1’ discussed above. Typically, a 40-min time slot is scheduled for which the scan time is typically 30 min, with the remainder used for patient preparation, and on- and off-boarding of the patient from the scanner. Depending upon the findings and indication, small FOV axial T2 TSE, STIR (Short Tau Inversion Recovery) or DWI, and/or sag T1 and STIR through spine are also obtained. The use of these advanced MR imaging sequences represents the second type of exam and constitutes “multiparametric PET/MRI exam” requiring a 60-min time slot. The third type of exam “advanced WB FDG PET/MR exam” consists of a basic WB PET/MR exam and an additional dedicated focused MR exam, similar to the protocol ‘2’ discussed above.
Patient preparation
In general, there is no dedicated patient preparation that is specific to PET/MR. Similar to PET/CT, patients are requested to be NPO for 4 hours prior to the exam, and are counseled regarding radiation safety and radioactivity. Patients are also screened similar to conventional MR exam with an MR safety questionnaire and gadolinium contrast safety questionnaire (for contrast enhanced exams). Finally, there is an additional dedicated PET/MR scan questionnaire that is completed by the patients (Fig. 4).
Fig. 4.
Dedicated PET/MR screening questionnaire.
Regarding PET, the most important topics to discuss with patients are (1) dietary restrictions prior to the exam to restrict the amount of blood-stream glucose; (2) recent injections or trauma that might confound metabolic activity; (3) strenuous activity; and (4) careful evaluation of diabetic medications. From an MRI perspective, the most important safety issues to discuss are (1) metallic implants or piercings and (2) valves, prostheses etc. We are quite cautious regarding documentation of MR-compatible implants/prostheses, such that if we cannot confirm that the device or implant is MR-compatible, we do not proceed with the examination. Overall, there is a very little difference regarding patient safety/preparation between PET/MRI examinations versus PET/CT and MRI examinations.
Post-processing and data requirements
As with any hybrid/combined imaging evaluation, image viewing and post-processing are of utmost importance. Several different software packages exist that are offered both by the PET/MR system manufacturers as well as separate third party entities.
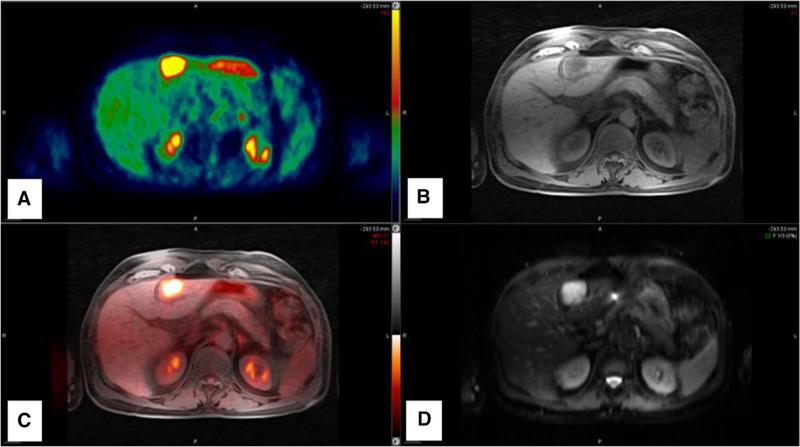
At NYU Medical Center, we utilize our PET/CT workstations for PET/MR readout sessions. Fusion software is used in a similar fashion, although, different from PET/CT, one MR sequence is utilized for fusion with PET. We routinely select the T1 weighted radial free-breathing acquisition (STAR VIBE, Siemens Health Care) for this purpose (Fig. 5). It has become clear that more work is required to optimize these work stations in order to support a higher degree of flexibility required to accommodate various MR acquisitions including functional techniques such as diffusion and perfusion weighted imaging.
Fig. 5.
Sample fusion image set for interpretation. A Color PET images. B Radial T1-W GRE (VIBE). C Fused overlay images. D High b value diffusion weighted image. Images show a hyper-metabolic metastatic lesion in segment 3 of the liver that is T1 hypo-intense and high signal intensity on high b value images.
There are increased data size requirements in comparison to PET/CT by nature of the fact that so many more sequences are performed during whole-body as well as during targeted organ MR acquisitions. Overall, hybrid PET/MR examinations have typical size requirements ranging from 500 MB to 2 GB. The PET sequences and post-processed sequences are similar in file size to those from a PET/CT examination.
Interpretation and reporting
At our institution, NYU Medical Center, interpretation currently occurs in a “joint readout” fashion and typically involves an “MR imager” and a nuclear medicine imager; the MR imager is often a subspecialist from the abdominal, neuroradiology, or breast imaging departments. Although this is not efficient, we believe that this is optimal while learning a new modality. We do however predict that, similar to the PET/CT model, a “hybrid” trained individual will interpret these examinations alone—though a significant amount of training may be necessary. Currently, separate PET and MR reports are produced from each joint readout session for clinical cases that were ordered and performed as separate PET and MR examinations. However, this model is already changing to the extent that combined reports are now being produced for certain types of examinations and indications at our institution (such as bladder cancer). Similar to the PET/CT model, we believe that this will eventually be the standard format.
At the Cleveland Clinic, teams of imaging physicians, consisting of members of both the nuclear medicine and diagnostic radiology departments, have worked together for nearly 4 years to become qualified and credentialed as single authors for interpreting diagnostic WB PET/CT examinations. Thus, the transition to reading PET/MR examinations has been relatively easy. Typically, a “basic” FDG PET/MR scan is single authored either by a diagnostic radiologist who has PET competency or by one of several nuclear medicine physicians. Many of the nuclear medicine physicians are comfortable with basic WB FDG PET/MR examinations whereas others co-author “basic” PET/MR examinations in collaboration with MR trained radiologists. Several radiologists at the Center of PET and Molecular Imaging are trained in both MR and PET and read out the majority of the “multiparametric” and “advanced” diagnostic PET/MRI scan as single authors (in consultation with the nuclear medicine physician if needed). Exams performed for primary bone and soft tissue malignancies and myeloma are co-authored with a musculoskeletal radiologist, and examinations employing contrast enhanced head and neck acquisitions are co-authored with a sub-specialized neuroradiologist. FDG PET/MR examinations performed on pediatric patients are read in collaboration with subspecialized pediatric radiologists.
Pre-certification and reimbursement
For clinical studies, unique billing codes for PET/MR do not exist. The Centers for Medicare and Medicaid Services (CMS) has indicated recently that they will cover up to 4 (baseline + 3 follow up scans) PET examinations, which can be performed as part of a hybrid (i.e., PET/CT or PET/MR) examination, regardless of the technique used to acquire ”anatomic” data (i.e., CT or MR) [6]. Furthermore, for those patients in whom PET and diagnostic dedicated MR is indicated, these examinations can be performed as whole-body PET/MR including a dedicated MR examination as discussed above with charges generated for diagnostic PET and diagnostic MR examinations.
Conclusion
PET/MR represents an exciting new hybrid technology with a broad range of clinical applications that are still being investigated [7–9]. At both NYU Medical Center and at the Cleveland Clinic, the experience has been mostly positive, with gradual transition from research to clinical implementation for selected applications. Although a model already exists in PET/CT for hybrid imaging, there are several differences which must be addressed prior to establishing a clinical service.
Attenuation correction: Although Dixon sequences are currently used in clinical practice, investigation is underway in order to analyze the reproducibility and reliability of these sequences and develop newer methods to improve attenuation correction.
Given that these hybrid examinations involve large whole-body PET data sets in conjunction with near if not full MR imaging examinations, the data requirements will increase as this technology is adapted. Large-scale institutions and small practices alike must consider the ramifications of this change.
How will personnel, including technologists and radiologists/nuclear medicine physicians, be trained for administering, acquiring, and interpreting these examinations? We believe that ‘hybrid’ trained technologist and the interpreting physician will be best suited to perform these tasks.
These ‘hybrid’ technologists and physicians may require additional certification and training, and it will be important for various organizations such as ABR, ACR, and other state and federal organizations to come together to address these issues.
While FDG PET/CT remains the workhorse for functional oncologic and neurodegenerative diseases, early experience shows that PET/MR has a clear complementary role in the imaging algorithm. PET/MR could potentially play a significant role in several disease diagnosis and management algorithms. Here we have highlighted some of the consideration for establishing a PET/MR service based on our clinical experience with simultaneous PET/MR hybrid system.
References
- 1. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm258700.html.
- 2. http://www.medgadget.com/2014/08/ges-new-signa-petmr-with-time-of-flight-tech-under-review-by-fda.html.
- 3. http://www3.gehealthcare.com/en/products/categories/molecular_imaging/pet_ct_and_mr_trimodality_imaging#tabs/tab8F8D8C24FA804C47AC2348D98A4F1971.
- 4.Gold LS, et al. Imaging Techniques for Treatment Evaluation for Metastatic Breast Cancer (Internet) Agency for Healthcare Research and Quality (US); Rockville (MD): Oct, 2014. Report No.: 14-EHC044-EF. [PubMed] [Google Scholar]
- 5.Buchbender C, et al. Oncologic PET/MRI, part 1: tumors of the brain, head and neck, chest, abdomen and pelvis. J Nucl Med. 2012;53(6):928–938. doi: 10.2967/jnumed.112.105338. doi:10.2967/jnumed.112.105338. [DOI] [PubMed] [Google Scholar]
- 6. http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=263&NcaName=Positron+Emission+Tomography+(FDG)+for+Solid+Tumors&TimeFrame=7&DocType=All&bc=AQAAIAAACAAAAA%3d%3d&.
- 7.Catalano OA, et al. Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: initial experience in 134 patients—a hypothesis-generating exploratory study. Radiology. 2013;269(3):857–869. doi: 10.1148/radiol.13131306. doi:10.1148/radiol.13131306. [DOI] [PubMed] [Google Scholar]
- 8.Drzezga A. First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med. 2012;53(6):845–855. doi: 10.2967/jnumed.111.098608. doi:10.2967/jnumed.111.098608. [DOI] [PubMed] [Google Scholar]
- 9.Moy L, et al. Role of fusion of prone FDG-PET and magnetic resonance imaging of the breasts in the evaluation of breast cancer. Breast J. 2010;16(4):369–376. doi: 10.1111/j.1524-4741.2010.00927.x. doi:10.1111/j.1524-4741.2010.00927.x. [DOI] [PubMed] [Google Scholar]