Highlights
-
•
Rats were trained under a progressive-ratio schedule of sucrose or corn oil reinforcement.
-
•
A mathematical model of progressive-ratio schedule performance was used to analyse the data.
-
•
The model’s incentive value parameter was greater for the corn oil than for the sucrose reinforcer.
-
•
Removal of the food restriction condition resulted in reduction of the incentive value parameter.
-
•
Δ9-Tetrahydrocannabinol selectively increased the incentive value of sucrose but not corn oil.
Keywords: Progressive-ratio schedule, Mathematical Principles of Reinforcement, Mathematical model, Food deprivation, Sucrose, Corn oil, Δ9-Tetrahydrocannabinol, Incentive value, Rat
Abstract
Rats’ performance on a progressive-ratio schedule maintained by sucrose (0.6 M, 50 μl) and corn oil (100%, 25 μl) reinforcers was assessed using a model derived from Killeen’s (1994) theory of schedule-controlled behaviour, ‘Mathematical Principles of Reinforcement’. When the rats were maintained at 80% of their free-feeding body weights, the parameter expressing incentive value, a, was greater for the corn oil than for the sucrose reinforcer; the response-time parameter, δ, did not differ between the reinforcer types, but a parameter derived from the linear waiting principle (T0), indicated that the minimum post-reinforcement pause was longer for corn oil than for sucrose. When the rats were maintained under free-feeding conditions, a was reduced, indicating a reduction of incentive value, but δ was unaltered. Under the food-deprived condition, the CB1 cannabinoid receptor agonist Δ9-tetrahydrocannabinol (THC: 0.3, 1 and 3 mg kg−1) increased the value of a for sucrose but not for corn oil, suggesting a selective enhancement of the incentive value of sucrose; none of the other parameters was affected by THC. The results provide new information about the sensitivity of the model’s parameters to deprivation and reinforcer quality, and suggest that THC selectively enhances the incentive value of sucrose.
1. Introduction
In ratio schedules of reinforcement, the subject is required to emit a specified number of responses, N, to obtain a reinforcer. In progressive-ratio schedules, N is systematically increased, usually from one reinforcer to the next (Hodos, 1961; Stafford and Branch, 1998), but sometimes after batches of two or more reinforcers (Baunez et al., 2002; Randall et al., 2012) or between successive sessions (Griffiths et al., 1978; Czachowski and Samson, 1999). Performance on progressive-ratio schedules is characterised by rapid responding under low ratios which peters out as N is increased. The ratio at which the subject stops responding, the breakpoint, is widely regarded as a measure of the subject’s motivation or the incentive value of the reinforcer (Hodos, 1961; Hodos and Kalman, 1963 for review, see Ping-Teng et al., 1996; Killeen et al., 2009).
Despite its widespread use, several authors have expressed doubts about the specificity of the breakpoint (Arnold and Roberts 1997; Killeen et al., 2009; Bradshaw and Killeen, 2012), pointing out that it is sensitive not only to changes in the incentive properties of reinforcers (Rickard et al., 2009; Gosnell et al., 2010) but also to non-motivational manipulations such as changes in the response requirement (Skjoldager et al., 1993; Aberman et al., 1998) and the ratio step size (Covarrubias and Aparicio, 2008). It has also been noted that the breakpoint shows considerable variability, being derived from a single point in time while data from the rest of the session are ignored, and that its definition is arbitrary, there being no consensus as to the time that must elapse without a response before responding may be said to have stopped (Arnold and Roberts, 1997; Killeen et al., 2009).
Many of the shortcomings of the breakpoint may be avoided by the use of quantitative models of performance on progressive-ratio schedules, for example the model recently proposed by Bradshaw and Killeen (2012). This model was derived from Killeen’s (1994) general theory of schedule-controlled behaviour, the Mathematical Principles of Reinforcement (MPR), according to which schedule-controlled responding is determined by an excitatory effect of reinforcers on behaviour, biological constraints on responding, and the efficiency with which schedules couple responses to reinforcers. In addition, the progressive-ratio model invokes the linear waiting principle (Wynne et al., 1996) to predict the escalating duration of the post-reinforcement pause in successive ratios, thereby yielding a dynamic account of performance on this schedule. The linear waiting principle expresses the finding that the post-reinforcement pause on trial i, TP,i, is linearly related to the total inter-reinforcement interval on trial i-1, TTOT,i-1:
| (1) |
where the parameters T0 and k represent the minimum post-reinforcement pause and the slope of the linear waiting function. The progressive-ratio model contains two key equations that define running response rate, RRUN, and overall response rate, ROVERALL:
| (2) |
| (3) |
The parameter a (‘specific activation’), which is defined as the duration of behavioural activation induced by a single reinforcer, is regarded as an index of incentive value. δ is the minimum time needed to execute a response (the reciprocal of the maximum response rate), and is regarded as a measure of the biological limitations on responding (Killeen, 1994; Reilly, 2003; Covarrubias and Aparicio, 2008; Sanabria et al., 2008; Bradshaw and Killeen, 2012).
Several lines of evidence support these interpretations of a and δ. Consistent with the notion that a is an index of incentive value, it has been found that this parameter is monotonically related to the volume of a sucrose-solution reinforcer (Rickard et al., 2009: data re-analysed by Bradshaw and Killeen, 2012). Recently, Olarte-Sánchez et al. (2013) compared the values of a for corn oil and sucrose reinforcers; their findings were consistent with extant evidence that sucrose is less efficacious than corn oil on a volume-for-volume basis, but more efficacious on a calorie-for-calorie basis (Naleid et al., 2008). Valencia-Torres et al. (2014) found that diabetes induced by systemic treatment with streptozotocin was associated with a reduction of a, consistent with an antihedonic effect of this treatment (Nefs et al., 2012). D1 and D2 dopamine receptor antagonists also reduce a, consistent with the purported antihedonic effect of these drugs (Olarte-Sánchez et al., 2012: data re-analysed by Bradshaw and Killeen, 2012; Olarte-Sánchez et al., 2013). Some drugs with known sedative properties, including clozapine and cyproheptadine, have been found to increase the response-time parameter δ (Olarte-Sánchez et al., 2012: data re-analysed by Bradshaw and Killeen, 2012).
The experiment described in this paper further explored the utility of the progressive-ratio model. The aims were firstly to examine the sensitivity of the parameters of the model to the food deprivation condition and the type of reinforcer used, and secondly to examine the effect of Δ9-tetrahydrocannabinol (THC), a principal constituent of cannabis resin with high affinity for CB1 cannabinoid receptors (Gaoni and Mechoulam, 1964; Howlett, 2002; Ledent et al., 1999; Matsuda et al., 1990), on the parameters of the model. Since, ex hypothesi, a represents the incentive value of a reinforcer, it was expected that the value of this parameter would be greater under conditions of food deprivation than under free-feeding conditions. Moreover, in view of the known orexigenic effect of THC (Abel, 1975; De Luca et al., 2012; Higgs et al., 2003; Williams et al., 1998; Williams and Kirkham, 2002a,b), it was expected that this drug would induce an increase in the value of a. However, in apparent conflict with the latter prediction, Olarte-Sánchez et al. (2012) recently reported that THC had no effect on the value of a for food-pellet reinforcers. The present experiment extended these findings by examining the effect of THC on performance on progressive-ratio schedules maintained by sucrose and corn oil reinforcers. In addition, since Olarte-Sánchez et al. (2012) analysed their data using an earlier model derived from MPR, designed to account for performance on fixed-ratio schedules (Killeen, 1994), a re-analysis of their data was carried out using the new progressive-ratio model.
2. Methods
The experiment was carried out in accordance with UK Home Office regulations governing experiments on living animals.
2.1. Subjects
Twenty-four female Wistar rats (Charles River, UK) approximately 4 months old and weighing 250–300 g at the start of the experiment were used. They were housed individually under a constant cycle of 12 h light and 12 h darkness (light on 0600–1800 h), and were maintained at 80% of their initial free-feeding body weights (see below) by providing a limited amount of standard rodent diet after each experimental session. Tap water was freely available in the home cages.
2.2. Apparatus
The rats were trained in operant conditioning chambers (CeNeS Ltd. Cambridge, UK) of internal dimensions 25 × 25 × 22 cm. One wall of the chamber contained a central recess covered by a hinged Perspex flap, into which a peristaltic pump delivered the liquid reinforcer (see below). An aperture located 5 cm above and 2.5 cm to one side of the recess (left for half the subjects; right for the other half) allowed insertion of a motorised retractable lever (CeNeS Ltd. Cambridge, UK) into the chamber. The lever could be depressed by a force of approximately 0.2 N. The chamber was enclosed in a sound-attenuating chest with additional masking noise generated by a rotary fan. No houselight was present during the sessions. An Acorn microcomputer programmed in Arachnid BASIC (CeNeS Ltd. Cambridge, UK) located in an adjacent room controlled the schedule and recorded the behavioural data.
2.3. Behavioural training
Two weeks before starting the experiment the food deprivation regimen was introduced and the rats were gradually reduced to 80% of their free-feeding body weights. They were randomly allocated to two groups that underwent training with different reinforcers: 50 μl of a 0.6 M solution of sucrose in distilled water (n = 12), and 25 μl of undiluted corn oil (n = 12). The rats were first trained to press the lever for the liquid reinforcer, and were then exposed to an fixed-ratio 1 schedule for 3 days followed by fixed-ratio 5 for a further 3 days. Thereafter, they underwent daily training sessions under the progressive-ratio schedule. The progressive-ratio schedule was based on the exponential progression: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, …, derived from the formula (5 × e0.2n) − 5, rounded to the nearest integer, where n is the position in the ratio sequence (Roberts and Richardson, 1992). Sessions took place at the same time each day during the light phase of the daily cycle (between 0800 and 1300 h) 7 days a week. At the start of each session, the lever was inserted into the chamber; the session was terminated by withdrawal of the lever 40 min later.
2.4. Drug treatment
Injections of THC were given on Tuesdays and Fridays, and injections of the vehicle alone on Mondays and Thursdays; no injections were given on Wednesdays, Saturdays or Sundays. Each rat was tested five times with each dose of THC, the order of treatments being counterbalanced across animals according to a Latin square design. THC (Δ9-tetahydrocannabinol, obtained from Tocris Bioscience, Bristol, UK) was dissolved in a mixture of ethanol and Tween (1:1) and diluted with sterile water to give the desired concentration. It was injected intraperitoneally (2.5 ml kg−1; 25-gauge needle) 30 min before the start of the experimental session. The doses of THC were selected on the basis of previous experience of the effect of this drug on performance on the progressive-ratio schedule (Olarte-Sánchez et al., 2012).
2.5. Experimental procedure
The experiment consisted of two phases. First, while the rats were maintained at 80% of their free-feeding body weights (‘food-deprived condition’), the effect of THC (0.3, 1 and 3 mg kg−1) was tested. Then the rats were given free access to standard laboratory chow (RM1 rodent diet: SDS Ltd., UK) in their home cages (‘free-feeding condition’) while daily training under the progressive-ratio schedule was continued.
2.6. Data analysis
Overall response rate (ROVERALL) was calculated for each ratio by dividing the number of responses by the total time taken to complete the ratio, including the post-reinforcement pause, measured from the end of the preceding reinforcer delivery until the emission of the last response of the ratio (Bizo and Killeen, 1997). The first ratio (a single response) and any ratios that had not been completed at the end of the session were excluded from the analysis. Running rate (RRUN) was calculated by dividing the number of responses by the ‘run-time’ (i.e. the time taken to complete the ratio, excluding the post-reinforcement pause: Bizo et al., 2001). Post-reinforcement pause duration was measured from the end of the reinforcer delivery until the emission of the first response of the following ratio.
The breakpoint was defined as the last ratio to be completed before 5 min elapsed without any responding, or, in cases where this criterion was not met within the session, the highest completed ratio (Olarte-Sánchez et al., 2012).
The progressive-ratio model comprising Eqs. (2) and (3) was fitted to the running and overall response rate data obtained from individual rats, and estimates of the four parameters, T0, k, a and δ, were derived using the ‘Solver’ facility of Excel (Microsoft Corporation); goodness of the combined fit of Eqs. (2) and (3) to the overall and running response rate data was expressed as R2 (Bradshaw and Killeen 2012).
The model was fitted to the data obtained from each rat in the last ten sessions in which no active treatment was administered under the food-deprived and free-feeding conditions, and estimates of the four parameters were derived. These estimates were analysed by separate two-factor analyses of variance with reinforcer type as a between-groups factor and deprivation condition as a within-subject factor, followed, in the case of a significant interaction, by post hoc comparisons of the two groups within conditions, and the two conditions within groups, using Student’s t-test.
The model was also fitted to the data obtained from each rat in the sessions in which injections of THC or its vehicle were administered, and estimates of the four parameters were derived. These estimates were analysed by separate one-factor analyses of variance with treatment condition as a within-subject factor, followed, in the case of a significant effect of treatment, by comparison of each dose of THC with the vehicle-alone treatment using Dunnett’s test. The effect sizes revealed by the analyses of variance were expressed as partial η2 (η2p).
The same statistical methods as were used to analyse parameters of the model were also used to analyse the breakpoints.
In addition to the results of the present experiment, the same analytical methods were used to re-analyse the data reported by Olarte-Sánchez et al. (2012) on the effect of THC on performance maintained by a progressive-ratio schedule of food-pellet reinforcement. These data were obtained from 12 female Wistar rats maintained under the same conditions, trained under the same progressive-ratio schedule, and tested with the same doses of THC as those used in the present experiment. A significance criterion of p < 0.05 (two-tailed) was adopted in all statistical analyses.
3. Results
3.1. Comparison of performance maintained by sucrose and corn oil reinforcers
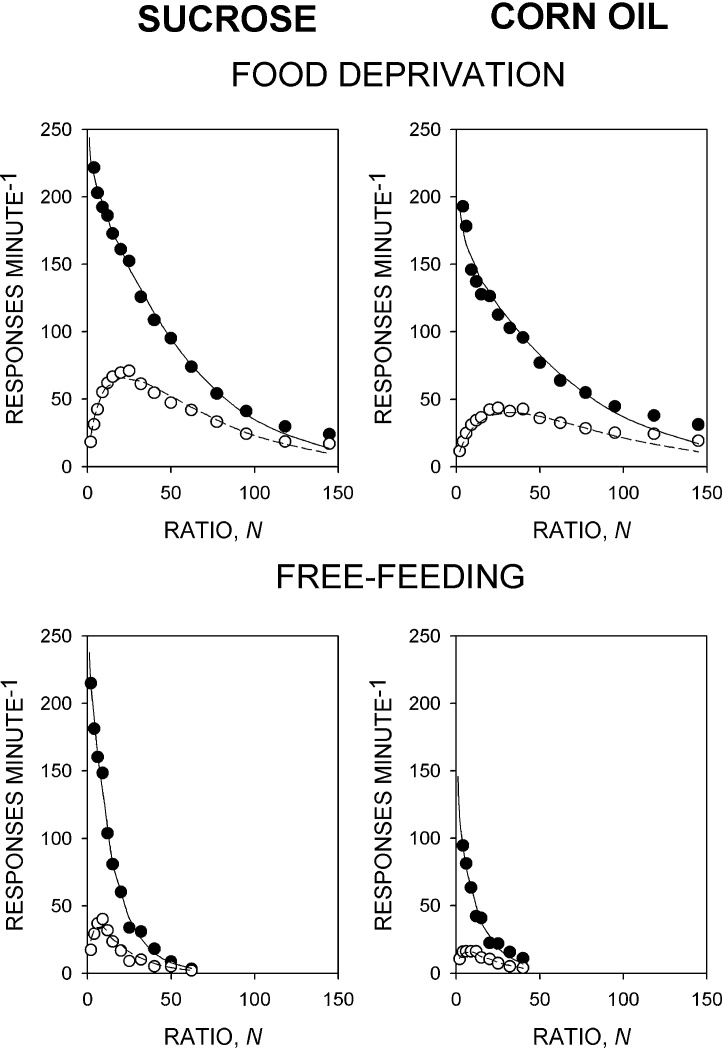
Fig. 1 shows the mean response rate data from the two groups in sessions in which no drug treatment was administered under the food-deprived and free-feeding conditions. In both groups and under both conditions, running response rate declined monotonically towards zero, whereas overall response rate rose to a peak before declining towards zero. Under the food-deprived condition, the peak of the overall response rate function was lower and the slope of the declining phase shallower in the corn oil-reinforced group than in the sucrose-reinforced group. In both groups response rates declined more steeply under the free-feeding condition than under the food-deprived condition. The progressive-ratio model provided a good description of the group mean overall and running response rate data obtained from both groups (sucrose-reinforced group: R2 = 0.995 [food-deprived], 0.994 [free-feeding]; corn oil-reinforced group: R2 = 0.983 [food-deprived], 0.988 [free-feeding]).
Fig. 1.
Performance on the progressive-ratio schedule maintained by the sucrose-solution and corn oil reinforcers (left and right columns) under the food-deprived and free-feeding conditions (upper and lower rows). Ordinates, response rate; abscissae, response/reinforcer ratio, N. Points are group mean data: filled symbols indicate running response rate, unfilled symbols overall response rate. The curves are best-fit functions defined by Eqs. (2) and (3).
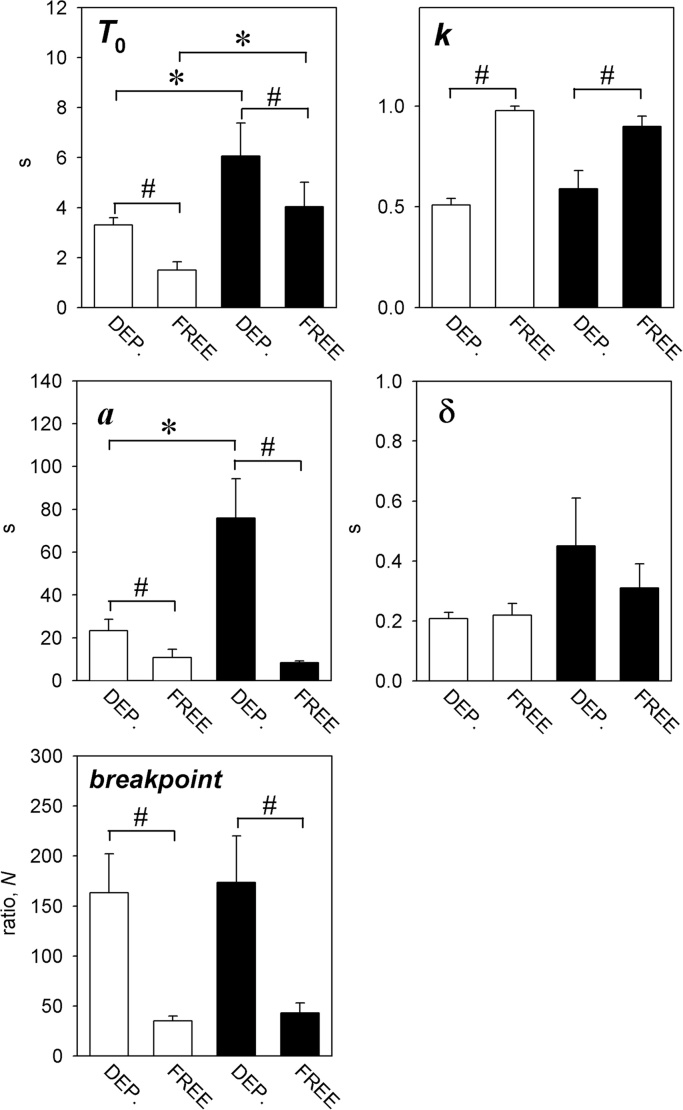
Fig. 2 shows the mean (+SEM) estimates of the parameters of the model derived from the individual rats in the two groups under the two deprivation conditions. The data from one rat in the corn oil-reinforced group which did not respond reliably under the free-feeding condition were omitted, leaving 11 rats in the corn oil-reinforced group and 12 in the sucrose-reinforced group. Analysis of variance of the values of T0 showed significant main effects of reinforcer type [F(1,21) = 9.1, p < 0.01, η2p = 0.32] and deprivation condition [F(1,21) = 9.6, p < 0.01, η2p = 0.30], reflecting the higher values of this parameter obtained under the food-deprived than the free-feeding condition, and the lower values seen in the sucrose-reinforced group compared to the corn oil-reinforced group; the interaction was not statistically significant [F < 1]. In the case of k, there was a significant main effect of deprivation condition [F(1,21) = 47.9, p < 0.001; η2p = 0.69], reflecting higher values of this parameter seen under the free-feeding condition than under the food-deprived condition; there was no significant main effect of group [F < 1] and no significant interaction [F(1,21) = 2.1, NS, η2p = 0.16]. In the case of a, there were significant main effects of both reinforcer type [F(1,21) = 19.5, p < 0.001, η2p = 0.69] and deprivation condition [F(1,21) = 6.5, p < 0.05, η2p = 0.24], and a significant interaction effect [F(1,21) = 9.1, p < 0.05; η2p = 0.30]. Multiple comparisons revealed that the free-feeding condition was associated with a reduction of the value of a, compared to the food-deprived condition, in the case of both reinforcer types. Under the food-deprived condition, the value of a was greater for corn oil than for sucrose; however, under the free-feeding condition, there was no significant difference between the values of a for the two reinforcers. In the case of δ, analysis of variance revealed no significant main effect of either reinforcer type [F(1,21) = 4.1, NS, η2p = 0.16] or deprivation condition [F < 1], and no significant interaction [F < 1].
Fig. 2.
Parameters of the progressive-ratio model, and the breakpoint, for performance maintained by the sucrose-solution (empty columns) and corn oil (filled columns) reinforcers under the food-deprived (DEP) and free-feeding (FREE) conditions. Columns show group mean values + SEM. Significant differences are denoted by horizontal lines: differences between reinforcer types, *p < 0.05; differences between deprivation conditions, #p < 0.05. T0 was greater for corn oil than for sucrose under both deprivation conditions, and was greater under the food-deprived condition than under the free-feeding condition for both reinforcer types. k was greater under the free-feeding condition than under the food-deprived condition for both reinforcer types, but did not differ significantly between reinforcer types. a was greater under the food-deprived condition than under the free-feeding condition for both reinforcer types, and was greater for corn oil than for sucrose only under the food-deprived condition. The value of δ did not differ significantly between reinforcer types or deprivation conditions. The breakpoint was higher under the food-deprived condition than under the free-feeding condition for both reinforcer types.
Also shown in Fig. 2 are the breakpoint data. Analysis of variance showed a significant main effect of deprivation condition [F(1,21) = 23.8, p < 0.001, η2p = 0.52], reflecting the higher breakpoints obtained under the food-deprived condition than under the free-feeding condition in the case of both the sucrose and the corn oil reinforcer. There was no significant main effect of reinforcer type [F < 1] and no significant interaction [F < 1].
3.2. Effect of THC on performance under the progressive-ratio schedule
3.2.1. Sucrose-reinforced group
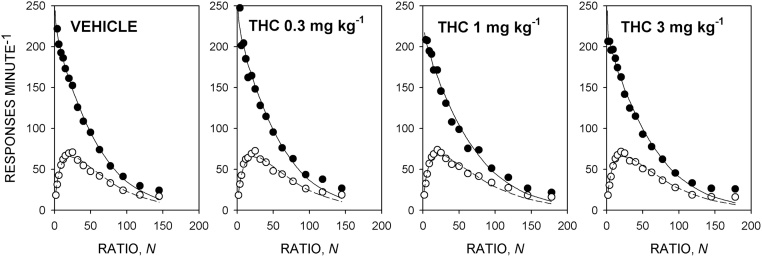
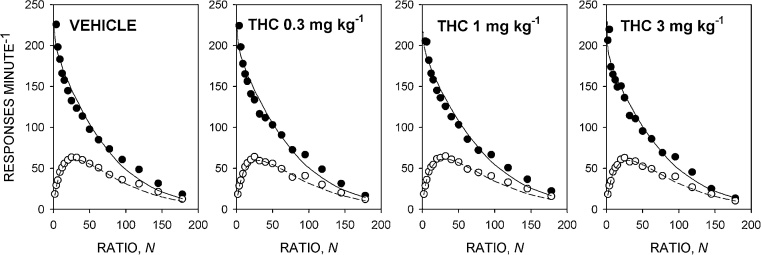
Fig. 3 shows the group mean response rate data and Table 1 shows the mean ± SEM values of the parameters of the model derived from the individual rats. There was no significant effect of THC on the value of T0 [F(3,33) = 1.9, NS, η2p = 0.15] or k [F(3,33) = 1.4, NS, η2p = 0.11]. There was a significant effect of treatment on a [F(3,33) = 2.9, p < 0.05, η2p = 0.21]; the linear contrast effect was significant [F(1,11) = 6.7, p < 0.05, η2p = 0.38]. Multiple comparisons showed that the value of a was significantly increased by THC 1 and 3 mg kg−1 compared to the vehicle-alone treatment, reflecting the somewhat shallower slopes of the descending limbs of the response rate functions obtained with these doses (Fig. 3). THC had no significant effect on the value of δ [F(3,33) = 1.7, NS, η2p = 0.14]. There was no significant effect of THC on the breakpoint [F(3,33) = 1.4, NS, η2p = 0.12].
Fig. 3.
Effect of THC 0.3, 1 and 3 mg kg−1 on performance on the progressive-ratio schedule maintained by the sucrose-solution reinforcer under the food-deprived condition. Ordinates, response rate; abscissae, response/reinforcer ratio, N. Points are group mean data: filled symbols indicate running response rate, unfilled symbols overall response rate. The curves are best-fit functions defined by Eqs. (2) and (3).
Table 1.
Sucrose reinforcer: effects of THC on the parameters of the progressive-ratio model, and the breakpoint, in rats maintained under the food-deprived condition (group mean values ± SEM).
| Parameter | Vehicle | THC 0.3 mg kg−1 | THC 1 mg kg−1 | THC 3 mg kg−1 |
|---|---|---|---|---|
| T0, s | 3.33 ± 0.29 | 3.72 ± 0.65 | 2.62 ± 0.68 | 3.19 ± 0.34 |
| k | 0.51 ± 0.03 | 0.53 ± 0.04 | 0.54 ± 0.04 | 0.48 ± 0.05 |
| a, s | 23.4 ± 5.4 | 24.4 ± 5.4 | 31.1 ± 5.6a | 30.2 ± 7.4a |
| δ, s | 0.21 ± 0.02 | 0.2 ± 0.03 | 0.25 ± 0.03 | 0.24 ± 0.02 |
| R2 | 0.94 ± 0.01 | 0.9 ± 0.03 | 0.91 ± 0.02 | 0.92 ± 0.02 |
| Breakpoint | 163.3 ± 38.8 | 170.5 ± 38.8 | 176.3 ± 43.1 | 157.1 ± 31.6 |
Significantly different from vehicle control condition, P < 0.05.
3.2.2. Corn oil-reinforced group
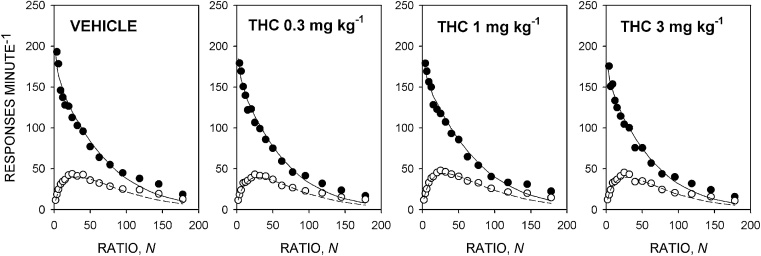
Fig. 4 shows the group mean response rate data and Table 2 shows the mean ± SEM values of the parameters of the model derived from the individual rats. THC had no significant effect on any of the parameters of the model [T0: F < 1; k: F(3,33) = 1.5, NS, η2p = 0.12; a: F < 1; δ: F < 1], or on the breakpoint [F(3,33) = 2.9, NS, η2p = 0.22].
Fig. 4.
Effect of THC 0.3, 1 and 3 mg kg−1 on performance on the progressive-ratio schedule maintained by the corn oil reinforcer under the food-deprived condition. Ordinates, response rate; abscissae, response/reinforcer ratio, N. Points are group mean data: filled symbols indicate running response rate, unfilled symbols overall response rate. The curves are best-fit functions defined by Eqs. (2) and (3).
Table 2.
Corn oil reinforcer: effects of THC on the parameters of the progressive-ratio model, and the breakpoint, in rats maintained under the food-deprived condition (group mean values ± SEM).
| Parameter | Vehicle | THC 0.3 mg kg−1 | THC 1 mg kg−1 | THC 3 mg kg−1 |
|---|---|---|---|---|
| T0, s | 5.63 ± 1.33 | 5.62 ± 1.29 | 6.14 ± 1.56 | 6.35 ± 1.56 |
| k | 0.63 ± 0.09 | 0.62 ± 0.09 | 0.58 ± 0.1 | 0.56 ± 0.1 |
| a, s | 70.2 ± 17.7 | 73 ± 19.7 | 64.3 ± 18 | 67.7 ± 18.6 |
| δ, s | 0.44 ± 0.15 | 0.47 ± 0.12 | 0.37 ± 0.12 | 0.45 ± 0.15 |
| R2 | 0.88 ± 0.03 | 0.86 ± 0.04 | 0.84 ± 0.04 | 0.81 ± 0.05 |
| Breakpoint | 173.7 ± 46.5 | 166.4 ± 46.7 | 177.3 ± 48 | 152.4 ± 38.7 |
3.2.3. Food pellet-reinforced group (re-analysis of data reported by Olarte-Sánchez et al., 2012)
Fig. 5 shows the group mean response rate data and Table 3 shows the mean ± SEM values of the parameters of the model derived from the individual rats. THC had no significant effect on any of the parameters of the model [T0: F(3,33) = 1.5, NS, η2p = 0.12; k: F(3,33) = 1.8, NS, η2p = 0.14; a: F < 1; δ: F(3,33) = 1.3, NS, η2p = 0.10], or on the breakpoint [F < 1].
Fig. 5.
Effect of THC 0.3, 1 and 3 mg kg−1 on performance on the progressive-ratio schedule maintained by food-pellet reinforcer under the food-deprivation condition (re-analysis of data reported by Olarte-Sánchez et al., 2012), Ordinates, response rate; abscissae, response/reinforcer ratio, N. Points are group mean data: filled symbols indicate running response rate, unfilled symbols overall response rate. The curves are best-fit functions defined by Eqs. (2) and (3).
Table 3.
Food-pellet reinforcer: Effects of THC on the parameters of the progressive-ratio model, and the breakpoint, in rats maintained under the food-deprived condition (group mean values ± SEM).
| Parameter | Vehicle | THC 0.3 mg kg−1 | THC 1 mg kg−1 | THC 3 mg kg−1 |
|---|---|---|---|---|
| T0, s | 4.81 ± 0.94 | 5.92 ± 1.2 | 6.14 ± 1.17 | 4.5 ± 0.56 |
| k | 0.5 ± 0.06 | 0.51 ± 0.06 | 0.44 ± 0.05 | 0.51 ± 0.07 |
| a, s | 28.1 ± 4.8 | 26.8 ± 4.9 | 26.2 ± 5.4 | 26.5 ± 5.1 |
| δ, s | 0.23 ± 0.03 | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.23 ± 0.02 |
| R2 | 0.96 ± 0.01 | 0.93 ± 0.02 | 0.94 ± 0.01 | 0.93 ± 0.02 |
| Breakpoint | 127.4 ± 18.2 | 126.1 ± 19.6 | 134 ± 19.9 | 127.4 ± 17.5 |
4. Discussion
In agreement with previous findings (Bezzina et al., 2015; Olarte-Sánchez et al., 2013; Valencia-Torres et al., 2014 also earlier data re-analysed by Bradshaw and Killeen, 2012), the present results indicate that operant behaviour maintained by progressive-ratio schedules is well described by the mathematical model of performance on this schedule (see Section 1). The results also provide new information about the sensitivity of the four parameters of the model to schedule manipulations and acute treatment with THC, a putative orexigenic drug.
4.1. Effect of deprivation level
The values of a were substantially reduced when the rats were tested under the free-feeding condition, compared to the values obtained under the food-deprived condition, indicating a reduction of the incentive values of both reinforcers when home cage feeding was not restricted.
It has, of course, been known for many years that food deprivation enhances the efficacy of food reinforcers (Clark, 1958; Hillman et al., 1953; Horenstein, 1951; Skinner, 1936). However, although most current theories of schedule-controlled behaviour (e.g. Herrnstein, 1970; Killeen, 1994) assume that deprivation enhances reinforcer value, the exact form of this relationship remains unknown. Herrnstein’s (1970) response-strength equation defines a hyperbolic relation between response rate, R, and reinforcement rate, r, thus:
| (4) |
where Rmax and KH are free parameters.1 Herrnstein (1970, 1974) interpreted KH (r0 in his notation) as the rate of extraneous (unobserved) reinforcement, expressed in units of the reference (food) reinforcer. According to this interpretation, the finding that an increase in the severity of food deprivation causes a reduction of the value of KH for food-reinforced responding (Bradshaw et al., 1983; Heyman and Monaghan, 1987) implies that deprivation enhances the efficacy or value of food reinforcers. Killeen’s (1994) MPR theory also assumes that deprivation level is a determinant of reinforcer value, where value (a) is defined as the duration of behavioural activation induced by a single reinforcer. The present finding of a reduction of a following a reduction of the severity of food deprivation is clearly in accord with this assumption. However, it is important to emphasise that neither Herrnstein’s (1970) nor Killeen’s (1994) theory makes specific predictions about the form of the relation between the level of deprivation and reinforcer value. Further analysis based on systematic manipulation of deprivation conditions is needed to address this issue.
The value of δ did not differ between the food-deprived and free-feeding conditions, suggesting that the deprivation condition did not affect the motor aspects of performance. However the two parameters expressing the linear waiting principle, T0 and k, did differ between the two conditions, the value of T0 being smaller and that of k larger under the food-deprived condition than under the free-feeding condition. There do not appear to have been any systematic investigations of the sensitivity of the linear waiting function to the level of food deprivation; this may be an issue worth pursuing in future experiments, using procedures such as the response-initiated delay schedule which reveal linear waiting more directly than the progressive-ratio schedule (Wynne and Staddon, 1988 see Staddon, 2014, for review).
The finding that the food-deprived condition was associated with higher breakpoints than the free-feeding condition is consistent with many earlier observations of performance on progressive-ratio schedules (Ferguson and Paule, 1997; Hodos, 1961; Hodos and Kalman, 1963; Jenks and Higgs, 2010; Rusted et al., 1998; Skjoldager et al., 1993). This effect has generally been interpreted in terms of a motivation-enhancing effect of deprivation. Whilst the present finding that the value of a was higher under the more severe deprivation condition is consistent with this interpretation, it should be noted that, unlike a, the breakpoint is sensitive to ‘non-motivational’ manipulations such as the response requirement and the ratio step size (Arnold and Roberts, 1997; Skoljdager et al., 1993; Covarrubias and Aparacio, 2008), and therefore does not constitute a specific index of motivation or incentive value (see Section 1).
4.2. Comparison of sucrose and corn oil reinforcers
As reported previously (Olarte-Sánchez et al., 2013), the value of a was higher for 25 μl of corn oil than for 50 μl of 0.6 M sucrose in the food-deprived condition. Olarte-Sánchez et al. (2013) noted that although corn oil was evidently more efficacious than 0.6 M sucrose on a volume-for-volume basis, sucrose was the more efficacious reinforcer on a calorie-for-calorie basis. Interestingly, under the free-feeding condition, the values of a derived for the two reinforcers did not differ significantly from one another. This suggests that the relationship between deprivation level and reinforcer value may differ between different types of reinforcer.
The values of δ did not differ significantly between the sucrose and corn oil reinforcers. However, as previously reported by Olarte-Sánchez et al. (2013), the parameters expressing the minimum post-reinforcement pause (T0) did differ significantly between the two reinforcers, possibly reflecting the occurrence of more protracted post-prandial orofacial grooming following ingestion of the more viscous reinforcer (see also Bradshaw and Killeen, 2012).
The inclusion of separate parameters to represent response time and post-reinforcement pausing is a feature of the new progressive-ratio model not shared by earlier models derived from MPR, for example the model of performance on fixed-ratio schedules:
| (5) |
where ζ is a parameter representing the coupling of responses to reinforcers and a and δ have the same meanings as in Eq. (2) (Killeen, 1994). This equation, which has been applied extensively to performance on progressive-ratio schedules (Bezzina et al., 2008a,b; Covarrubias and Aparicio, 2008; den Boon et al., 2012; Ho et al., 2003; Kheramin et al., 2005; Killeen et al., 2009; Olarte-Sánchez et al., 2012; Rickard et al., 2009; Zhang et al., 2005a,b), defines the maximum response rate as 1/δ and makes no allowance for the inclusion of the post-reinforcement pause in the overall response rate. Incorporation of the linear waiting parameters in the new model provides a basis for estimating δ without contaminating it with post-reinforcement pausing.
The present results may have some bearing on an ongoing controversy about the sensitivity of the asymptotic response rate in Eq. (4) (Rmax) to reinforcer manipulations. According to Herrnstein (1970, 1974), this parameter (k according to his nomenclature) represents the totality of behaviour, expressed in units of the reference response. At very high rates of reinforcement, the reference response swamps all other behaviours, causing the rate of measured operant responding to approach its maximum value, Rmax. Reinforcer-related variables such as the magnitude or type of reinforcer are assumed to affect the rate of operant responding entirely via changes in the value of KH, and are not expected to influence Rmax (Herrnstein, 1974). Evidence related to this prediction has been inconsistent, some workers reporting uniform values of Rmax across different sizes and types of reinforcer (Bradshaw et al., 1981; Heyman and Monaghan, 1987, 1994; Petry and Heyman, 1994), and others reporting systematic effects of these variables on the value of Rmax (Belke, 1998; Bradshaw et al., 1978; Dallery et al., 2000; Harper and McLean, 1992; McDowell and Dallery, 1999; Shah et al., 1991). Recent work on the fine structure of responding on variable-interval schedules suggests a way of resolving this difficulty. It has become increasingly evident that overall response rate on these schedules reflects several factors, including the minimum time needed to execute a response, pausing between responses, pausing between bouts of responses, and post-reinforcement pausing (Brackney et al., 2011; Cheung et al., 2012; Killeen et al., 2002; Shull, 2004, 2005; Shull et al., 2004; Smith et al., 2014). All these factors are potentially confounded in the overall response rate (Cheung et al., 2012), and hence in any unitary index of response capacity derived solely from ROVERALL, such as Rmax in Eq. (4), and δ as defined by Eq. (5). Although caution is needed in generalizing findings across different schedules, the successful decomposition of the determinants of maximum response rate in the new progressive-ratio model into response time (δ) and post-reinforcement pause time (T0, k) suggests that a similar decomposition of Rmax may be in order. Furthermore, the present finding that T0 but not δ was affected by the quality of the reinforcer raises the possibility that the effects of reinforcer quality on recovered values of Rmax in some previous experiments with variable-interval schedules (Belke, 1998; Bradshaw et al., 1978; Dallery et al., 2000; Shah et al., 1991) may reflect differences in post-reinforcement pausing rather than differences in response time. If this is the case, then fitting Eq. (4) to RRUN rather than ROVERALL should reduce the effect of reinforcer quality on Rmax.
It should be noted that the progressive-ratio model does not take into account the possible contributions of the length of response bouts and the rate of bout initiation to RRUN. Any such contribution would presumably be absorbed by the recovered value of δ, which would therefore need to be further decomposed if the role of response bouts is to be isolated from that of response time (see Brackney et al., 2011). Further work is needed to establish whether the bout-and-pause pattern that characterises variable-interval performance is also a feature of progressive-ratio responding. The present findings offer indirect evidence that this may not be the case, since deprivation level and reinforcer quality did not affect δ, whereas such reinforcer-related variables are known to affect bout initiation rate in variable-interval schedules (Brackney et al., 2011; Shull, 2004, 2011; Shull et al., 2004).
There was no significant difference between the breakpoints seen with sucrose and corn oil. This contrasts with the substantial difference between the values of a associated with the two reinforcers. However, in a previous study employing identical reinforcers to those used in the present experiment, Olarte-Sánchez et al. (2013) observed higher breakpoints with the corn oil than with the sucrose reinforcer. The reason for this discrepancy is not clear, although, as noted by Rickard et al. (2009), the influence of motivational manipulations on the breakpoint may be less reliable than their effects on a when, as in the present experiment, overall response rates are also affected.
4.3. Effects of THC
It is well known that THC and other CB1 receptor agonists, including the endocannabinoids anandamide and 2-arachidonoyl glycerol (2-AG), can induce hyperphagia in rats and mice (Brown et al., 1977; Gluck and Ferraro, 1974; Hao et al., 2000; Higgs et al., 2003, 2005; Kirkham and Williams, 2001; Koch, 2001; Williams and Kirkham, 1999). This effect is especially pronounced in the case of sweet and fatty foods (DiPatrizio and Simansky, 2008; Foltin et al., 1988; Higgs et al., 2003, 2005; Jones and Kirkham, 2012; Koch, 2001; Shinohara et al., 2009; Sofia and Knobloch, 1976; Ward and Dykstra, 2005), leading to the suggestion that CB1 receptors may play an important role in determining the incentive values of sapid foodstuffs (Arnone et al., 1997; Higgs et al., 2003, 2005; Simiand et al., 1998; Wakley and Rassmussen, 2009; Williams and Kirkham, 2002a). The ability of CB1 receptor agonists to increase and antagonists to reduce the breakpoint in progressive-ratio schedules has been cited in support of this suggestion (Hernandez and Cheer, 2012; Higgs et al., 2005; Jones and Kirkham, 2012; Maccioni et al., 2008; Rasmussen and Huskinson, 2008; Solinas and Goldberg, 2005; Wakley and Rasmussen, 2009; Ward and Dykstra, 2005).
The progressive-ratio model is well suited to examine the effects of drugs on the incentive value of reinforcers because it allows separate quantification of motivational and motor processes, which are often confounded in univariate indices such as the breakpoint (Bezzina et al., 2015). The present results are consistent with the proposal that CB1 receptors may be involved in determining the incentive values of palatable foods, since acute treatment with THC resulted in a selective increase in the value of a for sucrose, none of the other parameters of the progressive-ratio model being significantly affected. As discussed above, a selective increase in the value of a is uniquely indicative of an increase in the incentive value of the reinforcer rather than an impairment of motor performance (Bradshaw and Killeen, 2012). In the present experiment, the breakpoint was not significantly affected by THC, suggesting that this index may be less sensitive to THC than the parameter a.
A somewhat unexpected finding of this experiment was that THC’s effect on a occurred only in the case of performance maintained by the sucrose reinforcer, no effect being apparent in the case of performance maintained by either corn oil (present results) or food pellets (re-analysis of results obtained by Olarte-Sánchez et al., 2012). The food pellets used by Olarte-Sánchez et al. (2012) (TestDiet 5TUM 45 mg pellets) have a low sugar content, the total mono- and disaccharide content amounting to approximately 2.3 mg per pellet (calculated from datasheet: TestDiet, 2011). Taking the relative sweetness of the various sugar constituents into account (Schallenberger, 1993), the sucrose equivalent of a single 45 mg 5TUM pellet is approximately 1.7 mg, compared to 10.27 mg in the case of the sucrose reinforcer used in this experiment (0.6 M, 50 μl). Taken together, therefore, these results suggest that while CB1 receptor stimulation may enhance the reinforcing value of sweet foods, it may have relatively little effect on the value of other foodstuffs.
It is well established that CB1 receptor agonists can enhance the unrestricted intake of both fatty and sweet foodstuffs (DiPatrizio and Simansky, 2008; Koch, 2001); however, less is known about the effects of these drugs on operant behaviour maintained by sweet and fatty reinforcers. Indeed, most previous studies of the effect of these drugs on performance on progressive-ratio schedules used either sucrose or sweetened food pellets as the reinforcer (Hernandez and Cheer, 2012; Higgs et al., 2005; Jones and Kirkham, 2012; Solinas and Goldberg, 2005; Wakley and Rasmussen, 2009). However, the present results are consistent with a report by Ward and Dykstra (2005) that CB1 receptor agonists and antagonists had more pronounced effects on responding maintained by a sweet reinforcer than on responding maintained by corn oil.
The present findings and those of Ward and Dykstra (2005) raise the possibility that the ‘incentive-enhancing’ effect of CB1 receptor agonists may not be entirely attributable to an involvement of these receptors in a general ‘reward system’ (DeLuca et al., 2012; Panagis et al., 2014). There is evidence that CB1 receptors are linked to glutamatergic neurotransmission in the mesocortical/ventral striatal circuit that is believed to regulate the efficacy of divers reinforcers including food, opiates and psychostimulants (Bellocchio et al., 2010). However, CB1 receptors are also present in the taste buds, and stimulation of these receptors selectively enhances the sensation of sweetness (Yoshida et al., 2013; Yoshida and Ninomiya, 2010). The possibility that stimulation of this peripheral receptor population may underlie the selective effect of THC on the incentive value of sucrose may merit further investigation.
5. Conclusions
In agreement with previous findings (see above), the results of this experiment indicate that the progressive-ratio model provides a good description of performance on this schedule. The model’s four parameters proffer a means of classifying and quantifying the effects of behavioural interventions and drugs on performance. The sensitivity of the ‘specific activation’ parameter, a, to the level of food deprivation and the quality and quantity of reinforcers lends support to the proposal that this parameter is a valid metric of incentive value (Bradshaw and Killeen, 2012; Reilly, 2003). Moreover, the lack of effect of these ‘motivational’ interventions on the ‘response time’ parameter, δ, encourages confidence in the utility of this parameter as a measure of ‘motor capacity’ (Killeen, 1994; Bradshaw and Killeen, 2012). An important feature of the model is the decomposition of maximum response rate, allowing post-reinforcement pausing to be treated separately from purely motor constraints on responding. This has enabled the intuitively reasonable attribution of the relatively low maximum overall response rate seen with the more viscous reinforcer (corn oil) to post-prandial behaviours such as orofacial grooming, rather than to motor incapacity (Olarte-Sánchez et al., 2013).
The progressive-ratio model was derived to account for performance on one particular schedule. It is therefore not a competitor of equations with more general applicability, such as Herrnstein’s (1970) response-strength equation. Nevertheless, the benefits derived from decomposing the maximum response rate in the progressive-ratio model suggests that a similar manoeuvre may be in order in the case of Herrnstein’s (1970) equation (Bradshaw, 1994). It is suggested that this may help to resolve the ongoing controversy about the sensitivity (or otherwise) of Rmax to motivational manipulations (Heyman and Monaghan, 1987; McDowell, 2013).
Finally, the effect of THC seen in this experiment suggests that this drug may preferentially enhance the incentive value of sweet tasting reinforcers (Ward and Dykstra, 2005). The possibility that this may reflect an effect of THC on peripheral taste receptors needs further investigation.
Acknowledgements
This work was supported by the Schools of Community Health Sciences and Psychology, University of Nottingham. C.M. Olarte-Sánchez was supported by a U.K. Medical Research Council (MRC/DTA) scholarship. L. Valencia-Torres was supported by a scholarship from the National Science Council of Mexico (CONACYT). We are grateful to Mrs V.K. Bak and Mr R.W. Langley for skilled technical assistance.
Footnotes
This nomenclature differs from that used by Herrnstein (1970). Rmax and KH are algebraically identical to Herrnstein’s (1970)k and ro; however, unlike Herrnstein’s parameters, Rmax and KH are theoretically neutral. Rmax expresses the maximum reponse rate and KH the reinforcement rate corresponding to Rmax/2 (Bradshaw et al., 1976; Shah et al., 1991; for discussion, see McDowell, 2013). In most experimental applications of Eq. (4), R has been taken to refer to ROVERALL (see text for further discussion). It should be noted that the term k in Eq. (1) is not related to the parameter of the same name in Herrnstein’s (1970) model.
Contributor Information
C.M. Olarte-Sánchez, Email: colarte-sanchez@abdn.ac.uk.
L. Valencia-Torres, Email: lourdes.valencia-torres@abdn.ac.uk.
H.J. Cassaday, Email: helen.cassaday@nottingham.ac.uk.
C.M. Bradshaw, Email: C.M.Bradshaw@hotmail.ac.uk.
E. Szabadi, Email: elemer.szabadi@nottingham.ac.uk.
References
- Abel E.L. Cannabis: effects on hunger and thirst. Behav. Biol. 1975;15:255–281. doi: 10.1016/s0091-6773(75)91684-3. [DOI] [PubMed] [Google Scholar]
- Aberman J.E., Ward S.J., Salamone J.D. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol. Biochem. Behav. 1998;61:341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Arnold J.M., Roberts D.C.S. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol. Biochem. Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Arnone M., Maruani J., Chaperon F., Thiebot M.H., Poncelet M., Soubrié P., LeFur G. Selective inhibition of sucrose and ethanol intake by SR. 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Baunez C., Amalric M., Robbins T.W. Enhanced food-related motivation after bilateral lesions of the subthalamic nucleus. J. Neurosci. 2002;22:562–568. doi: 10.1523/JNEUROSCI.22-02-00562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke T.W. Qualitatively different reinforcers and parameters of Herrnstein’s (1970) response-strength equation. Anim. Learn. Behav. 1998;26:235–242. [Google Scholar]
- Bellocchio L., Lafenêtre P., Cannich A., Cota D., Puente N., Grandes P., Chaouloff F., Piazza P.V., Marsicano G. Bimodal control of stimulated food intake by the endocannabinoid system. Nature Neurosci. 2010;13:281–283. doi: 10.1038/nn.2494. [DOI] [PubMed] [Google Scholar]
- Bezzina G., Body S., Cheung T.H.C., Hampson C.L., Bradshaw C.M., Glennon J.C., Szabadi E. Evidence for a role of 5-HT2C receptors in the motor aspects of performance, but not the efficacy of food reinforcers, in a progressive ratio schedule. Psychopharmacology. 2015;232:699–711. doi: 10.1007/s00213-014-3700-5. [DOI] [PubMed] [Google Scholar]
- Bezzina G., Body S., Cheung T.H.C., Hampson C.L., Deakin J.F.W., Anderson I.M., Szabadi E., Bradshaw C.M. Effect of quinolinic acid-induced lesions of the nucleus accumbens core on performance on a progressive ratio schedule of reinforcement: implications for inter-temporal choice. Psychopharmacology. 2008;197:339–350. doi: 10.1007/s00213-007-1036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzina G., den Boon F.S., Hampson C.L., Cheung T.H.C., Body S., Bradshaw C.M., Szabadi E., Anderson I.M., Deakin J.F.W. Effect of quinolinic acid-induced lesions of the subthalamic nucleus on performance on a progressive-ratio schedule of reinforcement: a quantitative analysis. Behav. Brain Res. 2008;195:223–230. doi: 10.1016/j.bbr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizo L.A., Kettle L.C., Killeen P.R. Rats don’t always respond faster for more food. Anim. Learn. Behav. 2001;29:66–78. [Google Scholar]
- Bizo L.A., Killeen P.R. Models of ratio schedule performance. J. Exp. Psychol. Anim. Behav. Process. 1997;23:351–367. doi: 10.1037//0097-7403.23.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackney R.J., Cheung T.H.C., Neisenwander J.L., Sanabria F. The isolation of motivational, motoric, and schedule effects on operant performance: a modeling approach. J. Exp. Anal. Behav. 2011;96:17–36. doi: 10.1901/jeab.2011.96-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw C.M. Mathematical principles of reinforcement. Behav. Brain Sci. 1994;17:136–137. Commentary on Killeen P.R. [Google Scholar]
- Bradshaw C.M., Killeen P.R. A theory of behaviour on progressive ratio schedules, with applications in behavioural pharmacology. Psychopharmacology. 2012;222:549–564. doi: 10.1007/s00213-012-2771-4. [DOI] [PubMed] [Google Scholar]
- Bradshaw C.M., Ruddle H.V., Szabadi E. Relationship between response rate and reinforcement frequency in variable-interval schedules: II. Effect of the volume of sucrose reinforcement. J. Exp. Anal. Behav. 1981;35:263–269. doi: 10.1901/jeab.1981.35-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw C.M., Szabadi E., Bevan P. Behavior of humans in variable-interval schedules of reinforcement. J. Exp. Anal. Behav. 1976;26:135–141. doi: 10.1901/jeab.1976.26-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw C.M., Szabadi E., Bevan P. Relationship between response rate and reinforcement frequency in variable-interval schedules. The effect of the concentration of sucrose reinforcement. J. Exp. Anal. Behav. 1978;29:447–452. doi: 10.1901/jeab.1978.29-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw C.M., Szabadi E., Ruddle H.V., Pears E. Herrnstein’s equation: effect of deprivation level on performance in variable-interval schedule. Behav. Anal. Lett. 1983;3:267–273. [Google Scholar]
- Brown J.E., Kassouny M., Cross J.K. Kinetic studies of food intake and sucrose solution preference by rats treated with low doses of delta-9-tetrahydro-cannabinol. Behav. Biol. 1977;20:104–110. doi: 10.1016/s0091-6773(77)90606-x. [DOI] [PubMed] [Google Scholar]
- Cheung T.H.C., Neisenwander J.L., Sanabria F. Extinction under a behavioral microscope: Isolating the sources of decline in operant response rate. Behav. Process. 2012;90:111–123. doi: 10.1016/j.beproc.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark F.C. The effect of deprivation and frequency of reinforcement on variable interval responding. J. Exp. Anal. Behav. 1958;1:221–228. doi: 10.1901/jeab.1958.1-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias P., Aparicio C.F. Effects of reinforcer quality and step size on rats’ performance under progressive ratio schedules. Behav. Process. 2008;78:246–252. doi: 10.1016/j.beproc.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Czachowski C.L., Samson H.H. Breakpoint determination and ethanol self-administration using an across-session progressive ratio procedure in the rat. Alcoholism: Clin. Exp. Res. 1999;23:1580–1586. [PubMed] [Google Scholar]
- Dallery J., McDowell J.J., Lancaster J.S. Falsification of matching theory’s account of single-alternative responding: Herrnstein’s k varies with sucrose concentration. J. Exp. Anal. Behav. 2000;73:23–43. doi: 10.1901/jeab.2000.73-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M.A., Solinas M., Bimpisidis Z., Goldberg S.R., Di Chiara G. Cannabinoid facilitation of behavioral and biochemical hedonic taste responses. Neuropharmacology. 2012;63:161–168. doi: 10.1016/j.neuropharm.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Boon F.S., Body S., Hampson C.L., Bradshaw C.M., Szabadi E., de Bruin N. Effects of amisulpride and aripiprazole on progressive-ratio schedule performance: comparison with clozapine and haloperidol. J. Psychopharmacol. 2012;26:1231–1243. doi: 10.1177/0269881111421974. [DOI] [PubMed] [Google Scholar]
- DiPatrizio N.V., Simansky K.J. Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J. Neurosci. 2008;28:9702–9709. doi: 10.1523/JNEUROSCI.1171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin R.W., Fischman M.W., Byrne M.F. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite. 1988;11:1–14. doi: 10.1016/s0195-6663(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Ferguson S.A., Paule M.G. Progressive ratio performance varies with body weight in rats. Behav. Process. 1997;40:177–182. doi: 10.1016/s0376-6357(97)00786-9. [DOI] [PubMed] [Google Scholar]
- Gaoni Y., Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964;86:1646–1647. [Google Scholar]
- Gluck J.P., Ferraro D.P. Effects of delta9-THC on food and water intake of deprivation experienced rats. Behav. Biol. 1974;11:395–401. doi: 10.1016/s0091-6773(74)90684-1. [DOI] [PubMed] [Google Scholar]
- Gosnell B.A., Mitra A., Avant R.A., Anker J.J., Carroll M.E., Levine A.S. Operant responding for sucrose by rats bred for high or low saccharin consumption. Physiol. Behav. 2010;99:529–533. doi: 10.1016/j.physbeh.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R.R., Brady J.V., Snell J.D. Progressive-ratio performance maintained by drug infusions: comparison of cocaine, diethylpropion, chlorphentermine, and fenfluramine. Psychopharmacology. 1978;56:5–13. doi: 10.1007/BF00571401. [DOI] [PubMed] [Google Scholar]
- Hao S.Z., Avraham Y., Mechoulam R., Berry E.M. Low dose anandamide affects food intake cognitive function, neurotransmitter and corticosterone levels in diet-restricted mice. Eur. J. Pharmacol. 2000;392:147–156. doi: 10.1016/s0014-2999(00)00059-5. [DOI] [PubMed] [Google Scholar]
- Harper D.N., McLean A.P. Resistance to change and the law of effect. J. Exp. Anal. Behav. 1992;57:317–337. doi: 10.1901/jeab.1992.57-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez G., Cheer J.F. Effect of CB1 receptor blockade on food-reinforced responding and associated nucleus accumbens neuronal activity in rats. J. Neurosci. 2012;32:11467–11477. doi: 10.1523/JNEUROSCI.1833-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein R.J. On the law of effect. J. Exp. Anal. Behav. 1970;13:243–266. doi: 10.1901/jeab.1970.13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein R.J. Formal properties of the matching law. J. Exp. Anal. Behav. 1974;21:159–164. doi: 10.1901/jeab.1974.21-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman G.M., Monaghan M.M. Effects of changes in response requirement and deprivation on the parameters of the matching law equation. New data and review. J. Exp. Psychol. Anim. Behav. Process. 1987;13:384–394. [Google Scholar]
- Heyman G.M., Monaghan M.M. Reinforcer magnitude (sucrose concentration) and the Matching Law theory of response strength. J. Exp. Anal. Behav. 1994;61:501–506. doi: 10.1901/jeab.1994.61-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs S., Williams C.M., Kirkham T.C. Cannabinoid influences on palatability: microstructural analysisof sucrose drinking after ∆9-tetrahydrocannabinol, anandamide, 2-arachidonoyl glycerol and SR. 141716. Psychopharmacology. 2003;165:370–377. doi: 10.1007/s00213-002-1263-3. [DOI] [PubMed] [Google Scholar]
- Higgs S., Barber D.J., Cooper A.J., Terry P. Differential effects of two cannabinoid receptor agonists on progressive ratio responding for food and free-feeding in rats. Behav. Pharmacol. 2005;16:389–393. doi: 10.1097/00008877-200509000-00011. [DOI] [PubMed] [Google Scholar]
- Hillman B., Hunter W.S., Kimble G.A. The effect of drive level on the maze performance of the white rat. J. Comp. Physiol. Psychol. 1953;46:87–89. doi: 10.1037/h0058030. [DOI] [PubMed] [Google Scholar]
- Ho M.-Y., Body S., Kheramin S., Bradshaw C.M., Szabadi E. Effects of 8-OH-DPAT and WAY-100635 on performance on a time-constrained progressive-ratio schedule. Psychopharmacology. 2003;167:137–144. doi: 10.1007/s00213-002-1375-9. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hodos W., Kalman G. Effects of increment size and reinforcer volume on progressive ratio performance. J. Exp. Anal. Behav. 1963;6:387–392. doi: 10.1901/jeab.1963.6-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein B.R. Performance of conditioned responses as a function of strength of hunger drive. J. Comp. Physiol. Psychol. 1951;44:210–224. doi: 10.1037/h0059362. [DOI] [PubMed] [Google Scholar]
- Howlett A.C. The cannabinoid receptors. Prostagland. Other Lipid Mediat. 2002;68–69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Jenks R.A., Higgs S. Effects of dieting status and cigarette deprivation on progressive ratio responding for cigarette puffs by young women smokers. J. Psychopharmacol. 2010;25:530–537. doi: 10.1177/0269881110389346. [DOI] [PubMed] [Google Scholar]
- Jones E.K., Kirkham T.C. Noladin ether a putative endocannabinoid, enhances motivation to eat after acute systemic administration in rats. Br. J. Pharmacol. 2012;166:1815–1821. doi: 10.1111/j.1476-5381.2012.01888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheramin S., Body S., Miranda Herrera F., Bradshaw C.M., Szabadi E., Deakin J.F.W., Anderson I.M. The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: implications for models of inter-temporal choice. Behav. Brain Res. 2005;156:145–152. doi: 10.1016/j.bbr.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Killeen P.R. Mathematical principles of reinforcement. Behav. Brain Sci. 1994;17:105–172. [Google Scholar]
- Killeen P.R., Hall S.S., Reilly M.P., Kettle L.C. Molecular analyses of the principal components of response strength. J. Exp. Anal. Behav. 2002;78:127–160. doi: 10.1901/jeab.2002.78-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen P.R., Posadas-Sanchez D., Johansen E.B., Thrailkil E.A. Progressive ratio schedules of reinforcement. J. Exp. Psychol. Anim. Behav. Process. 2009;35:35–50. doi: 10.1037/a0012497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham T.C., Williams C.M. Endogenous cannabinoids and appetite. Nutr. Res. Rev. 2001;14:65–86. doi: 10.1079/NRR200118. [DOI] [PubMed] [Google Scholar]
- Koch J.E. ∆9-THC stimulates food intake in Lewis rats: effects on chow: high-fat and sweet high-fat diets. Nutr. Neurosci. 2001;4:179–187. doi: 10.1016/s0091-3057(01)00467-1. [DOI] [PubMed] [Google Scholar]
- Ledent C., Valverde O., Cossu G., Petitet F., Aubert J.F., Beslot F., Bohme G.A., Imperato A., Pedrazzini T., Roques B.P., Vassart G., Fratta W., Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Maccioni P., Pes D., Carai M.A., Gessa G.L., Colombo G. Suppression by the cannabinoid CB1 receptor antagonist rimonabant, of the reinforcing and motivational properties of a chocolate-flavoured beverage in rats. Behav. Pharmacol. 2008;19:197–209. doi: 10.1097/FBP.0b013e3282fe8888. [DOI] [PubMed] [Google Scholar]
- Matsuda L.A., Lolait S.J., Brownstein M.J., Young A.C., Bonner T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McDowell J.J. On the theoretical and empirical status of the matching law and matching theory. Psychol. Bull. 2013;139:1000–1028. doi: 10.1037/a0029924. [DOI] [PubMed] [Google Scholar]
- McDowell J.J., Dallery J. Falsification of matching theory: Changes in the asymptote of Herrnstein’s hyperbola as a function of water deprivation. J. Exp. Anal. Behav. 1999;72:251–268. doi: 10.1901/jeab.1999.72-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naleid A.M., Grimm J.W., Kessler D.A., Sipols A.J., Aliakbari S., Bennett J.L., Wells J., Figlewicz D.P. Deconstructing the vanilla milkshake: the dominant effect of sucrose on self-administration of nutrient-flavor mixtures. Appetite. 2008;50:128–138. doi: 10.1016/j.appet.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefs G., Pouwer F., Denollet J., Kramer H., Wijnands-van Gent C.J., Pop V.J. Suboptimal glycemic control in type 2 diabetes: a key role for anhedonia? J. Psychiatr. Res. 2012;46:549–554. doi: 10.1016/j.jpsychires.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Olarte-Sánchez C.M., Valencia-Torres L., Body S., Cassaday H.J., Bradshaw C.M., Szabadi E., Goudie A.J. A clozapine-like effect of cyproheptadine on progressive-ratio schedule performance. J. Psychopharmacol. 2012;26:857–870. doi: 10.1177/0269881111408961. [DOI] [PubMed] [Google Scholar]
- Olarte-Sánchez C.M., Valencia-Torres L., Cassaday H.J., Bradshaw C.M., Szabadi E. Effects of SKF-83566 and haloperidol on performance on progressive ratio schedules maintained by sucrose and corn oil reinforcement: quantitative analysis using a new model derived from the Mathematical Principles of Reinforcement (MPR) Psychopharmacology. 2013;230:617–630. doi: 10.1007/s00213-013-3189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagis G., Mackey B., Vlachow S. Cannabinoid regulation of brain reward processing with an emphasis on the role of CB1 receptors: a step back into the future. Front. Psychiatry. 2014;5:article 7. doi: 10.3389/fpsyt.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry N.M., Heyman G.M. Effects of qualitatively different reinforcers on the parameters of the response strength equation. J. Exp. Anal. Behav. 1994;61:97–106. doi: 10.1901/jeab.1994.61-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping-Teng C., Lee E.S., Konz S.A., Richardson N.R., Roberts D.C.S. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Randall P.A., Pardo M., Nunes E.J., López Cruz L., Vemuri V.K., Makriyannis A., Baqi Y., Müller, Correa C.E., Salamone M. Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PLoS One. 2012;7(10):e47934. doi: 10.1371/journal.pone.0047934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen E.B., Huskinson S.L. Effects of rimonabant on behavior maintained by progressive ratio schedules of sucrose reinforcement in obese Zucker (fa/fa) rats. Behav. Pharmacol. 2008;19:735–742. doi: 10.1097/FBP.0b013e3283123cc2. [DOI] [PubMed] [Google Scholar]
- Reilly M.P. Extending mathematical principles of reinforcement into the domain of behavioral pharmacology. Behav. Process. 2003;62:75–88. doi: 10.1016/s0376-6357(03)00027-5. [DOI] [PubMed] [Google Scholar]
- Rickard J.F., Body S., Zhang Z., Bradshaw C.M., Szabadi E. Effect of reinforcer magnitude on performance maintained by progressive-ratio schedules. J. Exp. Anal. Behav. 2009;91:75–87. doi: 10.1901/jeab.2009.91-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D.C.S., Richardson N.R. Self-administration of psychostimulants using progressive ratio schedules of reinforcement. In: Boulton A., Baker G., Wu P.H., editors. vol. 24. Humana; New York: 1992. pp. 233–269. (Neuromethods, Animal Models of Drug Addiction). [Google Scholar]
- Rusted J.M., Mackee A., Williams R., Willner P. Deprivation state but not nicotine content of the cigarette affects responding by smokers on a progressive ratio task. Psychopharmacology. 1998;140:411–417. doi: 10.1007/s002130050783. [DOI] [PubMed] [Google Scholar]
- Sanabria F., Acosta J.I., Killeen P.R., Neisewander J.L., Bizo L.A. Modeling the effects of fluoxetine on food-reinforced behavior. Behav. Pharmacol. 2008;19:61–70. doi: 10.1097/FBP.0b013e3282f3df9b. [DOI] [PubMed] [Google Scholar]
- Schallenberger R.S. Chapman and Hall; Glasgow: 1993. Taste Chemistry. [Google Scholar]
- Shah K., Bradshaw C.M., Szabadi E. Relative and absolute reinforcement frequency as determinants of choice in concurrent variable-interval schedules. Q. J. Exp. Psychol. 1991;43B:25–38. [PubMed] [Google Scholar]
- Shinohara Y., Inui T., Yamamoto T., Shimura T. Cannabinoid in the nucleus accumbens enhances the intake of palatable solution. Neuroreport. 2009;20:1382–1385. doi: 10.1097/WNR.0b013e3283318010. [DOI] [PubMed] [Google Scholar]
- Shull R.L. Bouts of responding on variable-interval schedules: effects of deprivation level. J. Exp. Anal. Behav. 2004;81:155–167. doi: 10.1901/jeab.2004.81-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull R.L. The sensitivity of response rate to the rate of variable-interval reinforcement for pigeons and rats: a review. J. Exp. Anal. Behav. 2005;84:99–110. doi: 10.1901/jeab.2005.03-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull R.L. Bouts changeovers, and units of operant behavior. Eur. J. Behav. Anal. 2011;12:49–72. [Google Scholar]
- Shull R.L., Grimes J.A., Bennett J.A. Bouts of responding: the relation between bout rate and the rate of variable-interval reinforcement. J. Exp. Anal. Behav. 2004;81:65–83. doi: 10.1901/jeab.2004.81-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simiand J., Keane M., Keane P.E., Soubrié P. SR. 141716 a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav. Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- Skinner B.F. Conditioning and extinction and their relation to drive. J. Gen. Psychol. 1936;6:22–37. [Google Scholar]
- Skjoldager P., Pierre P.J., Mittleman G. Reinforcer magnitude and progressive ratio responding in the rat effects of increased effort, prefeeding, and extinction. Learn. Motiv. 1993;24:303–343. [Google Scholar]
- Smith T.T., McLean A.P., Shull R.L., Hughes C.E., Pitts R.C. Concurrent performance as bouts of behavior. J. Exp. Anal. Behav. 2014;102:102–125. doi: 10.1002/jeab.90. [DOI] [PubMed] [Google Scholar]
- Sofia R.D., Knobloch L.C. Comparative effects of various naturally occurring cannabinoids on food: sucrose and water consumption by rats. Pharmacol. Biochem. Behav. 1976;4:591–599. doi: 10.1016/0091-3057(76)90202-1. [DOI] [PubMed] [Google Scholar]
- Solinas M., Goldberg S.R. Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology. 2005;30:2035–2045. doi: 10.1038/sj.npp.1300720. [DOI] [PubMed] [Google Scholar]
- Staddon J.E.R. Psychology Press; Philadelphia, PA: 2014. The New Behaviorism: Mind, Mechanism and Society. [Google Scholar]
- Stafford D., Branch M.N. Effects of step size and break-point criterion on progressive-ratio performance. J. Exp. Anal. Behav. 1998;70:123–138. doi: 10.1901/jeab.1998.70-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TestDiet. Technical data sheet on grain-based rodent tablet 5TUM, 2011. www.testdiet.com/cs/groups/lolweb/@testdiet/documents/web_content/mdrf/mdi2/edisp/ducm04_026411.pdf.
- Valencia-Torres L., Bradshaw C.M., Bouzas A., Hong E., Orduña V. Effect of streptozotocin-induced diabetes on performance on a progressive ratio schedule. Psychopharmacology. 2014;231:2375–2384. doi: 10.1007/s00213-013-3401-5. [DOI] [PubMed] [Google Scholar]
- Wakley A.A., Rasmussen E.B. Effects of cannabinoid drugs on the reinforcing properties of food in gestationally undernourished rats. Pharmacol. Biochem. Behav. 2009;94:30–36. doi: 10.1016/j.pbb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Ward S.J., Dykstra L.A. The role of CB1 receptors in sweet versus fat reinforcement: effect of CB1 receptor deletion, CB1 receptor antagonism (SR 141716A) and CB1 receptor agonism (CP-55940) Behav. Pharmacol. 2005;16:381–388. doi: 10.1097/00008877-200509000-00010. [DOI] [PubMed] [Google Scholar]
- Williams C.M., Kirkham T.C. Anandamide induces overeating:mediation by central cannabinoid receptors. Psychopharmacology. 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- Williams C.M., Kirkham T.C. Observational analysis of feeding induced by ∆9-THC and anandamide. Physiol. Behav. 2002;76:241–250. doi: 10.1016/s0031-9384(02)00725-4. [DOI] [PubMed] [Google Scholar]
- Williams C.M., Kirkham T.C. Reversal of ∆9-THC hyperphagia by SR. 141716 and naloxone but not dexfenfluramine. Pharmacol. Biochem. Behav. 2002;71:333–340. doi: 10.1016/s0091-3057(01)00694-3. [DOI] [PubMed] [Google Scholar]
- Williams C.M., Rogers P.J., Kirkham T.C. Hyperphagia in pre-fed rats following oral ∆9-THC. Physiol. Behav. 1998;65:343–346. doi: 10.1016/s0031-9384(98)00170-x. [DOI] [PubMed] [Google Scholar]
- Wynne C.D.L., Staddon J.E.R. Typical delay determines waiting time on periodic-food schedules: static and dynamic tests. J. Exp. Anal. Behav. 1988;50:197–210. doi: 10.1901/jeab.1988.50-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne C.D.L., Staddon J.E.R., Delius J.D. Dynamics of waiting in pigeons. J. Exp. Anal. Behav. 1996;65:603–618. doi: 10.1901/jeab.1996.65-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R., Niki M., Jyotaki M., Sanematsu K., Shigemura N., Ninomiya Y. Modulation of sweet responses of taste receptor cells. Semin. Cell Dev. Biol. 2013;24:226–231. doi: 10.1016/j.semcdb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Yoshida R., Ninomiya Y. New insights into the signal transmission from taste cells to gustatory nerve fibers. Int. Rev. Cell Mol. Biol. 2010;279C:101–134. doi: 10.1016/S1937-6448(10)79004-3. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Rickard J.F., Asgari K., Body S., Bradshaw C.M., Szabadi E. Quantitative analysis of the effects of some atypical and conventional antipsychotics on progressive ratio schedule performance. Psychopharmacology. 2005;179:489–497. doi: 10.1007/s00213-004-2049-6. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Rickard J.F., Asgari K., Body S., Bradshaw C.M., Szabadi E. Comparison of the effects of clozapine and 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) on progressive ratio schedule performance: evidence against the involvement of 5-HT1A receptors in the behavioural effects of clozapine. Psychopharmacology. 2005;181:381–391. doi: 10.1007/s00213-005-2258-7. [DOI] [PubMed] [Google Scholar]