Abstract
Purpose
We compared efficacy of trastuzumab versus no trastuzumab in patients with small (≤ 2 cm) human epidermal growth factor receptor 2 (HER2) –positive breast cancer treated in randomized trials.
Methods
A meta-analysis was conducted using data from five of the six adjuvant trastuzumab trials. Efficacy end points were disease-free survival (DFS) and overall survival (OS). Separate analyses were prospectively planned for hormone receptor (HR) –positive and HR-negative cohorts. Random effect models and Yusuf-Peto fixed effects models assessed the impact of heterogeneity on baseline hazards and treatment effects across studies. Peto-Pike cumulative incidence estimates were stratified by study and nodal status.
Results
Median follow-up time was 8 years. For 2,263 patients with HR-positive disease, 8-year cumulative incidence rates comparing trastuzumab versus no trastuzumab were 17.3% versus 24.3% (P < .001) for DFS and 7.8% versus 11.6% (P = .005) for OS, respectively; for 1,092 HR-positive patients with zero or one positive lymph nodes, results were 12.7% versus 19.4% (P = .005) for DFS and 5.3% versus 7.4% (P = .12) for OS, respectively. For 1,957 patients with HR-negative disease, 8-year cumulative incidence rates were 24.0% versus 33.4% (P < .001) for DFS and 12.4% versus 21.2% (P < .001) for OS, respectively; for 1,040 HR-negative patients with zero or one positive lymph nodes, results were 20.4% versus 26.3% (P = .05) for DFS and 8.2% versus 12.2% (P = .084) for OS, respectively.
Conclusion
Women with HER2-positive tumors ≤ 2 cm in the randomized trastuzumab trials derived substantial DFS and OS benefit from adjuvant trastuzumab. Trastuzumab-treated patients with HR-positive disease and ≤ one positive lymph node may be candidates for trials assessing less aggressive treatment approaches.
INTRODUCTION
In 2014, approximately 232,670 invasive breast cancers will be diagnosed in the United States and 465,000 in Europe,1,2 and approximately 20% of patients with invasive breast cancer have human epidermal growth factor receptor 2 (HER2) –positive disease. HER2 is a member of the family of ErbB tyrosine kinase receptors, mediating tumor progression.3 Before the development of trastuzumab, a monoclonal antibody directed against HER2, patients with HER2-positive breast cancer had a high risk of disease recurrence and reduced survival.4 By late 2006, trastuzumab and chemotherapy were standard of care in the adjuvant and metastatic settings in women with HER2-positive breast cancer, based on a significant benefit in progression-free survival and overall survival (OS).5–11 However, approximately 16% to 22% of women with HER2-positive breast cancer treated in the adjuvant setting will experience relapse12; thus, strategies to overcome trastuzumab resistance are being studied, including the use of dual HER2 blockade and/or novel targeted therapies.13–19 To date, four HER2-targeted agents, trastuzumab, lapatinib, pertuzumab, and trastuzumab emtansine (T-DM1), have been approved for use in patients with metastatic HER2- positive breast cancer, and trials have been conducted or are ongoing in both adjuvant and neoadjuvant settings.20–22
Combining dual HER2-targeted therapy and chemotherapy versus trastuzumab and chemotherapy in the first-line setting resulted in a substantial improvement in progression-free survival and OS in patients with HER2-positive metastatic breast cancer23; therefore, adjuvant trials are ongoing. However, we hypothesized that there is a subgroup of patients with small HER2-positive breast cancer who have a favorable prognosis when treated with chemotherapy and trastuzumab, with or without endocrine therapy. Therefore, these patients may not contribute a sufficient number of events to justify inclusion in trials evaluating additional therapy. In an initial meta-analysis, we identified a subgroup of patients with small HER2-positive tumors ≤ 2 cm with hormone receptor (HR) –positive disease with ≤ one positive lymph node who have an excellent prognosis when treated with chemotherapy and trastuzumab, with or without endocrine therapy. We then conducted an individual patient data meta-analysis to compare the efficacy of adjuvant trastuzumab versus no trastuzumab for patients with HER2-positive breast cancer and tumors ≤ 2 cm. Given increasing recognition that HR-positive and HR-negative breast cancers are different diseases,24 all analyses were performed separately for these two cohorts.
METHODS
Search Strategy
Randomized clinical trials (RCTs) were identified by a PubMed search and by examining the reference list of published trials, review articles, and editorials on chemotherapy and trastuzumab for patients with resected, early-stage HER2-positive breast cancer. For the PubMed search, the following keywords or corresponding medical subject heading terms were used: “trastuzumab” “HER2-positive breast cancer,” “adjuvant,” “chemotherapy,” and “randomized controlled trials.” The database was searched for articles published between 1995 and 2013.
Selection of Trials and Data Collection
The search identified the following six randomized trials to be included in the meta-analysis: Herceptin Adjuvant (HERA), North Central Cancer Treatment Group (NCCTG) N9831, National Surgical Adjuvant Breast and Bowel Project (NSABP) B31, Breast Cancer International Research Group 006, Programme Adjuvant Cancer Sein (PACS) -04, and Finland Herceptin Study (FinHER). Investigators from five of the six trials agreed to provide data for this meta-analysis. We were unable to obtain permission to include patient-level data from Breast Cancer International Research Group 006. Data analyzed from the remaining five trials included publication details, methodologic components and trial characteristics, sample size, eligibility criteria, interventions, follow-up duration, and measures of outcome. The trial data were reviewed for internal consistency.
Statistical Methods
We included patients with resected HER2-positive tumors measuring ≤ 2 cm who either received or did not receive 9 weeks, 1 year, or 2 years of trastuzumab. Efficacy end points were disease-free survival (DFS), considering events as defined by each trial (Appendix and Appendix Table A1, online only), and OS. We prospectively planned to analyze HR-positive and HR-negative cohorts separately. Log-rank tests for each study were performed as a descriptive tool. The effect of treatment was assessed using Yusuf-Peto fixed effects meta-analysis methodology, and Cox modeling was used to assess the effect of baseline covariates. The study-to-study heterogeneity of treatment effects was assessed by mixed effects models and by the inclusion of a treatment × study interaction in the fixed effects Cox models. In the presence of significant heterogeneity across studies, the mean estimate from the mixed effects model is presented. Cumulative incidence estimates for graphic display of DFS events and death in both HR cohorts were produced using the Peto-Pike method, constant in 2-year intervals, with the analysis stratified by study and nodal status.
An intent-to-treat approach produced a conservative effect estimate, because a substantial number of patients crossed over from the control arm to the trastuzumab arm when positive DFS results were reported in 2005. Analyses were performed at Frontier Science Scotland by three statisticians (I.B., E.H., and C.C.) using SAS version 9.3 (SAS Institute, Cary, NC) and R 3.0.1 (www.r-project.org) software. All tests were two-sided.
Subgroup Analyses
Subgroup analyses were performed to assess the influence of the following variables on DFS and OS outcomes in patients who participated in the five RCTs included in this analysis: trastuzumab therapy (yes or no), HR status (positive or negative), and number of positive lymph nodes (zero to one, two to three, or ≥ four nodes). The prospective analysis plan considered patients with ≤ one positive node to warrant analysis as a low-risk cohort, because analyses of the node-negative cohort are almost exclusively based on events in HERA.
RESULTS
Study Inclusion and Characteristics
Table 1 lists the characteristics of the five RCTs included in this meta-analysis (HERA, NCCTG N9831, NSABP B31, PACS-04, and FinHER). Only three trials enrolled patients with node-negative HER2-positive breast cancer (HERA, NCCTG N9831, and FinHER). Trastuzumab was given sequentially to chemotherapy in HERA and PACS-04, concurrently in FinHER and NSABP B31, and either sequentially or concurrently in NCCTG N9831. The duration of trastuzumab therapy ranged from 9 weeks in FinHER to up to 2 years in one of the two trastuzumab arms of the HERA trial. The majority of trastuzumab-treated patients (73%) received 1 year of treatment. The chemotherapy regimens also varied; however, most patients (96%) received anthracycline-based treatment (mandated in B31, N9831, and PACS-04; 94% of patients in HERA received anthracyclines). The median follow-up data in the trials were mature, and median follow-up time ranged from 5.0 years in PACS-04 to 9.4 years in NSABP B31.
Table 1.
Trials Included in This Meta-Analysis
| Trial | HER2-Positive Tumors (No.) | HER2-Positive Tumors ≤ 2 cm (No.) | HR-Positive, HER2-Positive Tumors ≤ 2 cm (No.) | HR-Negative, HER2-Positive Tumors ≤ 2 cm (No.) | Timing of Trastuzumab | Duration of Trastuzumab | Chemotherapy Regimen | Median Follow-Up (years) |
|---|---|---|---|---|---|---|---|---|
| HERA | 5,102 | 2,003 | 1,054 | 949 | Sequential | 1 or 2 years | Any–94% A; 26% A and T | 8.0 |
| NCCTG N9831 | 3,505 | 1,062 | 546 | 516 | Concurrent or Sequential | 1 year | AC→T AC→wTH AC→wT→H |
8.7 |
| NSABP B31 | 3,222 | 840 | 476 | 364 | Concurrent | 1 year | AC→T AC→TH |
9.4 |
| PACS-04 | 528 | 235 | 146 | 89 | Sequential | 1 year | FEC→H DE→H |
5.0 |
| FinHER | 232 | 81 | 41 | 40 | Concurrent | 9 weeks | D±H→FEC V±H→FEC |
5.6 |
| Total | 12,589 | 4,221 | 2,263 | 1,958 |
Abbreviations: A, doxorubicin; AC, doxorubicin and cyclophosphamide; D, docetaxel; DE, epirubicin and docetaxel; FEC, fluorouracil, epirubicin, cyclophosphamide; FinHER, Finland Herceptin Study; H, trastuzumab; HER2, human epidermal growth factor receptor 2; HERA, Herceptin Adjuvant Study; HR, hormone receptor; NCCTG, North Central Cancer Treatment Group; NSABP, National Surgical Adjuvant Breast and Bowel Project; PACS, Programme Adjuvant Cancer Sein; T, paclitaxel; V, vinorelbine.
Description of Study Participants
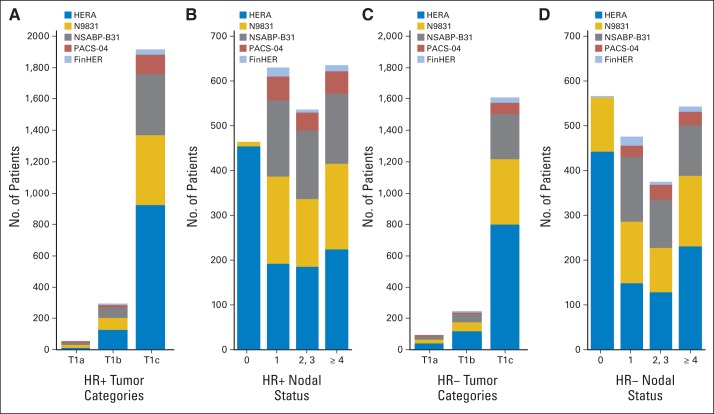
Patient characteristics are shown in Figure 1. HER2-positive tumors ≤ 2 cm were identified in 4,220 patients. In total, 2,588 patients received adjuvant trastuzumab and 1,632 did not. Overall, 2,263 patients had HR-positive disease, 94.1% of whom received endocrine therapy, and the majority of patients (n = 1,821; 80%) had T1c disease. Almost all of the HR-positive, node-negative patients were from HERA. One thousand nine hundred fifty-seven patients had HR-negative disease ≤ 2 cm, only 3.3% of whom received endocrine therapy, and most of the patients (n = 1,607; 82%) had T1c disease. Again, the majority of patients with node-negative disease (87%) came from HERA.
Fig 1.
Patient characteristics by hormone receptor (HR) status. (A) HR-positive tumor categories; (B) HR-positive nodal status; (C) HR-negative tumor categories; (D) HR-negative nodal status. FinHER, Finland Herceptin Study; HERA, Herceptin Adjuvant Study; NSABP, National Surgical Adjuvant Breast and Bowel Project; PACS, Programme Adjuvant Cancer Sein.
Study-to-Study Heterogeneity
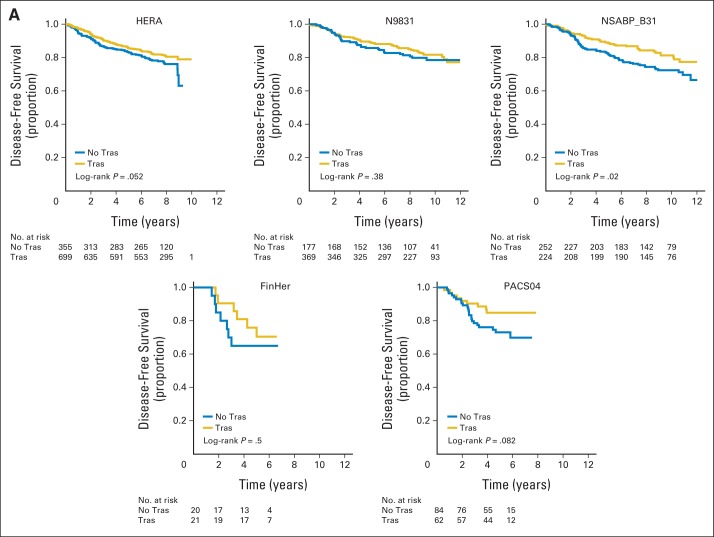
Study-to-study heterogeneity was not statistically significant in patients with HR-positive disease (P = .76); the hazard ratio for the effect of trastuzumab was 0.70 (95% CI, 0.58 to 0.84; Fig 2A). There was evidence of significant study-to-study heterogeneity in patients with HR-negative disease (P = .01; Fig 2B). This finding may be influenced by the degree of cross over between the trials (55% of patients crossed over in HERA, compared with 12% in N9831 and 13% in NSABP B31). Other factors potentially influencing this observation include concurrent versus sequential administration of trastuzumab relative to chemotherapy, differences in tumor biology, and chance. The hazard ratio for DFS comparing trastuzumab versus no trastuzumab from the mixed effects model (using COXME in R), with random effect for trial to account for heterogeneity, was 0.66 (95% CI, 0.49 to 0.88) in the HR-negative cohort. There was clear evidence of heterogeneity between trials, and a diminished treatment effect was noted over time in HERA.
Fig 2.
Disease-free survival for (A) hormone receptor (HR) –positive and (B) HR-negative disease treated with or without trastuzumab (Tras). FinHER, Finland Herceptin Study; HERA, Herceptin Adjuvant Study; NSABP, National Surgical Adjuvant Breast and Bowel Project; PACS, Programme Adjuvant Cancer Sein.
HR-Positive Disease
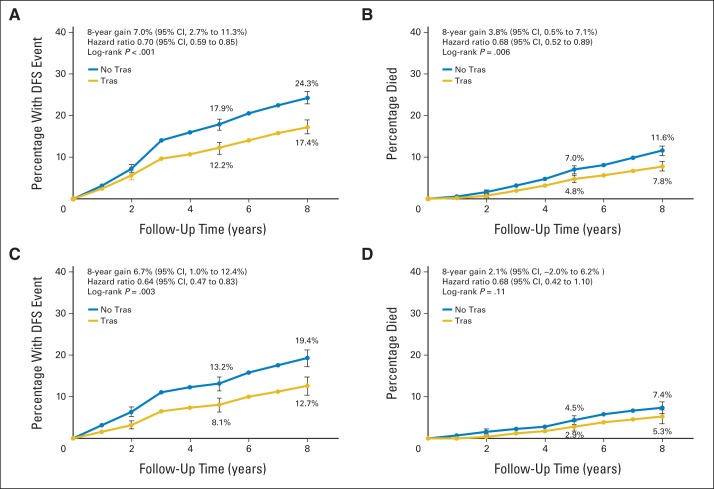
The cumulative incidence of a DFS event at 8 years for patients with HR-positive disease and tumors ≤ 2 cm (all nodal groups) was 17.3% for patients assigned to receive trastuzumab versus 24.3% for those who did not receive trastuzumab, resulting in an absolute gain in DFS of 7.0% (P < .001; Fig 3A). The cumulative incidence of death was 7.8% in patients assigned to receive trastuzumab compared with 11.6% in patients who did not receive trastuzumab, which resulted in a 3.8% absolute gain in OS at 8 years (P = .005; Fig 3B). For the 1,092 patients with HR-positive disease, tumors measuring ≤ 2 cm, and ≤ one positive lymph node, the cumulative incidence of recurrence at 8 years was 12.7% for patients who received trastuzumab versus 19.4% for patients who did not, which resulted in an absolute DFS gain of 6.7% (P = .005; Fig 3C). The cumulative incidence of death was 5.3% and 7.4% in patients who did and did not receive trastuzumab, respectively, which resulted in a 2.1% absolute gain in OS (P = .12; Fig 3D).
Fig 3.
Cumulative incidence of a disease-free survival (DFS) event and death for patients with hormone receptor–positive disease and tumors ≤ 2 cm. (A) DFS event among all 2,263 patients; (B) overall survival (OS) event among all 2,263 patients; (C) DFS event among 1,092 patients with ≤ one positive lymph node; (D) OS event among 1,092 patients with ≤ one positive lymph node. Hazard ratios come from fixed effects model stratified by study.
HR-Negative Disease
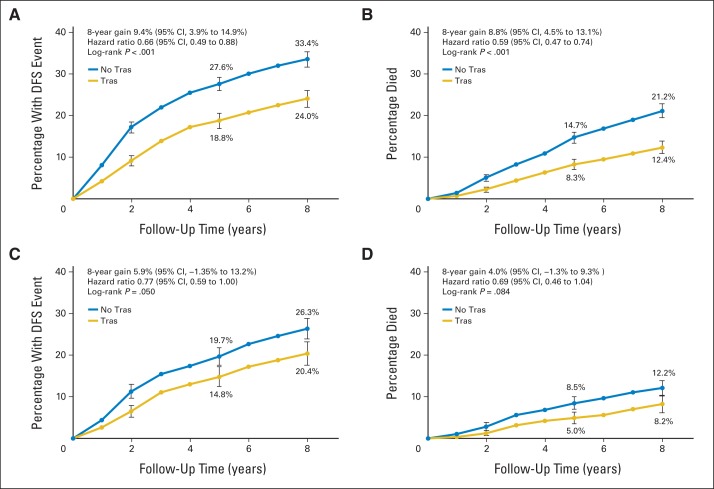
The cumulative incidence of a DFS event at 8 years in patients with HR-negative disease with tumors ≤ 2 cm was 24.5% for patients treated with trastuzumab versus 33.5% for patients who were not, resulting in a DFS gain of 9.4% (P < .001; Fig 4A). At 8 years, 12.6% of patients assigned to receive adjuvant trastuzumab had died versus 21.2% of patients who did not receive trastuzumab, resulting in an OS gain of 8.8% (P < .001; Fig 4B). For the 1,040 patients with HR-negative disease, tumors measuring ≤ 2 cm, and ≤ one positive lymph node, the cumulative incidence of recurrence at 8 years was 20.4% for patients assigned to receive trastuzumab versus 26.3% for patients who did not receive trastuzumab, which resulted in an absolute DFS gain of 5.9% (P = .05; Fig 4C). The cumulative incidence of death was 8.2% and 12.2% in patients who did and did not receive trastuzumab, respectively, which resulted in a 4.0% absolute gain in OS (P = .084; Fig 4D). Although the proportional benefit of trastuzumab at 8 years of median follow-up was similar in HR-positive and HR-negative groups, the risk of recurrence and the magnitude of treatment effect seemed to differ over follow-up time. Patients with HR-negative disease who did not receive trastuzumab experienced relapse earlier than patients with HR-positive disease, and a greater degree of separation between the curves was noted during early follow-up.
Fig 4.
Cumulative incidence of a disease-free survival (DFS) event and death for patients with hormone receptor–negative disease and tumors ≤ 2 cm. (A) DFS event among all 1,957 patients; (B) overall survival (OS) event among all 1,957 patients; (C) DFS event among 1,040 patients with ≤ one positive lymph node; (D) OS event among 1,040 patients with ≤ one positive lymph node. The hazard ratio in panel A comes from the mixed effects model, which accounts for study-to-study heterogeneity, whereas the hazard ratios in panels B, C, and D come from the fixed effects model stratified by study.
DISCUSSION
In this analysis, we demonstrated that patients with HER2-positive tumors ≤ 2 cm benefited substantially from adjuvant trastuzumab in terms of DFS and OS. However, almost all of the patients included in our meta-analysis had T1c disease and, therefore, were a highly selected subgroup. There is no definitive evidence available from five adjuvant trastuzumab trials, or from the present meta-analysis, regarding the utility of adjuvant trastuzumab in patients with T1a-b node-negative tumors because the majority of these patients were excluded based on eligibility criteria.25 Overall, subgroup analyses on the large adjuvant trastuzumab clinical trials suggest that patients with small, node-negative, HER2-positive tumors also derive benefit from adjuvant trastuzumab, a finding that is supported by the results of our meta-analysis. However, current guidelines suggest that not all patients with stage I HER2-positive breast cancer warrant treatment with trastuzumab and/or adjuvant chemotherapy (eg, patients with T1aN0 disease). Across the five trials studied in our meta-analysis, only 75 patients had T1a-b node-negative disease, likely because they had other adverse biologic features. Therefore, it would be difficult to extrapolate the DFS and OS results to every patient with T1a and T1b node-negative HER2-positive disease seen in the clinical setting.
Although we acknowledge the clinical interest in DFS and OS outcomes for patients with node-negative, HER2-positive tumors ≤ 2 cm, our analysis plan did not specify a separate analysis for this subset because there were few events, and almost all of them came from HERA. Trastuzumab was given sequentially in HERA, so results may not reflect current practice and could potentially be misleading. Therefore, the management of small HER2-positive, node-negative breast cancer remains controversial. The general recommendation is that patients with tumors ≥ 0.5 cm with features associated with biologic aggressiveness, such as lymphovascular invasion, estrogen receptor (ER) negativity, grade 3 histology, or lymph node positivity, should be considered for treatment with adjuvant chemotherapy and trastuzumab.26,27 It is unlikely that prospective trials will be performed to address whether chemotherapy and trastuzumab are indicated for all patients with small HER2-positive breast cancer for several reasons. First, small HER2-positive, node-negative tumors are rare, and the rate of events is quite low in this patient cohort.28–32 Therefore, a large sample size would be required to confirm efficacy of trastuzumab in this setting. Because previous retrospective studies have demonstrated that trastuzumab is beneficial in patients with node-negative, HER2-positive disease, it would be difficult to include a non–trastuzumab-containing treatment arm. In addition, several retrospective studies have demonstrated that HER2 positivity is still an important predictor of subsequent relapse and has a less favorable prognosis in patients with T1a-b N0M0 tumors.28–32
Interestingly, the proportional benefit of trastuzumab was similar for HR-positive and HR-negative disease, but patterns and incidence of relapse seem to differ over follow-up time. This implies that patients with HR-positive and HR-negative disease have different diseases and potentially should be considered separately in future research trials. Potential reasons for observed differences in patterns of incidence and relapse include cross-talk between the ER and HER2 pathways and the role of endocrine therapy to reduce the risk of relapse over time in patients with ER-positive disease. In addition, the use of endocrine therapy combined with HER2-targeted therapy represents a type of dual blockade for patients with both HER2-positive and HR-positive disease, which is not available for the HR-negative, HER2-positive subset. The effect of this additional adjuvant therapy modality could blunt study-to-study differences associated with timing of chemotherapy and HER2-targeted therapy.
In our meta-analysis, the majority of patients received sequential trastuzumab, and for patients with HR-positive disease, testing for study-to-study heterogeneity did not reach statistical significance. By contrast, testing for study-to-study heterogeneity in patients with HR-negative disease did reach statistical significance. In this subgroup, it was noted that patients treated with trastuzumab derived greater benefit versus patients who did not receive trastuzumab in NSABP B31 compared with HERA. These findings might be influenced by the differences in cross over between the two trials. Other potential factors influencing this observation included concurrent (NSABP B31) versus sequential (HERA) administration of trastuzumab, differences in tumor biology, and chance. In addition, patients in HERA were enrolled after receiving chemotherapy (trastuzumab started a median of 8.4 months after diagnosis) so that patients suffering early recurrences, predominantly those with HR-negative disease, would not have enrolled onto HERA. For patients with HR-negative disease, statistically significant differences in DFS and OS existed across the trials, which may suggest inferior outcomes if trastuzumab is not used concurrently with chemotherapy. However, other prognostic factors, such as the interaction of ER status and HER2 status, increased tumor burden, and number of positive lymph nodes may have greater impact on DFS and OS outcomes for patients with HR-negative disease.
Whether all patients with small HER2-positive tumors need to be treated with both adjuvant chemotherapy and 1 year of trastuzumab is an important question. Tolaney et al33 recently published results from the prospective single-arm Adjuvant Paclitaxel and Trastuzumab (APT) trial, in which 410 patients with resected, node-negative, HER2-positive breast cancer ≤ 3 cm were treated with 12 weeks of trastuzumab and paclitaxel, followed by trastuzumab monotherapy to complete 1 year of treatment. The 3-year rate of survival free from invasive disease was 98.7% (95% CI, 97.6% to 99.8%), indicating that this regimen is a reasonable option for the patients included in APT.33 Of note, APT enrolled a greater percentage of patients with HR-positive disease (67%) than in the adjuvant trastuzumab trials (51% to 54%). Furthermore, 41.6% of patients in the APT trial had tumors ≤ 1 cm compared with virtually no node-negative tumors ≤ 1 cm in the adjuvant trastuzumab trials, limiting cross-study comparisons. Because only 16 patients (9%) had tumors measuring 2.0 to 3.0 cm in the APT trial, the results apply to those with smaller tumors.
In our previous meta-analysis of patients with HER2-positive tumors ≤ 2 cm, we identified a subgroup of patients treated with chemotherapy, trastuzumab, and endocrine therapy who have an excellent prognosis, with a 5-year DFS of 91% and OS of 97%.34 These patients may not be suitable for trials evaluating additional therapy, and these results may serve as a benchmark for trials evaluating (chemo)therapy reduction strategies. Interestingly, in the NEOSPHERE trial, 16.8% of patients treated with dual HER2-targeted therapy without chemotherapy in the neoadjuvant setting had a pathologic complete response, and for the subset of patients with ER-negative disease in this group, the pathologic complete response rate was 27.3%.35 The phase II ATEMPT trial is randomly assigning women with stage I HER2-positive breast cancer to paclitaxel and trastuzumab followed by 1 year of trastuzumab (the regimen evaluated in APT) or trastuzumab emtansine.36 Because the event rate for both arms will likely be low, differences in toxicity between the two regimens is an important question that will be answered. Conversely, patients with high-risk HER2-positive disease may derive additional benefit from dual HER2 blockade, chemotherapy, and/or endocrine therapy in the (neo)adjuvant setting. Therefore, careful patient selection is important when deciding on a treatment regimen to avoid unnecessary toxicity, patient inconvenience, and cost without compromising outcomes.
In summary, patients with HER2-positive tumors ≤ 2 cm derive significant benefit from the addition of trastuzumab to their treatment regimens, although the majority of patients included in our analyses had T1c, node-positive disease and were thus a selected group. The proportional benefit was similar for patients with HR-positive and HR-negative disease, although timing of relapses and absolute magnitude of benefit seemed to differ over time. The benefit of trastuzumab was also seen in patients with HR-positive, HER2-positive breast cancers measuring ≤ 2 cm with ≤ one positive lymph node, who had an especially good prognosis when treated with chemotherapy, trastuzumab, and endocrine therapy (5-year DFS of 91% and OS of 97%). These patients may not contribute a sufficient number of events to justify inclusion in trials evaluating additional therapy. Future and ongoing trials should aim to reduce treatment-related morbidity for this patient cohort without compromising DFS and OS outcomes.
Glossary Terms
- DM1:
a derivative of maytansine 1 and a cytotoxic microtubule inhibitor trastuzumab emtansine (T-DM1), an antibody-drug conjugate composed of the cytotoxic microtubule inhibitor DM1 and the human epidermal growth factor receptor 2–targeted humanized monoclonal antibody trastuzumab. Also referred to as Kadcyla.
- HER2/neu (human epidermal growth factor receptor 2):
also called ErbB2. HER2/neu belongs to the epidermal growth factor receptor (EGFR) family and is overexpressed in several solid tumors. Like EGFR, it is a tyrosine kinase receptor whose activation leads to proliferative signals within the cells. On activation, the human epidermal growth factor family of receptors are known to form homodimers and heterodimers, each with a distinct signaling activity. Because HER2 is the preferred dimerization partner when heterodimers are formed, it is important for signaling through ligands specific for any members of the family. It is typically overexpressed in several epithelial tumors.
- lapatinib:
a dual tyrosine kinase inhibitor. Lapatinib has been developed as an inhibitor of the tyrosine kinase activities of ErbB1 (EGFR) and ErbB2. Like other tyrosine kinase inhibitors, it competes with ATP binding to the intracellular regions of the receptors that are activated after tyrosine phosphorylation.
- pertuzumab:
a humanized monoclonal antibody that binds to HER2 at a site used by the receptor to form dimers with other receptors (the dimerization site) belonging to this family. Signaling via all HER2 dimers is, therefore, inhibited. Also referred to as Omnitarg. See HER2/neu (human epidermal growth factor receptor 2) and ErbB.
- trastuzumab:
a humanized anti-ErbB2 monoclonal antibody approved for treating patients whose breast cancers overexpress the ErbB2 protein or demonstrate ErbB2 gene amplification. It is currently being tested in combination with other therapies.
Appendix
Definitions of Disease-Free Survival Abstracted From Publications
In the Herceptin Adjuvant Study (HERA), disease-free survival (DFS) was defined as time from random assignment to the first occurrence of any of the following DFS events: recurrence of breast cancer at any site; the development of ipsilateral or contralateral breast cancer, including ductal carcinoma in situ but not lobular carcinoma in situ; second nonbreast malignant disease other than basal cell or squamous cell carcinoma of the skin or carcinoma in situ of the cervix; or death from any cause without documentation of a cancer-related event.
In the North Central Cancer Treatment Group N9831 and National Surgical Adjuvant Breast and Bowel Project B31 studies, events determining DFS were local, regional, and distant recurrence; contralateral breast cancer, including ductal carcinoma in situ; other second primary cancers; and death before recurrence or a second primary cancer.
In the Programme Adjuvant Cancer Sein (PACS) -04 study, DFS was defined as time from first random assignment to one of the following events: local or regional recurrence, distant metastases, contralateral breast cancer, or death from any cause, whichever came first.
In the Finland Herceptin Study (FinHER), recurrence-free survival was defined as time from date of random assignment to the detection (with histologic or cytologic confirmation or with radiologic evidence) of local, distant, or contralateral invasive breast cancer or death, whichever occurred first.
In the Breast Cancer International Research Group 006 study, DFS was defined as the time from random assignment to breast cancer recurrence, a second primary cancer (excluding contralateral ductal carcinoma in situ), or death from any cause, whichever came first.
Table A1.
Definition of Disease-Free Survival Event by Trial
| Trial | Local Recurrence | Regional Recurrence | Distant Metastases | Contralateral Breast Cancer (including DCI) | Other Second Primary Cancer | Death |
|---|---|---|---|---|---|---|
| HERA | Yes | Yes | Yes | Yes | Yes | Yes |
| NCCTG N9831 | Yes | Yes | Yes | Yes | Yes | Yes |
| NSABP B31 | Yes | Yes | Yes | Yes | Yes | Yes |
| PACS-04 | Yes | Yes | Yes | Yes* | No | Yes |
| FinHER | Yes | Yes | Yes | Yes* | No | Yes |
Abbreviations: DCI, ductal carcinoma in situ; FinHER, Finland Herceptin Study; HERA, Herceptin Adjuvant Study; NCCTG, North Central Cancer Treatment Group; NSABP, National Surgical Adjuvant Breast and Bowel Project; PACS, Programme Adjuvant Cancer Sein.
Invasive disease only.
Footnotes
Processed as a Rapid Communication manuscript.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00045032, ISRCTN76560285., NCT00054587, NCT00004067, NCT00005970
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Ciara C. O'Sullivan, Edith A. Perez, Heikki Joensuu, Joseph P. Costantino, Priya Rastogi, Dimitrios Zardavas, Jo Anne Zujewski, Richard D. Gelber
Administrative support: Ciara C. O'Sullivan
Provision of study materials or patients: Edith A. Perez, Marc Spielman, Heikki Joensuu, Priya Rastogi, Suzette Delaloge, Martine Piccart-Gebhart
Collection and assembly of data: Marc Spielmann, Edith A. Perez, Heikki Joensuu, Joseph P. Costantino, Suzette Delaloge, Priya Rastogi, Dimitrios Zardavas, Karla V. Ballman, Evandro de Azambuja, Martine Piccart-Gebhart, Richard D. Gelber
Data analysis and interpretation: Ciara C. O'Sullivan, Ian Bradbury, Christine Campbell, Marc Spielmann, Edith A. Perez, Eileen Holmes, Evandro de Azambuja, Martine Piccart-Gebhart, Jo Anne Zujewski, Richard D. Gelber
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Efficacy of Adjuvant Trastuzumab for Patients With Human Epidermal Growth Factor Receptor 2–Positive Early Breast Cancer and Tumors ≤ 2 cm: A Meta-Analysis of the Randomized Trastuzumab Trials
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Ciara C. O'Sullivan
No relationship to disclose
Ian Bradbury
No relationship to disclose
Christine Campbell
No relationship to disclose
Marc Spielmann
Honoraria: Roche
Consulting or Advisory Role: Roche, Novartis
Edith A. Perez
No relationship to disclose
Heikki Joensuu
Consulting or Advisory Role: Blueprint Medicines, Orion Pharma, Ariad Pharmaceutical
Joseph P. Costantino
Consulting or Advisory Role: Celgene
Research Funding: AstraZeneca (Inst)
Suzette Delaloge
Honoraria: Roche, Novartis, Pfizer
Consulting or Advisory Role: Roche, Novartis, Pfizer
Research Funding: Roche (Inst), AstraZeneca (Inst), Pfizer (Inst), Puma (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Roche, Novartis
Priya Rastogi
No relationship to disclose
Dimitrios Zardavas
No relationship to disclose
Karla V. Ballman
No relationship to disclose
Eileen Holmes
No relationship to disclose
Evandro de Azambuja
Travel, Accommodations, Expenses: Roche
Martine Piccart-Gebhart
Consulting or Advisory Role: Amgen, Astellas, AstraZeneca, Bayer, Eli Lilly, Invivis, MSD, Novartis, Pfizer, Roche-Genentech, Sanofi, Symphogen, Synthon, Verastem
Research Funding: Amgen (Inst), Astellas (Inst), AstraZeneca (Inst), Bayer (Inst), Eli Lilly (Inst), Invivis (Inst), MSD (Inst), Novartis (Inst), Pfizer (Inst), Roche-Genentech (Inst), Sanofi (Inst), Symphogen (Inst), Synthon (Inst), Verastem (Inst)
Jo Anne Zujewski
No relationship to disclose
Richard D. Gelber
Research Funding: AstraZeneca (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Roche (Inst), Celgene (Inst), Merck (Inst), Pfizer (Inst)
REFERENCES
- 1.American Cancer Society. What are the key statistics about breast cancer? http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics.
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 4.Slamon D, Eiermann W, Robert N, et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients: BCIRG 006 Study. 2008 San Antonio Breast Cancer Symposium; December 10-14, 2008; San Antonio, TX. (abstr 62) [Google Scholar]
- 5.Perez EA, Dueck AC, McCullough AE, et al. Impact of PTEN protein expression on benefit from adjuvant trastuzumab in early-stage human epidermal growth factor receptor 2-positive breast cancer in the North Central Cancer Treatment Group N9831 trial. J Clin Oncol. 2013;31:2115–2122. doi: 10.1200/JCO.2012.42.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spielmann M, Roché H, Delozier T, et al. Trastuzumab for patients with axillary-node-positive breast cancer: Results of the FNCLCC-PACS 04 trial. J Clin Oncol. 2009;27:6129–6134. doi: 10.1200/JCO.2009.23.0946. [DOI] [PubMed] [Google Scholar]
- 8.Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: Final results of the FinHer Trial. J Clin Oncol. 2009;27:5685–5692. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 9.Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): An open-label, randomised controlled trial. Lancet. 2013;382:1021–1028. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- 10.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 11.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 12.Jelovac D, Wolff AC. The adjuvant treatment of HER2-positive breast cancer. Curr Treat Options Oncol. 2012;13:230–239. doi: 10.1007/s11864-012-0186-4. [DOI] [PubMed] [Google Scholar]
- 13.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 14.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 15.Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zardavas D, Cameron D, Krop I, et al. Beyond trastuzumab and lapatinib: New options for HER2-positive breast cancer. http://meetinglibrary.asco.org/content/237-132. [DOI] [PubMed]
- 18.Zardavas D, Bozovic-Spasojevic I, de Azambuja E. Dual human epidermal growth factor receptor 2 blockade: Another step forward in treating patients with human epidermal growth factor receptor 2-positive breast cancer. Curr Opin Oncol. 2012;24:612–622. doi: 10.1097/CCO.0b013e328358a29a. [DOI] [PubMed] [Google Scholar]
- 19.Saini KS, Azim HA, Jr, Metzger-Filho O, et al. Beyond trastuzumab: New treatment options for HER2-positive breast cancer. Breast. 2011;20(suppl 3):S20–S27. doi: 10.1016/S0960-9776(11)70289-2. [DOI] [PubMed] [Google Scholar]
- 20.ClinicalTrials.gov. A randomized multicenter, double-blind, placebo-controlled comparison of chemotherapy plus trastuzumab plus placebo versus chemotherapy plus trastuzumab plus pertuzumab as adjuvant therapy in patients with operable HER2-positive primary breast cancer. APHINITY trial. https://clinicaltrials.gov/ct2/show/NCT01358877.
- 21.ClinicalTrials.gov. ALTTO (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) Study; BIG 2-06/N063D. https://clinicaltrials.gov/ct2/show/NCT00490139.
- 22.ClinicalTrials.gov. NeoALTTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) Study. https://clinicaltrials.gov/ct2/show/NCT00553358.
- 23.Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis MJ. HER2-positive breast cancer, intrinsic subtypes, and tailoring therapy. J Natl Cancer Inst. 2014;106:8. doi: 10.1093/jnci/dju212. [DOI] [PubMed] [Google Scholar]
- 25.Vaz-Luis I, Ottesen RA, Hughes ME, et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: A multi-institutional study. J Clin Oncol. 2014;32:2142–2150. doi: 10.1200/JCO.2013.53.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burstein HJ, Winer EP. Refining therapy for human epidermal growth factor receptor 2-positive breast cancer: T stands for trastuzumab, tumor size, and treatment strategy. J Clin Oncol. 2009;27:5671–5673. doi: 10.1200/JCO.2009.24.2222. [DOI] [PubMed] [Google Scholar]
- 27.Joerger M, Thürlimann B, Huober J. Small HER2-positive, node-negative breast cancer: Who should receive systemic adjuvant treatment? Ann Oncol. 2011;22:17–23. doi: 10.1093/annonc/mdq304. [DOI] [PubMed] [Google Scholar]
- 28.Curigliano G, Viale G, Bagnardi V, et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol. 2009;27:5693–5699. doi: 10.1200/JCO.2009.22.0962. [DOI] [PubMed] [Google Scholar]
- 29.Tovey SM, Brown S, Doughty JC, et al. Poor survival outcomes in HER2-positive breast cancer patients with low-grade, node-negative tumours. Br J Cancer. 2009;100:680–683. doi: 10.1038/sj.bjc.6604940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joensuu H, Isola J, Lundin M, et al. Amplification of erbB2 and erbB2 expression are superior to estrogen receptor status as risk factors for distant recurrence in pT1N0M0 breast cancer: A nationwide population-based study. Clin Cancer Res. 2003;9:923–930. [PubMed] [Google Scholar]
- 31.Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27:5700–5706. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chia S, Norris B, Speers C, et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol. 2008;26:5697–5704. doi: 10.1200/JCO.2007.15.8659. [DOI] [PubMed] [Google Scholar]
- 33.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372:134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Sullivan CC, Holmes E, Spielmann M, et al. The prognosis of small HER2-positive breast cancers: A meta-analysis of the randomized trastuzumab trials. 36th Annual CTRC-AACR San Antonio Breast Cancer Symposium; December 10-14, 2013; San Antonio, TX. (abstr S6-03) [Google Scholar]
- 35.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 36.ClinicalTrials.gov. T-DM1 vs paclitaxel/trastuzumab for breast (ATEMPT Trial) https://clinicaltrials.gov/ct2/show/NCT01853748.