Abstract
Purpose
Lapatinib plus trastuzumab improves outcomes relative to lapatinib alone in heavily pretreated, human epidermal growth factor receptor 2–positive metastatic breast cancer (MBC). We tested the combination in the earlier-line setting and explored the predictive value of [18F]fluorodeoxyglucose positron emission tomography ([18F]FDG-PET) for clinical outcomes.
Patients and Methods
Two cohorts were enrolled (cohort 1: no prior trastuzumab for MBC and ≥ 1 year from adjuvant trastuzumab, if given; cohort 2: one to two lines of chemotherapy including trastuzumab for MBC and/or recurrence < 1 year from adjuvant trastuzumab). The primary end point was objective response rate by RECIST v1.0; secondary end points included clinical benefit rate (complete response plus partial response plus stable disease ≥ 24 weeks) and progression-free survival. [18F]FDG-PET scans were acquired at baseline, week 1, and week 8. Associations between metabolic response and clinical outcomes were explored.
Results
Eighty-seven patients were registered (85 were evaluable for efficacy). The confirmed objective response rate was 50.0% (95% CI, 33.8% to 66.2%) in cohort 1 and 22.2% (95% CI, 11.3% to 37.3%) in cohort 2. Clinical benefit rate was 57.5% (95% CI, 40.9% to 73.0%) in cohort 1 and 40.0% (95% CI, 25.7% to 55.7%) in cohort 2. Median progression-free survival was 7.4 and 5.3 months, respectively. Lack of week-1 [18F]FDG-PET/computed tomography ([18F]FDG-PET/CT) response was associated with failure to achieve an objective response by RECIST (negative predictive value, 91% [95% CI, 74% to 100%] for cohort 1 and 91% [95% CI, 79% to 100%] for cohort 2).
Conclusion
Early use of lapatinib and trastuzumab is active in human epidermal growth factor receptor 2–positive MBC. Week-1 [18F]FDG-PET/CT may allow selection of patients who can be treated with targeted regimens and spared the toxicity of chemotherapy.
INTRODUCTION
Human epidermal growth factor receptor 2 (HER2/neu) plays a central role in tumorigenesis.1,2 In the first-line setting, trastuzumab monotherapy produces objective responses in approximately one third of patients with HER2-positive metastatic breast cancer (MBC); response rates are higher when trastuzumab is combined with chemotherapy.3,4 Preclinical models suggest synergy between trastuzumab and the HER2-directed tyrosine kinase inhibitor lapatinib.5 At the time this study was conceived, phase I data were available for the doublet.6 Since then, a phase III study comparing lapatinib with lapatinib plus trastuzumab demonstrated improvements in progression-free survival (PFS) and overall survival (OS) with the combination in heavily pretreated patients who had received a median of three prior trastuzumab-based regimens.7,8
The cost of [18F]fluorodeoxyglucose positron emission tomography/computed tomography ([18F]FDG-PET/CT) is covered by the Centers for Medicare & Medicaid Services and third-party insurers for monitoring response to treatment in patients with distant metastasis. However, because of the lack of prospective data regarding the utility of [18F]FDG-PET/CT over CT alone in MBC, the 2014 National Comprehensive Cancer Network guidelines “generally discourage” [18F]FDG-PET/CT “except in those situations in which other staging studies are equivocal or suspicious.”9 An advantage of [18F]FDG-PET/CT over anatomic imaging such as CT is its ability to detect changes in tumor metabolism, often before changes in tumor size, and provide an early indicator of efficacy. Although this paradigm is widely used in some disease settings (eg, lymphoma), prospective data in MBC are lacking. Given that glucose uptake is regulated by the PI3K/AKT pathway, which itself is strongly activated by HER2, we hypothesized that effective HER2 inhibition should result in a reduction in [18F]FDG uptake in responsive tumors.10
We evaluated the combination of trastuzumab and lapatinib in patients with HER2-positive MBC earlier in their course of disease (zero to two prior lines of therapy). As a secondary end point, we evaluated the predictive value of early metabolic imaging with respect to traditional clinical outcomes.
PATIENTS AND METHODS
Patients
The study enrolled patients with MBC who met the following key criteria: histologically or cytologically confirmed invasive breast cancer, age ≥ 18 years, Eastern Cooperative Oncology Group performance status 0 to 2, at least one measurable lesion by RECIST (Response Evaluation Criteria in Solid Tumors) v1.0, and primary and/or metastatic tumor with HER2 overexpression (3+ by immunohistochemistry) or gene amplification (fluorescent in situ hybridization ratio ≥ 2.0). Adequate organ function, including left ventricular ejection fraction (LVEF) ≥ 50%, was required. Prior lapatinib was prohibited. Prior trastuzumab, pertuzumab, and/or trastuzumab emtansine were allowed. Patients with active brain metastases were excluded; stable brain metastases were allowed. A washout period of 2 weeks from chemotherapy was required.
Study Design
This was a nonrandomized phase II study. Cohort 1 included patients without prior trastuzumab for MBC. Adjuvant or neoadjuvant trastuzumab was allowed, if the interval from trastuzumab completion to recurrence exceeded 1 year. Cohort 2 included patients with one to two lines of chemotherapy for metastatic disease with at least one trastuzumab-containing regimen or patients who recurred within 12 months of adjuvant or neoadjuvant trastuzumab with up to one line of metastatic trastuzumab-based therapy. A baseline research biopsy for correlative studies was required; results of these analyses will be reported separately. Initially, patients received lapatinib 1,000 mg orally once per day and intravenous trastuzumab 2 mg/kg (following a 4-mg/kg loading dose) once per week. In July 2009, the study was amended to allow trastuzumab 6 mg/kg (after an 8-mg/kg loading dose) once every 3 weeks. Lapatinib dose modifications were per protocol according to a predefined algorithm. Adverse events (AEs) were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Cardiac evaluations (echocardiogram or multiple gated acquisition scans) were obtained every 8 weeks and then every 16 weeks if a patient had three consecutive normal assessments. Tumor assessments were performed every 8 weeks, but they decreased to every 12 weeks for patients who had been on study for 12 or more cycles. Magnetic resonance imaging scans of the brain at baseline and every 8 weeks were required for patients with a history of brain metastases. The institutional review board for each participating institution approved the study protocol. All patients provided written informed consent.
Assessment of [18F]FDG-PET/CT Scans
Whole-body [18F]FDG-PET/CT scans were obtained at baseline, after 1 week, and after 8 weeks of treatment, in accordance with National Cancer Institute guidelines for [18F]FDG-PET/CT as an indicator of therapeutic response.11 Scanner qualification, central quality assurance, and image analysis were performed by investigators at Dana-Farber Cancer Institute who were blinded to clinical outcome data. Up to five target lesions were assessed by using European Organisation for Research and Treatment of Cancer criteria: complete metabolic response, complete metabolic resolution of [18F]FDG uptake in all lesions, partial metabolic response (PMR), ≥ 25% reduction in maximum standardized uptake value (SUVmax), progressive metabolic disease, more than 25% increase in SUVmax and/or new [18F]FDG-avid lesion(s), or stable metabolic disease (SMD), if the patient did not meet criteria for either metabolic response or progression.12
Study End Points
The primary study end point was objective response (complete response [CR] plus partial response [PR]) by investigator assessment using RECIST v1.0. Secondary end points included safety, PFS, site(s) of first progression, clinical benefit rate (CR plus PR plus stable disease ≥ 24 weeks), and OS. The association between metabolic response and RECIST response was a secondary correlative end point.
Statistical Analysis
The study used Simon's two-stage minimax designs with a one-sided α = .05 and 80% power to detect the acceptable response rates. Sample size was calculated to distinguish between a response rate of 25% versus 45% in cohort 1 and 10% versus 25% in cohort 2. The observed response rates were to be reported with 95% CIs for the two-stage designs.13 Post hoc exploratory analyses of objective response rate (ORR) and PFS by hormone receptor (HR) status were performed. In the case of discordance in HR status between primary tumor and metastasis, HR results from the metastatic sample were used in the analysis.
AEs were tabulated by grade for all events and for events deemed possibly, probably, or definitely attributed to protocol therapy. Estimation for PFS and OS were calculated by using the Kaplan-Meier product-limit method. All estimates for secondary end points were computed by using single-stage 95% CIs.
For [18F]FDG-PET/CT analyses, the protocol prespecified plan was to combine cohorts 1 and 2. Because of the differences in response rates and PFS, we chose to analyze cohorts 1 and 2 separately; this decision was made before analysis of the [18F]FDG-PET/CT data. Kappa statistics and McNemar's test were used to evaluate the agreement between week-1 and week-8 metabolic response. The association between week-1 metabolic response and objective response by RECIST v1.0 is summarized by positive predictive value and negative predictive value with 95% CIs calculated by using Wald's method. Landmark analysis was used to evaluate PFS; only patients who had a follow-up [18F]FDG-PET/CT and were progression-free at week 1 were included in the analysis, and progression-free intervals were recalculated from that start date.14 Statistical analyses were performed by using SAS 9.4 (SAS Institute, Cary, NC) and R version 2.6.1.15
RESULTS
Patients and Treatment
Eighty-seven patients were registered between May 2007 and October 2010. One patient canceled before start of protocol therapy and is not included in the analyses. One patient was found not to have MBC on review of her baseline biopsy after initiation of protocol therapy and is included in the safety (n = 86) but not the efficacy (n = 85) analyses. Retrospective review of patient medical records later determined that some patients had received more than the allowed number of prior regimens. All analyses are based on initial cohort assignment per treating investigator. In both cohorts, the number of responses in the first stages (10 of 17 in cohort 1 and six of 22 in cohort 2) were sufficient to continue enrollment onto the second stage.
Baseline characteristics are provided in Table 1. Cohort 1 included a higher proportion of patients with de novo metastatic disease than cohort 2 (39% v 20%) and fewer patients who had received neoadjuvant trastuzumab (20% v 47%).
Table 1.
Patient, Tumor, and Treatment Characteristics
| Characteristic | All (N = 86) | Cohort 1 (n = 41) | Cohort 2 (n = 45) |
|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |
| Median age, years (range) | 52 (32-83) | 55 (32-77) | 50 (33-83) |
| Sex | |||
| Female | 86 (100) | 41 (100) | 45 (100) |
| Male | 0 (0) | 0 (0) | 0 (0) |
| Race | |||
| White | 79 (92) | 36 (88) | 43 (96) |
| Black or African American | 4 (5) | 2 (5) | 2 (4) |
| Asian | 1 (1) | 1 (2) | 0 (0) |
| Other | 2 (2) | 2 (5) | 0 (0) |
| Ethnicity | |||
| Hispanic or Latino | 4 (5) | 3 (7) | 1 (2) |
| Non-Hispanic | 78 (91) | 35 (85) | 43 (96) |
| Unknown | 4 (5) | 3 (7) | 1 (2) |
| ECOG PS at baseline | |||
| 0 | 56 (65) | 26 (63) | 30 (67) |
| 1 | 28 (33) | 14 (34) | 14 (31) |
| 2 | 2 (2) | 1 (2) | 1 (2) |
| Stage at initial diagnosis | |||
| I | 9 (10) | 4 (10) | 5 (11) |
| II | 22 (26) | 11 (27) | 11 (24) |
| III | 28 (33) | 10 (24) | 18 (40) |
| IV | 25 (29) | 16 (39) | 9 (20) |
| Unknown | 2 (2) | 0 (0) | 2 (4) |
| Hormone receptor status | |||
| Primary tumor | |||
| ER positive and/or PgR positive | 52 (60) | 27 (66) | 25 (56) |
| ER negative and PgR negative | 32 (37) | 13 (32) | 19 (42) |
| Unknown/not done | 2 (2) | 1 (2) | 1 (2) |
| Metastatic lesion | |||
| ER positive and/or PgR positive | 41 (48) | 16 (39) | 25 (56) |
| ER negative and PgR negative | 33 (38) | 17 (41) | 16 (36) |
| Unknown/not done | 12 (14) | 8 (20) | 4 (9) |
| HER2 status | |||
| Primary tumor | |||
| Positive | 70 (81) | 33 (80) | 37 (82) |
| Negative/equivocal | 10 (12) | 5 (12) | 5 (11) |
| Unknown/not done | 6 (7) | 3 (7) | 3 (7) |
| Metastatic lesion | |||
| Positive | 73 (85) | 33 (80) | 40 (89) |
| Negative/equivocal | 1 (1) | 0 (0) | 1 (2) |
| Unknown/not done | 12 (14) | 8 (20) | 4 (9) |
| Disease-free interval, years | |||
| 0 (de novo metastatic breast cancer) | 25 (29) | 16 (39) | 9 (20) |
| < 2 | 22 (26) | 8 (20) | 14 (31) |
| ≥ 2 | 39 (45) | 17 (41) | 22 (49) |
| Median No. of metastatic disease sites (range)* | 3 (1-6) | 3 (1-6) | 2 (1-5) |
| Disease site | |||
| CNS | 6 (7) | 1 (2) | 5 (11) |
| Lung | 32 (37) | 20 (49) | 12 (27) |
| Pleural effusion | 7 (8) | 3 (7) | 4 (9) |
| Liver | 38 (44) | 20 (49) | 18 (40) |
| Bone | 39 (45) | 19 (46) | 20 (44) |
| Breast or chest wall | 46 (53) | 22 (54) | 24 (53) |
| Lymph nodes | 59 (69) | 32 (78) | 27 (60) |
| Other | 11 (13) | 6 (15) | 5 (11) |
| Adjuvant or neoadjuvant hormonal therapy | 32 (37) | 11 (27) | 21 (47) |
| Adjuvant or neoadjuvant chemotherapy | 46 (53) | 19 (46) | 27 (60) |
| Anthracycline | 42 (49) | 17 (41) | 25 (56) |
| Taxane | 38 (44) | 14 (34) | 24 (53) |
| Trastuzumab | 29 (34) | 8 (20) | 21 (47) |
| No. of lines of chemotherapy for metastasis or recurrence* | |||
| None | 46 (53) | 39 (95) | 7 (16) |
| 1 | 21 (24) | 1 (2) | 20 (44) |
| 2 | 15 (17) | 0 (0) | 15 (33) |
| ≥ 3 | 4 (5) | 1 (2) | 3 (7) |
| Prior chemotherapy for metastasis or recurrence | |||
| Trastuzumab | 39 (45) | 1 (2) | 38 (84) |
| Trastuzumab-emtansine | 1 (1) | 1 (2) | 0 (0) |
| Pertuzumab | 0 (0) | 0 (0) | 0 (0) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PgR, progesterone receptor.
Retrospective review of patient records later determined that some patients had received more than the allowed number of prior regimens. All analyses were based on initial cohort assignment per treating investigator.
The median follow-up for all surviving patients was 37.1 months (maximum, 72.8 months). The reasons for discontinuation of protocol therapy were progression in non-CNS only (n = 68; 79%), isolated CNS progression (n = 5; 6%), progression in both non-CNS and CNS (n = 2; 2%), treatment-related toxicity (n = 1; 1%), physician or patient decision (n = 5; 6%), or review of baseline biopsy indicating the patient did not have MBC (n = 1; 1%). At the time of data lock (November 30, 2013), four patients were still receiving protocol therapy, all in cohort 1, with duration exceeding 3 years. Of these patients, one had received adjuvant trastuzumab for stage III breast cancer, and the other three were trastuzumab-naive (see Appendix Table A1, online only). Among 85 patients evaluable for efficacy, 49 deaths were observed. Surviving patients were censored at the last time point that vital status was known: 15 patients were known to be alive at database lock, 14 patients completed 2 years of follow-up after discontinuing therapy, five patients were lost to follow-up, and two patients withdrew consent.
Efficacy
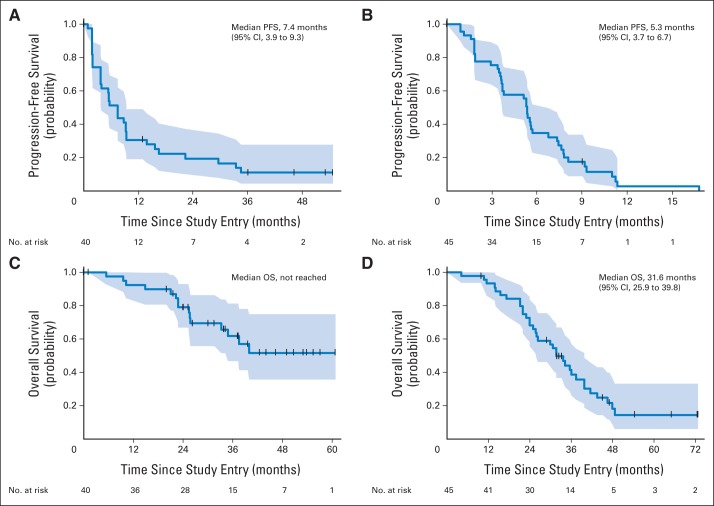
Confirmation of response ≥ 4 weeks later was required for a CR or PR. As delineated in Table 2, the confirmed ORR was 50.0% (95% CI, 33.8% to 66.2%) in cohort 1 and 22.2% (95% CI, 11.3% to 37.3%) in cohort 2. Median PFS was 7.4 months (95% CI, 3.9 to 9.3 months) in cohort 1 and 5.3 months (95% CI, 3.7 to 6.7 months) in cohort 2 (Fig 1). In cohort 1, median OS was not reached; survival rate at 3 years was 61.7% (95% CI, 46.9% to 81.2%). In cohort 2, median OS was 31.6 months (95% CI, 25.9 to 39.8 months) with survival rate at 3 years of 38.6% (95% CI, 26.2% to 56.9%). The clinical benefit rate was 57.5% (95% CI, 40.9% to 73.0%) in cohort 1 and 40.0% (95% CI, 25.7% to 55.7%) in cohort 2 (Table 2). ORR and PFS according to HR status are presented in Table 2. Because this was a post hoc analysis, we chose not to formally compare outcomes by HR status but instead to present the data descriptively. Among patients with HR-negative tumors treated in the first-line setting, ORR was 63.2% (95% CI, 38.4% to 83.7%). ORR was 38.1% (95% CI, 18.1% to 61.6%) among patients with HR-positive tumors receiving first-line therapy.
Table 2.
Summary Table of Efficacy Outcomes
| Outcome | Cohort 1 (n = 40) |
Cohort 2 (n = 45) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR Positive (n = 21) |
HR Negative (n = 19) |
Total (n = 40) |
HR Positive (n = 26) |
HR Negative (n = 19) |
Total (n = 45) |
|||||||
| No. (%) | 95% CI | No. (%) | 95% CI | No. (%) | 95% CI | No. (%) | 95% CI | No. (%) | 95% CI | No. (%) | 95% CI | |
| Response (RECIST v1.0) | ||||||||||||
| Confirmed CR | 2 (9.5) | 1 (5.3) | 3 (7.5) | 0 (0) | 0 (0) | 0 (0) | ||||||
| Confirmed PR | 6 (28.6) | 11 (57.8) | 17 (42.5) | 6 (23.1) | 4 (21.1) | 10 (22.2) | ||||||
| Unconfirmed PR | 1 (4.8) | 1 (5.3) | 2 (5.0) | 4 (15.4) | 0 (0) | 4 (8.9) | ||||||
| SD ≥ 24 weeks | 3 (14.3) | 0 (0) | 3 (7.5) | 5 (19.2) | 3 (15.8) | 8 (17.8) | ||||||
| SD < 24 weeks | 2 (9.5) | 2 (10.5) | 4 (10.0) | 5 (19.2) | 8 (42.1) | 13 (28.8) | ||||||
| PD | 6 (28.6) | 4 (21.1) | 10 (25.0) | 6 (23.1) | 4 (21.1) | 10 (22.2) | ||||||
| PD in CNS by RECIST | 1* (4.8) | 0 (0) | 1 (2.5) | 1 (3.8) | 0 (0) | 1 (2.2) | ||||||
| Clinical PD | 0 (0) | 1 (5.3) | 1 (2.5) | 1 (3.8) | 1 (5.3) | 2 (4.4) | ||||||
| Symptomatic deterioration | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||
| Not evaluated | 1 (4.8) | 0 (0) | 1 (2.5) | 0 (0) | 0 (0) | 0 (0) | ||||||
| Confirmed ORR† (CR + PR) | 8 (38.1) | 18.1 to 61.6 | 12 (63.2) | 38.4 to 83.7 | 20 (50.0) | 33.8 to 66.2 | 6 (23.1) | 9.0 to 43.6 | 4 (21.1) | 6.1 to 45.6 | 10 (22.2) | 11.3 to 37.3 |
| CBR (CR + PR + SD ≥ 24 weeks) | 11 (52.4) | 29.8 to 74.3 | 12 (63.2) | 38.4 to 83.7 | 23 (57.5) | 40.9 to 73.0 | 11 (42.3) | 23.4 to 63.1 | 7 (36.8) | 16.3 to 61.6 | 18 (40.0) | 25.7 to 55.7 |
| Time to event outcomes | ||||||||||||
| Median PFS, months | 6.5 | 3.7 to 16.6 | 8.8 | 3.9 to inf | 7.4 | 3.9 to 9.3 | 5.4 | 3.6 to 7.6 | 5.1 | 3.7 to inf | 5.3 | 3.7 to 6.7 |
| Median OS, months | 40.0 | 33.3 to inf | Not reached | — | Not reached | — | 26.1 | 23.8 to 41.4 | 36.0 | 30.7 to inf | 31.6 | 25.9 to 39.8 |
NOTE. Data were analyzed separately for cohorts 1 and 2 and by hormone receptor (HR) status. Patients who achieved both an unconfirmed partial response (PR) and stable disease (SD) ≥ 24 weeks are included in the group with SD ≥ 24 weeks (n = 2).
Abbreviations: CBR, clinical benefit rate; CR, complete response; inf, infinity; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival.
One patient with both CNS and non-CNS PD.
CIs for ORR are based on the two-stage design.
Fig 1.
Progression-free survival (PFS) for (A) cohort 1, no prior trastuzumab in the advanced setting (n = 40), and (B) cohort 2, up to two lines of chemotherapy including trastuzumab for metastatic breast cancer (n = 45). Overall survival (OS) for (C) cohort 1 and (D) cohort 2. Shaded areas depict the 95% CIs.
Safety
The most common AEs were diarrhea, fatigue, and rash (Appendix Table A2, online only). No grade 4 toxicities were reported. Grade 3 diarrhea was reported in less than 10% of patients. Four patients (5%) experienced a decline in LVEF to below 50%, with a lowest value of 45%. All four patients recovered their LVEF and continued protocol therapy. No grade 3 or 4 cardiac events were reported. Dose holds and dose reductions were required in less than 10% of patients, most commonly for diarrhea or rash.
[18F]FDG-PET/CT Metabolic Assessments
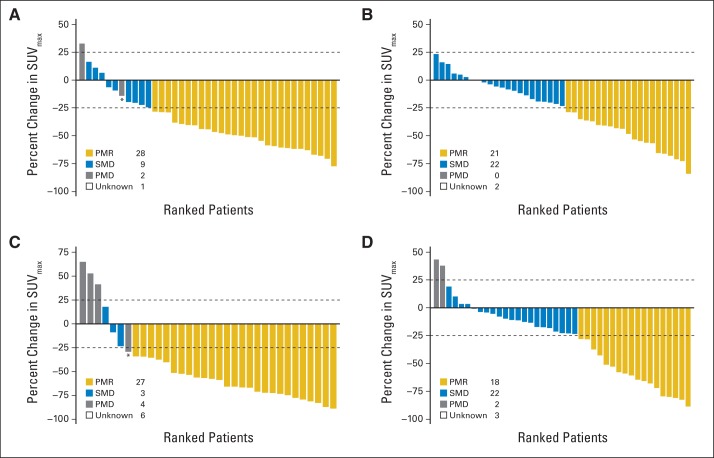
Week-1 PMR was observed in 28 (71.8%) of 39 patients in cohort 1 and 21 (48.8%) of 43 of patients in cohort 2 (Appendix Fig A1, online only). Week-8 PMR was observed in 28 (82.5%) of 34 patients in cohort 1 and 18 (42.9%) of 42 patients in cohort 2. Week-1 and week-8 metabolic responses showed substantial agreement (κ, 0.66) and no shift in the rates of response was detected in the paired measures (McNemar's test P = .29; Appendix Table A3 and Appendix Table A4, online only).
In cohort 1, 19 (67.9%) of 28 patients who achieved a week-1 metabolic response subsequently achieved a confirmed objective response by RECIST v1.0 compared with one (9.1%) of 11 patients with SMD or progressive metabolic disease at week 1 (Table 3; Fig 2, and Appendix Fig A2, online only). In cohort 2, seven (33%) of 21 patients who achieved a week-1 PMR went on to achieve a confirmed objective response compared with two (9.1%) of 22 of patients with week-1 SMD. These data suggest a strong negative predictive value (NPV; 91% [95% CI, 74% to 100%] for cohort 1 and 91% [95% CI, 79% to 100%] for cohort 2) of week-1 metabolic response for objective response, with positive predictive value varying by cohort (68% [95% CI, 51% to 85%] in cohort 1 and 33% [95% CI, 13% to 53%] in cohort 2). Similar results were seen when evaluating week-1 metabolic response for prediction of clinical benefit (Appendix Table A5, online only) and when evaluating week-8 metabolic response (Appendix Table A6, online only).
Table 3.
Association Between Week-1 Metabolic Response and Objective Response by RECIST v1.0
| Week-1 Metabolic Response | Total | Objective Response |
Predictive Value |
||||
|---|---|---|---|---|---|---|---|
| No. of Responders (%) | No. of Nonresponders (%) | PPV (%) | 95% CI | NPV (%) | 95% CI | ||
| Cohort 1 | |||||||
| PMR | 28 | 19 (68) | 9 (32) | 68 | 51 to 85 | 91 | 74 to 100 |
| SMD/PMD | 11 | 1 (9) | 10 (91) | ||||
| Unknown | 1 | 0 (0) | 1 (100) | ||||
| Total | 40 | 20 (50) | 20 (50) | ||||
| Cohort 2 | |||||||
| PMR | 21 | 7 (33) | 14 (67) | 33 | 13 to 53 | 91 | 79 to 100 |
| SMD | 22 | 2 (9) | 20 (91) | ||||
| Unknown | 2 | 1 (50) | 1 (50) | ||||
| Total | 45 | 10 (22) | 35 (78) | ||||
NOTE. Patients were tabulated by objective response (responder v nonresponder) and metabolic response (partial metabolic response [PMR] v stable metabolic disease [SMD]/progressive metabolic disease [PMD]). Objective response was based on RECIST v1.0 (responder: confirmed complete response or partial response; nonresponder: unconfirmed partial response, stable disease, progressive disease, or unknown). Metabolic response was based on European Organisation for Research and Treatment of Cancer criteria.
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
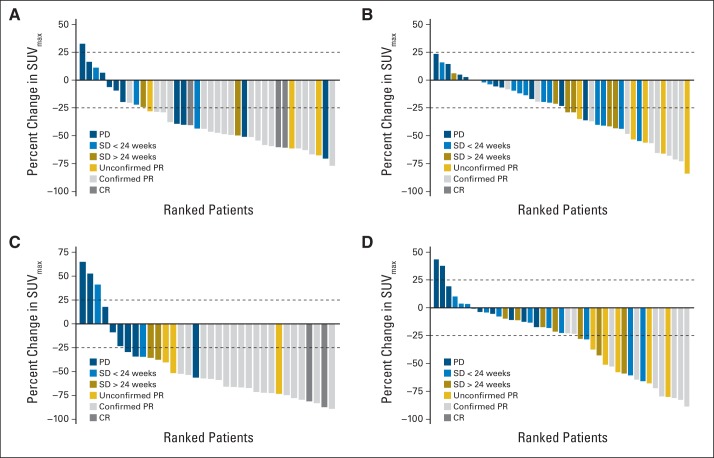
Fig 2.
Relationship between change in maximum standardized uptake value (ΔSUVmax) and objective response by RECIST v1.0. Paired [18F]fluorodeoxyglucose positron emission tomography/computed tomography scan data were available for 82 patients at week 1 and for 76 patients at week 8. One patient's best overall response was not evaluable and was excluded from the figure. Dashed lines denote European Organisation for Research and Treatment of Cancer cutoffs for metabolic response (−25%) and progression (+25%). Color codes indicate objective response for each patient according to RECIST v1.0. (A) Cohort 1 and (B) cohort 2 ΔSUVmax at week 1 v baseline. (C) Cohort 1 and (D) cohort 2 ΔSUVmax at week 8 v baseline. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Landmark analyses were performed to describe the associations between week-1 metabolic response and PFS (Fig 3). In cohort 1, patients with week-1 PMR experienced a median interval of 8.8 months (95% CI, 5.3 to 29.1 months) until progression versus 1.6 months (95% CI, 1.4 months to infinity) in patients without a week-1 PMR. In cohort 2, patients with a week-1 PMR experienced a median interval of 5.3 months (95% CI, 5.1 to 7.9 months) until progression, whereas patients without a week-1 PMR experienced a median interval of 3.2 months (95% CI, 1.6 to 5.3 months; Fig 3).
Fig 3.
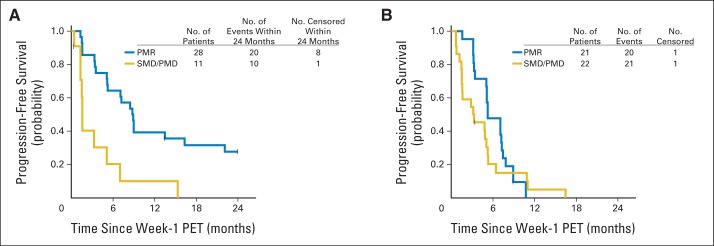
Association between week-1 metabolic response by [18F]fluorodeoxyglucose positron emission tomography (PET) /computed tomography and progression-free survival (PFS). Patients were grouped according to those with a partial metabolic response (PMR) by European Organisation for Research and Treatment of Cancer criteria, defined as > 25% decrease in maximum standardized uptake value relative to baseline v those with either stable metabolic disease (SMD) or progressive metabolic disease (PMD). PFS was calculated from the date of the landmark (week 1). (A) Cohort 1 patients with a week-1 PMR had a median PFS of 8.8 months (95% CI, 5.3 to 29.1 months) and those without a week-1 PMR had a median PFS of 1.6 months (95% lower CI, 1.4 months). (B) Cohort 2 patients with a week-1 PMR had a median PFS of 5.3 months (95% CI, 5.1 to 7.9 months) and those without a week-1 PMR had a median PFS of 3.2 months (95% CI, 1.6 to 5.3 months).
DISCUSSION
We evaluated trastuzumab plus lapatinib in patients with HER2-positive MBC and observed a response rate of 50.0% (95% CI, 33.8% to 66.2%) in the first-line and 22.2% (95% CI, 11.3% to 37.3%) in the second- and third-line settings. Fifty-seven percent of patients in cohort 1 and 40.0% of patients in cohort 2 achieved clinical benefit. Our data compare favorably with that for single-agent trastuzumab or lapatinib, in which first-line response rates between 24% and 35% have been reported.3,16 Furthermore, we demonstrated that lack of metabolic response by [18F]FDG-PET/CT at week 1 was highly predictive of poor response by RECIST v1.0 (NPV, 91% [95% CI, 74% to 100%] for cohort 1 and 91% [95% CI, 79% to 100%] for cohort 2). Notably, in cohort 1, lack of week-1 metabolic response was associated with median PFS of only 1.6 months compared with 8.8 months in patients with an early metabolic response.
Our study was initiated in 2007, but since then, the treatment landscape for HER2-positive breast cancer has changed dramatically. Data from the CLEOPATRA trial (A Study to Evaluate Pertuzumab + Trastuzumab + Docetaxel vs. Placebo + Trastuzumab + Docetaxel in Previously Untreated HER2-positive Metastatic Breast Cancer) have demonstrated the value of first-line pertuzumab when added to trastuzumab/taxane.17 In second-line therapy, trastuzumab emtansine improves PFS and OS compared with lapatinib plus capecitabine.18 For patients newly diagnosed with HER2-positive MBC, these regimens now represent standard options.19 In the adjuvant setting, although there was optimism for the addition of lapatinib to trastuzumab based on improvements in pathologic complete response (pCR) in preoperative trials,20–24 as well as a high rate of pCR and near-pCR with lapatinib and trastuzumab without chemotherapy,25 the ALTTO (Adjuvant Lapatinib And/Or Trastuzumab Treatment Optimisation) Study; BIG 2-06/N063D trial did not demonstrate concomitant gains in disease-free survival or OS when lapatinib was incorporated into trastuzumab-based adjuvant therapy.26 Despite these disappointing results in patients with early-stage disease, we believe the activity of lapatinib and trastuzumab in our study is notable, particularly given the favorable toxicity profile, and remains relevant in the metastatic setting. Of note, four (10%) of 40 patients in cohort 1 remained on protocol therapy more than 3 years after study entry.
Because the number of treatment options and their cost for patients with HER2-positive breast cancer continues to increase, a key question is how best to tailor therapies to individual patients. In the metastatic setting, predictive tests for clinical benefit could spare patients unnecessary toxicity and cost from ineffective therapies and maximize the likelihood of meaningful improvements from treatment. In the early-stage setting, predictive tests may reduce both under- and overtreatment.
To date, minimal prospective data are available regarding testing of the incremental utility of advanced imaging techniques such as [18F]FDG-PET/CT in MBC relative to conventional CT imaging.27 An exception to this is a recent phase IB study of buparlisib in patients with HR-positive/HER2-negative tumors.28 The study reported a relationship between day 15 [18F]FDG-PET/CT and time on treatment; however, only 17 patients were evaluable for metabolic response. To the best of our knowledge, ours is the largest prospective study to date reporting on correlations between early metabolic response and clinical outcomes in patients with uniformly treated HER2-positive advanced breast cancer. We believe the high NPV is of interest and, if confirmed, it may allow clinicians to use a widely available imaging modality to discontinue ineffective therapies. Consistent with the role of HER2/PI3K/AKT in glucose uptake, the absence of a week-1 metabolic response in nonresponding patients suggests that the combination of lapatinib and trastuzumab was unable to inhibit HER2 function in these cancers. Future trials might consider evaluating targeted combinations with an early “metabolic look” to determine whether the addition of cytotoxic agents and/or an early treatment switch is indicated. We acknowledge that such trials will be expensive to launch. We also acknowledge that switching therapies on the basis of other early predictive tests, such as circulating tumor cell burden, as assessed in the S0500 (Treatment Decision Making Based on Blood Levels of Tumor Cells in Women With Metastatic Breast Cancer Receiving Chemotherapy) trial, has not been shown to improve OS.29 However, we would argue that OS is not the only end point of importance to patients and that methods for identifying patients who can do well with less toxic regimens will be increasingly important as the number of treatment regimens for MBCs (with their attendant AEs) continue to expand.
Our data are also consistent with evidence in the preoperative setting linking early metabolic response with pCR.30,31 In the Neo ALTTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) Study, the rate of pCR was higher among patients who achieved a week-2 metabolic response compared with patients without a week-2 metabolic response (n = 68 with baseline and week-2 data; pCR, 42% v 21%; P = .12).31 Our study supports continued exploration of this approach in preoperative and metastatic trials. The Translational Breast Cancer Research Consortium is exploring the value of preoperative [18F]FDG-PET/CT for pCR with an all-biologic regimen of trastuzumab and pertuzumab (Translational Breast Cancer Research Consortium [TBCRC] 026; [NCT01937117]; Pertuzumab and Trastuzumab as Neoadjuvant Treatment in Patients With HER2-Positive Breast Cancer); related initiatives by the Breast International Group are under way to elucidate whether evaluation of early metabolic response can reduce the need for cytotoxic chemotherapy in lieu of targeted approaches.
Our study has several limitations. First, the treatment landscape for HER2-positive breast cancer continues to evolve. Although we believe that our data support lapatinib and trastuzumab as a valid option in the metastatic setting, we recognize that other options, such as pertuzumab-trastuzumab-taxane combinations may be preferred in many circumstances.19 We acknowledge that the [18F]FDG-PET/CT analyses are exploratory and will need to be confirmed before early metabolic response can influence treatment recommendations in routine clinical practice. In addition, novel methods for assessing metabolic response, such as PET Response Criteria in Solid Tumors (PERCIST), have been developed.32 In the future, we plan to explore the performance of PERCIST and other metabolic parameters versus European Organisation for Research and Treatment of Cancer criteria in our data set. Finally, our results regarding the predictive value of early metabolic response may or may not be generalizable to other regimens, including chemotherapy and other targeted therapies.
In summary, the combination of lapatinib and trastuzumab is active and well tolerated in patients with HER2-positive MBC who have received up to two lines of therapy for advanced disease. Use of early metabolic imaging as a clinically relevant biomarker merits additional investigation, particularly in studies with molecularly targeted therapies. Finally, several molecular correlative analyses of metastatic specimens are under way in the context of this study to elucidate mechanisms of HER2 resistance.
Acknowledgment
We acknowledge Robyn T. Burns, PhD, Translational Breast Cancer Research Consortium patient advocates, Elizabeth S. Lawler, and all of the highly dedicated clinical research coordinators at each participating site for their untiring efforts on behalf of this study.
Presented in part at the 47th Annual American Society of Clinical Oncology Meeting, Chicago, IL, June 3-7, 2011, and the 34th Annual San Antonio Breast Cancer Symposium, San Antonio, TX, December 6-10, 2011.
Glossary Terms
- HER2/neu (human epidermal growth factor receptor 2):
also called ErbB2. HER2/neu belongs to the epidermal growth factor receptor (EGFR) family and is overexpressed in several solid tumors. Like EGFR, it is a tyrosine kinase receptor whose activation leads to proliferative signals within the cells. On activation, the human epidermal growth factor family of receptors are known to form homodimers and heterodimers, each with a distinct signaling activity. Because HER2 is the preferred dimerization partner when heterodimers are formed, it is important for signaling through ligands specific for any members of the family. It is typically overexpressed in several epithelial tumors.
- negative predictive value:
the probability of a negative test result being truly negative.
- pathologic complete response:
the absence of any residual tumor cells in a histologic evaluation of a tumor specimen.
- PI3K/AKT pathway:
signal transduction pathways involving the signaling molecules phosphatidylinositol-3 kinase (PI3K) and AKT, where PI3K generates phosphorylated inositides at the cell membrane, which are required for the recruitment and activation of AKT, a transforming serine-threonine kinase involved in cell survival.
- positive predictive value:
the probability of a positive test result being truly positive.
- RECIST (Response Evaluation Criteria in Solid Tumors):
a model proposed by the Response Evaluation Criteria Group by which a combined assessment of all existing lesions, characterized by target lesions (to be measured) and nontarget lesions, is used to extrapolate an overall response to treatment.
Appendix
Table A1.
Clinical Characteristics of Patients Given Protocol Therapy for More Than 3 Years
| Patient ID | Stage at Diagnosis | Hormone Receptor Status |
Neoadjuvant Chemotherapy | Neoadjuvant Trastuzumab | Prior Metastatic Therapy | |
|---|---|---|---|---|---|---|
| Primary Tumor | Recurrence/Metastasis | |||||
| 034 | IV | ER positive/PgR positive | ER negative/PgR negative | None | No | No |
| 042 | II | ER negative/PgR negative | Not done/not done | Anthracycline and taxane based | No | No |
| 064 | III | ER positive/PgR positive | Not done/not done | Anthracycline based | No | No |
| 087 | III | ER negative/PgR negative | ER negative/PgR negative | Anthracycline and taxane based | Yes | No |
Abbreviations: ER, estrogen receptor; ID, identification; PgR, progesterone receptor.
Table A2.
Summary of AEs With at Least 10% Incidence (all grades or any grade 3 or 4) Deemed Related (definite, probable, possible) to Protocol Therapy, Cohorts 1 and 2 Combined
| AE | Maximum Grade |
|||
|---|---|---|---|---|
| Total | 1 (mild) | 2 (moderate) | 3 (severe) | |
| Diarrhea without prior colostomy | 55 | 41 | 8 | 6 |
| Fatigue | 45 | 38 | 6 | 1 |
| Acne/acneiform rash | 35 | 21 | 14 | 0 |
| Nausea | 22 | 21 | 1 | 0 |
| AST | 21 | 17 | 4 | 0 |
| Hyperglycemia | 14 | 13 | 1 | 0 |
| Anorexia | 13 | 11 | 2 | 0 |
| Vomiting | 12 | 10 | 2 | 0 |
| ALT | 10 | 6 | 4 | 0 |
| Nail changes | 10 | 7 | 3 | 0 |
| Headache | 9 | 8 | 1 | 0 |
| Hemoglobin | 9 | 8 | 1 | 0 |
| Mucositis/stomatitis by examination of oral cavity | 9 | 7 | 2 | 0 |
| Rash/desquamation | 9 | 6 | 3 | 0 |
| GI, other | 1 | 0 | 0 | 1 |
| Hypokalemia | 1 | 0 | 0 | 1 |
NOTE. All patients who received at least one dose of protocol therapy were included in this analysis (n = 86). No grade 4 toxicities were reported on study.
Abbreviation: AE, adverse event.
Table A3.
No. of Patients With [18F]FDG-PET/CT Scan Data by Time Point and Cohort
| Time Point | Cohort 1 | Cohort 2 | Total |
|---|---|---|---|
| Baseline | 39 | 44 | 83 |
| Week 1 | 39 | 43 | 82 |
| Week 8 | 34 | 42 | 76 |
| Week 1 and week 8 | 34 | 41 | 75 |
NOTE. Eighty-seven patients were enrolled onto the trial (cohort 1, 41; cohort 2, 46). Per protocol, patients underwent [18F]fluorodeoxyglucose positron emission tomography/computed tomography ([18F]FDG-PET/CT) scans at baseline, week 1, and week 8. One patient who did not start protocol therapy and one patient who did not have metastatic breast cancer were excluded from all [18F]FDG-PET/CT analyses.
Table A4.
Cross-Tabulation of Week-1 v Week-8 Metabolic Response
| Week-1 PET Response | Week-8 PET Response |
||||
|---|---|---|---|---|---|
| PMR | SMD | PMD | Unknown | Total | |
| Cohort 1 | |||||
| PMR | 24 | 1 | 1 | 2 | 28 |
| SMD | 3 | 1 | 3 | 2 | 9 |
| PMD | 0 | 1 | 0 | 1 | 2 |
| Unknown | 0 | 0 | 0 | 1 | 1 |
| Total | 27 | 3 | 4 | 6 | 40 |
| Cohort 2 | |||||
| PMR | 16 | 5 | 0 | 0 | 21 |
| SMD | 2 | 16 | 2 | 2 | 22 |
| PMD | 0 | 0 | 0 | 0 | 0 |
| Unknown | 0 | 1 | 0 | 1 | 2 |
| Total | 18 | 22 | 2 | 3 | 45 |
NOTE. Seventy-five patients (cohort 1, 34; cohort 2, 41) were evaluable for comparison of week-1 v week-8 metabolic response by [18F]fluorodeoxyglucose positron emission tomography/computed tomography according to European Organisation for Research and Treatment of Cancer criteria.
Abbreviations: PET, positron emission tomography; PMD, progressive metabolic disease; PMR, partial metabolic response; SMD, stable metabolic disease.
Table A5.
Association Between Week-1 Metabolic Response and Clinical Benefit by RECIST v1.0
| Week-1 Metabolic Response | Clinical Benefit No. (%) |
Total | Predictive Value |
||
|---|---|---|---|---|---|
| Yes | No | PPV (%) | NPV (%) | ||
| Cohort 1 | |||||
| PMR | 21 (75.0) | 7 (25.0) | 28 | 75 | 82 |
| SMD/PMD | 2 (18.2) | 9 (81.8) | 11 | ||
| Unknown | 0 (0.0) | 1 (100.0) | 1 | ||
| Total | 23 (57.5) | 17 (42.5) | 40 | ||
| Cohort 2 | |||||
| PMR | 12 (57.1) | 9 (42.9) | 21 | 57 | 77 |
| SMD | 5 (22.7) | 17 (77.3) | 22 | ||
| Unknown | 1 (50.0) | 1 (50.0) | 2 | ||
| Total | 18 (40.0) | 27 (60.0) | 45 | ||
NOTE. Patients were tabulated by clinical benefit (clinical benefit v no clinical benefit) and metabolic response (partial metabolic response [PMR] v stable metabolic disease [SMD]/progressive metabolic disease [PMD]). Clinical benefit was defined as confirmed complete response plus confirmed partial response plus stable disease ≥ 24 weeks. Patients with unconfirmed complete response, unconfirmed partial response, stable disease < 24 weeks, and progressive disease as best response were considered nonresponders for this analysis. Metabolic response was based on European Organisation for Research and Treatment of Cancer criteria.
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
Table A6.
Association Between Week-8 Metabolic Response and Objective Response by RECIST v1.0
| Week-8 Metabolic Response | Total | Objective Response No. (%) |
Predictive Values |
||||
|---|---|---|---|---|---|---|---|
| Responder | Nonresponder | PPV (%) | 95% CI | NPV (%) | 95% CI | ||
| Cohort 1 | |||||||
| PMR | 27 | 19 (70) | 8 (30) | 70 | 53 to 88 | 100 | 100 to 100 |
| SMD/PMD | 7 | 0 (0) | 7 (100) | ||||
| Unknown | 6 | 1 (17) | 5 (83) | ||||
| Total | 40 | 20 (50) | 20 (50) | ||||
| Cohort 2 | |||||||
| PMR | 18 | 7 (39) | 11 (61) | 39 | 16 to 61 | 88 | 74 to 100 |
| SMD | 24 | 3 (13) | 21 (88) | ||||
| Unknown | 3 | 0 (0) | 3 (100) | ||||
| Total | 45 | 10 (22) | 35 (78) | ||||
NOTE. Patients were tabulated by objective response (responder v nonresponder) and metabolic response (partial metabolic response [PMR] v stable metabolic disease [SMD]/progressive metabolic disease [PMD]). Objective response was based on RECIST v1.0 (responder: confirmed complete response or partial response; nonresponder: unconfirmed partial response, stable disease, progressive disease, or unknown). Metabolic response was based on European Organisation for Research and Treatment of Cancer criteria.
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
Fig A1.
Waterfall plots of change in maximum standardized uptake value (ΔSUVmax) relative to baseline. Dashed lines denote European Organisation for Research and Treatment of Cancer cutoffs for metabolic response (−25%) and progression (+25%). Asterisks denote patients who were considered metabolic progressors on the basis of new lesion(s). (A) Cohort 1 and (B) cohort 2 ΔSUVmax at week 1 v baseline; (C) cohort 1 and (D) cohort 2 ΔSUVmax at week 8 v baseline. PMD, progressive metabolic disease; SMD, stable metabolic disease; PMR, partial metabolic response.
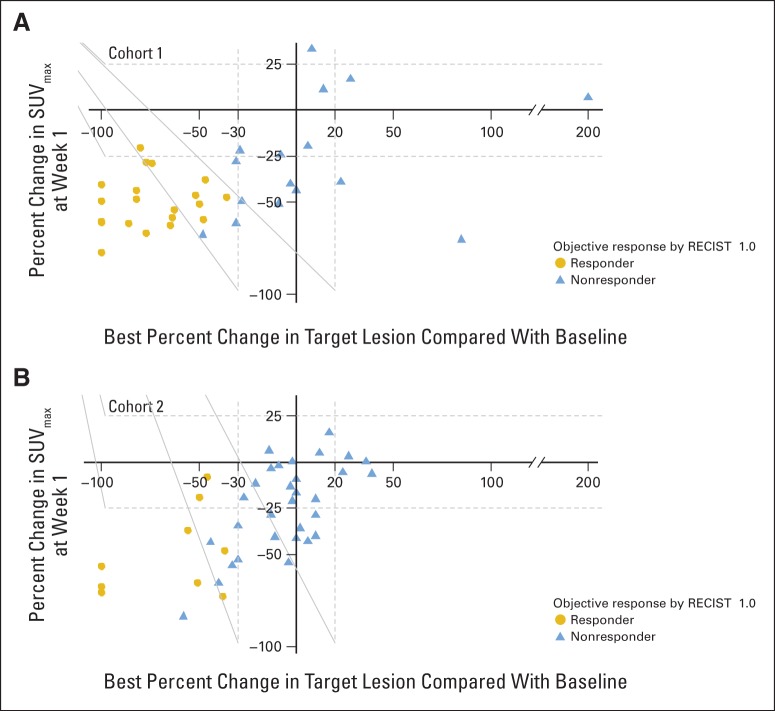
Fig A2.
Relationship between percentage change in maximum standardized uptake value (SUVmax) at week 1 and best percentage change of target lesions. Each individual patient is plotted according to percentage change in SUVmax by [18F]fluorodeoxyglucose positron emission tomography/computed tomography (CT) comparing baseline and week 1 on the y-axis and according to best percentage change in sum of longest dimensions of target lesions relative to baseline by CT imaging on the x-axis. Seven patients were considered RECIST nonresponders despite reductions of more than 30% in the sum of target lesions. Of these patients, six had an initial partial response that was not confirmed on subsequent imaging 8 weeks later: one had stable disease > 24 weeks and one had stable disease on initial restaging and more than 30% on second restaging but in the setting of a new lesion and was therefore deemed a nonresponder.
Footnotes
See accompanying editorial on page 2591
Written on behalf of the Translational Breast Cancer Research Consortium.
Supported by the AVON Foundation, Breast Cancer Research Foundation, Susan G. Komen for the Cure, GlaxoSmithKline, Grant No. CA089393 from the National Cancer Institute (NCI) Specialized Program of Research Excellence (SPORE) at Dana-Farber/Harvard Cancer Center, Grant No. CA089393-10S1 from the NCI SPORE Avon Supplement, The Nancy and Randy Berry Junior Faculty Fund (N.U.L.), Dunkin' Donuts Rising Star Program (N.U.L.), and the CJL Foundation (W.T.B.).
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
The funding sources for the study were not involved in the collection, analysis, or interpretation of the data. GlaxoSmithKline received a draft of the manuscript before publication; however, it was not involved in writing the report or in the decision to submit it for publication. N.U.L. had full access to all the data in the study and had final responsibility for the decision to submit the article for publication.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00470704.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Nancy U. Lin, Jeffrey T. Yap, Ingrid A. Mayer, Carla I. Falkson, Timothy J. Hobday, E. Claire Dees, Andrea L. Richardson, Rita Nanda, Mothaffar F. Rimawi, Antonio C. Wolff, Ian E. Krop, Eric P. Winer, Annick D. Van den Abbeele
Financial support: Nancy U. Lin, Ian E. Krop, Eric P. Winer
Administrative support: Nicole Ryabin
Provision of study materials or patients: Nancy U. Lin, Ingrid A. Mayer, Carla I. Falkson, Timothy J. Hobday, E. Claire Dees, Rita Nanda, Mothaffar F. Rimawi, Carlos L. Arteaga, Ian E. Krop, Eric P. Winer
Collection and assembly of data: Nancy U. Lin, Hao Guo, Jeffrey T. Yap, Ingrid A. Mayer, Carla I. Falkson, Timothy J. Hobday, E. Claire Dees, Andrea L. Richardson, Rita Nanda, Mothaffar F. Rimawi, Nicole Ryabin, Ian E. Krop, Eric P. Winer, Annick D. Van den Abbeele
Data analysis and interpretation: Nancy U. Lin, Hao Guo, Jeffrey T. Yap, Ingrid A. Mayer, Carla I. Falkson, Timothy J. Hobday, E. Claire Dees, Andrea L. Richardson, Rita Nanda, Mothaffar F. Rimawi, Julie S. Najita, William T. Barry, Carlos L. Arteaga, Antonio C. Wolff, Ian E. Krop, Eric P. Winer, Annick D. Van den Abbeele
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Study of Lapatinib in Combination With Trastuzumab in Patients With Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer: Clinical Outcomes and Predictive Value of Early [18F]Fluorodeoxyglucose Positron Emission Tomography Imaging (TBCRC 003)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Nancy U. Lin
Consulting or Advisory Role: Novartis, GlaxoSmithKline, Genentech
Research Funding: Genentech, GlaxoSmithKline, Array BioPharma, Novartis, Geron, Kadmon
Hao Guo
No relationship to disclose
Jeffrey T. Yap
No relationship to disclose
Ingrid A. Mayer
Consulting or Advisory Role: Novartis, Genentech/Roche
Research Funding: Novartis
Carla I. Falkson
No relationship to disclose
Timothy J. Hobday
No relationship to disclose
E. Claire Dees
Consulting or Advisory Role: Novartis (I), Amgen (I)
Research Funding: Novartis, Genentech/Roche, Bayer HealthCare Pharmaceuticals, Eli Lilly/ImClone Systems, Pfizer, Millennium Pharmaceuticals, Agensys
Andrea L. Richardson
Consulting or Advisory Role: Myriad Genetics
Patents, Royalties, Other Intellectual Property: Myriad Genetics
Rita Nanda
No relationship to disclose
Mothaffar F. Rimawi
Research Funding: GlaxoSmithKline, AstraZeneca
Nicole Ryabin
No relationship to disclose
Julie S. Najita
No relationship to disclose
William T. Barry
No relationship to disclose
Carlos L. Arteaga
No relationship to disclose
Antonio C. Wolff
Consulting or Advisory Role: Mersana Therapeutics
Research Funding: Myriad Genetics (Inst), Genentech (Inst)
Ian E. Krop
Employment: Vertex Pharmaceuticals (I)
Stock or Other Ownership: Vertex Pharmaceuticals (I)
Consulting or Advisory Role: Amgen
Research Funding: Genentech
Travel, Accommodations, Expenses: Bayer HealthCare Pharmaceuticals
Eric P. Winer
Consulting or Advisory Role: Verastem
Research Funding: Genentech/Roche (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Genentech/Roche, Novartis
Annick D. Van Den Abbeele
No relationship to disclose
REFERENCES
- 1.Di Fiore PP, Pierce JH, Kraus MH, et al. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987;237:178–182. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- 2.Muller WJ, Sinn E, Pattengale PK, et al. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54:105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- 3.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 6.Storniolo AM, Pegram MD, Overmoyer B, et al. Phase I dose escalation and pharmacokinetic study of lapatinib in combination with trastuzumab in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2008;26:3317–3323. doi: 10.1200/JCO.2007.13.5202. [DOI] [PubMed] [Google Scholar]
- 7.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 8.Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: Final results from the EGF104900 study. J Clin Oncol. 2012;30:2585–2592. doi: 10.1200/JCO.2011.35.6725. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network (NCCN) NCCN Guidlines: Breast Cancer Version 3.2014. 2014.
- 10.Garrett JT, Olivares MG, Rinehart C, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A. 2011;108:5021–5026. doi: 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of [18F]FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–1066. [PubMed] [Google Scholar]
- 12.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations—European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson EN, Brown BW. Confidence limits for probability of response in multistage phase II clinical trials. Biometrics. 1985;41:741–744. [PubMed] [Google Scholar]
- 14.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 15.R Development Core Team. A language and environment for statistical computing, R Foundation for Statistical Computing. Vienna: Austria; 2007. [Google Scholar]
- 16.Gomez HL, Doval DC, Chavez MA, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol. 2008;26:2999–3005. doi: 10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- 17.Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2011;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giordano SH, Temin S, Kirshner JJ, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:2078–2099. doi: 10.1200/JCO.2013.54.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guarneri V, Frassoldati A, Bottini A, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: Results of the randomized phase II CHER-LOB study. J Clin Oncol. 2012;30:1989–1995. doi: 10.1200/JCO.2011.39.0823. [DOI] [PubMed] [Google Scholar]
- 21.Robidoux A, Tang G, Rastogi P, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): An open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1183–1192. doi: 10.1016/S1470-2045(13)70411-X. [DOI] [PubMed] [Google Scholar]
- 22.Bonnefoi H, Jacot W, Saghatchian M, et al. Neoadjuvant treatment with docetaxel plus lapatinib, trastuzumab, or both followed by an anthracycline-based chemotherapy in HER2-positive breast cancer: Results of the randomised phase II EORTC 10054 study. Ann Oncol. 2015;26:325–332. doi: 10.1093/annonc/mdu551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey LA, Berry DA, Olilia D, et al. Clinical and translational results of CALGB 40601: A neoadjuvant phase III trial of weekly paclitaxel and trastuzumab with or without lapatinib for HER2-positive breast cancer. J Clin Oncol. 2013;(suppl 15s):31. abstr 500. [Google Scholar]
- 24.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rimawi MF, Mayer IA, Forero A, et al. Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. J Clin Oncol. 2013;31:1726–1731. doi: 10.1200/JCO.2012.44.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piccart-Gebhart MJ, Holmes AP, Baselga J, et al. First results from the phase III ALTTO trial (BIG 2-06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T→L), or their combination (T+L) in the adjuvant treatment of HER2-positive early breast cancer (EBC) J Clin Oncol. 2014;(suppl 5s):32. abstr LBA4. [Google Scholar]
- 27.Groheux D, Espié M, Giacchetti S, et al. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266:388–405. doi: 10.1148/radiol.12110853. [DOI] [PubMed] [Google Scholar]
- 28.Mayer IA, Abramson VG, Isakoff SJ, et al. Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2014;32:1202–1209. doi: 10.1200/JCO.2013.54.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smerage JB, Barlow WE, Hortobagyi GN, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol. 2014;32:3483–3489. doi: 10.1200/JCO.2014.56.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coudert B, Pierga JY, Mouret-Reynier MA, et al. Use of [(18)F]-FDG PET to predict response to neoadjuvant trastuzumab and docetaxel in patients with HER2-positive breast cancer, and addition of bevacizumab to neoadjuvant trastuzumab and docetaxel in [(18)F]-FDG PET-predicted non-responders (AVATAXHER): An open-label, randomised phase 2 trial. Lancet Oncol. 2014;15:1493–1502. doi: 10.1016/S1470-2045(14)70475-9. [DOI] [PubMed] [Google Scholar]
- 31.Gebhart G, Gámez C, Holmes E, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: Results from Neo-ALTTO. J Nucl Med. 2013;54:1862–1868. doi: 10.2967/jnumed.112.119271. [DOI] [PubMed] [Google Scholar]
- 32.Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]