Abstract
Purpose
Given concerns that dexrazoxane may reduce treatment efficacy, induce second cancers, and thus compromise overall survival among children, we examined long-term overall and cause-specific mortality and disease relapse rates from three randomized clinical trials.
Patients and Methods
Children's Oncology Group trials P9404 (T-cell acute lymphoblastic leukemia/lymphoma; n = 537), P9425 (intermediate/high-risk Hodgkin lymphoma; n = 216), and P9426 (low-risk Hodgkin lymphoma; n = 255) were conducted between 1996 and 2001. Each trial randomly assigned patients to doxorubicin with or without dexrazoxane. The dexrazoxane:doxorubicin dose ratio was 10:1, and the cumulative protocol-specified doxorubicin dose was 100 to 360 mg/m2. Dexrazoxane was given as an intravenous bolus before each doxorubicin dose. Data from all three trials were linked with the National Death Index to determine overall and cause-specific mortality by dexrazoxane status.
Results
Among 1,008 patients (507 received dexrazoxane) with a median follow-up of 12.6 years (range, 0 to 15.5 years), 132 died (67 received dexrazoxane). Overall mortality did not vary by dexrazoxane status (12.8% with dexrazoxane at 10 years v 12.2% without; hazard ratio [HR], 1.03; 95% CI, 0.73 to 1.45). Findings were similar when each trial was examined separately. Dexrazoxane also was not significantly associated with differential causes of death. The original cancer caused 76.5% of all deaths (HR, 0.90; 95% CI, 0.61 to 1.32) followed by second cancers (13.6% of deaths; HR, 1.24; 95% CI, 0.49 to 3.15). Specifically, dexrazoxane was not associated with deaths from acute myeloid leukemia/myelodysplasia or cardiovascular events.
Conclusion
Among pediatric patients with leukemia or lymphoma, after extended follow-up, dexrazoxane use did not seem to compromise long-term survival.
INTRODUCTION
Premature cardiovascular disease is one of the leading causes of morbidity and mortality among survivors of childhood cancer.1,2 Given the widespread use of anthracyclines and their associated cardiotoxicity, developing an effective cardioprotective strategy is important.3,4 Studies in adults show that dexrazoxane (DRZ) minimizes the development of clinical and subclinical heart failure after anthracycline therapy.3,5 However, studies in children have not been definitive.6–8
To determine the efficacy of DRZ in contemporary anthracycline-containing protocols, the Children's Oncology Group (COG) conducted three phase III trials that randomly assigned patients to DRZ as part of initial cancer therapy: P9404 (T-cell acute lymphoblastic leukemia/lymphoma), P9425 (intermediate- and high-risk Hodgkin lymphoma [HL]), and P9426 (low-risk HL). The outcomes of these trials have been published individually, and they showed that DRZ did not appear to affect overall survival or event-free survival9–11 and may have some protective cardiovascular effects.12
However, concerns regarding how DRZ interacts with cancer therapies and its possible association with an increased risk of second cancers have limited its use among children.13–16 To better assess the effect of DRZ on mortality, we aggregated the overall and cause-specific mortality data from all three studies, in which the median follow-up time is now more than 12 years (on average, > 4 years longer than the individual study reports),9–11 and secondarily, we also examined the overall rates of original disease relapse.
PATIENTS AND METHODS
Patients and Treatments
Between 1996 and 2001, P9404, P9425, and P9426 enrolled 1,008 patients across 133 institutions (Table 1).9–11 Patients were randomly assigned to treatment with or without DRZ. DRZ was given as an intravenous bolus before each doxorubicin dose in a DRZ:doxorubicin dose ratio of 10:1. Individual doxorubicin doses ranged from 25 to 30 mg/m2 depending on the trial, with cumulative doses ranging from 100 to 360 mg/m2 across the three trials. No other anthracyclines were part of treatment. In P9425, patients receiving DRZ also received it at the same 10:1 dose ratio with their day-8 bleomycin dose (1 week after doxorubicin). Although COG did not record actual prescribed doses, study chairs reviewed all chemotherapy roadmaps (> 90% compliance per B. Asselin and C. Schwartz; personal communication, December 2014), and COG performs institutional audits to further ensure compliance with study protocols.
Table 1.
Overview of COG Randomized Trials of Dexrazoxane
| Characteristic | P94049 | P942510 | P942611 |
|---|---|---|---|
| Patients | |||
| Diagnosis | T-cell acute lymphoblastic leukemia/lymphoma | Intermediate-/high-risk Hodgkin lymphoma | Low-risk Hodgkin lymphoma |
| No. of patients enrolled | 537 | 216 | 255 |
| Median age at diagnosis, years (range) | 9.6 (1.0-22.0) | 14.8 (3.7-20.8) | 14.0 (2.1-20.9) |
| Treatment | DFCI 87-01 backbone, with or without high-dose methotrexate,* with or without dexrazoxane | Response-adapted, 3 to 5 courses ABVE-PC, with or without dexrazoxane | Response-adapted, 2 to 4 courses ABVE alone, with or without dexrazoxane |
| Cumulative doxorubicin dose, mg/m2 | 360† | 180-300‡ | 100-200‡ |
| Radiotherapy dose (target), Gy | 18 (cranial) | 21 (involved region) | 25.5 (involved field) |
| Median time since diagnosis, years (range)§ | 12.4 (0-15.5) | 13.0 (0-14.7) | 12.4 (1.4-15.0) |
Abbreviations: ABVE-PC, doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide; COG, Children's Oncology Group; DFCI, Dana-Farber Cancer Institute.
High-dose methotrexate randomization closed early after 436 patients; the remaining 101 patients were assigned exclusively to the high-dose methotrexate arm.
Although COG did not record actual doses received, even if a patient experienced delays during treatment or required dose reductions, the protocol required patients to still receive up to this cumulative dose.
Dose varied depending on initial response, and accordingly, the total number of chemotherapy courses. COG did not record actual doses received.
For US patients, death or December 31, 2011, whichever occurred first; for patients outside the United States, death, date last seen reported to COG, or December 31, 2011, whichever occurred first.
The three studies were also designed to answer additional questions. P9404 determined whether adding high-dose methotrexate to a Dana-Farber Cancer Institute 87-01 protocol backbone would improve event-free survival among children with T-cell acute lymphoblastic leukemia/lymphoma.9 P9425 determined the efficacy of dose-dense doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide (ABVE-PC) given in a response-based approach (three courses if early response was rapid and five courses if response was slow) followed by involved region consolidative radiotherapy (21 Gy) among intermediate- and high-risk patients with HL.10 Results were compared with historical data. P9426 determined the efficacy of giving ABVE alone in a response-based approach (two courses if response was complete and four courses if response was partial or disease was stable) followed by involved-field consolidation radiotherapy (25.5 Gy) among patients with low-risk HL.11 Results were compared with historical data. Participants or their legal guardians provided written informed consent in accordance with the Declaration of Helsinki, and all studies were approved by the relevant institutional review boards.
Outcomes
Our primary outcomes were overall and cause-specific mortality, particularly death as a result of the original cancer, second cancer, or a cardiovascular cause. Of secondary interest was risk of relapse (including progression of tumor without achieving initial remission), given concerns that DRZ might reduce the efficacy of chemotherapy. Data for all three studies were aggregated by the COG Statistical and Data Center on June 27, 2013. Although the three studies ended accrual between 2000 and 2001, formal data reporting by participating sites continued until June 2008 for P9425 and P9426, and data reporting is ongoing for P9404. This analysis used the most recent P9404 data set (September 2011). Participating sites provided information on any relapse, second cancer, and death (including whether the cause was attributed to the original tumor, treatment toxicity, infection, hemorrhage, or other causes).
To enhance COG follow-up data on mortality (Appendix Table A1, online only), we submitted COG data from US sites (name, birth date, sex, race, state of residence [state where the COG site was located if the patient's residence was unknown], date of death, and the date the patient was last seen) to the National Death Index (NDI) to determine overall and cause-specific mortality through December 31, 2011 (most recent year for which data were available at the time of data linkage). Because unique identifiers (eg, Social Security number) were not available to COG, the NDI performed probabilistic matching with true matches determined by NDI on the basis of the number of matching elements and the importance of each element as determined by their relative uniqueness in NDI files and in National Health Interview Survey data.17,18 For deaths not previously reported to COG, two investigators (D.R.D. and E.J.C.) blinded to DRZ status reviewed all matches made by NDI (both those that NDI deemed true and those likely to be false) to ensure that all true matches would be included (all were) and that false matches would not be included (none were). NDI provided information on the underlying cause of death and up to 20 contributing causes coded by using the International Classification of Diseases, revision 9 (ICD-9; before 1999) or revision 10 (ICD-10; 1999 onwards).
COG and NDI data were then combined to form the following seven cause-of-death categories: original tumor without history of relapse, original tumor with history of relapse (or bone marrow transplant), second cancer, cardiovascular cause not immediately attributable to active/recent cancer therapy, other acute treatment-related toxicity, other cause not elsewhere classified, and unknown. Three investigators (C.L.S., E.J.C., and S.H.A.) blinded to DRZ status reviewed all death records and determined the probable cause of death by consensus. When COG and NDI data were discordant, preference was typically given to COG data. For each death, a primary cause was assigned and, if relevant, secondary and tertiary causes also were assigned. Patients determined to have died from second cancers preferentially had the second cancer coded as the underlying cause of death. Any NDI record in which the tumor histology differed from that of the original cancer was reviewed in detail.
Statistical Analysis
All analyses were conducted by DRZ intent-to-treat status. We examined the cumulative incidence (at 10-year follow-up) and hazard ratios (HRs) for overall mortality, cause-specific mortality, and relapse. For overall mortality, we used Kaplan-Meier methods to estimate cumulative incidence and Cox proportional hazards models to estimate the HR. For other outcomes, we used methods that accounted for competing events (death as a result of any cause for relapse; death as a result of other causes for cause-specific mortality).19,20 Because data from NDI were available only through 2011, we set December 31, 2011, as the date of last follow-up for our mortality analyses. COG did not report any instances of relapse or deaths beyond that date because most participants had ceased active follow-up many years earlier. In our analysis of relapse risk, patients were censored at their last COG contact date. Because NDI provided information only on US residents, participants from non-US COG sites (n = 106; 54 received DRZ) were censored on their COG date of last contact or December 31, 2011, whichever occurred earlier. Proportionality of Cox models was assessed via Schoenfeld residuals, and for competing risks models, by testing the interaction of DRZ with analysis time. In subanalyses, we modeled relapse as a time-dependent covariate because it is often associated with both higher original cancer mortality and other adverse outcomes. We also examined outcomes for each trial (P9404, P9425, and P9426) separately and COG or NDI-defined deaths separately. Alpha was set at .05, and all tests were two-tailed. Data were analyzed with STATA version 13 (STATA, College Station, TX).
RESULTS
Among the entire study population (n = 1,008), patient demographics, including follow-up duration, were similar by DRZ status (Table 2; Appendix Table A2, online only). Over a median follow-up of 12.6 years (range, 0 to 15.5 years), 174 patients (17.3%) experienced relapse or progressive disease, and 132 died (13.1%; Table 3). Of these deaths, 125 were ascertained from COG records and 104 were ascertained from NDI; 97 were in both data sources. Among 28 deaths recorded by COG but not by NDI, 14 (seven received DRZ) were reported by institutions outside the United States for which NDI data were not available. All 104 deaths (52 received DRZ) from NDI occurred in patients treated at US sites. The NDI linkage identified seven deaths (two received DRZ) previously unknown to COG.
Table 2.
Characteristics of Patients Enrolled Onto COG Randomized Trials of DRZ
| Characteristic | Trial | Received DRZ (n = 507) No. (%) | Did Not Receive DRZ (n = 501) No. (%) |
|---|---|---|---|
| Female sex | 159 (31.4) | 152 (30.3) | |
| Race/ethnicity | |||
| White non-Hispanic | 322 (63.5) | 324 (64.7) | |
| Black | 74 (14.6) | 88 (17.6) | |
| Hispanic | 20 (3.9) | 16 (3.2) | |
| Asian/Native American | 6 (1.2) | 2 (0.4) | |
| Other | 8 (1.6) | 9 (1.8) | |
| Unknown | 77 (15.2) | 62 (12.4) | |
| Cancer diagnosis | |||
| T-cell acute lymphoblastic leukemia | P9404 | 187 (36.9) | 175 (34.9) |
| Lymphoblastic lymphoma | P9404 | 86 (17.0) | 89 (17.8) |
| Hodgkin lymphoma | 234 (46.2) | 237 (47.3) | |
| Low risk | P9426 | 127 | 128 |
| Intermediate or high risk | P9425 | 107 | 109 |
| Median age at treatment, years (range) | 12.6 (1.0-22.0) | 12.6 (1.3-21.0) | |
| < 5 | 61 (12.0) | 60 (12.0) | |
| 5-9 | 126 (24.9) | 109 (21.8) | |
| 10-14 | 162 (32.0) | 181 (36.1) | |
| 15+ | 158 (31.2) | 151 (30.1) | |
| Median age at last follow-up, years (range)* | 23.5 (3.4-36.6) | 23.8 (3.0-35.2) | |
| Median time since diagnosis, years (range)* | 12.6 (0-15.5) | 12.6 (0-15.5) | |
| P9404 | 12.6 (0-15.5) | 12.4 (0-15.5) | |
| P9425 | 13.1 (0-14.6) | 13.0 (1.6-14.7) | |
| P9426 | 12.4 (1.4-15.0) | 12.4 (1.8-14.8) |
Abbreviations: COG, Children's Oncology Group; DRZ, dexrazoxane.
For US patients, death or December 31, 2011, whichever occurred first; for patients outside the United States, death, date last seen reported to COG, or December 31, 2011, whichever occurred first. In our analysis of relapse risk, follow-up was limited to date last seen as reported to COG, death, or December 31, 2011, whichever occurred first (patients exposed to DRZ: median, 8.1 years [range, 0-15.0 years]; patients not exposed to DRZ: median, 8.0 years [range, 0-14.7 years]).
Table 3.
Outcomes of the COG Randomized Trials of DRZ
| Outcome | No. of Patients |
Received DRZ (n = 507) |
Did Not Receive DRZ (n = 501) |
HR (95% CI)* | |
|---|---|---|---|---|---|
| With DRZ | Without DRZ | No. (%) | No. (%) | ||
| Relapse/progressive disease | 79 (15.6) | 95 (19.0) | 0.81 (0.60 to 1.08) | ||
| P9404† | 273 | 264 | 50 (18.3) | 63 (23.9) | 0.75 (0.52 to 1.08) |
| P9245† | 107 | 109 | 15 (14.0) | 15 (13.8)) | 1.01 (0.50 to 2.06) |
| P9426† | 127 | 128 | 14 (11.0) | 17 (13.3) | 0.81 (0.40 to 1.64) |
| Overall deaths | 67 (13.2) | 65 (13.0) | 1.03 (0.73 to 1.45) | ||
| Clinical trials data (COG) only | 65 (12.8) | 60 (12.0) | 1.07 (0.75 to 1.52) | ||
| National Death Index only‡ | 52 (11.5) | 52 (11.6) | 1.00 (0.68 to 1.47) | ||
| P9404† | 273 | 264 | 53 (19.4) | 54 (20.5) | 0.95 (0.65 to 1.39) |
| P9245† | 107 | 109 | 9 (8.4) | 7 (6.4) | 1.34 (0.50 to 3.59) |
| P9426† | 127 | 128 | 5 (3.9) | 4 (3.1) | 1.26 (0.34 to 4.70) |
| Cause-specific mortality§ | |||||
| Original cancer | 48 (9.5) | 53 (10.6) | 0.90 (0.61 to 1.32) | ||
| Second cancer | 10 (2.0) | 8 (1.6) | 1.24 (0.49 to 3.15) | ||
| Other toxicity | 7 (1.4) | 3 (0.6) | 2.31 (0.60 to 8.95) | ||
| Other specified | 1 (0.2) | 1 (0.2) | — | ||
| Unknown | 1 (0.2) | 0 | — | ||
Abbreviations: COG, Children's Oncology Group; DRZ, dexrazoxane.
Cox regression used for overall mortality; competing risk models20 used for relapse/progressive disease and cause-specific mortality.
Proportions in this row based on denominator of those treated on each arm of the specific study listed.
NDI only links to US deaths, and all 104 deaths identified were for patients treated at US COG sites (n = 902; patients exposed to DRZ: n = 453).
No cardiovascular cause assigned as underlying cause of death.
Overall, DRZ exposure was not associated with an increased hazard of relapse (HR, 0.81; 95% CI, 0.60 to 1.08) or death (HR, 1.03; 95% CI, 0.73 to 1.45; Table 3). At 10 years, the cumulative incidence of relapse was 16.1% for those treated with DRZ and 19.1% for those without (difference, −3.0%; 95% CI, −7.9% to 0.2%). Overall mortality was 12.8% for those treated with DRZ and 12.2% for those without (difference, 0.6%; 95% CI, −3.5% to 4.7%; Fig 1). Findings were similar for deaths ascertained separately by COG and by NDI, and neither relapse nor overall mortality rates differed significantly by DRZ status in any individual trial (Table 3).
Fig 1.
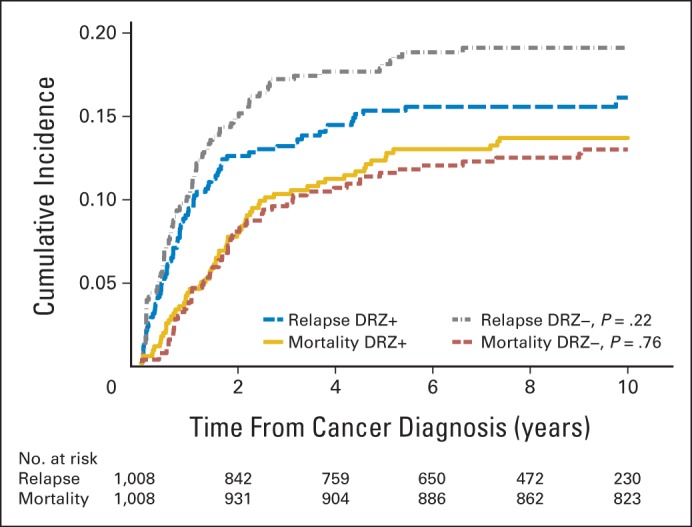
Cumulative incidence of relapse and overall mortality in the combined Children's Oncology Group randomized trials of dexrazoxane (DRZ). Cumulative incidences at 10 years were not significantly different by DRZ status for either outcome. DRZ+, exposed to DRZ; DRZ−, not exposed to DRZ.
The original cancer caused 76.5% of all deaths, and second cancers caused 13.6% (Table 3; Fig 2; numbers at risk are the same as for overall mortality in Figure 1). However, the proportion of deaths in either category did not differ by DRZ status (HR, 0.90; 95% CI, 0.61 to 1.32 for the original cancer; HR, 1.24; 95% CI, 0.49 to 3.15 for second cancers). The risk of death as a result of second cancers remained unchanged, even when relapse was modeled as a time-dependent variable (HR, 1.25; 95% CI, 0.49 to 3.19). From NDI records, two new second cancer deaths not known to COG were included (both in patients not exposed to DRZ). These two deaths (as a result of acute myeloid leukemia/myelodysplastic syndrome [AML/MDS] and an unspecified bronchus/lung tumor) occurred 3.4 and 6.1 years after COG's date of last known follow-up, respectively (Table 4). If only COG data were considered, the risk for death as a result of second cancers was greater but remained statistically nonsignificant (HR, 1.77; 95% CI, 0.59 to 5.28; P = .31).
Fig 2.
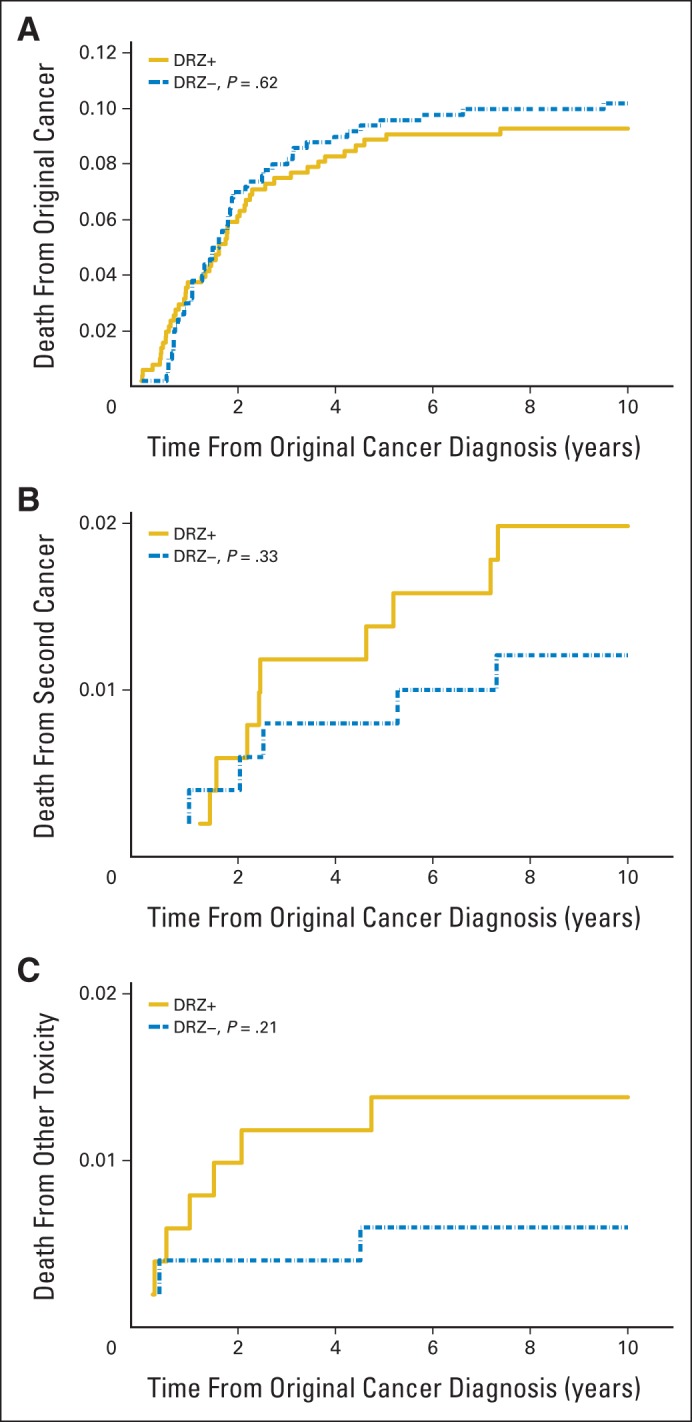
Cumulative mortality through 10 years attributed to (A) original cancer, (B) second cancer, and (C) other toxicity by dexrazoxane (DRZ) status. Cumulative mortality at 10 years was not significantly different by DRZ status for any of the three outcomes. The y-axis scales differ. DRZ+, exposed to DRZ; DRZ−, not exposed to DRZ.
Table 4.
Second Cancer Deaths in COG Randomized Trials of DRZ
| Type of Second Cancer | Received DRZ (n = 507) | Did Not Receive DRZ (n = 501) |
|---|---|---|
| Hematologic malignancy | ||
| Acute myeloid leukemia/myelodysplastic syndrome | 7 | 5* |
| P9404 | 3 | 3* |
| P9425 | 1 | 1 |
| P9426 | 3 | 1 |
| Non-Hodgkin lymphoma | 1† | 1† |
| Other malignancies | ||
| Astrocytoma | 1† | — |
| Atypical teratoid rhabdoid brain tumor | — | 1‡ |
| Chest/lung Ewing sarcoma | 1‡ | — |
| Bronchus/lung tumor not otherwise specified | — | 1‡§ |
| Total | 10 | 8 |
Abbreviations: COG, Children's Oncology Group; DRZ, dexrazoxane.
One case based on National Death Index records alone and not reported to COG; death occurred > 3 years after COG date of last known follow-up.
Original cancers were acute lymphoblastic leukemias.
Original cancers were Hodgkin lymphomas diagnosed > 12 years earlier and > 6 years after COG date of last known follow-up.
Based on National Death Index records alone and not reported to COG.
Of the 18 deaths attributed to second cancers (10 received DRZ; Table 4), 12 were from AML/MDS (seven received DRZ), two were new non-Hodgkin lymphomas (one received DRZ), and four were new solid tumors (two received DRZ). The 10-year AML/MDS mortality rate was 1.4% for those treated with DRZ and 0.8% for those without (difference, 0.6%; 95% CI, −0.7% to 1.9%; HR, 1.39; 95% CI, 0.44 to 4.37). From COG data alone, 13 patients with AML/MDS were reported (nine received DRZ); 11 were deceased. The NDI linkage contributed one additional patient (no DRZ). If all patients (not just deceased) with incident AML/MDS on the basis of COG data alone were examined (n = 13), the HR associated with DRZ was 2.21 (95% CI, 0.68 to 7.18; P = .19).
Among 10 deaths attributed to other treatment-related toxicities (seven received DRZ; HR, 2.31; 95% CI, 0.60 to 8.95), infection was reported as the underlying reason in seven (five received DRZ). No patient died as a result of a cardiovascular cause, although it was listed as a secondary cause for four patients, including one with acute myocardial infarct and one with stroke (both received DRZ), and two with cardiomyopathy/heart failure (neither received DRZ). The underlying cause of death in these four patients was the original tumor.
DISCUSSION
After more than 12 years of follow-up, overall mortality did not differ by DRZ status in three pediatric leukemia and lymphoma trials (n = 1,008). DRZ also did not appear to interfere with cancer treatment efficacy, in terms of original cancer mortality or overall risk of relapse. Although the risk for secondary cancer mortality (mainly as a result of AML/MDS) was greater among those exposed to DRZ, the overall number of events was small, and the differences were not statistically significant.
DRZ use among children has been limited and controversial.1,14–16 Several randomized trials, primarily featuring adult patients with breast cancer, found that DRZ clearly decreased the risk of heart failure following anthracycline-based therapy.3 However, data for childhood cancer survivors have not been definitive, because heart failure can develop over a much longer time period in children compared with adult patients with cancer; a protracted follow-up would be required to observe a clinically significant difference. In a recent analysis from the Childhood Cancer Survivor Study (CCSS), survivors who were predicted (on the basis of sex, treatment age, and anthracycline and chest radiotherapy doses) to be at high risk for clinical heart failure at the end of cancer therapy had a 41-fold relative risk of heart failure compared with siblings but a cumulative incidence of only 12% at age 40 years.21 For predicted moderate-risk individuals (12-fold relative risk v siblings), the cumulative incidence at age 40 was only 2%. However, extended follow-up of the CCSS cohort through age 50 showed that the absolute risks increased significantly with additional age.2 Although the patients in the three trials we analyzed would all be classified as moderate to high risk in the CCSS prediction models, given that the median age of survivors in our study was only 24 years at last follow-up, our study would have been underpowered to detect an important difference in cardiac mortality, even if DRZ was an effective cardioprotectant.
Studies of DRZ cardioprotection in children have therefore focused on intermediate end points. Wexler et al6 found that randomly assigned patients with sarcoma (n = 38) who received DRZ had significantly smaller decreases in left ventricular ejection fraction during treatment than those who did not, which enabled the DRZ group to receive higher cumulative doxorubicin doses. In the Dana-Farber ALL Consortium 95-01 protocol (n = 206), Lipshultz et al7 reported that those who received DRZ had significantly fewer episodes of troponin increases compared with controls. Five years after diagnosis, pathologic left ventricular remodeling (per echocardiographic changes) was less common in the DRZ group than in the controls.8
Given that DRZ is also a weak topoisomerase II inhibitor, theoretically it could reduce the efficacy of other chemotherapies targeting this protein (eg, anthracyclines and epipodophyllotoxins).13 Of the three trials we examined, P9425 had previously reported a borderline association between DRZ and slow early-tumor response (P = .07).10 However, DRZ status was not associated with an increased risk of relapse in the three trials we studied, although late relapses may not have been reliably recorded by COG as a result of loss of follow-up. Other randomized trials of children6,8 and adults3,14 have not found DRZ to be associated with reduced treatment efficacy.
DRZ use in children also has been limited by concerns that it may induce second cancers as a topoisomerase inhibitor.13,15 This report included all children with HL initially reported by Tebbi et al13 but with longer follow-up, allowing us to further evaluate the potential risks presented in that study. In this updated analysis, we found no significant association with death as a result of second cancers, but there were relatively few events (18, including 12 deaths as a result of AML/MDS), although our analysis remains one of the largest to have considered this association. A focus on mortality also underestimates the true incidence of second cancers.12,13 Although our NDI linkage may not be perfect because of the lack of a unique identifier, it was one way that we could attempt to comprehensively ascertain mortality because more than two thirds of patients had not been seen at their original COG center for more than 3 years (Appendix Table A1). Given the lack of a national cancer registry, no such mechanism was available to similarly ascertain all incident second cancers. Attempting to combine new information on second cancer mortality with prior data on second cancer incidence is problematic, because such efforts will miss individuals with new incident cancers who did not die. However, for the particular concern over possible associations between DRZ exposure and secondary AML/MDS,13 a mortality-focused analysis with a median follow-up of more than 10 years would be unlikely to miss a strong association given that secondary AML/MDS, particularly those instances attributed to topoisomerase inhibitor exposure, has a short median time of onset (typically < 5 years) and is associated with high mortality.22
Other studies in children have not observed an increased risk of second cancers after treatment with DRZ. This includes 553 patients with ALL who were exposed to DRZ on consecutive Dana-Farber ALL Consortium trials, including 95-01, which reported an overall 5-year cumulative incidence of second cancers of 0.24% based on a single patient with AML.23 A retrospective study that used 12 years of administrative data from 43 US children's hospitals estimated a secondary AML rate of 0.21% among more than 1,400 patients exposed to DRZ (v 0.55% among patients who were not exposed to DRZ; adjusted odds ratio, 0.4; P = .12).24 In our study, the 10-year second cancer mortality rate was 1.2% among patients not exposed to DRZ and 2.0% among patients exposed to DRZ, which may reflect exposure of all patients to radiotherapy and chemotherapy (etoposide, doxorubicin, and/or cyclophosphamide) associated with an increased risk of second cancers. Among childhood cancers, HL survivors historically have the highest second cancer mortality rates, two- to three-fold that of acute lymphoblastic leukemia survivors.25 Long-term HL survivors also have exceptionally high rates of late cardiac mortality, with annual rates estimated to be more than twice the mortality resulting from second cancers.25
Given that second cancers and symptomatic cardiac disease appear to be by far the two most common categories of serious late effects (in terms of both absolute and relative risks) among long-term childhood cancer survivors as a group (not just HL survivors), with cumulative incidences of each approaching 20% by age 50 years,2 any strategy that offers the promise of reduced cardiotoxicity without being offset by second cancers is highly attractive. Other strategies to reduce anthracycline-related cardiotoxicity such as alternative formulations (including liposomal), although promising in studies of adults, have generally been inadequately studied in pediatric patients and are not clearly protective.1,4,26,27
In conclusion, we found no significant association between DRZ use and increased mortality, second cancer–related mortality (including AML/MDS), or relapse of the original cancer. Random assignment of patients to first-line treatment with DRZ strengthened the validity of our results. Studying the long-term effects of DRZ on both incident second cancers and cardiac health will be important in determining the overall cost-effectiveness of DRZ as a cardioprotectant. These are the primary goals of a new COG study, ALTE11C2 (Effects of Dexrazoxane Hydrochloride on Biomarkers Associated With Cardiomyopathy and Heart Failure After Cancer Treatment [HEART]), which will prospectively ascertain current cardiovascular health of individuals treated on P9404, P9425, and P9426, including echocardiographic changes.
Acknowledgment
We thank Lu Chen, Doojduen Villaluna, and Nancy Blythe for their assistance in assembling the data set.
Appendix
Table A1.
Currency of Patient Follow-Up Based on COG Data Among Those Not Known to Be Deceased as of December 31, 2011
| Interval Since Date Last Seen (years)* | P9404† (n = 430) No. (%) | P9425‡ (n = 200) No. (%) | P9426‡ (n = 246) No. (%) | Total (N = 876) No. (%) |
|---|---|---|---|---|
| < 2 | 206 (47.9) | 4 (2.0) | 16 (6.5) | 226 (25.8) |
| 2-2.9 | 45 (10.5) | 1 (0.5) | 0 | 46 (5.3) |
| 3-4.9 | 61 (14.2) | 100 (50.0) | 115 (46.7) | 276 (31.5) |
| 5-6.9 | 65 (15.1) | 53 (26.5) | 64 (26.0) | 182 (20.8) |
| ≥ 7 | 53 (12.3) | 42 (21.0) | 51 (20.7) | 146 (16.7) |
Abbreviation: COG, Children's Oncology Group.
In relation to December 31, 2011.
Study remains open for data collection.
Data collection ended June 30, 2008.
Table A2.
Characteristics of Patients Enrolled Onto COG Randomized Trials of DRZ, by Individual Trial
| Characteristic | P9404 |
P9425 |
P9426 |
|||
|---|---|---|---|---|---|---|
| Received DRZ (n = 273) No. (%) | Did Not Receive DRZ (n = 264) No. (%) | Received DRZ (n = 107) No. (%) | Did Not Receive DRZ (n = 109) No. (%) | Received DRZ (n = 127) No. (%) | Did Not Receive DRZ (n = 128) No. (%) | |
| Female sex | 69 (25.3) | 61 (23.1) | 37 (34.6) | 39 (35.8) | 53 (41.7) | 52 (40.6) |
| Race/ethnicity | ||||||
| White non-Hispanic | 184 (67.4) | 168 (63.6) | 66 (61.7) | 82 (75.2) | 72 (56.7) | 74 (57.8) |
| Black | 41 (15.0) | 55 (20.8) | 13 (12.1) | 15 (13.8) | 20 (15.7) | 18 (14.1) |
| Hispanic | 8 (2.9) | 8 (3.0) | 5 (4.7) | 1 (0.9) | 7 (5.5) | 7 (5.5) |
| Asian/Native American | 4 (1.5) | 2 (0.8) | 1 (0.9) | 0 | 1 (0.8) | 0 |
| Other | 3 (1.1) | 3 (1.1) | 2 (1.9) | 2 (1.8) | 3 (2.4) | 4 (3.1) |
| Unknown | 33 (12.1) | 28 (10.6) | 20 (18.7) | 9 (8.3) | 24 (18.9) | 25 (19.5) |
| Median age at treatment, years (range) | 9.3 (1.0-21.9) | 9.8 (1.3-21.0) | 14.8 (3.7-20.0) | 14.9 (5.6-20.8) | 14.2 (2.1-20.9) | 13.9 (3.7-19.7) |
| < 5 | 51 (18.7) | 54 (20.5) | 3 (2.8) | 0 | 7 (5.5) | 6 (4.7) |
| 5-9 | 98 (35.9) | 81 (30.7) | 8 (7.5) | 9 (8.3) | 20 (15.7) | 19 (14.8) |
| 10-14 | 67 (24.5) | 83 (31.4) | 45 (42.1) | 46 (42.2) | 50 (39.4) | 52 (40.6) |
| 15+ | 57 (20.9) | 46 (17.4) | 51 (47.7) | 54 (49.5) | 50 (39.4) | 51 (39.8) |
| Median age at last follow-up, years (range)* | 20.1 (3.4-36.7) | 20.7 (3.0-35.2) | 26.9 (9.2-34.2) | 27.8 (14.0-34.7) | 25.6 (13.8-35.6) | 25.3 (14.2-32.3) |
Abbreviations: COG, Children's Oncology Group; DRZ, dexrazoxane.
For US patients, death or December 31, 2011, whichever occurred first; for non-US patients, death, date last seen reported to COG, or December 31, 2011, whichever occurred first.
Footnotes
Supported by Grants No. U10 CA09543 and K07 CA151775 from the US National Institutes of Health, by St Baldrick's Foundation, and by the Leukemia and Lymphoma Society for the Children's Oncology Group study (Effects of Dexrazoxane Hydrochloride on Biomarkers Associated With Cardiomyopathy and Heart Failure After Cancer Treatment [HEART (ALTE11C2)]).
Presented in part at the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014.
The content of this research is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01790152.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Eric J. Chow
Administrative support: Eric J. Chow
Provision of study materials or patients: Barbara L. Asselin, Cindy L. Schwartz, Smita Bhatia, Louis S. Constine, Steven E. Lipshultz
Collection and assembly of data: Eric J. Chow, Barbara L. Asselin, Cindy L. Schwartz, Smita Bhatia, Saro H. Armenian
Data analysis and interpretation: Eric J. Chow, David R. Doody, Wendy M. Leisenring, Sanjeev Aggarwal, K. Scott Baker, Smita Bhatia, Louis S. Constine, David R. Freyer, Steven E. Lipshultz, Saro H. Armenian
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Late Mortality After Dexrazoxane Treatment: A Report From the Children's Oncology Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Eric J. Chow
No relationship to disclose
Barbara L. Asselin
Consulting or Advisory Role: Jazz Pharmaceuticals, Sigma Tau Pharmaceuticals
Speakers' Bureau: Jazz Pharmaceuticals
Cindy L. Schwartz
No relationship to disclose
David R. Doody
No relationship to disclose
Wendy M. Leisenring
Research Funding: Merck
Sanjeev Aggarwal
No relationship to disclose
K. Scott Baker
No relationship to disclose
Smita Bhatia
No relationship to disclose
Louis S. Constine
No relationship to disclose
David R. Freyer
No relationship to disclose
Steven E. Lipshultz
Consulting or Advisory Role: Clinigen Group
Research Funding: Roche Diagnostics, Pfizer
Travel, Accommodations, Expenses: Clinigen Group
Saro H. Armenian
No relationship to disclose
REFERENCES
- 1.Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions—A scientific statement from the American Heart Association. Circulation. 2013;128:1927–1995. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the Childhood Cancer Survivor Study. J Clin Oncol. 2014;32:1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dalen EC, Caron HN, Dickinson HO, et al. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2011;6:CD003917. doi: 10.1002/14651858.CD003917.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armenian SH, Gelehrter SK, Chow EJ. Strategies to prevent anthracycline-related congestive heart failure in survivors of childhood cancer. Cardiol Res Pract. 2012;2012:713294. doi: 10.1155/2012/713294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasinoff BB, Hellmann K, Herman EH, et al. Chemical, biological and clinical aspects of dexrazoxane and other bisdioxopiperazines. Curr Med Chem. 1998;5:1–28. [PubMed] [Google Scholar]
- 6.Wexler LH, Andrich MP, Venzon D, et al. Randomized trial of the cardioprotective agent ICRF-187 in pediatric sarcoma patients treated with doxorubicin. J Clin Oncol. 1996;14:362–372. doi: 10.1200/JCO.1996.14.2.362. [DOI] [PubMed] [Google Scholar]
- 7.Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145–153. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- 8.Lipshultz SE, Scully RE, Lipsitz SR, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: Long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010;11:950–961. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asselin BL, Devidas M, Wang C, et al. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: A randomized study by the Children's Oncology Group (POG 9404) Blood. 2011;118:874–883. doi: 10.1182/blood-2010-06-292615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: The results of P9425. Blood. 2009;114:2051–2059. doi: 10.1182/blood-2008-10-184143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tebbi CK, Mendenhall NP, London WB, et al. Response-dependent and reduced treatment in lower risk Hodgkin lymphoma in children and adolescents, results of P9426: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2012;59:1259–1265. doi: 10.1002/pbc.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asselin B, Devidas M, Zhou T, et al. Cardioprotection and safety of dexrazoxane (DRZ) in children treated for newly diagnosed T-cell acute lymphoblastic leukemia (T-ALL) or advanced stage lymphoblastic leukemia (T-LL) J Clin Oncol. 2012;30(suppl):607s. doi: 10.1200/JCO.2015.60.8851. abstr 9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol. 2007;25:493–500. doi: 10.1200/JCO.2005.02.3879. [DOI] [PubMed] [Google Scholar]
- 14.Hensley ML, Hagerty KL, Kewalramani T, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: Use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009;27:127–145. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- 15.European Medicines Agency. June 24, 2011, Press Release: European Medicines Agency recommends restricting the use of dexrazoxane-containing medicines. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2011/06/news_detail_001288.jsp&mid=WC0b01ac058004d5c1.
- 16.Walker DM, Fisher BT, Seif AE, et al. Dexrazoxane use in pediatric patients with acute lymphoblastic or myeloid leukemia from 1999 and 2009: Analysis of a national cohort of patients in the Pediatric Health Information Systems database. Pediatr Blood Cancer. 2013;60:616–620. doi: 10.1002/pbc.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics. Hyattsville, MD: 2013. National Death Index user's guide. http://www.cdc.gov/nchs/data/ndi/NDI_Users_Guide.pdf. [Google Scholar]
- 18.Rogot E, Sorlie P, Johnson NJ. Probabilistic methods in matching census samples to the National Death Index. J Chronic Dis. 1986;39:719–734. doi: 10.1016/0021-9681(86)90155-4. [DOI] [PubMed] [Google Scholar]
- 19.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data (ed 2) Hoboken, NJ: John Wiley and Sons; 2002. [Google Scholar]
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 21.Chow EJ, Chen Y, Kremer LC, et al. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol. 2015;33:394–402. doi: 10.1200/JCO.2014.56.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatia S. Therapy-related myelodysplasia and acute myeloid leukemia. Semin Oncol. 2013;40:666–675. doi: 10.1053/j.seminoncol.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vrooman LM, Neuberg DS, Stevenson KE, et al. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: A report from the Dana-Farber Cancer Institute ALL Consortium. Eur J Cancer. 2011;47:1373–1379. doi: 10.1016/j.ejca.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seif AE, Walker DM, Li Y, et al. Dexrazoxane exposure and risk of secondary acute myeloid leukemia in pediatric oncology patients. Pediatr Blood Cancer. 2015;62:704–709. doi: 10.1002/pbc.25043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Dalen EC, van der Pal HJ, Caron HN, et al. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database Syst Rev. 2009;4:CD005008. doi: 10.1002/14651858.CD005008.pub3. [DOI] [PubMed] [Google Scholar]
- 27.van Dalen EC, Michiels EM, Caron HN, et al. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev. 2010;5:CD005006. doi: 10.1002/14651858.CD005006.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]


