Abstract
Purpose
Low-grade serous carcinoma of the ovary (LGSOC) or peritoneum (LGSPC) is a rare subtype of ovarian or peritoneal cancer characterized by young age at diagnosis and relative resistance to chemotherapy. The purpose of this study is to report our updated experience with women diagnosed with LGSOC or LGSPC to assess the validity of our original observations.
Patients and Methods
Eligibility criteria for patients from our database were: stage I to IV LGSOC or LGSPC, original diagnosis before January 2012, and adequate clinical information. All patients were included in progression-free survival, overall survival, and multivariable Cox regression analyses. A subset analysis was performed among patients with stage II to IV low-grade serous carcinoma treated with primary surgery followed by platinum-based chemotherapy.
Results
We identified 350 eligible patients. Median progression-free survival was 28.1 months; median overall survival was 101.7 months. In the multivariable analysis, compared with women age ≤ 35 years, those diagnosed at age > 35 years had a 43% reduction in likelihood of dying (hazard ratio, 0.53; 95% CI, 0.37 to 0.74; P < .001). Having disease present at completion of primary therapy was associated with a 1.78 increased hazard of dying compared with being clinically disease free (P < .001). Similar trends were noted in the smaller patient cohort. In this cohort, women with LGSPC had a 41% decreased chance of dying (hazard ratio, 0.59; 95% CI, 0.36 to 0.98; P = .04) compared with those with LGSOC.
Conclusion
Women age < 35 years with low-grade serous carcinoma and those with persistent disease at completion of primary therapy have the worst outcomes. Patients with LGSPC seem to have a better prognosis than those with LGSOC.
INTRODUCTION
Ten years ago, our group first proposed the concept of a binary system for grading ovarian serous carcinoma.1 Since then, the clinical behavior of low-grade serous carcinoma of the ovary (LGSOC) or peritoneum (LGSPC) has been well characterized, and the two-tier grading system has become widely accepted.2–12 Concomitantly, our understanding of the molecular biology and genetics of low-grade serous carcinoma has greatly expanded.13–24
In 2006, we reported our clinical experience with women with newly diagnosed stage II to IV LGSOC.3 The salient features of this cohort included: relatively young age at diagnosis, relative resistance to chemotherapy, and prolonged overall survival (OS). In subsequent publications, we presented our experience with patients with LGSOC who had received neoadjuvant chemotherapy and those with LGSPC.5,8 In 2007, we established a longitudinal database to systematically capture demographic and clinical information on all women diagnosed with low-grade serous tumors seen at our institution. The purpose of this study is to present our experience with a larger cohort of women diagnosed with LGSOC or LGSPC to re-evaluate the validity of our original observations.
PATIENTS AND METHODS
An institutional review board–approved longitudinal database—the Low-Grade Serous Tumor Database—was established in 2007, and patients seen at MD Anderson Cancer Center with the following diagnoses are eligible for inclusion in the database: ovarian tumors of low malignant potential, primary peritoneal tumors of low malignant potential, LGSOC, and LGSPC. Data collection is both retrospective and prospective in nature. All patients included in the database have signed informed consent. A consent waiver for those patients who had not been seen for ≥ 1 year from the date of protocol activation was granted by the institutional review board. Eligibility criteria for patients included in this study were: stage I to IV LGSOC or LGSPC, original diagnosis before January 1, 2012, and adequate clinical information. Two distinct groups of patients were analyzed: first, all patients who met these eligibility criteria, and second, a subset of eligible patients who met the same eligibility criteria contained in our initial report (ie, stage II to IV low-grade serous carcinoma and treatment with primary surgery followed by platinum-based chemotherapy).3 The major difference in this report is that we included both LGSOC and LGSPC.
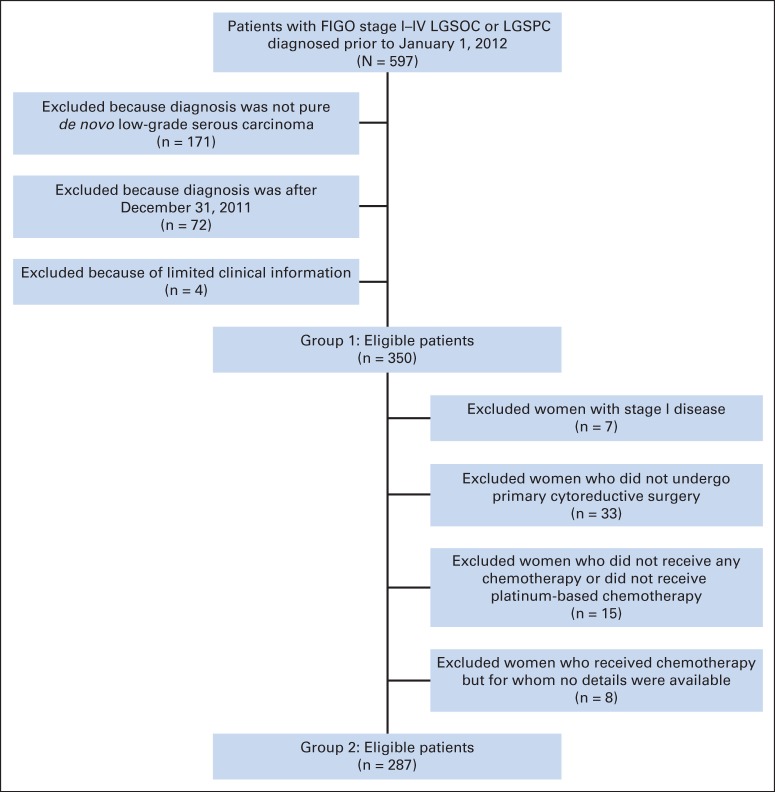
Review of the database identified 597 potentially eligible patients. We excluded 247 patients for the following reasons: diagnosis other than pure de novo low-grade serous carcinoma (n = 171), diagnosis after December 31, 2011 (n = 72), or limited clinical information (n = 4). For the more homogeneous subset analyses, patients were excluded if they: had stage I disease (n = 7), did not undergo primary cytoreductive surgery (n = 33), did not receive chemotherapy (n = 11) or received chemotherapy but no details were available (n = 8), or did not receive platinum-based chemotherapy (n = 4; Appendix Fig A1, online only).
Pathology slides of all patients were reviewed by MD Anderson gynecologic pathologists and documented as LGSOC or LGSPC. Excluded from this study were patient cases of nonserous histotypes, serous tumors of low malignant potential, low-grade serous carcinoma after a diagnosis of serous tumor of low malignant potential, and high-grade serous carcinoma. Criteria for diagnosis of low-grade serous carcinoma have been previously reported.1,8
Database elements included demographic information, gynecologic history, obstetric history, details of primary and secondary surgical procedures, International Federation of Gynecology and Obstetrics stage, perioperative studies, details of systemic therapies, cancer antigen 125 (CA-125) data, disease status at completion of primary treatment, date of initial disease progression, disease status at date of last contact, and date of last contact or death.
Statistical analyses were performed using SPSS software (version 21; SPSS, Chicago, IL). Progression-free survival (PFS) was calculated from the date of primary surgery (or date of tissue diagnosis) to date of disease progression or recurrence or date of last contact or death resulting from ovarian cancer. OS was calculated from the date of primary surgery (or date of tissue diagnosis) to date of last contact or death resulting from any cause. The cumulative distributions of OS and PFS durations were estimated using the Kaplan-Meier method.25 The log-rank test was used to compare differences between survival curves. Cox proportional hazards regression was used to model the association of important clinical variables and PFS and OS. Variables with P values < .25 on univariable analysis were included in the multivariable models. All P values were two sided. P values < .05 were considered statistically significant.
RESULTS
Study Population Analyses
A total study population of 350 patients was identified. Demographic and clinical characteristics are listed in Table 1. As noted, most patients were white, had LGSOC, had stage III disease, and received postoperative chemotherapy.
Table 1.
Characteristics of Patients With Low-Grade Serous Carcinoma of Ovary or Peritoneum (N = 350)
| Characteristic | No. | % |
|---|---|---|
| Age at diagnosis, years | ||
| Median | 46.5 | |
| Mean | 46.9 | |
| Range | 11.9 to 81.1 | |
| BMI at diagnosis, kg/m2a | ||
| Median | 26.9 | |
| Mean | 27.9 | |
| Range | 16.8 to 52.8 | |
| Pretreatment CA-125b | ||
| Median | 146.3 | |
| Mean | 401.1 | |
| Range | 6.0 to 10,000.0 | |
| Race | ||
| White | 294 | 84.0 |
| Black | 19 | 5.4 |
| Hispanic | 31 | 8.9 |
| Other | 6 | 1.7 |
| Smoking historyc | ||
| Never | 216 | 61.7 |
| Former or current | 133 | 38.0 |
| Parityd | ||
| Nulliparous | 94 | 26.9 |
| Parous | 254 | 726 |
| Site | ||
| Ovary | 275 | 78.6 |
| Peritoneum | 75 | 21.4 |
| Surgery | ||
| None | 5 | 1.4 |
| Primary cytoreductive | 317 | 90.6 |
| Interval cytoreductive | 28 | 8.0 |
| Residual disease at completion of primary cytoreductive surgerye | ||
| No gross residual | 53 | 16.7 |
| Gross residual | 199 | 62.8 |
| Residual disease at completion of interval cytoreductive surgeryf | ||
| No gross residual | 6 | 21.4 |
| Gross residual | 18 | 64.3 |
| FIGO stageg | ||
| I | 7 | 2.0 |
| II | 8 | 2.3 |
| III | 292 | 83.4 |
| IV | 29 | 8.3 |
| Primary treatmenth | ||
| Adjuvant chemotherapy | 305 | 87.4 |
| Neoadjuvant chemotherapy | 31 | 8.9 |
| No chemotherapy | 13 | 3.7 |
| Chemotherapy | ||
| Platinum | 324 | 98.5 |
| Nonplatinum | 5 | 1.5 |
| Disease status at completion of primary treatmenti | ||
| NED | 169 | 57.3 |
| Disease present | 126 | 42.7 |
Abbreviations: BMI, body mass index; CA-125, cancer antigen 125; FIGO, International Federation of Gynecology and Obstetrics; NED, no evidence of disease.
Not available for 126 patients.
Not available for 145 patients.
Not available for one patient.
Missing for two patients.
Not available for 65 patients.
Missing for four patients.
Not available for 14 patients.
Modality of chemotherapy was missing for one patient; 10 of 305 patients who received adjuvant chemotherapy also received concurrent hormonal therapy; one of 13 patients who did not undergo chemotherapy was treated with hormonal agent.
Not available for 55 patients.
Median follow-up time for the entire group was 72.4 months. Median PFS was 28.1 months (95% CI, 24.0 to 32.2), and median OS was 101.7 months (95% CI, 91.0 to 112.4). At the time of analysis, 55 women (15.7%) were alive and disease free, 103 (29.4%) were alive with disease, six (1.7%) were alive with unknown disease status, 177 (50.6%) were dead as a result of disease, and nine were dead as a result of other causes.
Of the 295 patients for whom clinical information was available at completion of primary treatment, 169 (57.3%) were clinically disease free based on the following examinations, tests, or record statements: abdominopelvic computed tomography, serum CA-125, or physical examination. A total of 242 patients received platinum and taxane–based chemotherapy, and 148 patients received maintenance therapy before first recurrence or progression; of this number, 62 patients received hormonal agents, 63 received chemotherapy, and 14 received hormonal therapy and chemotherapy.
Neither progression nor recurrence occurred in 52 women; they were censored. Women with LGSPC had significantly longer PFS and OS compared with women with LGSOC (PFS: 36.2 v 25.4 months; P = .02; and OS: 129.0 v 95.2 months; P = .01, respectively). Survival outcomes differed by age. Women diagnosed at age > 35 years had a longer median PFS compared with women diagnosed at age ≤ 35 years (32.6 months; 95% CI, 26.8 to 38.5; and 18.8 months; 95% CI, 14.2 to 23.5; P < .001, respectively). Detailed outcomes for PFS and OS of the 350 women are listed in in Appendix Table A1 (online only).
Univariable analysis for PFS showed that primary site, stage (ll v lll or lV), age group (≤ 35 v > 35 years), race (white v nonwhite), parity (nulliparous v parous), and clinical disease status at the end of primary therapy (no evidence of disease v disease present) met the criteria of P < .25 for inclusion in the multivariable analysis. Only age and disease status at the end of primary therapy remained significant in the multivariable setting. Compared with women age ≤ 35 years, those age > 35 years at diagnosis had a lower likelihood of progression or recurrence (hazard ratio [HR], 0.55; 95% CI, 0.38 to 0.79; P ≤ .001). Women with disease present at the end of primary therapy had a 1.79 greater chance of progression or recurrence compared with those without evidence of disease at the end of primary treatment (95% CI, 1.30 to 2.46; P < .001). The results of univariable and multivariable Cox proportional hazards regression for PFS for the overall study population of 350 patients are listed in Appendix Table A2 (online only).
Univariable analysis for OS showed that primary site, age group, maintenance therapy, race, and disease status at the end of primary therapy met the criteria of P < .25 for inclusion in the multivariable analysis. After adjusting for these variables in the multivariable analysis, only age group and disease status at the end of primary therapy remained statistically significant. Women who were older than age 35 years at diagnosis had just under half the risk of dying compared with women who were age 35 years or younger at diagnosis (HR, 0.53; 95% CI, 0.37 to 0.74; P < .001). Presence of disease at completion of primary therapy was associated with a 1.78 increased hazard of dying compared with no clinical evidence of disease at the end of primary treatment (95% CI, 1.30 to 2.45; P < .001). Results of univariable and multivariable Cox proportional hazards regression for OS for the overall study population of 350 patients are available in Appendix Table A3 (online only).
Subgroup Analysis
A subgroup analysis was conducted among 287 eligible patients who were diagnosed with stage II to IV LGOSC or LGPSC and treated with primary cytoreductive surgery followed by platinum-based chemotherapy. Table 2 lists clinical and demographic information for this group.
Table 2.
Characteristics of Patients With Low-Grade Serous Carcinoma of Ovary or Peritoneum Treated With Primary Cytoreductive Surgery Followed by Platinum-Based Chemotherapy (n = 287)
| Characteristic | No. | % |
|---|---|---|
| Age at diagnosis, years | ||
| Median | 46.1 | |
| Mean | 46.3 | |
| Range | 11.9 to 79.8 | |
| BMI at diagnosis, kg/m2* | ||
| Median | 26.9 | |
| Mean | 28.1 | |
| Range | 16.8 to 52.8 | |
| Pretreatment CA-125† | ||
| Median | 142.1 | |
| Mean | 432.8 | |
| Range | 6.0 to 10,000.0 | |
| No. of cycles of chemotherapy | ||
| Median | 6.0 | |
| Mean | 6.5 | |
| Range | 1.0 to 16.0 | |
| Race | ||
| White | 241 | 84.0 |
| Black | 16 | 5.6 |
| Hispanic | 25 | 8.7 |
| Other | 5 | 1.7 |
| Smoking history‡ | ||
| Never | 188 | 65.5 |
| Former or current | 98 | 34.1 |
| Parity§ | ||
| Nulliparous | 80 | 27.9 |
| Parous | 205 | 71.4 |
| Site | ||
| Ovary | 227 | 79.1 |
| Peritoneum | 60 | 20.9 |
| FIGO stage‖ | ||
| II | 8 | 2.8 |
| III | 251 | 87.5 |
| IV | 22 | 7.7 |
| Residual disease at completion of primary cytoreductive surgery¶ | ||
| No gross residual | 49 | 17.1 |
| Gross residual | 187 | 65.2 |
| Type of initial systemic therapy | ||
| Adjuvant chemotherapy | 278 | 96.9 |
| Adjuvant chemotherapy plus hormonal therapy | 9 | 3.1 |
| Clinical disease status on completion of primary therapy# | ||
| No evidence of disease | 152 | 53.0 |
| Disease present | 102 | 35.5 |
Abbreviations: BMI, body mass index; CA-125, cancer antigen 125; FIGO, International Federation of Gynecology and Obstetrics.
Not available for 112 patients.
Missing for 103 patients.
Not available for one patient.
Not available for two patients.
Missing for six patients.
Not available for 51 patients.
Not available for 33 patients.
Median follow-up time was 72.5 months. Median PFS was 25.3 months (95% CI, 21.6 to 29.0), and median OS was 97.8 months (95% CI, 88.6 to 107.1). At the time of analysis, 38 women (13.2%) were alive and disease free, 85 (29.6%) were alive with disease, three (1%) were alive with unknown disease status, 153 (53.3%) were dead as a result of disease, and eight (2.8%) were dead as a result of other causes.
Differences in PFS and OS are listed in Table 3. Neither progression nor recurrence occurred in 33 women; they were censored. Women with LGSPC had significantly longer PFS and OS compared with women with LGSOC (36.2 v 23.8 months; P = .007; 127.0 v 90.0 months; P = .007, respectively). Women diagnosed at age > 35 years had longer PFS than younger women (31.2 months; 95% CI, 25.0 to 37.4; and 17.8 months; 95% CI, 15.5 to 21.2; P < .001, respectively). Similarly, women in the older age group had longer OS than women diagnosed at age ≤ 35 years (102.9 months; 95% CI, 80.7 to 125.2; and 72.8 months; 95% CI, 51.2 to 94.4; P < .001, respectively).
Table 3.
Median PFS and OS of Patients With Low-Grade Serous Carcinoma of Ovary or Peritoneum Treated With Primary Cytoreductive Surgery Followed by Platinum-Based Chemotherapy (n = 287)
| Variable | No. of Patients | PFSa |
OS |
||||
|---|---|---|---|---|---|---|---|
| Months | 95% CI | P | Months | 95% CI | P | ||
| Age, years | < .001 | < .001 | |||||
| ≤ 35 | 78 | 17.8 | 15.5 to 20.2 | 72.8 | 51.2 to 94.4 | ||
| > 35 | 209 | 31.2 | 25.0 to 37.4 | 102.9 | 80.7 to 125.2 | ||
| Race | .28 | .37 | |||||
| White | 241 | 24.5 | 20.9 to 28.1 | 93.6 | 83.3 to 103.8 | ||
| Nonwhite | 46 | 31.2 | 13.7 to 48.7 | 127.0 | 85.3 to 168.7 | ||
| Parityb | .11 | .34 | |||||
| Nulliparous | 80 | 20.6 | 15.0 to 26.2 | 97.8 | 78.6 to 117.1 | ||
| Parous | 205 | 26.4 | 22.0 to 30.8 | 97.5 | 87.1 to 107.9 | ||
| Smoking historyc | .65 | .71 | |||||
| Never | 188 | 24.4 | 22.0 to 26.8 | 86.8 | 43.8 to 129.8 | ||
| Ever | 98 | 30.0 | 23.5 to 36.5 | 97.5 | 88.0 to 107.0 | ||
| BMI, kg/m2d | .86 | .88 | |||||
| Normal | 66 | 29.7 | 19.5 to 40.0 | 103.5 | 89.5 to 117.5 | ||
| Overweight | 52 | 30.0 | 14.3 to 45.7 | 102.8 | 64.1 to 141.5 | ||
| Obese | 57 | 34.4 | 25.3 to 43.4 | 134.4 | 84.3 to 184.5 | ||
| Pretreatment CA-125e | .01 | .52 | |||||
| < 35 | 28 | 41.0 | 21.1 to 60.9 | 134.4 | 52.9 to 216.0 | ||
| > 35 | 156 | 22.0 | 18.6 to 25.5 | 91.3 | 82.6 to 100.00 | ||
| FIGO stagef | .44 | .71 | |||||
| II | 8 | 22.5 | 0.00 to 57.1 | 86.8 | 43.8 to 129.8 | ||
| III to IV | 273 | 24.7 | 21.5 to 27.9 | 97.5 | 88.0 to 107.0 | ||
| Site | .007 | .01 | |||||
| Ovary | 241 | 23.8 | 20.7 to 26.8 | 90.0 | 76.8 to 130.1 | ||
| Peritoneal | 46 | 36.2 | 20.6 to 51.7 | 127.0 | 91.0 to 163.0 | ||
| Residual disease (primary CRS)g | .57 | .69 | |||||
| No gross residual | 49 | 23.4 | 16.9 to 30.0 | 97.8 | 72.2 to 123.5 | ||
| Gross residual | 187 | 23.9 | 19.7 to 28.1 | 93.1 | 82.2 to 104.0 | ||
| Chemotherapy | .25 | .35 | |||||
| Adjuvant chemotherapy | 278 | 25.2 | 21.6 to 28.8 | 97.5 | 87.8 to 107.2 | ||
| Chemotherapy (any) plus hormonal therapy | 9 | 32.2 | 9.4 to 55.0 | — | — | ||
| Maintenanceh | .46 | .03 | |||||
| None or missing information | 159 | 26.4 | 20.0 to 32.7 | 112.9 | 86.2 to 139.5 | ||
| Hormonal therapy | 50 | 25.9 | 13.4 to 38.3 | 100.6 | 80.9 to 120.4 | ||
| Chemotherapy | 57 | 23.8 | 21.5 to 26.1 | 79.5 | 71.2 to 87.9 | ||
| Chemotherapy (any) plus hormonal therapy | 12 | 19.7 | 0.0 to 42.9 | 87.5 | 30.8 to 144.2 | ||
| Disease status at completion of primary treatmenti | < .001 | < .001 | |||||
| NED | 152 | 33.4 | 27.1 to 39.7 | 112.9 | 88.1 to 137.6 | ||
| Disease present | 102 | 17.2 | 12.6 to 21.8 | 74.6 | 64.8 to 84.5 | ||
Abbreviations: BMI, body mass index; CA-125, cancer antigen 125; CRS, cytoreductive surgery; FIGO, International Federation of Gynecology and Obstetrics; NED, no evidence of disease; OS, overall survival; PFS, progression-free survival.
Eighteen patients were not included in PFS calculations, because exact dates of recurrence were not documented in medical record, or there was interval of time for which no clinical data were available to document patient was diagnosed with recurrent disease.
Missing for two patients.
Not available for one patient.
Missing for 112 patients.
Missing for 103 patients.
Not available for six patients.
Missing for 51 patients.
Considered miscellaneous in nine patients.
Not available for 33 patients.
Univariable and multivariable Cox proportional hazards regression results for PFS and OS are listed in Tables 4 and 5, respectively. Variables with P < .25 on univariable analysis for PFS included primary site of disease, chemotherapy (adjuvant v adjuvant plus hormones), disease status at completion of primary therapy, age group (≤ 35 v > 35 years), parity (nulliparous v parous), and CA-125 at diagnosis (≤ 35 v > 35 U/mL). After adjusting for these in the multivariable setting, only age, primary site, and disease status at end of primary therapy remained significantly predictive of progression or recurrence. Compared with women age ≤ 35 years, those age > 35 years had a much lower likelihood of progression (HR, 0.55; 95% CI, 0.38 to 0.81; P = .002). Women with LGSPC had a lower risk of disease progression compared with women with LGSOC (HR, 0.65; 95% CI, 0.43 to 1.00; P = .048). Having disease present at completion of primary therapy was associated with a 1.93-fold chance of disease progression compared with no clinical evidence of disease at the end of primary treatment. When age was included as a continuous variable, the results did not change appreciably (primary site: HR, 0.63; 95% CI, 0.41 to 0.96; P = .03; and disease status at completion of primary therapy: HR, 2.02; 95% CI, 1.45 to 2.83; P < .001).
Table 4.
Univariable and Multivariable Cox Proportional Hazards for PFS of Patients With Low-Grade Serous Carcinoma of Ovary or Peritoneum Treated With Primary Cytoreductive Surgery Followed by Platinum-Based Chemotherapy (n = 287)a
| Variable | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age, years | ||||||
| ≤ 35 (reference) | — | — | — | — | ||
| > 35 | 0.51 | 0.37 to 0.71 | < .001 | 0.55 | 0.38 to 0.81 | .002 |
| Race | ||||||
| White (reference) | — | — | ||||
| Nonwhite | 0.82 | 0.58 to 1.17 | .28 | |||
| Parityb | ||||||
| Nulliparous (reference) | — | — | ||||
| Parous | 0.79 | 0.60 to 1.06 | .11 | |||
| Smoking historyc | ||||||
| Never (reference) | — | — | ||||
| Ever | 0.97 | 0.72 to 1.23 | .65 | |||
| BMI, kg/m2d | .86 | |||||
| Normal (reference) | — | — | ||||
| Overweight | 1.00 | 0.66 to 1.54 | .99 | |||
| Obese | 0.91 | 0.61 to 1.36 | .63 | |||
| Pretreatment CA-125e | ||||||
| < 35 (reference) | — | — | ||||
| > 35 | 1.86 | 1.15 to 3.02 | .01 | |||
| FIGO stagef | ||||||
| II | — | — | ||||
| III to IV | 1.35 | 0.63 to 2.87 | .44 | |||
| Site | ||||||
| Ovary (reference) | — | — | — | — | ||
| Peritoneal | 0.63 | 0.45 to 0.88 | .007 | 0.65 | 0.43 to 1.00 | .048 |
| Residual disease (primary CRS)g | ||||||
| No gross residual | — | — | ||||
| Gross residual | 1.11 | 0.78 to 1.58 | .57 | |||
| Chemotherapy | ||||||
| Adjuvant chemotherapy | — | — | ||||
| Chemotherapy (any) plus hormonal therapy | 0.60 | 0.25 to 1.45 | .25 | |||
| Maintenance | .46 | |||||
| None or missing information | — | — | ||||
| Hormonal therapy | 0.92 | 0.64 to 1.32 | .66 | |||
| Chemotherapy | 1.25 | 0.91 to 1.73 | .17 | |||
| Chemotherapy plus hormonal therapy | 1.10 | 0.58 to 2.10 | .77 | |||
| Disease status at completion of primary treatmenth | ||||||
| NED (reference) | — | — | — | — | ||
| Disease present | 1.92 | 1.45 to 2.54 | < .001 | 1.93 | 1.38 to 2.71 | < .001 |
Abbreviations: BMI, body mass index; CA-125, cancer antigen 125; CRS, cytoreductive surgery; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; NED, no evidence of disease; PFS, progression-free survival.
Eighteen patients were not included in PFS calculations, because exact dates of recurrence were not documented in medical record, or there was interval of time for which no clinical data were available to document patient was diagnosed with recurrent disease.
Not available for two patients.
Missing for one patient.
Not available for 112 patients.
Missing for 103 patients.
Not available for six patients.
Missing for 51 patients.
Not available for 33 patients.
Table 5.
Univariable and Multivariable Cox Proportional Hazards for OS of Patients With Low-Grade Serous Carcinoma of Ovary or Peritoneum Treated With Primary Cytoreductive Surgery Followed by Platinum-Based Chemotherapy (n = 287)
| Variable | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age, years | ||||||
| ≤ 35 (reference) | — | — | — | — | ||
| > 35 | 0.51 | 0.37 to 0.71 | < .001 | 0.55 | 0.39 to 0.79 | .001 |
| Race | ||||||
| White (reference) | — | — | ||||
| Nonwhite | 0.83 | 0.54 to 1.26 | .37 | |||
| Parity* | ||||||
| Nulliparous (reference) | — | — | ||||
| Parous | 0.85 | 0.60 to 1.19 | .34 | |||
| Smoking history† | ||||||
| Never (reference) | — | — | ||||
| Ever | 0.89 | 0.64 to 1.24 | .48 | |||
| BMI, kg/m2‡ | .88 | |||||
| Normal (reference) | — | — | ||||
| Overweight | 1.07 | 0.61 to 1.87 | .82 | |||
| Obese | 0.91 | 0.53 to 1.59 | .75 | |||
| Pretreatment CA-125§ | ||||||
| < 35 (reference) | — | — | ||||
| > 35 | 1.21 | 0.68 to 2.17 | .52 | |||
| FIGO stage | ||||||
| II (reference) | — | — | ||||
| III to IV | 1.16 | 0.53 to 2.52 | .71 | |||
| Site | ||||||
| Ovary (reference) | — | — | — | — | ||
| Peritoneal | 0.55 | 0.34 to 0.88 | .01 | 0.59 | 0.36 to 0.98 | .04 |
| Residual disease (primary CRS)‖ | ||||||
| No gross residual (reference) | — | — | ||||
| Gross residual | 1.09 | 0.72 to 1.66 | .69 | |||
| Chemotherapy | ||||||
| Adjuvant chemotherapy (reference) | — | — | ||||
| Chemotherapy (any) plus hormonal therapy | 0.52 | 0.13 to 2.10 | .36 | |||
| Maintenance | .03 | |||||
| None or missing information (reference) | — | — | ||||
| Hormonal therapy | 1.05 | 0.64 to 1.73 | .86 | |||
| Chemotherapy | 1.72 | 1.19 to 2.48 | .004 | |||
| Chemotherapy plus hormonal therapy | 1.38 | 0.64 to 2.98 | .42 | |||
| Disease status at completion of primary treatment¶ | ||||||
| NED (reference) | — | — | — | — | ||
| Disease present | 2.02 | 1.44 to 2.85 | < .001 | 1.99 | 1.41 to 2.81 | < .001 |
Abbreviations: BMI, body mass index; CA-125, cancer antigen 125; CRS, cytoreductive surgery; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; NED, no evidence of disease; OS, overall survival.
Missing for two patients.
Not available for one patient.
Not available for 112 patients.
Not available for 103 patients.
Missing for 51 patients.
Not available for 33 patients.
Variables that resulted in P < .25 by univariable Cox proportional hazards regression for OS included primary site, age group, maintenance therapy, and clinical disease status on completion of chemotherapy. On multivariable analysis, maintenance therapy fell out of the model. The final model showed that a diagnosis of LGSPC conferred a decreased chance of dying (HR, 0.59; 95% CI, 0.36 to 0.98; P = .04), whereas presence of disease at completion of primary therapy was associated with an almost two-fold chance of dying compared with no clinical evidence of disease at completion of primary therapy (HR, 1.99; 95% CI, 1.41 to 2.81; P < .001). Women who were diagnosed at age > 35 years had a lower risk of dying compared with younger patients (HR, 0.55; 95% CI, 0.39 to 0.79; P < .001). Figure 1 shows the Kaplan-Meier survival curves by age group (log-rank P < .001). When age was included as a continuous variable, only primary site and clinical disease status at completion of primary therapy remained in the model (HR, 0.56; 95% CI, 0.34 to 0.92; P = .02; and HR, 2.05; 95% CI, 1.46 to 2.89; P < .001, respectively).
Fig 1.
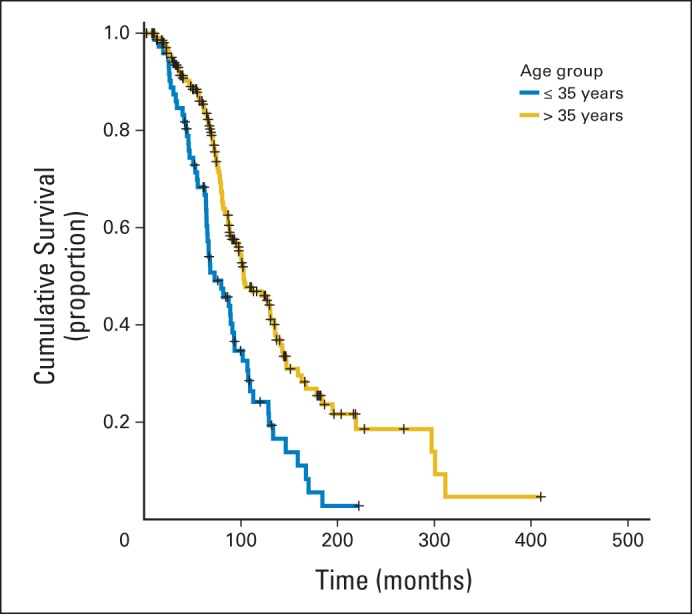
Overall survival by age group at diagnosis of 287 patients with stage II to IV low-grade serous carcinoma of ovary or peritoneum treated with primary cytoreductive surgery followed by platinum-based chemotherapy.
Because of the observation that women with LGSPC had better PFS and OS outcomes than those with LGSOC, we performed an exploratory analysis for the smaller, more homogeneous cohort (n = 287) focused on primary site of disease. There were statistically significant differences between patients with LGSOC and LGSPC for serum CA-125 level at time of diagnosis (173.5 v 54.5 U/mL; P = .007), body mass index (25.7 v 28.8 kg/m2; P = .002), and age (median, 44.3 v 48.0 years; P = .015). There were no significant differences in the proportion of patients with LGSOC and LGSPC for serum CA-125 at diagnosis above normal, disease-free status at completion of primary therapy, or age category. There was a statistically significant difference in the proportion of patients with LGSOC versus LGSPC with respect to no gross residual and gross residual disease. A higher percentage of patients with LGSPC had gross residual disease at the end of primary surgery compared with those with LGSOC (91.7% v 76.1%; P = .017). Similar trends were also observed for the entire cohort of 350 patients.
We grouped the entire cohort of 350 patients based on quartiles of OS times for which complete survival data were available: quartile one, 0 to 55.74; quartile two, 55.74 to 78.05; quartile three, 78.05 to 103.13; and quartile four, ≥ 108.13 months. We then compared patient characteristics of deceased patients in the lowest and highest quartiles. Significant differences between the two subgroups were observed for median OS (33.3 v 143.1 months; P < .001) and having no disease present at completion of primary therapy (37.2% v 81.1%; P < .001). Age differences between the two quartiles were also significant (P = .02). The proportion of patients age ≤ 35 years was significantly higher in the lowest OS quartile (68.8% v 31.2%; P = .018). In addition, when we reviewed the 15 patients in the highest quartile who were still alive and disease free, the only patient characteristics that seemed to reveal a trend were age at diagnosis and disease status at completion of primary therapy. All 15 patients were age ≥ 35 years, and 11 (85%) of 13 for whom disease status at completion of primary therapy was known were disease free.
DISCUSSION
The principal findings of this study confirm the results of our original publication, indicating that relative to patients with high-grade ovarian or peritoneal cancers, women with low-grade serous carcinoma are younger on average and have prolonged OS.3 In addition, we found that three factors seemed to have a significant influence on patient outcomes: disease status at completion of primary treatment, age, and primary site of disease. The findings related to these variables seemed to be consistent for both cohorts analyzed.
As in our original study, > 40% of women had persistent disease at completion of primary treatment, and this factor resulted in significantly shorter median PFS and median OS compared with those women who were clinically disease free at completion of primary treatment. In multivariable regression analyses for OS, HR regarding disease persistence was 1.77 for all 350 patients and 1.96 for the cohort of 287 patients. Because most ovarian cancer trials do not report disease status at completion of primary treatment, we cannot directly compare our study population with those in prospective clinical trials. However, it is our clinical impression that persistent disease rates > 40% may be higher than those observed in patients with metastatic ovarian cancer of all histologic subtypes. Such a high rate of disease persistence may reflect relative resistance to chemotherapy—an observation also supported by reports of chemotherapy in the neoadjuvant and recurrent settings.5,6 Although platinum-based chemotherapy remains the standard first-line treatment for these patients, the evidence strongly suggests that randomized clinical trials testing different agents in this setting are warranted.
In this larger long-term follow-up study, two new observations related to age at diagnosis and primary site of disease—ovary versus peritoneum—emerge. Patients age ≤ 35 years old, who comprised > 25% of the study population, had a significantly worse outcome for both PFS and OS compared with women age > 35 years. Interestingly, breast cancer studies have revealed a similar trend; younger patients with breast cancer have a worse prognosis compared with their older counterparts.26,27 In one study of women with invasive breast cancer identified from the British Columbia Cancer Agency Breast Cancer Outcomes Database, age < 40 years predicted inferior relapse-free survival and OS for luminal breast cancer (positive for estrogen and/or progesterone receptor and negative for human epidermal growth factor receptor 2).27 Cancello et al28 found that patients with luminal breast cancer age < 35 years had a significantly increased risk of recurrence and death when compared with older patients with similar characteristics. Freedholm et al26 found that among all age groups, the 5-year relative survival ratio was lowest in women age 20 to 34 years. The fact that a high percentage of metastatic low-grade serous carcinomas also have estrogen or progesterone receptor positivity indicates that there may be some relationship between hormonal receptor positivity and poor prognosis in young patients.10 In addition, it is possible that there may be some estrogenic effects in younger patients compared with older patients regardless of whether fertility-sparing surgery is performed. Such observations clearly warrant further study.
In addition, women with LGSPC had a statistically significantly superior median PFS and median OS compared with patients with LGSOC in both cohorts. This observation was unexpected, and whether it is real or simply chance remains to be elucidated. For ovarian and primary peritoneal cancers of all histologic subtypes, studies have reported conflicting survival comparisons, with some studies showing equivalent OS times and others indicating worse OS times for primary peritoneal cancer. Two recent, large population-based studies of serous histology revealed a significantly worse prognosis for primary peritoneal cancer compared with primary ovarian cancer.29,30
The limitations of an observational study such as this are well characterized and include incomplete data, a long study period, referral bias, inconsistent therapies, changing detection methods, and other confounding factors. Nevertheless, this report details information of a large cohort of women with a rare ovarian cancer and is hypothesis generating, potentially leading to advances that will make a difference for women with this condition.
In summary, our study not only strengthens the original observations of our group but also provides new insights into the influence of age at diagnosis, disease status at completion of primary treatment, and primary site of disease on patient outcomes. In particular, the finding that the youngest cohort seemed to have a significantly worse prognosis, in the context of a similar phenomenon in women with luminal breast cancer, raises the issue of hormonal influences and will, it is hoped, encourage additional research in this area.
Appendix
Table A1.
Median PFS and OS of Patients With Low-Grade Serous Carcinoma of Ovary or Peritoneum (N = 350)
| Variable | No. of Patients | PFSa |
OS |
||||
|---|---|---|---|---|---|---|---|
| Months | 95% CI | P | Months | 95% CI | P | ||
| Age, years | < .001 | < .001 | |||||
| ≤ 35 | 88 | 18.8 | 14.2 to 23.5 | 79.7 | 58.2 to 101.3 | ||
| > 35 | 262 | 32.6 | 26.8 to 38.5 | 111.3 | 91.4 to 131.2 | ||
| Race | .11 | .06 | |||||
| White | 294 | 26.2 | 22.4 to 29.9 | 98.8 | 88.6 to 108.8 | ||
| Nonwhite | 56 | 41.6 | 23.4 to 59.8 | 129.0 | 72.2 to 185.8 | ||
| Parityb | .03 | .28 | |||||
| Nulliparous | 94 | 21.9 | 16.3 to 27.5 | 101.5 | 82.6 to 120.4 | ||
| Parous | 254 | 30.6 | 24.9 to 36.3 | 101.7 | 89.5 to 113.9 | ||
| Smoking historyc | .36 | .69 | |||||
| Never | 216 | 25.3 | 21.3 to 29.3 | 100.6 | 89.5 to 111.8 | ||
| Ever | 133 | 32.2 | 25.5 to 38.8 | 107.0 | 72.8 to 141.2 | ||
| BMI, kg/m2d | .72 | .98 | |||||
| Normal | 82 | 31.6 | 21.7 to 41.5 | 107.0 | 74.6 to 139.5 | ||
| Overweight | 73 | 36.2 | 17.2 to 55.2 | 107.6 | 74.8 to 140.4 | ||
| Obese | 69 | 37.6 | 26.5 to 48.7 | 132.4 | 90.4 to 174.4 | ||
| Pretreatment CA-125e | .002 | .40 | |||||
| < 35 | 35 | 44.1 | 0.00 to 91.0 | 134.4 | 66.0 to 202.7 | ||
| > 35 | 187 | 23.5 | 20.7 to 26.3 | 95.2 | 84.7 to 105.6 | ||
| FIGO stagef | .02 | .48 | |||||
| I to II | 15 | 66.9 | 0.00 to 57.1 | 104.7 | 46.8 to 162.5 | ||
| III to IV | 321 | 25.9 | 21.5 to 27.9 | 101.5 | 90.7 to 112.3 | ||
| Site | .02 | .01 | |||||
| Ovary | 275 | 25.4 | 21.4 to 29.3 | 95.2 | 84.8 to 105.5 | ||
| Peritoneal | 75 | 36.2 | 21.7 to 50.7 | 129.0 | 98.4 to 159.6 | ||
| Surgery | .60 | .93 | |||||
| None | 5 | 30.5 | 0.00 to 78.1 | 132.4 | 35.9 to 228.9 | ||
| Primary cytoreductive | 317 | 26.4 | 22.4 to 30.4 | 101.5 | 90.5 to 112.4 | ||
| Interval cytoreductive | 28 | 34.6 | 11.9 to 57.3 | 106.5 | 74.5 to 138.5 | ||
| Residual disease (primary CRS)g | .44 | .77 | |||||
| No gross residual | 59 | 25.4 | 18.9 to 31.9 | 98.8 | 78.6 to 119.1 | ||
| Gross residual | 217 | 25.4 | 19.4 to 31.3 | 95.2 | 83.6 to 106.8 | ||
| Chemotherapyh | .70 | .20 | |||||
| Adjuvant | 305 | 26.4 | 22.2 to 30.5 | 100.8 | 89.9 to 111.7 | ||
| Neoadjuvant | 31 | 33.6 | 8.6 to 58.7 | 88.3 | 52.8 to 123.8 | ||
| None | 13 | 30.5 | 17.0 to 44.1 | 135.7 | 44.2 to 227.1 | ||
| Maintenancei | .26 | .03 | |||||
| None or missing information | 202 | 30.0 | 24.3 to 35.8 | 113.9 | 91.7 to 136.1 | ||
| Hormonal therapy | 62 | 34.6 | 16.8 to 52.4 | 132.4 | 75.3 to 189.4 | ||
| Chemotherapy | 63 | 24.4 | 22.4 to 26.4 | 79.7 | 69.4 to 90.1 | ||
| Chemotherapy plus hormonal therapy | 14 | 19.7 | 0.0 to 44.8 | 86.5 | 76.3 to 96.7 | ||
| Disease status at completion of primary treatmentj | < .001 | < .001 | |||||
| NED | 169 | 36.2 | 28.9 to 43.5 | 114.3 | 91.7 to 136.9 | ||
| Disease present | 126 | 18.7 | 12.7 to 24.7 | 80.9 | 67.3 to 94.4 | ||
Abbreviations: BMI, body mass index; CA-125, cancer antigen 125; CRS, cytoreductive surgery; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; NED, no evidence of disease; OS, overall survival; PFS, progression-free survival.
Twenty-seven patients were not included in PFS calculations because exact dates of recurrence were not documented in medical record, or there was interval of time for which no clinical data were available to document patient was diagnosed with recurrent disease.
Missing for two patients.
Not available for one patient.
Missing for 126 patients.
Missing for 128 patients.
Not available for 14 patients.
Missing for 74 patients.
Medical record of one patient who received chemotherapy did not include detailed information.
Considered miscellaneous in nine patients; these patients were excluded.
Not available for 55 patients.
Table A2.
Univariable and Multivariable Cox Proportional Hazards for PFS of Patients With Low-Grade Serous Carcinoma of Ovary or Peritoneum (N = 350)
| Variable | Univariable |
Multivariablea |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age, years | ||||||
| ≤ 35 | — | — | — | — | ||
| > 35 | 0.49 | 0.38 to 0.66 | < .001 | 0.55 | 0.38 to 0.79 | .001 |
| Race | ||||||
| White (reference) | — | — | ||||
| Nonwhite | 0.76 | 0.55 to 1.06 | .11 | |||
| Parityb | ||||||
| Nulliparous (reference) | — | — | ||||
| Parous | 0.74 | 0.57 to 0.97 | .03 | |||
| Smoking historyc | ||||||
| Never (reference) | — | — | ||||
| Ever | 0.89 | 0.70 to 1.14 | .36 | |||
| BMI, kg/m2d | .72 | |||||
| Normal (reference) | — | — | ||||
| Overweight | 0.94 | 0.64 to 1.37 | .73 | |||
| Obese | 0.86 | 0.58 to 1.25 | .42 | |||
| Pretreatment CA-125e | ||||||
| < 35 (reference) | — | — | — | — | ||
| > 35 | 2.07 | 1.31 to 3.29 | .002 | 1.46 | 0.91 to 2.34 | .11 |
| FIGO stage | ||||||
| II (reference) | — | — | ||||
| III to IV | 2.20 | 1.13 to 4.29 | .02 | |||
| Site | ||||||
| Ovary (reference) | — | — | ||||
| Peritoneal | 0.70 | 0.51 to 0.95 | .02 | |||
| Surgery | .60 | |||||
| None | — | — | ||||
| Primary cytoreductive | 0.75 | 0.28 to 2.00 | .56 | |||
| Interval cytoreductive | 0.61 | 0.21 to 1.82 | .38 | |||
| Residual diseasef | ||||||
| No gross residual (any; reference) | — | — | ||||
| Gross residual | 1.14 | 0.82 to 1.59 | .45 | |||
| Chemotherapy typeg | .48 | |||||
| Adjuvant (reference) | — | — | ||||
| Neoadjuvant | 0.85 | 0.54 to 1.34 | .48 | |||
| Chemotherapy (any) plus hormonal therapy | 0.66 | 0.29 to 1.48 | .31 | |||
| Maintenance | .27 | |||||
| None or missing information (reference) | — | — | ||||
| Hormonal treatment | 0.84 | 0.60 to 1.17 | .30 | |||
| Chemotherapy | 1.21 | 0.89 to 1.64 | .23 | |||
| Chemotherapy plus hormonal therapy | 1.27 | 0.71 to 2.29 | .43 | |||
| Disease status at completion of primary treatmenth | ||||||
| NED (reference) | — | — | — | — | ||
| Disease present | 1.78 | 1.38 to 2.31 | < .001 | 1.79 | 1.30 to 2.46 | < .001 |
Abbreviations: BMI, body mass index; CA-125, cancer antigen 125; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; NED, no evidence of disease; PFS, progression-free survival.
When age was analyzed as continuous variable, presence of disease at completion of primary therapy was associated with significantly increased risk of progression (HR, 1.84; 95% CI, 1.41 to 2.40; P < .001), as was stage III to IV disease (HR, 2.13; 95% CI, 1.09 to 4.17; P = .03). Having low-grade serous carcinoma of peritoneum conferred decreased risk of progression (HR, 0.66; 95% CI, 0.47 to 0.95; P = .02), as did having children (HR, 0.75; 95% CI, 0.56 to 0.99; P = .045).
Missing for two patients.
Missing for one patient.
Not available for 126 patients.
Missing for 128 patients.
Not available for 69 patients.
Missing for one patient.
Not available for 43 patients.
Table A3.
Univariable and Multivariable Cox Proportional Hazards for OS of Patients With Low-Grade Serous Carcinoma of Ovary or Peritoneum (N = 350)
| Variable | Univariable |
Multivariablea |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age, years | ||||||
| ≤ 35 (reference) | — | — | — | — | ||
| > 35 | 0.49 | 0.36 to 0.67 | < .001 | 0.53 | 0.37 to 0.74 | < .001 |
| Race | ||||||
| White (reference) | — | — | ||||
| Nonwhite | 0.67 | 0.44 to 1.01 | .06 | |||
| Parityb | ||||||
| Nulliparous (reference) | — | — | ||||
| Parous | 0.84 | 0.61 to 1.15 | .28 | |||
| Smoking historyc | ||||||
| Never (reference) | — | — | ||||
| Ever | 0.97 | 0.70 to 1.27 | .69 | |||
| BMI, kg/m2d | .98 | |||||
| Normal (reference) | — | — | ||||
| Overweight | 1.05 | 0.63 to 1.74 | .86 | |||
| Obese | 1.00 | 0.60 to 1.68 | .99 | |||
| Pretreatment CA-125e | ||||||
| < 35 (reference) | — | — | ||||
| > 35 | 1.27 | 0.73 to 2.23 | .40 | |||
| FIGO stage | ||||||
| II (reference) | — | — | ||||
| III to IV | 1.29 | 0.63 to 2.66 | .48 | |||
| Site | ||||||
| Ovary (reference) | — | — | — | — | ||
| Peritoneal | 0.59 | 0.39 to 0.89 | .01 | 0.65 | 0.41 to 1.01 | .06 |
| Surgery | .93 | |||||
| None | — | — | ||||
| Primary cytoreductive | 0.85 | 0.27 to 2.66 | .78 | |||
| Interval cytoreductive | 0.91 | 0.26 to 3.18 | .89 | |||
| Residual diseasef | ||||||
| No gross residual (any; reference) | — | — | ||||
| Gross residual | 1.06 | 0.72 to 1.56 | .77 | |||
| Chemotherapy typeg | .71 | |||||
| Adjuvant (reference) | — | — | ||||
| Neoadjuvant | 1.07 | 0.63 to 1.81 | .81 | |||
| Chemotherapy (any) plus hormonal therapy | 0.63 | 0.20 to 1.98 | .43 | |||
| Maintenance | .03 | |||||
| None or missing information (reference) | — | — | ||||
| Hormonal therapy | 1.06 | 0.68 to 1.66 | .80 | |||
| Chemotherapy | 1.63 | 1.15 to 2.32 | .006 | |||
| Chemotherapy plus hormonal therapy | 1.62 | 0.82 to 3.22 | .17 | |||
| Disease status at completion of primary treatmenth | ||||||
| NED (reference) | — | — | — | — | ||
| Disease present | 1.80 | 1.31 to 2.47 | < .001 | 1.78 | 1.30 to 2.45 | < .001 |
Abbreviations: BMI, body mass index; CA-125, cancer antigen 125; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; NED, no evidence of disease; OS, overall survival.
When age was analyzed as continuous variable, presence of disease at completion of primary therapy was associated with significantly increased risk of dying (HR, 1.83; 95% CI, 1.33 to 2.52; P < .001), whereas having primary peritoneal as primary site conferred decreased risk of dying (HR, 0.63; 95% CI, 0.41 to 0.99; P = .046).
Missing for two patients.
Missing for one patient.
Not available for 126 patients.
Missing for 128 patients.
Missing for 69 patients.
Not available for one patient.
Not available for 43 patients.
Fig A1.
Flowchart of study population. FIGO, International Federation of Gynecology and Obstetrics; LGSOC, low-grade serous carcinoma of ovary; LGSPC, low-grade serous carcinoma of peritoneum.
Footnotes
Supported in part by the Sara Brown Musselman Fund for Serous Ovarian Cancer Research and MD Anderson Cancer Center Support Grant No. NIH/NCI P30 CA016672 from the National Cancer Institute, National Institutes of Health.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: David M. Gershenson, Diane C. Bodurka, Karen H. Lu, Kwong K. Wong, Charlotte C. Sun
Financial support: Anais Malpica
Collection and assembly of data: David M. Gershenson, Diane C. Bodurka, Lisa C. Nathan, Ljiljana Milojevic, Charlotte C. Sun
Data analysis and interpretation: David M. Gershenson, Kwong K. Wong, Anais Malpica, Charlotte C. Sun
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of Age and Primary Disease Site on Outcome in Women With Low-Grade Serous Carcinoma of the Ovary or Peritoneum: Results of a Large Single-Institution Registry of a Rare Tumor
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
David M. Gershenson
Stock or Other Ownership: Johnson & Johnson, Procter & Gamble, Biogen Idec
Patents, Royalties, Other Intellectual Property: Elsevier, Up To Date
Diane C. Bodurka
No relationship to disclose
Karen H. Lu
No relationship to disclose
Lisa C. Nathan
No relationship to disclose
Ljiljana Milojevic
No relationship to disclose
Kwong K. Wong
Research Funding: Amgen
Anais Malpica
No relationship to disclose
Charlotte C. Sun
Employment: Inform Genomics (I)
Leadership: Inform Genomics (I)
Stock or Other Ownership: Inform Genomics
REFERENCES
- 1.Malpica A, Deavers MT, Lu K, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Malpica A, Deavers MT, Tornos C, et al. Interobserver and intraobserver variability of a two-tier system for grading ovarian serous carcinoma. Am J Surg Pathol. 2007;31:1168–1174. doi: 10.1097/PAS.0b013e31803199b0. [DOI] [PubMed] [Google Scholar]
- 3.Gershenson DM, Sun CC, Lu KH, et al. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol. 2006;108:361–368. doi: 10.1097/01.AOG.0000227787.24587.d1. [DOI] [PubMed] [Google Scholar]
- 4.Seidman JD, Horkayne-Szakaly I, Cosin JA, et al. Testing of two binary grading systems for FIGO stage III serous carcinoma of the ovary and peritoneum. Gynecol Oncol. 2006;103:703–708. doi: 10.1016/j.ygyno.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Schmeler KM, Sun CC, Bodurka DC, et al. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 2008;108:510–514. doi: 10.1016/j.ygyno.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Gershenson DM, Sun CC, Bodurka D, et al. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol. 2009;114:48–52. doi: 10.1016/j.ygyno.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Vang R, Shih IeM, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma. Adv Anat Pathol. 2009;16:267–282. doi: 10.1097/PAP.0b013e3181b4fffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmeler KM, Sun CC, Malpica A, et al. Low-grade serous primary peritoneal carcinoma. Gynecol Oncol. 2011;121:482–486. doi: 10.1016/j.ygyno.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Schlumbrecht MP, Sun CC, Wong KK, et al. Clinicodemographic factors influencing outcomes in patients with low-grade serous ovarian carcinoma. Cancer. 2011;117:3741–3749. doi: 10.1002/cncr.25929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gershenson DM, Sun CC, Iyer RB, et al. Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 2011;125:661–666. doi: 10.1016/j.ygyno.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodurka DC, Deavers MT, Tian C, et al. Reclassification of serous ovarian carcinoma by a 2-tier system: A Gynecologic Oncology Group study. Cancer. 2012;118:3087–3094. doi: 10.1002/cncr.26618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali RH, Kalloger SE, Santos JL, et al. Stage II to IV low-grade serous carcinoma of the ovary is associated with a poor prognosis. Int J Gynecol Pathol. 2013;32:529–535. doi: 10.1097/PGP.0b013e31827630eb. [DOI] [PubMed] [Google Scholar]
- 13.Singer G, Kurman RJ, Chang HW, et al. Diverse tumorigenic pathways in ovarian serous carcinoma. Am J Pathol. 2002;160:1223–1228. doi: 10.1016/s0002-9440(10)62549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer G, Oldt R, 3rd, Cohen Y, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–486. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 15.Shih IeM, Kurman RJ. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonome T, Lee JY, Park DC, et al. Expression profiling of serous low malignant potential, low-grade, and high-grade tumors of the ovary. Cancer Res. 2005;65:10602–10612. doi: 10.1158/0008-5472.CAN-05-2240. [DOI] [PubMed] [Google Scholar]
- 17.O'Neill CJ, Deavers MT, Malpica A, et al. An immunohistochemical comparison between low-grade and high-grade ovarian serous carcinomas: Significantly higher expression of p53, MIB1, BCL2, HER-2/neu, and C-KIT in high-grade neoplasms. Am J Surg Pathol. 2005;29:1034–1041. [PubMed] [Google Scholar]
- 18.Sieh W, Köbel M, Longacre TA, et al. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013;14:853–862. doi: 10.1016/S1470-2045(13)70253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo KT, Guan B, Feng Y, et al. Analysis of DNA copy number alterations in ovarian serous tumors identifies new molecular genetic changes in low-grade and high-grade carcinomas. Cancer Res. 2009;69:4036–4042. doi: 10.1158/0008-5472.CAN-08-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong KK, Tsang YTM, Deavers MT, et al. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol. 2010;177:1611–1617. doi: 10.2353/ajpath.2010.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones S, Wang T-L, Kurman RJ, et al. Low-grade serous carcinomas of the ovary contain very few point mutations. J Pathol. 2011;226:413–420. doi: 10.1002/path.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King ER, Zu Z, Tsang YT, et al. The insulin-like growth factor 1 pathway is a potential therapeutic target for low-grade serous ovarian carcinoma. Gynecol Oncol. 2011;123:13–18. doi: 10.1016/j.ygyno.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grisham RN, Iyer G, Garg K, et al. BRAF Mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer. 2012;119:548–554. doi: 10.1002/cncr.27782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsang YT, Deavers MT, Sun CC, et al. KRAS (but not BRAF) mutations in ovarian serous borderline tumour are associated with recurrent low-grade serous carcinoma. J Pathol. 2013;231:449–456. doi: 10.1002/path.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 26.Fredholm H, Eaker S, Frisell J, et al. Breast cancer in young women: Poor survival despite intensive treatment. PLoS One. 2009;4:e7695. doi: 10.1371/journal.pone.0007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson S, Scott T, Speers C, et al. Breast cancer in young women: Have the prognostic implications of breast cancer subtypes changed over time? Breast Cancer Res Treat. 2014;147:617–629. doi: 10.1007/s10549-014-3125-1. [DOI] [PubMed] [Google Scholar]
- 28.Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (<35 years) with operable breast cancer. Ann Oncol. 2010;21:1974–1981. doi: 10.1093/annonc/mdq072. [DOI] [PubMed] [Google Scholar]
- 29.Usach I, Blansit K, Chen LM, et al. Survival differences in women with serous tubal, ovarian, peritoneal, and uterine carcinomas. Am J Obstet Gynecol. 2015;212:188.e1–188.e6. doi: 10.1016/j.ajog.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Schnack TH, Sørensen RD, Nedergaard L, et al. Demographic clinical and prognostic characteristics of primary ovarian, peritoneal and tubal adenocarcinomas of serous histology: A prospective comparative study. Gynecol Oncol. 2014;135:278–284. doi: 10.1016/j.ygyno.2014.08.020. [DOI] [PubMed] [Google Scholar]